Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges
Abstract
1. Introduction
2. T2D and Its Therapy
2.1. The Aberrant Glucose Metabolism in T2D
2.2. Clinical Therapeutic Drugs and Their Targets in T2D
3. Legume-Derived Peptides and Their Anti-Diabetic Activity
3.1. Native Legume Peptides
3.2. Legume Proteins
3.3. Hydrolytic Peptides
4. Potential Hypoglycemic Mechanism of Bioactive Legume Peptides
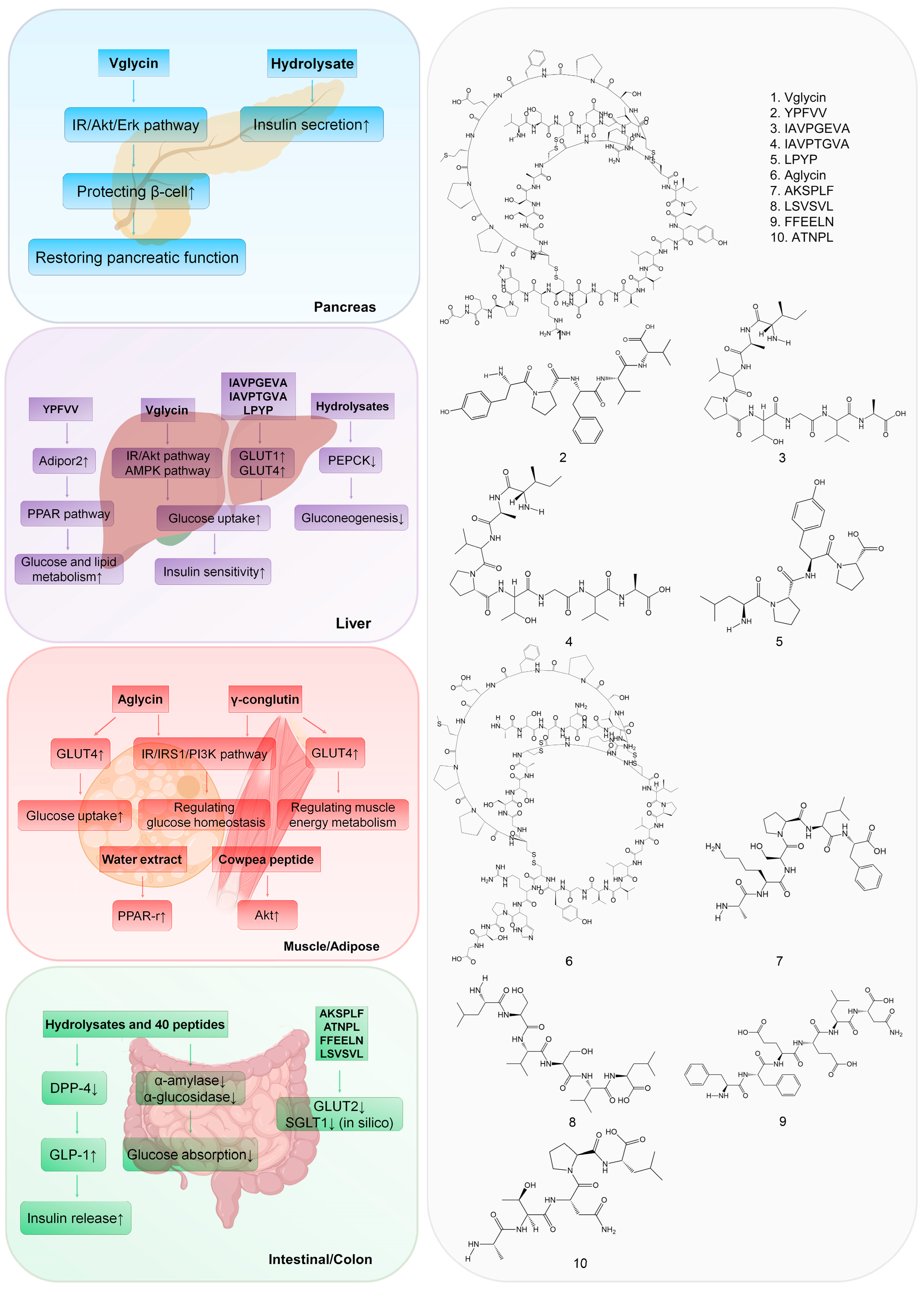
4.1. Targeting the Pancreas
4.2. Targeting the Liver
4.3. Targeting Muscle and Adipose Tissue
4.4. Targeting the Intestine and Colon
5. Challenges of Legume Peptides’ Development in T2D Treatment
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| AUC | area under curve |
| BG | blood glucose |
| BW | body weight |
| Cγ | γ-conglutin |
| DPP-4 | dipeptidyl peptidase IV |
| FA | fatty acid |
| FAS | fatty acid synthase |
| FBG | fasting blood glucose |
| FPG | fasting plasma glucose |
| GIP | gastric inhibitory peptide |
| GLP-1 | glucagon-like peptide-1 |
| GLUT4 | glucose transporter type 4 |
| HFD | high-fat diet |
| IC50 | half maximal inhibitory concentration |
| IR | insulin receptor |
| IRS1 | insulin receptor substrate 1 |
| ITT | insulin tolerance test |
| OGTT | oral glucose tolerance test |
| PPAR | peroxisome proliferator-activated receptor |
| REU | rosetta energy units |
| SD | Sprague Dawley |
| SGLT-2 | sodium-glucose cotransporter-2 |
| STZ | streptozotocin |
| T2D | type 2 diabetes |
| TG | triglyceride |
| TZDs | thiazolidinediones |
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef]
- Sterrett, J.J.; Bragg, S.; Weart, C.W. Type 2 Diabetes Medication Review. Am. J. Med. Sci. 2016, 351, 342–355. [Google Scholar] [CrossRef]
- Acquah, C.; Dzuvor, C.K.O.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2022, 62, 2158–2171. [Google Scholar] [CrossRef]
- Hewage, S.S.; Wu, S.; Neelakantan, N.; Yoong, J. Systematic review of effectiveness and cost-effectiveness of lifestyle interventions to improve clinical diabetes outcome measures in women with a history of GDM. Clin. Nutr. ESPEN 2020, 35, 20–29. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed. Available online: https://diabetesatlas.org/en/resources/ (accessed on 5 December 2022).
