Nutritional Treatment of Hypertriglyceridemia in Childhood: From Healthy-Heart Counselling to Life-Saving Diet
Abstract
1. Introduction
2. Hypertriglyceridemia
2.1. Triglyceride Metabolism
2.2. Classification of Hypertriglyceridemia
3. Hypertriglyceridemia in Childhood and Adolescence
4. Nutritional Intervention in Pediatric Patients with Hypertriglyceridemia
4.1. Macronutrients in the Treatment of HTG
- Fats: they are a concentrated energy source. Saturated fats and fatty acids have a negative influence on lipid profile, promoting an increase in atherogenic lipid fractions and in TG. They should be substituted with middle chain unsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) [18].
- Carbohydrates: they represent the main nutritional energy source for healthy children. In nutritional treatment of patients with HTG, daily intake of carbohydrates must be slightly reduced, especially simple sugars, with a preferential intake of complex carbohydrates. In the case of severe HTG, simple sugars must be almost totally eliminated, whereas in intermediate forms, the total daily intake of simple sugars should not exceed 10% of total daily energy intake [17].
- Proteins: daily intake of foods rich in proteins and poor in fats (such as lean meats, egg white, poultry, and others) can grant an adequate protein intake, maintaining the prescribed lipid restriction. Some studies, conducted in adult patients, have highlighted that a hyperproteic diet is linked to relevant reduction in TG plasma levels. However, studies conducted in pediatric patients (CHOP) have showed an increase in overweight and obesity in patients with a high daily protein intake [19,20].
4.2. Main Food Categories and Dietary Patterns
5. Nutritional Intervention in Primary and Secondary Forms of Hypertriglyceridemia
5.1. Familial Chylomicronemia Syndrome
5.2. Multifactorial Chylomicronemia Syndrome
5.3. Familial Combined Hyperlipidemia
5.4. Dysbetalipoproteinemia
5.5. Weight-Excess-Related Hypertriglyceridemia
6. Focus on Specific Nutrients
6.1. Long Chain Polyunsaturated Fatty Acids
6.2. Medium-Chain Triglycerides
6.3. Fructose
6.4. Stevia
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | Alfa linoleic acid |
| ApoA5 | Apolipoprotein A5 |
| ApoB | Apolipoprotein B |
| ApoC2 | Apolipoprotein C2 |
| ApoE | Apolipoprotein E |
| ARA | Arachidonic acid |
| ASCVD | Atherosclerotic cardiovascular disease |
| BMI | Body mass index |
| CM | Chylomicrons |
| DHA | Docosaexahenoic acid |
| EPA | Eicosapentaenoic acid |
| FAO | Food and agricultural organization |
| FCHL | Familial combined hyperlipidemia |
| FCS | Familial chylomicronemia syndrome |
| FFA | Free fatty acid |
| GPIHBP1 | Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 |
| HDL | High density lipoprotein |
| HTG | Hypertriglyceridemia |
| IDL | Intermediate density lipoprotein |
| LA | Linoleic acid |
| LCPUFA | Long chain polyunsaturated fatty acid |
| LDL | Low density lipoprotein |
| LDL-R | Low density lipoprotein receptor |
| LMF1 | Lipase maturation factor 1 |
| LPL | Lipoprotein lipase |
| MAFLD | Metabolic dysfunction associated fatty liver disease |
| MCS | Multifactorial chylomicronemia syndrome |
| MCT | Medium chain triglyceride |
| NAFLD | Non-alcoholic fatty liver disease |
| NCEP | National Cholesterol Expert Panel |
| PDAY | Pathological Determinants of Atherosclerosis in Youth |
| SINUPE | Società Italiana di Nutrizione Pediatrica |
| TG | Triglyceride |
