Characterizing Dietary Advanced Glycation End-Product (dAGE) Exposure and the Relationship to Colorectal Adenoma Recurrence: A Secondary Analysis
Abstract
1. Introduction
2. Materials and Methods
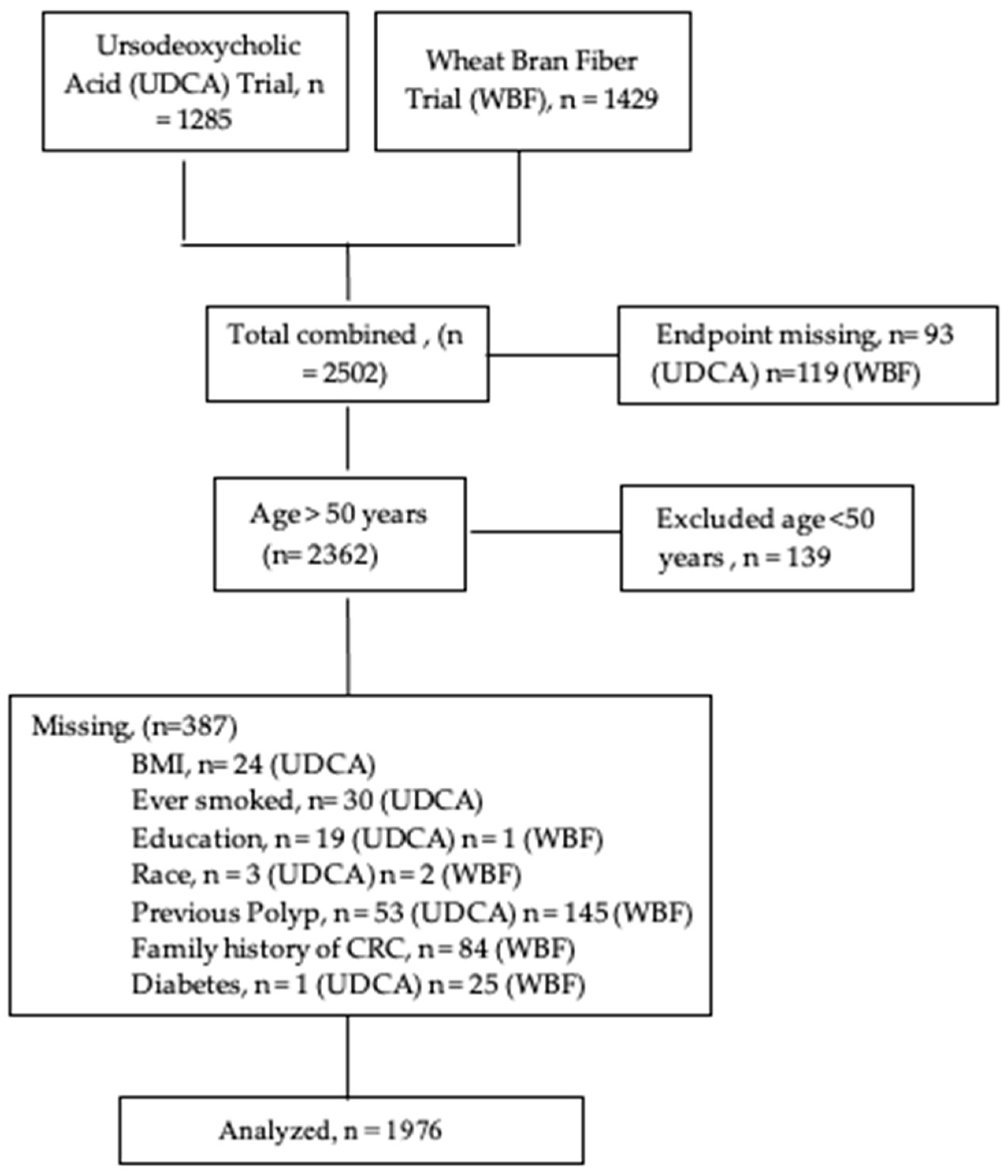
2.1. Study Design & Parent Studies
2.2. Study Sample
2.3. Data Collection
2.4. Data Availability Statement
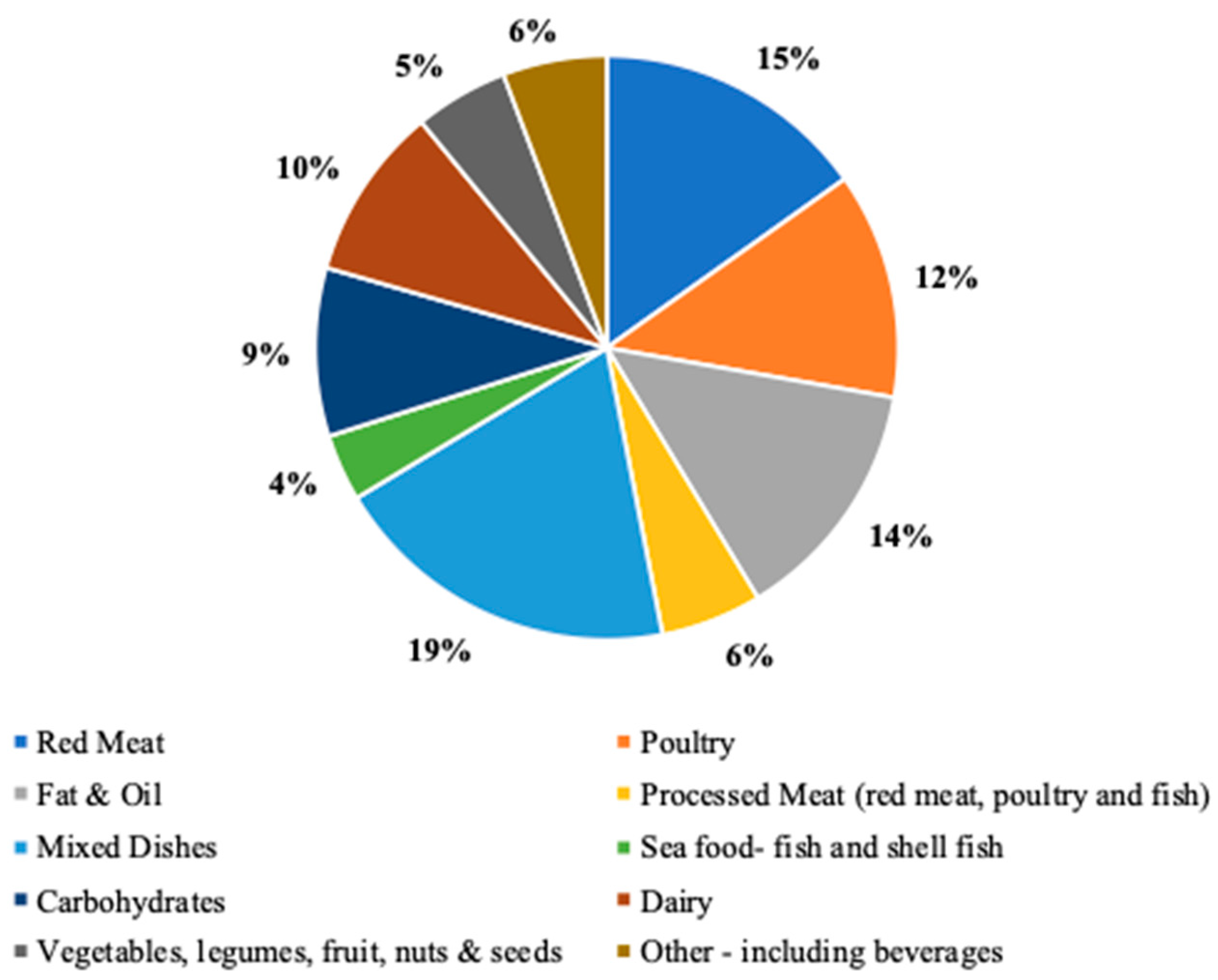
2.5. Dietary AGE intake Assessment
2.6. Outcome Variable: Adenoma Recurrence
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colorectal Cancer Facts & Figures 2020–2022. Available online: https://www.cancer.org/research/cancer-facts-statistics/colorectal-cancer-facts-figures.html (accessed on 13 March 2022).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Waluga, M.; Zorniak, M.; Fichna, J.; Kukla, M.; Hartleb, M. Pharmacological and Dietary Factors in Prevention of Colorectal Cancer. J. Physiol. Pharmacol. 2018, 69, 3. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef]
- Miller, P.E.; Lesko, S.M.; Muscat, J.E.; Lazarus, P.; Hartman, T.J. Dietary Patterns and Colorectal Adenoma and Cancer Risk: A Review of the Epidemiological Evidence. Nutr. Cancer 2010, 62, 413–424. [Google Scholar] [CrossRef]
- Øines, M.; Helsingen, L.M.; Bretthauer, M.; Emilsson, L. Epidemiology and Risk Factors of Colorectal Polyps. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 419–424. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Tantamango, Y.M.; Knutsen, S.F.; Beeson, W.L.; Fraser, G.; Sabate, J. Foods and Food Groups Associated With the Incidence of Colorectal Polyps: The Adventist Health Study. Nutr. Cancer 2011, 63, 565–572. [Google Scholar] [CrossRef]
- Modifiable Risk Factors for Colon Cancer—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0889855302000572?via%3Dihub (accessed on 19 February 2022).