- Mata-Cases, M.; Rodriguez-Sanchez, B.; Mauricio, D.; Real, J.; Vlacho, B.; Franch-Nadal, J.; Oliva, J. The Association Between Poor Glycemic Control and Health Care Costs in People with Diabetes: A Population-Based Study. Diabetes Care 2020, 43, 751–758. [Google Scholar] [CrossRef]
- Mullins, A.P.; Arjmandi, B.H. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Hashidume, T.; Sakano, T.; Mochizuki, A.; Ito, K.; Ito, S.; Kawarasaki, Y.; Miyoshi, N. Identification of soybean peptide leginsulin variants in different cultivars and their insulin-like activities. Sci. Rep. 2018, 8, 16847. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, Y.; Yan, D.; Jia, S.; Ostenson, C.G.; Chen, Z. The Soybean Peptide Vglycin Preserves the Diabetic beta-cells through Improvement of Proliferation and Inhibition of Apoptosis. Sci. Rep. 2015, 5, 15599. [Google Scholar] [CrossRef]
- Yao, C.-C.; Tong, Y.-X.; Jiang, H.; Yang, D.-R.; Zhang, X.-J.; Zhang, P.; Su, L.; Zhao, Y.-Y.; Chen, Z.-W. Native polypeptide vglycin prevents nonalcoholic fatty liver disease in mice by activating the AMPK pathway. J. Funct. Foods 2020, 73, 104110. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Bonner-Weir, S. Pancreatic beta Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018, 27, 57–67. [Google Scholar] [CrossRef]
- Weir, G.C.; Gaglia, J.; Bonner-Weir, S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endo. 2020, 8, 249–256. [Google Scholar] [CrossRef]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Moller, D.E. New drug targets for type 2 diabetes and the metabolic syndrome. Nature 2001, 414, 821–827. [Google Scholar] [CrossRef]
- Bailey, C.J. The Current Drug Treatment Landscape for Diabetes and Perspectives for the Future. Clin. Pharmacol. Ther. 2015, 98, 170–184. [Google Scholar] [CrossRef]
- Thule, P.M. Mechanisms of current therapies for diabetes mellitus type 2. Adv. Physiol. Educ. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, Z.; Paul, P.K.; Lu, Y.; Wu, W.; Qi, J. Oral delivery of proteins and peptides: Challenges, status quo and future perspectives. Acta Pharm. Sin. B 2021, 11, 2416–2448. [Google Scholar] [CrossRef]
- Yari, Z.; Behrouz, V.; Zand, H.; Pourvali, K. New insight into diabetes management: From glycemic index to dietary insulin index. Curr. Diabetes Rev. 2020, 16, 293–300. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Oral delivery of therapeutic protein/peptide for diabetes--future perspectives. Int. J. Pharm. 2013, 440, 48–62. [Google Scholar] [CrossRef]
- Soccio, R.E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar] [CrossRef]
- Akter, H. Review on diabetics and anti-diabetic drugs. AJPTI 2014, 2, 1–16. [Google Scholar]
- DeFronzo, R.A. Pharmacologic Therapy for Type 2 Diabetes Mellitus. Ann. Intern. Med. 1999, 131, 281–303. [Google Scholar] [CrossRef]
- Bailey, C.J.; Tahrani, A.A.; Barnett, A.H. Future glucose-lowering drugs for type 2 diabetes. Lancet Diabetes Endo. 2016, 4, 350–359. [Google Scholar] [CrossRef]
- Furman, B.L. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon 2012, 59, 464–471. [Google Scholar] [CrossRef]
- Liao, H.J.; Tzen, J.T.C. Investigating Potential GLP-1 Receptor Agonists in Cyclopeptides from Pseudostellaria heterophylla, Linum usitatissimum, and Drymaria diandra, and Peptides Derived from Heterophyllin B for the Treatment of Type 2 Diabetes: An In Silico Study. Metabolites 2022, 12, 549. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef]
- Scott, L.J. Liraglutide: A review of its use in adult patients with type 2 diabetes mellitus. Drugs 2014, 74, 2161–2174. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef]
- Ahren, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376. [Google Scholar] [CrossRef]
- Syed, Y.Y. Tirzepatide: First Approval. Drugs 2022, 82, 1213–1220. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ajabiya, J.; Teli, D.; Bojarska, J.; Apostolopoulos, V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules 2022, 27, 4315. [Google Scholar] [CrossRef]
- Hallakou-Bozec, S.; Vial, G.; Kergoat, M.; Fouqueray, P.; Bolze, S.; Borel, A.L.; Fontaine, E.; Moller, D.E. Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 664–673. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Chiglitazar: A novel pan-PPAR agonist. Sci. Bull. 2021, 66, 1497–1498. [Google Scholar] [CrossRef]
- Xu, H.-R.; Zhang, J.-W.; Chen, W.-L.; Ning, Z.-Q.; Li, X.-N. Pharmacokinetics, Safety and Tolerability of Chiglitazar, A Novel Peroxisome Proliferator-Activated Receptor (PPAR) Pan-Agonist, in Healthy Chinese Volunteers: A Phase I Study. Clin. Drug Investig. 2019, 39, 553–563. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Definition and Classification of Commodities: Pulses and Derived Products. 1994. Available online: http://www.fao.org/es/faodef/fdef04e.htm (accessed on 2 June 2015).