| WHO | World Health Organization |
References
- Virani, S.S.; Morris, P.B.; Agarwala, A.; Ballantyne, C.M.; Birtcher, K.K.; Kris-Etherton, P.M.; Ladden-Stirling, A.B.; Miller, M.; Orringer, C.E.; Stone, N.J. Expert Consensus Decision Pathway on the Management of ASCVD Risk Reduction in Patients With Persistent Hypetriglyceridemia A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 78, 960–993. [Google Scholar] [CrossRef]
- Societies, E.N.C.; Mach, F.; Baigent, C.; Taskinen, M.R. ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar]
- Laufs, U.; Parhofer, K.G.; Ginsberg, H.N.; Hegele, R. Clinical review on triglycerides. Eur. Heart J. 2019, 41, 99–109c. [Google Scholar] [CrossRef]
- Wiegman, A.; Gidding, S.S.; Watts, G.F.; Chapman, M.J.; Ginsberg, H.N.; Cuchel, M.; Ose, L.; Averna, M.; Boileau, C.; Borén, J.; et al. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur. Heart J. 2015, 36, 2425–2437. [Google Scholar] [CrossRef]
- Wolska, A.; Dunbar, R.L.; Freeman, L.A.; Ueda, M.; Amar, M.J.; Sviridov, D.O.; Remaley, A.T. Apolipoprotein C-II: New findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis 2017, 267, 49–60. [Google Scholar] [CrossRef]
- Berglund, L.; Brunzell, J.D.; Goldberg, A.C.; Goldberg, I.J.; Sacks, F.; Murad, M.H.; Stalenhoef, A.F.H. Evaluation and Treatment of Hypertriglyceridemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2012, 97, 2969–2989. [Google Scholar] [CrossRef]
- Hegele, R.; Ginsberg, H.N.; Chapman, M.J.; Nordestgaard, B.G.; Kuivenhoven, J.A.; Averna, M.; Borén, J.; Bruckert, E.; Catapano, A.L.; Descamps, O.S.; et al. The polygenic nature of hypertriglyceridaemia: Implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2013, 2, 655–666. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Shah, A.S.; Wilson, D.P. Primary hypertriglyceridemia in children and adolescents. J. Clin. Lipidol. 2015, 9, S20–S28. [Google Scholar] [CrossRef]
- Blackett, P.R.; Wilson, D.P.; McNeal, C.J. Secondary hypertriglyceridemia in children and adolescents. J. Clin. Lipidol. 2015, 9, S29–S40. [Google Scholar] [CrossRef]
- Christian, J.B.; Juneja, M.X.; Meadowcroft, A.M.; Borden, S.; Lowe, K.A. Prevalence, Characteristics, and Risk Factors of Elevated Triglyceride Levels in US Children. Clin. Pediatr. 2011, 50, 1103–1109. [Google Scholar] [CrossRef]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P., 3rd; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E. Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arter. Thromb Vasc Biol. 2000, 20, 836–845. [Google Scholar] [CrossRef]
- Obarzanek, E.; Kimm, S.Y.S.; Barton, B.A.; Van Horn, L.; Kwiterovich, P.O.; Simons-Morton, D.G.; Hunsberger, S.A.; Lasser, N.L.; Robson, A.M.; Franklin, F.A.; et al. Long-Term Safety and Efficacy of a Cholesterol-Lowering Diet in Children With Elevated Low-Density Lipoprotein Cholesterol: Seven-Year Results of the Dietary Intervention Study in Children (DISC). Pediatrics 2001, 107, 256–264. [Google Scholar] [CrossRef]
- Rask-Nissilä, L.; Jokinen, E.; Terho, P.; Tammi, A.; Hakanen, M.; Rönnemaa, T.; Viikari, J.; Seppänen, R.; Välimäki, I.; Helenius, H.; et al. Effects of diet on the neurologic development of children at 5 years of age: The STRIP project. J. Pediatr. 2002, 140, 328–333. [Google Scholar] [CrossRef]
- Pederiva, C.; Capra, M.; Viggiano, C.; Rovelli, V.; Banderali, G.; Biasucci, G. Early Prevention of Atherosclerosis: Detection and Management of Hypercholesterolaemia in Children and Adolescents. Life 2021, 11, 345. [Google Scholar] [CrossRef]