- Giovannucci, E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C. Physical Activity, Obesity, and Risk of Colorectal Adenoma in Women (United States). Cancer Causes Control 1996, 7, 253–263. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; Gapstur, S.; Patel, A.V.; Andrews, K.; Gansler, T. American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on Nutrition and Physical Activity for Cancer Prevention: Reducing the Risk of Cancer with Healthy Food Choices and Physical Activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Diet and Cancer Report. WCRF International. Available online: https://www.wcrf.org/diet-and-cancer/ (accessed on 19 February 2022).
- Gut Microbiota Imbalance and Colorectal Cancer. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4716055/ (accessed on 19 February 2022).
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef]
- Bultman, S.J. Interplay between Diet, Gut Microbiota, Epigenetic Events, and Colorectal Cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef] [PubMed]
- Mechanistic Evidence for Red Meat and Processed Meat Intake and Cancer Risk: A Follow-Up on the International Agency for Research on Cancer Evaluation of 2015. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6294997/ (accessed on 19 February 2022).
- The Association of Diet, Gut Microbiota and Colorectal Cancer: What We Eat May Imply What We Get. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5960467/ (accessed on 19 February 2022).
- The Intestinal Microbiota and Colorectal Cancer. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7734048/ (accessed on 19 February 2022).
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Li, Y.; Qian, H.; Ying, H.; Wang, L. Advanced Glycation End Products in Food and Their Effects on Intestinal Tract. Crit. Rev. Food Sci. Nutr. 2020, 2020, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Geicu, O.I.; Stanca, L.; Voicu, S.N.; Dinischiotu, A.; Bilteanu, L.; Serban, A.I.; Calu, V. Dietary AGEs Involvement in Colonic Inflammation and Cancer: Insights from an in Vitro Enterocyte Model. Sci. Rep. 2020, 10, 2754. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T. Effect of Diet-Derived Advanced Glycation End Products on Inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Azizian-Farsani, F.; Abedpoor, N.; Hasan Sheikhha, M.; Gure, A.O.; Nasr-Esfahani, M.H.; Ghaedi, K. Receptor for Advanced Glycation End Products Acts as a Fuel to Colorectal Cancer Development. Front. Oncol. 2020, 10, 552283. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Omofuma, O.O.; Turner, D.P.; Peterson, L.L.; Merchant, A.T.; Zhang, J.; Steck, S.E. Dietary Advanced Glycation End-Products (AGE) and Risk of Breast Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO). Cancer Prev. Res. 2020, 13, 601–610. [Google Scholar] [CrossRef]
- Aglago, E.K.; Mayén, A.-L.; Knaze, V.; Freisling, H.; Fedirko, V.; Hughes, D.J.; Jiao, L.; Eriksen, A.K.; Tjønneland, A.; Boutron-Ruault, M.-C.; et al. Dietary Advanced Glycation End-Products and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Nutrients 2021, 13, 3132. [Google Scholar] [CrossRef]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary Consumption of Advanced Glycation End Products and Pancreatic Cancer in the Prospective NIH-AARP Diet and Health Study12345. Am. J. Clin. Nutr. 2015, 101, 126–134. [Google Scholar] [CrossRef]