- Singhal, P.; Kaushik, G.; Mathur, P. Antidiabetic potential of commonly consumed legumes: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 655–672. [Google Scholar] [CrossRef]
- Kamran, F.; Reddy, N. Bioactive peptides from legumes: Functional and nutraceutical potential. Recent Adv. Food Sci. 2018, 1, 134–149. [Google Scholar]
- Jakubczyk, A.; Karas, M.; Rybczynska-Tkaczyk, K.; Zielinska, E.; Zielinski, D. Current Trends of Bioactive Peptides-New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef]
- Baldeon, M.E.; Felix, C.; Fornasini, M.; Zertuche, F.; Largo, C.; Paucar, M.J.; Ponce, L.; Rangarajan, S.; Yusuf, S.; Lopez-Jaramillo, P. Prevalence of metabolic syndrome and diabetes mellitus type-2 and their association with intake of dairy and legume in Andean communities of Ecuador. PLoS ONE 2021, 16, e0254812. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Tagliazucchi, D.; Babini, E.; Sefora Rutella, G.; Taneyo Saa, D.L.; Gianotti, A. Bioactive peptides from vegetable food matrices: Research trends and novel biotechnologies for synthesis and recovery. J. Funct. Foods 2016, 27, 549–569. [Google Scholar] [CrossRef]
- Becerra-Tomas, N.; Diaz-Lopez, A.; Rosique-Esteban, N.; Ros, E.; Buil-Cosiales, P.; Corella, D.; Estruch, R.; Fito, M.; Serra-Majem, L.; Aros, F.; et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin. Nutr. 2018, 37, 906–913. [Google Scholar] [CrossRef]
- Villegas, R.; Gao, Y.T.; Yang, G.; Li, H.L.; Elasy, T.A.; Zheng, W.; Shu, X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008, 87, 162–167. [Google Scholar] [CrossRef]
- Tang, J.; Wan, Y.; Zhao, M.; Zhong, H.; Zheng, J.S.; Feng, F. Legume and soy intake and risk of type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 111, 677–688. [Google Scholar] [CrossRef]
- Konishi, K.; Wada, K.; Yamakawa, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Tsuji, M.; Nagata, C. Dietary Soy Intake Is Inversely Associated with Risk of Type 2 Diabetes in Japanese Women but Not in Men. J. Nutr. 2019, 149, 1208–1214. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.; Elahi, F.; Yeon, S.J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.H. The Role of Bioactive Peptides in Diabetes and Obesity. Foods 2021, 10, 2220. [Google Scholar] [CrossRef]
- Marya; Khan, H.; Nabavi, S.M.; Habtemariam, S. Anti-diabetic potential of peptides: Future prospects as therapeutic agents. Life Sci. 2018, 193, 153–158. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Antidiabetic Food-Derived Peptides for Functional Feeding: Production, Functionality and In Vivo Evidences. Foods 2020, 9, 983. [Google Scholar] [CrossRef]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic effect of lupin seed gamma-conglutin in experimental animals and healthy human subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef]
- Méric, E.; Lemieux, S.; Turgeon, S.L.; Bazinet, L. Insulin and glucose responses after ingestion of different loads and forms of vegetable or animal proteins in protein enriched fruit beverages. J. Funct. Foods 2014, 10, 95–103. [Google Scholar] [CrossRef]
- Lu, J.; Zeng, Y.; Hou, W.; Zhang, S.; Li, L.; Luo, X.; Xi, W.; Chen, Z.; Xiang, M. The soybean peptide aglycin regulates glucose homeostasis in type 2 diabetic mice via IR/IRS1 pathway. J. Nutr. Biochem. 2012, 23, 1449–1457. [Google Scholar] [CrossRef]
- Jiang, H.; Feng, J.; Du, Z.; Zhen, H.; Lin, M.; Jia, S.; Li, T.; Huang, X.; Ostenson, C.G.; Chen, Z. Oral administration of soybean peptide Vglycin normalizes fasting glucose and restores impaired pancreatic function in Type 2 diabetic Wistar rats. J. Nutr. Biochem. 2014, 25, 954–963. [Google Scholar] [CrossRef]
- Yamada, Y.; Muraki, A.; Oie, M.; Kanegawa, N.; Oda, A.; Sawashi, Y.; Kaneko, K.; Yoshikawa, M.; Goto, T.; Takahashi, N.; et al. Soymorphin-5, a soy-derived mu-opioid peptide, decreases glucose and triglyceride levels through activating adiponectin and PPARalpha systems in diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E433–E440. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Parolari, A.; Magni, C.; Duranti, M. Lupin seed gamma-conglutin lowers blood glucose in hyperglycaemic rats and increases glucose consumption of HepG2 cells. Br. J. Nutr. 2012, 107, 67–73. [Google Scholar] [CrossRef]
- Mojica, L.; Gonzalez de Mejia, E.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Gonzalez-Montoya, M.; Hernandez-Ledesma, B.; Mora-Escobedo, R.; Martinez-Villaluenga, C. Bioactive Peptides from Germinated Soybean with Anti-Diabetic Potential by Inhibition of Dipeptidyl Peptidase-IV, alpha-Amylase, and alpha-Glucosidase Enzymes. Int. J. Mol. Sci. 2018, 19, 2883. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, H.; He, R.; Ma, Y. Purification and a molecular docking study of α-glucosidase-inhibitory peptides from a soybean protein hydrolysate with ultrasonic pretreatment. Eur. Food Res. Technol. 2018, 244, 1995–2005. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three Peptides from Soy Glycinin Modulate Glucose Metabolism in Human Hepatic HepG2 Cells. Int. J. Mol. Sci. 2015, 16, 27362–27370. [Google Scholar] [CrossRef]