- Luna-Castillo, K.P.; Olivares-Ochoa, X.C.; Hernández-Ruiz, R.G.; Llamas-Covarrubias, I.M.; Rodríguez-Reyes, S.C.; Betancourt-Núñez, A.; Vizmanos, B.; Martínez-López, E.; Muñoz-Valle, J.F.; Márquez-Sandoval, F.; et al. The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence. Nutrients 2022, 14, 1104. [Google Scholar] [CrossRef]
- Bowen, K.J.; Kris-Etherton, P.M.; West, S.G.; Fleming, J.A.; Connelly, P.W.; Lamarche, B.; Couture, P.; Jenkins, D.J.A.; Taylor, C.G.; Zahradka, P.; et al. Diets Enriched with Conventional or High-Oleic Acid Canola Oils Lower Atherogenic Lipids and Lipoproteins Compared to a Diet with aWestern Fatty Acid Profile in Adults with Central Adiposity. J. Nutr. 2019, 149, 471–478. [Google Scholar] [CrossRef]
- Mateo-Gallego, R.; Marco-Benedí, V.; Perez-Calahorra, S.; Bea, A.M.; Baila-Rueda, L.; Lamiquiz-Moneo, I.; de Castro-Orós, I.; Cenarro, A.; Civeira, F. Energy-restricted, high-protein diets more effectively impact cardiometabolic profile in overweight and obese women than lower-protein diets. Clin. Nutr. 2016, 36, 371–379. [Google Scholar] [CrossRef]
- Shah, M.; Adams-Huet, B.; Franklin, B.; Phillips, M.; Mitchell, J. The Effects of High-Protein and High-Monounsaturated Fat Meals on Postprandial Lipids, Lipoprotein Particle Numbers, Cytokines, and Leptin Responses in Overweight/Obese Subjects. Metab. Syndr. Relat. Disord. 2018, 16, 150–158. [Google Scholar] [CrossRef]
- Capra, M.; Pederiva, C.; Viggiano, C.; De Santis, R.; Banderali, G.; Biasucci, G. Nutritional Approach to Prevention and Treatment of Cardiovascular Disease in Childhood. Nutrients 2021, 13, 2359. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Food Based Dietary Guidelines; United Nations: New York, NY, USA, 2021. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/background/en/ (accessed on 10 February 2023).
- Brunzell, J.D.; Deeb, S.S. Familial Lipase Deficiency, ApoC-II Deficiency and Hepatic Lipase Deficiency. In The Metabolic Basis of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; p. 2789e816. [Google Scholar]
- Pugni, L.; Riva, E.; Pietrasanta, C.; Rabacchi, C.; Bertolini, S.; Pederiva, C.; Mosca, F.; Calandra, S. Severe Hypertriglyceridemia in a Newborn with Monogenic Lipoprotein Lipase Deficiency: An Unconventional Therapeutic Approach with Exchange Transfusion. JIMD Rep.-Case Res. Rep. 2013, 13, 59–64. [Google Scholar] [CrossRef]
- Rabacchi, C.; Pisciotta, L.; Cefalù, A.B.; Noto, D.; Fresa, R.; Tarugi, P.; Averna, M.; Bertolini, S.; Calandra, S. Spectrum of mutations of the LPL gene identified in Italy in patients with severe hypertriglyceridemia. Atherosclerosis 2015, 241, 79–86. [Google Scholar] [CrossRef]
- Connor, W.E.; DeFrancesco, C.A.; Connor, S.L. N-3 fatty acids from fish oil. Effects on plasma lipoproteins and hypertriglyceridemic patients. Ann. N. Y. Acad. Sci. 1993, 683, 16–34. [Google Scholar] [CrossRef]
- Williams, L.; Rhodes, K.S.; Karmally, W.; Welstead, L.A.; Alexander, L.; Sutton, L. Familial chylomicronemia syndrome: Bringing to life dietary recommendations throughout the life span. J. Clin. Lipidol. 2018, 12, 908–919. [Google Scholar] [CrossRef]
- Williams, L.; Wilson, D.P. Editorial commentary: Dietary management of familial chylomicronemia syndrome. J. Clin. Lipidol. 2016, 10, 462–465. [Google Scholar] [CrossRef]
- Dron, J.S.; Hegele, R.A. Genetics of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 455. [Google Scholar] [CrossRef]