- Jacobs, E.T.; Giuliano, A.R.; Roe, D.J.; Guillen-Rodrıguez, J.M.; Hess, L.M.; Alberts, D.S.; Martınez, M.E. Intake of Supplemental and Total Fiber and Risk of Colorectal Adenoma Recurrence in the Wheat Bran Fiber Trial. Cancer Epidemiol. Biomark. Prev. 2002, 11, 906–914. [Google Scholar]
- Alberts, D.S.; Martínez, M.E.; Hess, L.M.; Einspahr, J.G.; Green, S.B.; Bhattacharyya, A.K.; Guillen, J.; Krutzsch, M.; Batta, A.K.; Salen, G.; et al. Phase III Trial of Ursodeoxycholic Acid To Prevent Colorectal Adenoma Recurrence. JNCI J. Natl. Cancer Inst. 2005, 97, 846–853. [Google Scholar] [CrossRef]
- Martínez, M.E.; Reid, M.E.; Guillén-Rodríguez, J.; Marshall, J.R.; Sampliner, R.; Aickin, M.; Ritenbaugh, C.; Leeuwen, B.v.; Mason-Liddil, N.; Giuliano, A.; et al. Design and Baseline Characteristics of Study Participants in the Wheat Bran Fiber Trial. Cancer Epidemiol. Prev. Biomark. 1998, 7, 813–816. [Google Scholar]
- Martínez, M.E.; Marshall, J.R.; Graver, E.; Whitacre, R.C.; Woolf, K.; Ritenbaugh, C.; Alberts, D.S. Reliability and Validity of a Self-Administered Food Frequency Questionnaire in a Chemoprevention Trial of Adenoma Recurrence. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 941–946. [Google Scholar]
- Staten, L.K.; Taren, D.L.; Howell, W.H.; Tobar, M.; Poehlman, E.T.; Hill, A.; Reid, P.M.; Ritenbaugh, C. Validation of the Arizona Activity Frequency Questionnaire Using Doubly Labeled Water. Med. Sci. Sports Exerc. 2001, 33, 1959–1967. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced Glycoxidation End Products in Commonly Consumed Foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary Advanced Glycation End Products and Their Role in Health and Disease12. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- NDSR Software. NCC: Nutrition Coordinating Center. Available online: http://www.ncc.umn.edu/products/ (accessed on 19 February 2022).
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137, Erratum in Am. J. Epidemiol. 1989, 130, 1066. [Google Scholar] [CrossRef] [PubMed]
- Omofuma, O.O.; Peterson, L.L.; Turner, D.P.; Merchant, A.T.; Zhang, J.; Thomson, C.A.; Neuhouser, M.L.; Snetselaar, L.G.; Caan, B.J.; Shadyab, A.H.; et al. Dietary Advanced Glycation End-Products and Mortality after Breast Cancer in the Women’s Health Initiative. Cancer Epidemiol. Prev. Biomark. 2021, 30, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Córdova, R.; Mayén, A.; Knaze, V.; Aglago, E.K.; Schalkwijk, C.; Wagner, K.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Katzke, V.A.; et al. Dietary Intake of Advanced Glycation Endproducts (AGEs) and Cancer Risk across More than 20 Anatomical Sites: A Multinational Cohort Study. Cancer Commun. 2022, 42, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Barnich, N.; Nguyen, H. Microbiota, Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1310. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced Glycation Endproducts in Food and Their Effects on Health. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Collotta, D.; Gaudioso, G.; Le Berre, M.; Cento, A.S.; Ferreira Alves, G.; Chiazza, F.; Verta, R.; Bertocchi, I.; Manig, F.; et al. Effects of Exogenous Dietary Advanced Glycation End Products on the Cross-Talk Mechanisms Linking Microbiota to Metabolic Inflammation. Nutrients 2020, 12, 2497. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Chen, L.; Alsarraj, A.; Ramsey, D.; Duan, Z.; El-Serag, H.B. Plasma Soluble Receptor for Advanced Glycation End-Products and Risk of Colorectal Adenoma. Int. J. Mol. Epidemiol. Genet. 2012, 3, 294–304. [Google Scholar]
- Uribarri, J.; Cai, W.; Peppa, M.; Goodman, S.; Ferrucci, L.; Striker, G.; Vlassara, H. Circulating Glycotoxins and Dietary Advanced Glycation Endproducts: Two Links to Inflammatory Response, Oxidative Stress, and Aging. J. Gerontol. Biol. Sci. Med. Sci. 2007, 62, 427–433. [Google Scholar] [CrossRef]

| Quartile 1 n = 494 | Quartile 2 n = 494 | Quartile 3 n = 494 | Quartile 4 n = 494 | |
|---|---|---|---|---|
| Average CML-AGE intake (kU/1000 kcal) | <4126 | 4126–5470 | 5471–6475 | >6475 |
| Age (yr) | 68.9 ± 7.0 | 67.8 ± 7.2 | 66.8 ± 7.2 | 65.2 ± 7.6 |
| Male | 223 (45) | 306 (629) | 381 (77) | 435 (88) |
| Female | 271 (559) | 188 (38) | 113 (23) | 59 (12) |
| Marital Status, n (%) | ||||
| Single | 14 (3) | 6 (1) | 10 (2) | 9 (2) |
| Married/Cohabitating | 375 (76) | 413 (84) | 424 (86) | 446 (90) |
| Widow/Widower | 73 (15) | 43 (9) | 32 (7) | 21 (4) |
| Divorced/Separated | 31 (6) | 32 (6) | 27 (5) | 18 (4) |
| Race & Ethnicity, n (%) | ||||
| White | 468 (25) | 480 (26) | 477 (25) | 461 (24) |
| Black | 6 (0.7) | 1 (0.2) | 3 (0.2) | 2 (0.2) |
| Hispanic | 5 (0.6) | 5 (0.5) | 7 (0.8) | 23 (2.5) |
| American Indian/Alaskan | 4 (0.6) | 1 (0.1) | 0 (0) | 3 (0.2) |
| Asian | 7 (0.6) | 3 (0.3) | 2 (0.3) | 2 (0.2) |
| Other | 4 (0.5) | 4 (0.3) | 5 (0.5) | 3 (0.3) |
| Physical Activity (MET- Hours/week) | 18.22 ± 19.1 | 19.72 ± 42.9 | 17.84 ± 34.8 | 17.28 ± 18.26 |
| Educational Status, n (%) | ||||
| Elementary/Primary school completed | 5 (1) | 14 (3) | 8 (2) | 19 (4) |
| Some or all high school | 214 (43) | 202 (41) | 175 (35) | 168 (34) |
| At least one year of college | 275 (56) | 278 (56) | 311 (63) | 307 (62) |
| Family history of CRC a | 107 (22) | 104 (21) | 100 (20) | 112 (23) |
| Previous polyps b | 231 (47) | 216 (44) | 222 (45) | 223 (45) |
| Lifestyle Characteristics | ||||
| Current smoker | 58 (12) | 51 (10) | 63 (13) | 71(14) |
| Ever smoked | 308 (62) | 328 (66) | 344 (70) | 358 (72) |
| Aspirin c | 144 (29) | 144 (29) | 168 (34) | 132 (27) |
| BMI category, n (%) | ||||
| Underweight < 18.5 (kg/m2) | 5 (1) | 2 (1) | 4 (1) | 2 (1) |
| Normal 18.5–24.9 (kg/m2) | 173 (35) | 149 (30) | 130 (26) | 102 (21) |
| Overweight 25.0–29.9 (kg/m2) | 213 (43) | 224 (45) | 230 (47) | 203 (41) |
| Obese > 30.0 (kg/m2) | 103 (21) | 119 (24) | 130 (26) | 187 (38) |
| Health History | ||||
| Diabetes, n (%) | 42 (9) | 33 (7) | 51 (10) | 55 (11) |
| Dietary Intake mean ± SD | ||||
| Average CML-AGE intake (kU/100g) | 4960 ± 1170 | 7902 ± 749 | 10782 ± 948 | 17032 ± 4898 |