- Muñoz, E.B.; Luna-Vital, D.A.; Fornasini, M.; Baldeón, M.E.; Gonzalez de Mejia, E. Gamma-conglutin peptides from Andean lupin legume (Lupinus mutabilis Sweet) enhanced glucose uptake and reduced gluconeogenesis in vitro. J. Funct. Foods 2018, 45, 339–347. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, M.J.; Yang, H.J.; Park, S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition 2011, 27, 244–252. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E.; Gonzalez de Mejia, E.; Amaya-Llano, S.L. Hard-to-cook bean (Phaseolus vulgaris L.) proteins hydrolyzed by alcalase and bromelain produced bioactive peptide fractions that inhibit targets of type-2 diabetes and oxidative stress. Food Res. Int. 2015, 76, 839–851. [Google Scholar] [CrossRef]
- Barnes, M.J.; Uruakpa, F.O.; Udenigwe, C.C. Influence of Cowpea (Vigna unguiculata) Peptides on Insulin Resistance. J. Nutrit. Health Food Sci. 2015, 3, 1–3. [Google Scholar]
- Davis, J.; Higginbotham, A.; O’Connor, T.; Moustaid-Moussa, N.; Tebbe, A.; Kim, Y.C.; Cho, K.W.; Shay, N.; Adler, S.; Peterson, R.; et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 2007, 51, 42–52. [Google Scholar] [CrossRef]
- Nordentoft, I.; Jeppesen, P.B.; Hong, J.; Abudula, R.; Hermansen, K. Increased Insulin Sensitivity and Changes in the Expression Profile of Key Insulin Regulatory Genes and Beta Cell Transcription Factors in Diabetic KKAy-Mice after Feeding with a Soy Bean Protein Rich Diet High in Isoflavone Content. J. Agric. Food Chem. 2008, 56, 4377–4385. [Google Scholar] [CrossRef]
- Gonzalez-Santiago, A.E.; Vargas-Guerrero, B.; Garcia-Lopez, P.M.; Martinez-Ayala, A.L.; Dominguez-Rosales, J.A.; Gurrola-Diaz, C.M. Lupinus albus Conglutin Gamma Modifies the Gene Expressions of Enzymes Involved in Glucose Hepatic Production In Vivo. Plant Foods Hum. Nutr. 2017, 72, 134–140. [Google Scholar] [CrossRef]
- Magni, C.; Sessa, F.; Accardo, E.; Vanoni, M.; Morazzoni, P.; Scarafoni, A.; Duranti, M. Conglutin gamma, a lupin seed protein, binds insulin in vitro and reduces plasma glucose levels of hyperglycemic rats. J. Nutr. Biochem. 2004, 15, 646–650. [Google Scholar] [CrossRef]
- Vargas-Guerrero, B.; Garcia-Lopez, P.M.; Martinez-Ayala, A.L.; Dominguez-Rosales, J.A.; Gurrola-Diaz, C.M. Administration of Lupinus albus gamma conglutin (Cgamma) to n5 STZ rats augmented Ins-1 gene expression and pancreatic insulin content. Plant Foods Hum. Nutr. 2014, 69, 241–247. [Google Scholar] [CrossRef]
- Terruzzi, I.; Senesi, P.; Magni, C.; Montesano, A.; Scarafoni, A.; Luzi, L.; Duranti, M. Insulin-mimetic action of conglutin-gamma, a lupin seed protein, in mouse myoblasts. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 197–205. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.; Fang, L.; Qin, X.; Cai, M.; Gu, R.; Lu, J.; Wang, Y. Hypoglycemic effects and biochemical mechanisms of Pea oligopeptide on high-fat diet and streptozotocin-induced diabetic mice. J. Food Biochem. 2019, 43, e13055. [Google Scholar] [CrossRef]
- Valencia-Mejia, E.; Batista, K.A.; Fernandez, J.J.A.; Fernandes, K.F. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res. Int. 2019, 121, 238–246. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Mojica, L.; Johnson, M.H.; Chang, Y.K.; González de Mejía, E. Germination of Phaseolus vulgaris and alcalase hydrolysis of its proteins produced bioactive peptides capable of improving markers related to type-2 diabetes in vitro. Food Res. Int. 2015, 76, 150–159. [Google Scholar] [CrossRef]
- Dun, X.P.; Wang, J.H.; Chen, L.; Lu, J.; Li, F.F.; Zhao, Y.Y.; Cederlund, E.; Bryzgalova, G.; Efendic, S.; Jornvall, H.; et al. Activity of the plant peptide aglycin in mammalian systems. FEBS J. 2007, 274, 751–759. [Google Scholar] [CrossRef]
- Hirano, K.; Hirano, H. Interaction of a 43-kDa Receptor-like Protein with a 4-kDa Hormone-like Peptide in Soybean. Biochemistry 2004, 43, 12105–12112. [Google Scholar]
- Watanbe, Y. A peptide that stimulates phosphorylation of the plant insulin-binding protein Isolation, primary structure and cDNA cloning. Eur. J. Biochem. 1994, 224, 167–172. [Google Scholar] [CrossRef]
- Gressent, F.; Duport, G.; Rahioui, I.; Pauchet, Y.; Bolland, P.; Specty, O.; Rahbe, Y. Biological activity and binding site characteristics of the PA1b Entomotoxin on insects from different orders. J. Insect. Sci. 2007, 7, 12. [Google Scholar] [CrossRef]
- Domoney, C. Inhibitors of legume seeds. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 635–655. [Google Scholar]
- Mane, S.P.; Johnson, S.K.; Duranti, M.; Pareek, V.K.; Utikar, R.P. Lupin seed γ-conglutin: Extraction and purification methods—A review. Trends Food Sci. Technol. 2018, 73, 1–11. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Robles-Bolivar, P.; Alché, J.D.; Jimenez-Lopez, J.C. Narrow Leafed Lupin Beta-Conglutin Proteins Epitopes Identification and Molecular Features Analysis Involved in Cross-Allergenicity to Peanut and Other Legumes. GCB 2016, 2, e29. [Google Scholar] [CrossRef]