- Paquette, M.; Bernard, S.; Hegele, R.A.; Baass, A. Chylomicronemia: Differences between familial chylomicronemia syndrome and multifactorial chylomicronemia. Atherosclerosis 2019, 283, 137–142. [Google Scholar] [CrossRef]
- Holmes, K.W.; Kwiterovich, P.O., Jr. Treatment of dyslipidemia in children and adolescents. Curr. Cardiol. Rep. 2005, 7, 445–456. [Google Scholar] [CrossRef]
- Giovannini, M.; De Carlis, S. Raccomandazioni per la prevenzione in età pediatrica dell’aterosclerosi. Riv. Ital. Pediat. 2000, 26, 13–28. [Google Scholar]
- Jung, M.K.; Yoo, E.-G. Hypertriglyceridemia in Obese Children and Adolescents. J. Obes. Metab. Syndr. 2018, 27, 143–149. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Prevalence of abnormal lipid levels among youths: United States, 1999–2006. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 29–33. [Google Scholar]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1991. [Google Scholar] [CrossRef]
- Eslam, M.; Alkhouri, N.; Vajro, P.; Baumann, U.; Weiss, R.; Socha, P.; Marcus, C.; Lee, W.S.; Kelly, D.; Porta, G.; et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement. Lancet Gastroenterol. Hepatol. 2021, 6, 864–873. [Google Scholar] [CrossRef]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef]
- Morrison, J.; Glueck, C.J.; Woo, J.G.; Wang, P. Risk factors for cardiovascular disease and type 2 diabetes retained from childhood to adulthood predict adult outcomes: The Princeton LRC Follow-up Study. Int. J. Pediatr. Endocrinol. 2012, 2012, 6. [Google Scholar] [CrossRef]
- He, B.; Long, W.; Li, X.; Yang, W.; Chen, Y.; Zhu, Y. Sugar-Sweetened Beverages Consumption Positively Associated with the Risks of Obesity and Hypertriglyceridemia Among Children Aged 7–18 Years in South China. J. Atheroscler. Thromb. 2018, 25, 81–89. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Tofigh, A.M.; Jahangiri, L.; Nikniaz, Z.; Nikniaz, L. Sugar-sweetened beverages intake and the risk of obesity in children: An updated systematic review and dose–response meta-analysis. Pediatr. Obes. 2022, 17. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Public Meeting, Washington, DC, USA, 13 March 2020, Morning Session. Available online: https://static1.squarespace.com/static/5a4d5666bff20053c65b7ff2/t/5e73d1f7dca77767aa53472a/1584648696147/March+13%2C+2020+-+Morning+Session-+2020+Dietary+Guidelines+Advisory+Committee+Public+Meeting.pdf (accessed on 30 June 2021).
- Available online: https://www.foodnavigator.com/Article/2018/05/07/WHO-guidelines-on-saturated-fats-and-trans-fats# (accessed on 10 February 2023).
- Stelmach-Mardas, M.; Walkowiak, J. Dietary Interventions and Changes in Cardio-Metabolic Parameters in Metabolically Healthy Obese Subjects: A Systematic Review with Meta-Analysis. Nutrients 2016, 8, 455. [Google Scholar] [CrossRef]
- Banderali, G.; Capra, M.E.; Viggiano, C.; Biasucci, G.; Pederiva, C. Nutraceuticals in Paediatric Patients with Dyslipidaemia. Nutrients 2022, 14, 569. [Google Scholar] [CrossRef]
- Amatruda, M.; Ippolito, G.; Vizzuso, S.; Vizzari, G.; Banderali, G.; Verduci, E. Epigenetic Effects of n-3 LCPUFAs: A Role in Pediatric Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 2118. [Google Scholar] [CrossRef]
- Linee Guida per Una Sana Alimentazione Revisione. 2018; ISBN 9788833850375. Available online: https://www.Crea.Gov.It/En/Web/Alimenti-e-Nutrizione/-/Linee-Guida-per-Una-Sana-Alimentazione-2018 (accessed on 30 January 2023).