| Energy, kcal/day | 1287 ± 411 | 1700 ± 448 | 2073 ± 491 | 2729 ± 770.4 |
| Carbohydrate intake (g/day) | 197.2 ± 78.3 | 243.8 ± 88.4 | 282.3 ± 93.6 | 350.2 ± 127 |
| Protein intake (g/day) | 47.1 ± 14.8 | 62.44 ± 15.3 | 76.54 ± 16.7 | 103 ± 28.87 |
| Total fat intake (g/day) | 35.4 ± 11.6 | 52.64 ± 12.6 | 69.54 ± 15.2 | 101.5 ± 31.32 |
| Saturated (g/day) | 11.2 ± 4.26 | 16.83 ± 4.89 | 22.71 ± 6.31 | 34.06 ± 12.45 |
| Poly-unsaturated fat (g/day) | 7.82 ± 3.02 | 11.38 ± 3.27 | 14.57 ± 3.95 | 20.57 ± 6.84 |
| Mono-unsaturated (g/day) | 13.4 ± 4.5 | 20.13 ± 4.98 | 26.65 ± 6.14 | 38.78 ± 12.27 |
| Fruit intake (cups /day) | 2.04 ± 1.41 | 2.19 ± 1.67 | 2.184 ± 1.5 | 2.329 ± 1.594 |
| Vegetable intake (cups/day) | 1.45 ± 0.88 | 1.759 ± 0.85 | 2.098 ± 0.98 | 2.68 ± 1.295 |
| Total meat intake (oz./day) | 1.82 ± 0.75 | 2.74 ± 0.91 | 3.668 ± 1.2 | 5.296 ± 2.223 |
| Red meat (g/day) | 24.3 ± 13.6 | 41.01 ± 19.2 | 57.75 ± 26.2 | 92.57 ± 48.11 |
| Processed meat (g/day) | 5.23 ± 6.44 | 9.61 ± 9.51 | 15.26 ± 14.9 | 23.84 ± 25.18 |
| Total fiber intake (g/day) | 17.3 ± 8.52 | 20.46 ± 9.18 | 23.15 ± 9.1 | 27.95 ± 11.1 |
| Alcohol (g/day) | 5.1 ± 9.41 | 7.441 ± 14.5 | 9.751 ± 19 | 9.742 ± 14.81 |
| Added Sugar (tsp/day) | 7.93 ± 5.37 | 10.94 ± 6.88 | 13.32 ± 8.44 | 17.41 ± 11.74 |
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||
|---|---|---|---|---|---|---|---|
| Adenoma recurrence cases, n | 236 | 226 | 232 | 246 | |||
| Average CML-AGE intake (kU/1000 kcal) | <4126 | 4126–5470 | 5471–6475 | >6475 | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Unadjusted | Ref | 0.92 (0.72, 1.18) | 0.52 | 0.97 (0.75, 1.24) | 0.35 | 1.08 (0.84, 1.39) | 0.49 |
| Adjusted a | Ref | 0.89 (0.69, 1.14) | 0.80 | 0.90 (0.69, 1.16) | 0.41 | 1.00 (0.76, 1.31) | 0.52 |
| Fully Adjusted b | Ref | 0.91 (0.70, 1.19) | 0.52 | 0.91 (0.68, 1.22) | 0.98 | 1.02 (0.71, 1.48) | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfeir, M.; Jacobs, E.T.; Kohler, L.N.; Steck, S.E.; Yung, A.K.; Thomson, C.A. Characterizing Dietary Advanced Glycation End-Product (dAGE) Exposure and the Relationship to Colorectal Adenoma Recurrence: A Secondary Analysis. Nutrients 2023, 15, 1126. https://doi.org/10.3390/nu15051126
Sfeir M, Jacobs ET, Kohler LN, Steck SE, Yung AK, Thomson CA. Characterizing Dietary Advanced Glycation End-Product (dAGE) Exposure and the Relationship to Colorectal Adenoma Recurrence: A Secondary Analysis. Nutrients. 2023; 15(5):1126. https://doi.org/10.3390/nu15051126
Chicago/Turabian StyleSfeir, Maren, Elizabeth T. Jacobs, Lindsay N. Kohler, Susan E. Steck, Angela K. Yung, and Cynthia A. Thomson. 2023. "Characterizing Dietary Advanced Glycation End-Product (dAGE) Exposure and the Relationship to Colorectal Adenoma Recurrence: A Secondary Analysis" Nutrients 15, no. 5: 1126. https://doi.org/10.3390/nu15051126
APA StyleSfeir, M., Jacobs, E. T., Kohler, L. N., Steck, S. E., Yung, A. K., & Thomson, C. A. (2023). Characterizing Dietary Advanced Glycation End-Product (dAGE) Exposure and the Relationship to Colorectal Adenoma Recurrence: A Secondary Analysis. Nutrients, 15(5), 1126. https://doi.org/10.3390/nu15051126