- Jessica Capraro, A.C.; Luis, A.R.; Chiara, M.; Alessio, S.; Marcello, D. Assessment of the lupin seed glucose-lowering protein intestinal absorption by using in vitro and ex vivo models. Food Chem. 2011, 125, 1279–1283. [Google Scholar] [CrossRef]
- Claessens, M.; Saris, W.H.; Van Baak, M.A. Glucagon and insulin responses after ingestion of different amounts of intact and hydrolysed proteins. Br. J. Nutr. 2008, 100, 61–69. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. FQS 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Daliri, E.B.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Agyei, D. Bioactive Proteins and Peptides from Soybeans. Recent Pat. Food Nutr. Agric. 2015, 7, 100–107. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive Peptides. J. Aoac. Int. 2008, 9, 914–931. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Soybean- and Lupin-Derived Peptides Inhibit DPP-IV Activity on In Situ Human Intestinal Caco-2 Cells and Ex Vivo Human Serum. Nutrients 2018, 10, 1082. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Tye, G.J.; Gan, C.Y. The investigation of α-amylase inhibitory activity of selected Pinto bean peptides via preclinical study using AR42J cell. J. Funct. Foods 2017, 35, 641–647. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Identification of Pinto bean peptides with inhibitory effects on alpha-amylase and angiotensin converting enzyme (ACE) activities using an integrated bioinformatics-assisted approach. Food Chem. 2018, 267, 124–131. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karas, M.; Zlotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G. Optimization of enzymatic production of anti-diabetic peptides from black bean (Phaseolus vulgaris L.) proteins, their characterization and biological potential. Food Funct. 2016, 7, 713–727. [Google Scholar] [CrossRef]
- Huang, M.H.; Lin, J.L.; Wang, M.; Ma, Y.; Wang, J.F. High-level expression and activity determination of hypoglycemic peptide aglycin. Mod. Food Sci. Technol. 2020, 36, 143–149. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of alpha-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Luna-Vital, D.; de Mejia, E.G. Identification and Comparison of Peptides from Chickpea Protein Hydrolysates Using Either Bromelain or Gastrointestinal Enzymes and Their Relationship with Markers of Type 2 Diabetes and Bitterness. Nutrients 2020, 12, 3843. [Google Scholar] [CrossRef]
- Sato, K.; Miyasaka, S.; Tsuji, A.; Tachi, H. Isolation and characterization of peptides with dipeptidyl peptidase IV (DPPIV) inhibitory activity from natto using DPPIV from Aspergillus oryzae. Food Chem. 2018, 261, 51–56. [Google Scholar] [CrossRef]
- Mojica, L.; Chen, K.; de Mejia, E.G. Impact of commercial precooking of common bean (Phaseolus vulgaris) on the generation of peptides, after pepsin-pancreatin hydrolysis, capable to inhibit dipeptidyl peptidase-IV. J. Food. Sci. 2015, 80, H188–H198. [Google Scholar] [CrossRef]
- Mune Mune, M.A.; Minka, S.R.; Henle, T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activity of Bambara bean protein hydrolysates. Food Chem. 2018, 250, 162–169. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejia, E.G. Impact of germination and enzymatic hydrolysis of cowpea bean (Vigna unguiculata) on the generation of peptides capable of inhibiting dipeptidyl peptidase IV. Food Res. Int. 2014, 64, 799–809. [Google Scholar] [CrossRef]
- Castañeda-Pérez, E.; Jiménez-Morales, K.; Quintal-Novelo, C.; Moo-Puc, R.; Chel-Guerrero, L.; Betancur-Ancona, D. Enzymatic protein hydrolysates and ultrafiltered peptide fractions from Cowpea Vigna unguiculata L bean with in vitro antidiabetic potential. J. Iran. Chem. Soc. 2019, 16, 1773–1781. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54, S97–S107. [Google Scholar] [CrossRef]
- Guo, S.D. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J. Endocrinol. 2014, 220, T1–T23. [Google Scholar] [CrossRef]
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186. [Google Scholar] [CrossRef]
- Hilder, T.L.; Baer, L.A.; Fuller, P.M.; Fuller, C.A.; Grindeland, R.E.; Wade, C.E.; Graves, L.M. Insulin-independent pathways mediating glucose uptake in hindlimb-suspended skeletal muscle. J. Appl. Physiol. 2005, 99, 2181–2188. [Google Scholar] [CrossRef]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Daily, J.W., 3rd; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and alpha-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Bompard-Gilles, C.; Rousseau, P.; Rougé, P.; Payan, F. Substrate mimicry in the active center of a mammalian α amylase: Structural analysis of an enzyme–inhibitor complex. Structure 1996, 4, 1441–1452. [Google Scholar] [CrossRef]