- Su, T.-C.; Hwang, J.-J.; Huang, K.-C.; Chiang, F.-T.; Chien, K.-L.; Wang, K.-Y.; Charng, M.-J.; Tsai, W.-C.; Lin, L.-Y.; Vige, R.; et al. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Assess the Efficacy and Safety of Ethyl-Ester Omega-3 Fatty Acid in Taiwanese Hypertriglyceridemic Patients. J. Atheroscler. Thromb. 2017, 24, 275–289. [Google Scholar] [CrossRef]
- González-Périz, A.; Horrillo, R.; Ferre, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef]
- Nobili, V.; Bedogni, G.; Alisi, A.; Pietrobattista, A.; Risé, P.; Galli, C.; Agostoni, C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: Double-blind randomised controlled clinical trial. Arch. Dis. Child. 2011, 96, 350–353. [Google Scholar] [CrossRef]
- Ahmad, Z.; Wilson, D.P. Familial chylomicronemia syndrome and response to medium-chain triglyceride therapy in an infant with novel mutations in GPIHBP1. J. Clin. Lipidol. 2014, 8, 635–639. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Elisaf, M.S. Recommendations for severe hypertriglyceridemia treatment, are there new strategies? Curr. Vasc. Pharmacol. 2014, 12, 598–616. [Google Scholar] [CrossRef]
- Rouis, M.; Dugi, K.A.; Previato, L.; Patterson, A.P.; Brunzell, J.D.; Brewer, H.B.; Santamarina-Fojo, S. Therapeutic response tomedium-chain triglycerides and omega-3 fatty acids in a patient with the familial chylomicronemia syndrome. Arterioscler Thromb. Vasc. Biol. 1997, 17, 1400–1406. [Google Scholar] [CrossRef]
- White, J.S. Challenging the Fructose Hypothesis: New Perspectives on Fructose Consumption and Metabolism. Adv. Nutr. Int. Rev. J. 2013, 4, 246–256. [Google Scholar] [CrossRef]
- Busnatu, S.-S.; Salmen, T.; Pana, M.-A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Chong, M.F.-F.; Fielding, B.; Frayn, K.N. Mechanisms for the acute effect of fructose on postprandial lipemia. Am. J. Clin. Nutr. 2007, 85, 1511–1520. [Google Scholar] [CrossRef]
- Teff, K.L.; Grudziak, J.; Townsend, R.R.; Dunn, T.N.; Grant, R.; Adams, S.; Keim, N.L.; Cummings, B.P.; Stanhope, K.L.; Havel, P. Endocrine and Metabolic Effects of Consuming Fructose- and Glucose-Sweetened Beverages with Meals in Obese Men and Women: Influence of Insulin Resistance on Plasma Triglyceride Responses. J. Clin. Endocrinol. Metab. 2009, 94, 1562–1569. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjærg-Hansen, A. Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef]
- David Wang, D.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014, 232, 125–133. [Google Scholar] [CrossRef]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting Compared With Nonfasting Triglycerides and Risk of Cardiovascular Events in Women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef]
- Aeberli, I.; Zimmermann, M.B.; Molinari, L.; Lehmann, R.; L’Allemand, D.; Spinas, G.; Berneis, K. Fructose intake is a predictor of LDL particle size in overweight schoolchildren. Am. J. Clin. Nutr. 2007, 86, 1174–1178. [Google Scholar] [CrossRef]
- The Korea National Health and Nutrition Examination Survey (KNHNES). Dietary Intake Survey of Infant, Children and Adolescents; The Korea National Health and Nutrition Examination Survey: Cheongju, Korea, 2011.