- Nahoum, V.; Farisei, F.; Le-Berre-Anton, V.; Egloff, M.P.; Rouge, P.; Poerio, E.; Payan, F. A plant-seed inhibitor of two classes of alpha-amylases: X-ray analysis of Tenebrio molitor larvae alpha-amylase in complex with the bean Phaseolus vulgaris inhibitor. Acta Crystallogr. Sect. D Struct. Biol. 1999, 55, 360–362. [Google Scholar] [CrossRef]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef]
- Arulmozhiraja, S.; Matsuo, N.; Ishitsubo, E.; Okazaki, S.; Shimano, H.; Tokiwa, H. Comparative Binding Analysis of Dipeptidyl Peptidase IV (DPP-4) with Antidiabetic Drugs—An Ab Initio Fragment Molecular Orbital Study. PLoS ONE 2016, 11, e0166275. [Google Scholar] [CrossRef]
- Zizzari, A.T.; Pliatsika, D.; Gall, F.M.; Fischer, T.; Riedl, R. New perspectives in oral peptide delivery. Drug Discov. Today 2021, 26, 1097–1105. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zhao, R.; Zhang, X. Advances in oral peptide drug nanoparticles for diabetes mellitus treatment. Bioact. Mater. 2022, 15, 392–408. [Google Scholar] [CrossRef]
- Yap, P.G.; Gan, C.Y. In vivo challenges of anti-diabetic peptide therapeutics: Gastrointestinal stability, toxicity and allergenicity. Trends Food Sci. Technol. 2020, 105, 161–175. [Google Scholar] [CrossRef]
- Oseguera-Toledo, M.E. Proteins and bioactive peptides: Mechanism of action in diabetes management. Nutrafoods 2014, 13, 147–157. [Google Scholar] [CrossRef]
- Rosenstock, J.; Allison, D.; Birkenfeld, A.L.; Blicher, T.M.; Deenadayalan, S.; Jacobsen, J.B.; Serusclat, P.; Violante, R.; Watada, H.; Davies, M.; et al. Effect of Additional Oral Semaglutide vs Sitagliptin on Glycated Hemoglobin in Adults with Type 2 Diabetes Uncontrolled with Metformin Alone or with Sulfonylurea: The PIONEER 3 Randomized Clinical Trial. JAMA 2019, 321, 1466–1480. [Google Scholar] [CrossRef]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.L.; Hansen, O.K.H.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide Compared with Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef]
- Poudwal, S.; Misra, A.; Shende, P. Role of lipid nanocarriers for enhancing oral absorption and bioavailability of insulin and GLP-1 receptor agonists. J. Drug Target. 2021, 29, 834–847. [Google Scholar] [CrossRef]
| Target Organs/Tissues | Mechanism | Drug Class | Drug Name | Adverse Reaction | Drug Delivery |
|---|---|---|---|---|---|
| Pancreatic β-cell | β-cell mass restore, increasing insulin secretion | Sulfonylurea | Glibenclamide, glipizide, glimepiride, gliclazide | Cause hypoglycemia | Oral |
| Meglitinide | Repaglinide, nateglinide | Not cause hypoglycemia | Oral | ||
| Pancreatic α-cell | Increasing glucagon secretion | GLP-1 agonist | Exenatide, lixisenatide, liraglutide, dulaglutide, exenatide extend-release, semaglutide, albiglutide | Gastrointestinal and hypoglycemic reactions | Inject/Oral |
| Amylin analog | Pramlintide | Cause hypoglycemic | Inject | ||
| Biguanide | Metformin | Lactic acidemia and ketouria | Oral | ||
| Liver | Decreasing hepatic glucose production | Thiazolidinedione | Pioglitazone, rosiglitazone | Weight gain, edema, cardiac problems | Oral |
| Insulin | Rapid-, short-, intermediate-, long-acting insulin | Hypoglycemia and edema | Inject | ||
| Biguanide | Metformin | Lactic acidemia and ketouria | Oral | ||
| Muscle and adipose tissue | Increasing glucose uptake and glycolysis | Biguanide | Metformin | Lactic acidemia and ketouria | Oral |
| Thiazolidinedione | Pioglitazone, rosiglitazone | Weight gain, edema, cardiac problems | Oral | ||
| Insulin | Rapid-, short-, intermediate-, long-acting insulin | Hypoglycemia and edema | Inject | ||
| α-glucosidase inhibitor | Acarbose, miglitol, voglibose | Gastrointestinal reaction | Oral | ||
| Intestine | Increasing incretin activity, enhancing glucose absorption | DPP-4 inhibitor | Sitagliptin, saxagliptin, linagliptin, vidaglipti, alogliptin, teneligliptin, gemigliptin | Minor adverse drug reactions | Oral |
| GLP-1 agonist | Exenatide, lixisenatide, liraglutide, dulaglutide, exenatide extend-release, semaglutide, albiglutide | Gastrointestinal and hypoglycemic reactions | Inject/Oral | ||
| Biguanide | Metformin | Lactic acidemia and ketouria | Oral | ||
| Colon | Balancing gut microbiota | GLP-1 agonist | Exenatide, lixisenatide, liraglutide, dulaglutide, exenatide extend-release, semaglutide, albiglutide | Gastrointestinal and hypoglycemic reactions | Inject/Oral |
| Kidney | Inhibiting glucose reabsorption | SGLT-2 inhibitor | Dapagliflozin, canagliflozin, empagliflozin, ertugliflozin, Sotagliflozin | Urinary genital infection risk, ketoacidosis risk | Oral |
| Central nervous system | Lowing neurotransmitters | Dopamine-receptor agonist | Bromocriptine | Gastrointestinal reaction | Oral |
| Source | Consumption(g/d) | People Tested | HRs or RR (95% CIs) | Reference |
|---|---|---|---|---|
| Lentils | 6.6 | Caucasian | 0.67 (0.46, 0.98) | [47] |
| Chickpea | 5.0 | Caucasian | 0.68 (0.46, 1.00) | [47] |
| Soybean | 32.0 | Chinese women | 0.57 (0.48, 0.60) | [48] |