- Gungor, A.; Balamtekin, N.; Ozkececi, C.F.; Aydin, H.I. The Relationship between Daily Fructose Consumption and Oxidized Low-Density Lipoprotein and Low-Density Lipoprotein Particle Size in Children with Obesity. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 483–491. [Google Scholar] [CrossRef]
- DiStefano, J.K. Fructose-mediated effects on gene expression and epigenetic mechanisms associated with NAFLD pathogenesis. Cell. Mol. Life Sci. 2019, 77, 2079–2090. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Anker, C.C.B.; Rafiq, S.; Jeppesen, P.B. Effect of Steviol Glycosides on Human Health with Emphasis on Type 2 Diabetic Biomarkers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 1965. [Google Scholar] [CrossRef]
- Curi, R.; Alvarez, M.; Bazotte, R.B.; Botion, L.; Godoy, J.L.; Bracht, A. Effect of Stevia rebaudiana on glucose tolerance in normal adult humans. Braz. J. Med Biol. Res. 1986, 19, 771–774. [Google Scholar]
- Geuns, J.M.C.; Buyse, J.; Vankeirsbilck, A.; Temme, E.H.M. Metabolism of stevioside by healthy subjects. Exp. Biol. Med. 2007, 232, 164–173. [Google Scholar]
- Jeppesen, P.; Barriocanal, L.; Meyer, M.; Palacios, M.; Canete, F.; Benitez, S.; Logwin, S.; Schupmann, Y.; Benitez, G.; Jimenez, J. Efficacy and tolerability of oral stevioside in patients with type 2 diabetes: A long-term, randomized, double-blinded, placebo-controlled study. Diabetologia 2006, 49 (Suppl. S1), 511–512. [Google Scholar]
- Ritu, M.; Nandini, J. Nutritional composition of Stevia rebaudiana, a sweet herb, and its hypoglycaemic and hypolipidaemic effect on patients with non-insulin dependent diabetes mellitus. J. Sci. Food Agric. 2016, 96, 4231–4234. [Google Scholar] [CrossRef]
- Aswathiah, S.; Prabhu, S.K.; Lingaiah, R.; Ramanna, A.; Prabhu, J.S.; Pankaj, S.K.; Mehta, A.; Bapna, A.; Raghavan, G. Effect of a Novel Sugar Blend on Weight and Cardiometabolic Health among Healthy Indian Adults: A Randomized, Open-Label Study. Foods 2022, 11, 3545. [Google Scholar] [CrossRef]
- Merino, J.; Jablonski, K.A.; Mercader, J.M.; Kahn, S.E.; Chen, L.; Harden, M.; Delahanty, L.M.; Araneta, M.R.G.; Walford, G.A.; Jacobs, S.B.; et al. Interaction Between Type 2 Diabetes Prevention Strategies and Genetic Determinants of Coronary Artery Disease on Cardiometabolic Risk Factors. Diabetes 2019, 69, 112–120. [Google Scholar] [CrossRef]
- Arpón, A.; Milagro, F.I.; Razquin, C.; Corella, D.; Estruch, R.; Fitó, M.; Marti, A.; Martínez-González, M.A.; Ros, E.; Salas-Salvadó, J.; et al. Impact of Consuming Extra-Virgin Olive Oil or Nuts within a Mediterranean Diet on DNA Methylation in Peripheral White Blood Cells within the PREDIMED-Navarra Randomized Controlled Trial: A Role for Dietary Lipids. Nutrients 2017, 10, 15. [Google Scholar] [CrossRef]

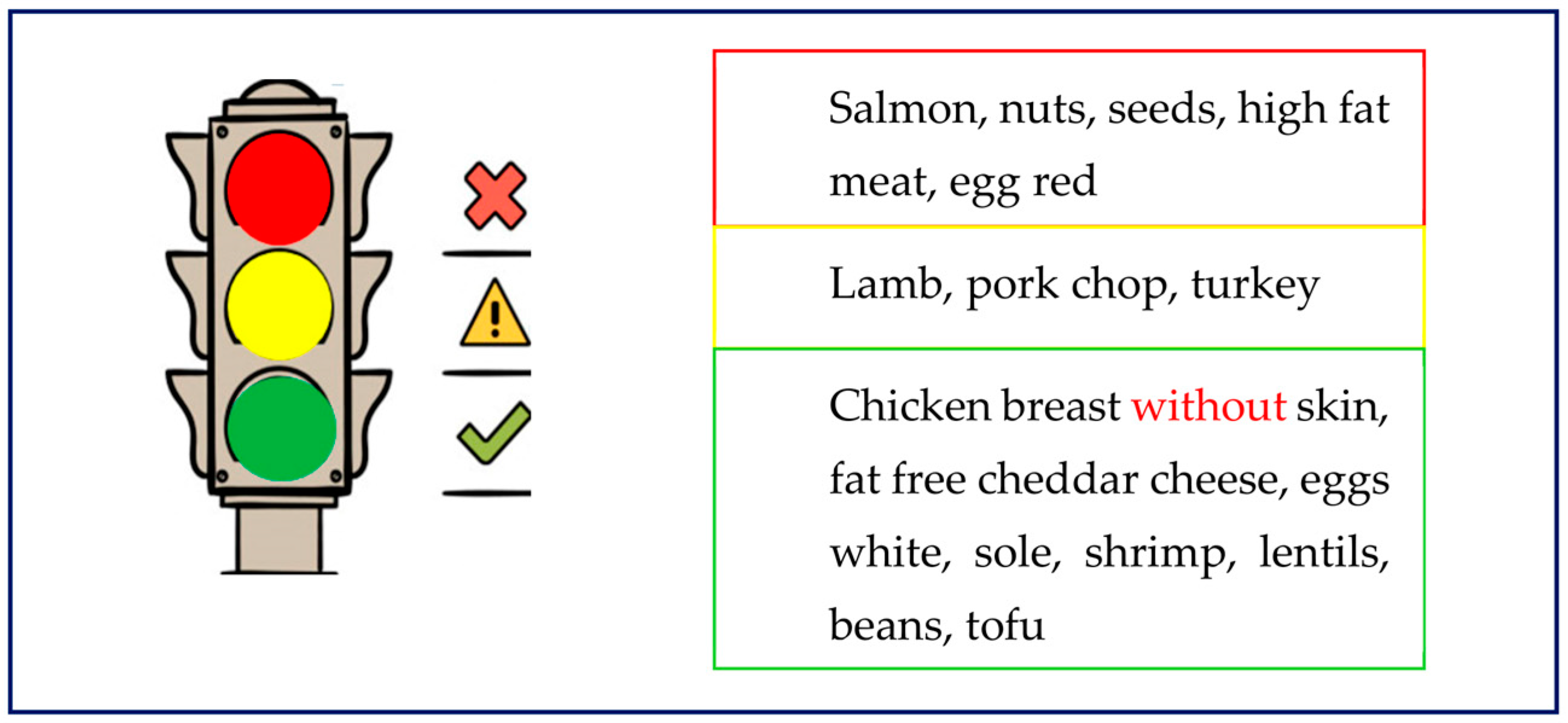
| The Endocrine Society | |
|---|---|
| Normal TG | <150 mg/dL |
| Mild HTG | 150–199 mg/dL |
| Moderate HTG | 200–999 mg/dL |
| Severe HTG | 1000–1999 mg/dL |
| Very severe HTG | ≥2000 mg/dL |
| The European Society Consensus Panel | |
|---|---|
| Normal | <150 mg/dL |
| HTG | 150–885 mg/dL |
| Severe HTG | ≥885 mg/dL |
| NCEP ATP III | |
|---|---|
| Normal TG | <150 mg/dL |
| Borderline-high TG | 150–199 mg/dL |
| High TG | 200–499 mg/dL |
| Very high TG | ≥500 mg/dL |
| Test | Acceptable | Borderline | High |
|---|---|---|---|
| Total cholesterol | <170 | 170–199 | ≥200 |
| LDL-cholesterol | <110 | 110–129 | ≥130 |
| Triglycerides | |||
| 0–9 years | <75 | 75–99 | ≥100 |
| 10–19 years | <90 | 90–129 | ≥130 |
| HDL-cholesterol | >45 | 40–45 | <40 |
| Forms of HTG | |
|---|---|
| Primary | Secondary |
|
|
| Age | Recommendation |
|---|---|
| Infant (0–12 months) |
|
| Toddler (12–24 months) |
|
| School-age children and adolescents |
|
| Dietary Recommendations for Pediatric Patients with Weight Excess | |
|---|---|
| Item | Specific Recommendation |
| Number of meals | 3 main meals and 2 snacks per day, breakfast every day |
| Type of carbohydrates | Substitute simple sugars with complex carbohydrtaes, |
| Type of snacks | Avoid high-energy and low-nutrient food, no sugar-sweetened beverages |
| Fruits/vegetables | 5 portions per day |