| Soy protein | 11.0−15.0 | Chinese/Japanese | 0.84 (0.75, 0.95) | [49] |
| Soy protein | 13.6 | Japanese women | 0.46 (0.30, 0.70) | [50] |
| Peptide | Amino Acid Sequence | Source | Model | Feeding/Treat Pattern | Hypoglycemic Effect and Index | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Aglycin | ASCNGVCSPFEMPPCGSSACRCIPVGLVVGYCRHPSG | Soybean | STZ/HFD-induced diabetic BALB/c mice, C2C12 cell | 50 mg/kg/d, 4 weeks | BG↓, OGTT↑, insulin tolerance↑, p-IR↑, p-IRS1↑, p-Akt↑, GLUT4↑, glucose uptake↑ | Increasing insulin receptor via IR/IRS1 pathway, enhancing glucose uptake | [56] |
| Vglycin | VSCNGVCSPFEMPPCGSSACRCIPYGLVVGNCRHPSG | Pea seed/germinating pea seed | STZ/HFD-induced diabetic rats, HepG2 cell, L02 cell | 4 weeks | BW↓, food intake↓, FPG↑, PARP↑, PDX1↑, GSK3α/β↑, GLUT4↑, p85-PI3-kinase↓, p-Akt↓ | Impairing glucose tolerance, restoring pancreatic function, enhancing insulin signaling by activating the IR/Akt pathway | [57] |
| T1D SD rat, STZ-induced T2D C57BL/6 mice, INS-1 832/13 cell | 80 mg/kg/d, 4 weeks, 8 weeks | BW↓, lee’s index↓, food intake↓, FPG↓, BG↓, ITT↑, pancreatic islet↑, plasma insulin↑, glucose-stimulated glucagon↓, pancreatic mass↑, β-cell area and mass↑, Ki67/PCNA immunostaining area↑, TUNEL-positive/total β-cells↓, Erk↑ | Promoting the proliferation of β-cells via the IR/Akt/Erk pathway | [14] | |||
| HFD-C57BL/6J mice, HepG2 cell | 15 weeks | BW↓, FPG↓, glucose tolerance↑, ITT↑, FA β-oxidation↑, FAS↓ | Improving insulin sensitivity and glucose tolerance, enhancing β-oxidation and inhibiting FAS via AMPK pathway and down-regulating the FAS | [15] | |||
| Soymorphin-5 | YPFVV | Soybean | KKAy mice | 10 mg/kg/d, 5 weeks | BG↓, plasma insulin↓, TG↓, adiponectin↑, liver TG↓, Adipor2↑, PPARα↑, AOX1↑, CPT1↑, UCP2 1↑ | Increasing insulin sensitivity, improving glucose and lipid metabolism via activation of the adiponectin and PPARα system | [58] |
| Soy protein | / | Soybean | ZDF rat | Soy protein, 11 weeks | BW↓, total adiposity↓, total and liver adiposity↓, glucose level↓, insulin↓, GLUT4↑, PPAR-r↑, FAS↓, GPDH↓ | Maintenance of peripheral (adipose tissue) insulin signaling | [68] |
| Soy protein | / | Soybean | KKAy mice | High content isoflavone soy protein, 9 weeks | FPG↓, insulin↓, TG↓, TC↓, GLUT2↑, GLUT3↑, Ins1↑, Ins2↑, IGF1↓, β2/Neurod1↓, cholecystokinin↓, LDLR↑ | Improving glucose and insulin sensitivity | [69] |
| γ-conglutin | / | Lupin bean | SD rat, HepG2 cell | 10 μmol/L, 24 or 48 h | Glucose consumption↑ | Maintaining glucose homeostasis | [59] |
| γ-conglutin | / | Lupin bean | STZ-induced Wistar rat | 150 mg/kg, 1 week | G6pc↓, Fbp1↓, Pck1↓ | Regulating glucose metabolism mainly through G6pc inhibition | [70] |
| γ-conglutin | / | Lupin bean | Glucose-administrated male rat | 50, 100 and 200 mg/kg BW, 0, 30, 60 and 90 min | BG↓, AUC↓ | Hypoglycemic effect | [54] |
| γ-conglutin | / | Lupin bean | Glucose-administrated male rat | 30, 60 and 120 mg/kg BW, 30, 60, 90, and 120 min | Plasma glucose↓ | Hypoglycemic effect | [71] |
| γ-conglutin | / | Lupin bean | STZ administrated rat | 120 mg/kg in saline, 1 week | Glucose↓, Ins1↑, pancreatic insulin↑, serum insulin↑ | Hypoglycemic effect | [72] |
| γ-conglutin | / | Lupin bean | C2C12 cell | 0.5 mg/mL,72 h | Plasma glucose↓, IRS1 protein↑, p85-PI3 kinase↑, Akt1 protein↑, eIF4E protein↑, p70S6 K↑, ERK1↑, ERK2↑, MHC protein↑, myogenin↑ | Regulating muscle energy metabolism, protein synthesis and MHC gene transcription through insulin signaling pathway | [73] |
| HPI/pure peptide | AKSPLF, ATNPL, FFEELN, LSVSVL | Black bean | STZ-induced Wistar rats, Caco-2 cell | 100, 150 and 200 mg HPI/kg/BW, 15 d, pure peptide (100 µM), HPI (10 mg/mL) | BW↓, BG↓, postprandial glucose level↓, insulin↓, GLP-1↑, OGTT↓, glucose absorption↓, GLUT2↓, SGLT1↓ | Reducing glucose absorption via blocking glucose transporters GLUT2 and SGLT1(in silico) | [60] |
| Synthetic peptide | IAVPGEVA, IAVPTGVA, LPYP | Soy glycinin | HepG2 cell | 50 and 100 µM | Glucose uptake↑, Akt↑, GSK3α/β↓, GLUT4↑, GLUT1↑ | Enhancing glucose uptake through GLUT1 and GLUT4 activation, Akt and AMPK pathway | [63] |
| Hydrolysate | / | Soy protein | Alloxan-induced male Kunming mice | 47.5 mg/kg/d, 3 weeks | FBG↓ | Hypoglycemic effect | [62] |
| Hydrolytic oligopeptide | / | Pea | STZ/HFD-induced diabetic mice | 800, 1600 and 3200 mg/kg BW, 4 weeks | FBG↓, BW↓, OGTT↑, serum insulin↑, TC↓, TG↓, HDL-C↑, fatty acid anion ↓, liver and muscle glycogen↑ | Enhancing insulin sensitivity | [74] |
| Protein/peptide fraction | <10 kDa | Common bean | Male Wistar rat | 0.5 and 5 mg/kg | Glucose level↓, glucose uptake↑ | Hypoglycemic activity | [75] |
| γ-conglutin hydrolysate | / | Lupin bean | Caco-2 cells, 3T3-L1 cell, HepG2 cell | 2 and 5 mg/mL | Glucose uptake↑, gluconeogenesis↓, PEPCK↓, GLUT4↑, HepG2 glucose production↓ | Inhibiting DPP-4, improving insulin receptor sensitivity, inhibiting hepatic gluconeogenesis through GLUT4 activation | [64] |