| Energy and macronutrients intake | Daily energy and macronutrient intake should fulfill the National Recommended Energy and Nutrient Intake Levels according to gender, age, and ideal weight for stature: protein 1 g/kg/day, carbohydrates 45–60% of total daily energy, lipids 20–30% of total daily energy, saturated fatty acids <10% of total daily energy |
| Food Item | Study | References |
|---|---|---|
| Sugar sweetened beverages |
| He et al. 2018 [41] |
| Farhangi et al. 2022 [42] | |
| Saturated fats |
| U.S. guidelines 2020 [43] WHO guidelines [44] |
| FAO guidelines 2021 [23] |
| Lipid and DHA Content in Fish, Seafood, and Meat | ||
|---|---|---|
| Food | Lipids (g) per 100 g of Food | DHA % of Total Lipids |
| Cod, deep frozen, roasted in oven | 0.9 | 38.65 |
| Round Sardinella, fresh | 4.5 | 29.29 |
| Tuna, fresh | 8.1 | 26.54 |
| Sole, fresh | 1.4 | 25.97 |
| Salmon, fresh | 12 | 11.27 |
| Liver, chicken, raw | 6.3 | 4.78 |
| Turkey, whole, with skin, raw | 6.9 | 4.54 |
| Beef, front part cuts | 7 | 1.50 |
| Beef, flank steak and brisket, lean only | 10.2 | 1.48 |
| Turkey, leg, with skin, raw | 6 | 1.48 |
| Beef, sirloin-steak, lean only | 5.2 | 1.34 |
| Beef, rib, lean only | 6.1 | 1.33 |
| Chicken, whole, with skin, raw | 10.6 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capra, M.E.; Biasucci, G.; Banderali, G.; Pederiva, C. Nutritional Treatment of Hypertriglyceridemia in Childhood: From Healthy-Heart Counselling to Life-Saving Diet. Nutrients 2023, 15, 1088. https://doi.org/10.3390/nu15051088
Capra ME, Biasucci G, Banderali G, Pederiva C. Nutritional Treatment of Hypertriglyceridemia in Childhood: From Healthy-Heart Counselling to Life-Saving Diet. Nutrients. 2023; 15(5):1088. https://doi.org/10.3390/nu15051088
Chicago/Turabian StyleCapra, Maria Elena, Giacomo Biasucci, Giuseppe Banderali, and Cristina Pederiva. 2023. "Nutritional Treatment of Hypertriglyceridemia in Childhood: From Healthy-Heart Counselling to Life-Saving Diet" Nutrients 15, no. 5: 1088. https://doi.org/10.3390/nu15051088
APA StyleCapra, M. E., Biasucci, G., Banderali, G., & Pederiva, C. (2023). Nutritional Treatment of Hypertriglyceridemia in Childhood: From Healthy-Heart Counselling to Life-Saving Diet. Nutrients, 15(5), 1088. https://doi.org/10.3390/nu15051088