| Peptide | / | Cowpea | Rat L6 skeletal muscle cell | Various doses | Akt↑ | Activating the insulin signaling pathway | [67] |
| Water extract peptide | <3 kDa | Fermented soybean | 3T3-L1 cell, Min6 cells NCI-H716 cell, Human embryo kidney 293 cell | 5 µg/mL | Glucose uptake↑, triacylglycerol↑, PPAR-r↑, β-cell viability↑, insulin secretion↑, cell proliferation↑, PDX1↑ | Increasing insulin sensitivity and exerting insulinotropic via PPAR-r activation | [65] |
| Hydrolysate/ fraction | <1 and 1–3 kDa | Hard-to-cook bean | INS-1E cell | 1 g Hydrolysate treatment | Increase insulin secretion up to 57% | Increasing insulin secretion | [66] |
| Hydrolysate | / | Common Bean(G0-0h) | INS-1E pancreatic β-cell | 2 mg SP/mL | Increase insulin secretion 45% from the basal state | Increasing insulin secretion | [76] |
| NO. | Peptide Sequence | MW(DA) | Source | Target | IC50, % | Reference |
|---|---|---|---|---|---|---|
| 1 | LSSLEMGSLGALFVCM | 1658.02 | Pinto bean | α-amylase | 0.31 mM | [92] |
| 2 | PLPLHMLP | 917.18 | Pinto bean | α-amylase | 5.92 mM | [92] |
| 3 | PPMHLP | 690.87 | Pinto bean | α-amylase | 6.08 mM | [92] |
| 4 | PPHMLP | 690.81 | Pinto bean | α-amylase | 23.33 ± 0.15 mM | [93] |
| 5 | PPHMGGP | 691.81 | Pinto bean | α-amylase | 6.14 mM | [92] |
| 6 | PLPWGAGF | 843.98 | Pinto bean | α-amylase | 6.64 mM | [92] |
| 7 | SPQSPPFATPLW | 1327.50 | Chickpea | α-amylase | −8.40 kcal/mol | [99] |
| 8 | FVVAEQAGNEEGFE | 1525.59 | Fermented bean seed | α-amylase | 0.04−0.65 μg/mL | [94] |
| 9 | SGGGGGGVAGAATASR | 1232.29 | Fermented bean seed | α-amylase | 0.59−2.12 μg/mL | [94] |
| 10 | GSGGGGGGGFGGPRR | 1232.29 | Fermented bean seed | α-amylase | 0.59−2.12 μg/mL | [94] |
| 11 | INEGSLLLPH | 1092.26 | Fermented bean seed | α-amylase | 0.04−0.65 μg/mL | [94] |
| 12 | GGYQGGGYGGNSGGGYGNRG | 1791.77 | Fermented bean seed | α-amylase | 0.59−2.12 μg/mL | [94] |
| 13 | GGSGGGGGSSSGRRP | 1232.24 | Fermented bean seed | α-amylase | 0.59−2.12 μg/mL | [94] |
| 14 | GDTVTVEFDTFLSR | 1586.72 | Fermented bean seed | α-amylase | 0.59−2.12 μg/mL | [94] |
| 15 | NEGEAH | 655.62 | Hard-to-cook bean | α-amylase | −12.84 REU | [66] |
| 16 | FFL | 425.53 | Hard-to-cook bean | α-amylase | −8.22 REU | [66] |
| 17 | WEVM | 563.67 | Black bean | α-amylase | 0.04 µM (ki) | [95] |
| 18 | AKSPLF | 661.79 | Black bean | α-amylase, DPP-4 | 0.03 µM (ki), 0.08 µM (ki) | [95] |
| 19 | QQEG | 460.44 | Hard-to-cook bean | α-amylase, DPP-4 | −7.03 REU, −7.29 REU | [66] |
| 20 | Aglycin 1 | 3743.40 | Soybean, pea | α-glucosidase | 36.48 μM | [96] |
| 21 | LLPLPVLK | 892.18 | Soybean | α-glucosidase | 237.43 ± 0.52 µM | [97] |
| 22 | SWLRL | 673.80 | Soybean | α-glucosidase | 182.05 ± 0.74 µM | [97] |
| 23 | WLRL | 586.73 | Soybean | α-glucosidase | 162.29 ± 0.74 µM | [97] |
| 24 | GSR | 318.33 | Soybean | α-glucosidase | 20.40 µM | [62] |
| 25 | EAK | 346.38 | Soybean | α-glucosidase | 520.20 µM | [62] |
| 26 | TTGGKGGK | 704.77 | Black bean | α-glucosidase | 0.27 µM (ki) | [95] |
| 27 | KKSSG | 505.57 | Common bean | α-glucosidase, DPP-4 | 49.34 ± 6.5%, 0.64 ± 0.16 mg/mL | [98] |
| 28 | GGGLHK | 567.65 | Common bean | α-glucosidase, DPP-4 | 46.10 ± 8.30%, 0.61 ± 0.10 mg/mL | [98] |
| 29 | CPGNK | 517.60 | Common bean | α-glucosidase, DPP-4 | 37.60 ± 6.8%, 0.87 ± 0.02 mg/mL | [98] |
| 30 | KTYGL | 580.68 | Common bean | α-glucosidase, DPP-4 | 36.30 ± 8.80%, 0.03 mg/mL | [98] |
| 31 | YVDGSGTPLT | 1009.08 | Chickpea | α-glucosidase, DPP-4 | −7.30 kcal/mol, −8.20 kcal/mol | [99] |
| 32 | IAVPTGVA | 726.87 | Soybean | DPP-4 | 223.20 µM (in situ) | [91] |
| 33 | LTFPGSAED | 935.97 | Lupin bean | DPP-4 | 207.50 µM (in situ) | [91] |
| 34 | EGLELLLLLLAG | 1253.52 | Black bean | DPP-4 | 0.06 µM (ki) | [95] |
| 35 | FEELN | 650.69 | Black bean | DPP-4 | 0.10 µM (ki) | [95] |
| 36 | RGPLVNPDPKPFL | 1449.72 | Common bean | DPP-4 | 14.04 kcal/mol | [76] |
| 37 | KL | 259.34 | Fermented soybean | DPP-4 | 41.40 ± 2.68 µg/mL | [100] |
| 38 | LR | 287.35 | Fermented soybean | DPP-4 | 598.02 ± 18.35 µg/mL | [100] |
| 39 | PHPATSGGGL | 892.97 | Chickpea | DPP-4 | −8.20 kcal/mol | [99] |
| 40 | LLSL | 444.57 | Hard-to-cook bean | DPP-4 | −11.75 REU | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.; Huang, H.; Li, H.; Wei, Y.; Yao, C. Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients 2023, 15, 1096. https://doi.org/10.3390/nu15051096
Hu K, Huang H, Li H, Wei Y, Yao C. Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients. 2023; 15(5):1096. https://doi.org/10.3390/nu15051096
Chicago/Turabian StyleHu, Kanghong, Huizhong Huang, Hanluo Li, Yanhong Wei, and Chenguang Yao. 2023. "Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges" Nutrients 15, no. 5: 1096. https://doi.org/10.3390/nu15051096
APA StyleHu, K., Huang, H., Li, H., Wei, Y., & Yao, C. (2023). Legume-Derived Bioactive Peptides in Type 2 Diabetes: Opportunities and Challenges. Nutrients, 15(5), 1096. https://doi.org/10.3390/nu15051096





