Dietary Supplementation of Cedryl Acetate Ameliorates Adiposity and Improves Glucose Homeostasis in High-Fat Diet-Fed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Design
2.2. Dosage Information
2.3. Oral Glucose Tolerance Test (OGTT) and Fasting Blood Glucose (FBG)
2.4. Insulin Tolerance Test (ITT)
2.5. Pyruvate Tolerance Test (PTT)
2.6. Serum Biochemical Analysis
2.7. Histopathological Analysis
2.8. Gut Microbiota Analysis
2.9. Real-Time Quantitative PCR (RT-qPCR)
2.10. Statistical Analysis
3. Results
3.1. CA Has a Significant Preventive Effect against HFD-Induced Body Weight Gain in Mice
3.2. CA Dramatically Decreases Visceral Fat Pad Weight, Attenuates Adipocyte Hypertrophy, and Improves Serum Lipid Profile in Mice
3.3. CA Improves Glucose Intolerance and Insulin Resistance in Mice
3.4. CA Inhibits Hepatic Gluconeogenesis in HFD-Fed Mice
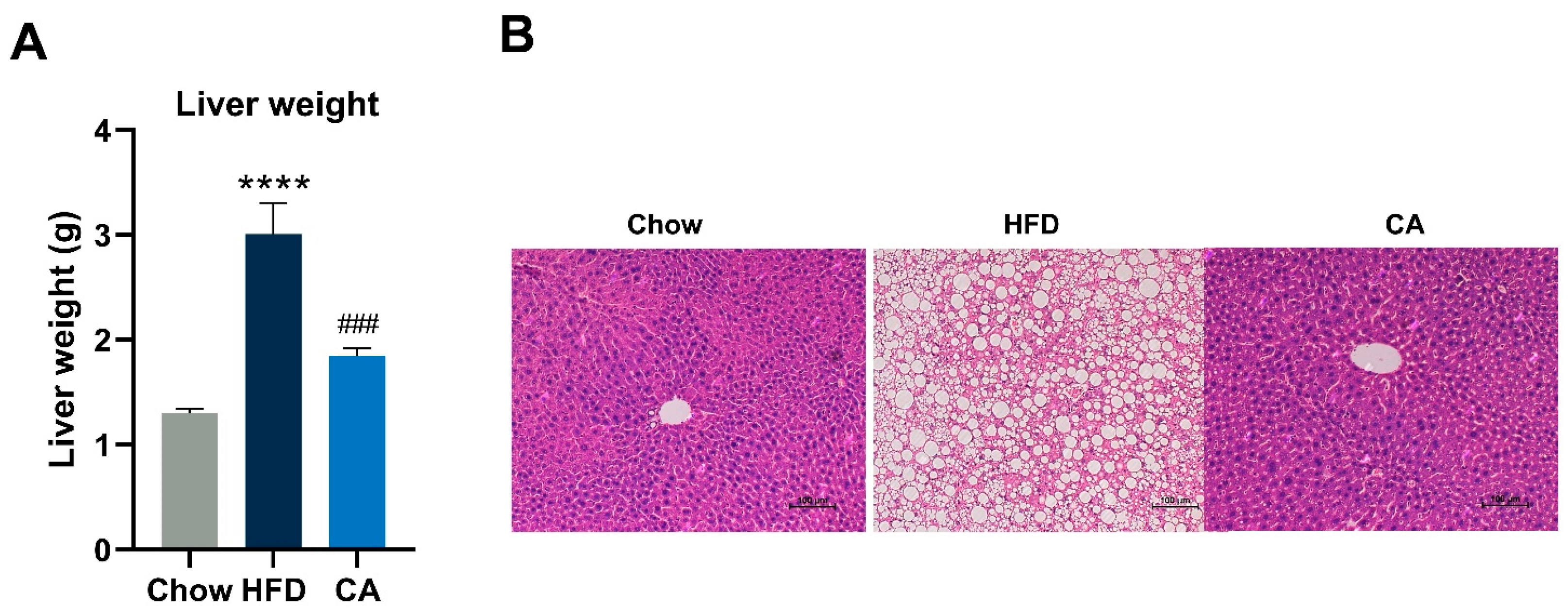
3.5. CA Protectes against Hepatic Lipid Accumulation in Mice
3.6. Limited Effects of CA on the Modifying Gut Microbiota in HFD-Fed Mice
3.7. CA Alters the Expression of Metabolic Genes in eWAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowley, V.E.F.; Yeo, G.S.H.; O’Rahilly, S. Obesity Therapy: Altering the Energy Intake-and-Expenditure Balance Sheet. Nat. Rev. Drug Discov. 2002, 1, 276–286. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 September 2022).
- Goedeke, L.; Perry, R.J.; Shulman, G.I. Emerging Pharmacological Targets for the Treatment of Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Type 2 Diabetes. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Pavanello, C.; Calabresi, L.; Ruscica, M. Nutraceutical Approaches to Metabolic Syndrome. Ann. Med. 2017, 49, 678–697. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Wang, Y.; Geng, R.; Fang, J.; Liu, Q.; Kang, S.G.; Zeng, W.C.; Huang, K.; Tong, T. Understanding the Mechanism Underlying the Anti-Diabetic Effect of Dietary Component: A Focus on Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–21. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Fang, J.; Geng, R.; Li, M.; Zhao, Y.; Kang, S.G.; Huang, K.; Tong, T. Oleuropein Ameliorates Advanced Stage of Type 2 Diabetes in Db/Db Mice by Regulating Gut Microbiota. Nutrients 2021, 13, 2131. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The Microbiota-Gut-Brain Axis in Obesity. Lancet Gastroenterol. 2017, 2, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Y.; Wang, Y.; Geng, R.; Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Eugenol, a Major Component of Clove Oil, Attenuates Adiposity, and Modulates Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2022, 66, e2200387. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yuk, H.G.; Ko, S.G.; Cho, S.G.; Moon, G.S. Gut Microbiome Prolongs an Inhibitory Effect of Korean Red Ginseng on High-Fat-Diet-Induced Mouse Obesity. Nutrients 2021, 13, 926. [Google Scholar] [CrossRef]
- Terzo, S.; Mule, F.; Caldara, G.F.; Baldassano, S.; Puleio, R.; Vitale, M.; Cassata, G.; Ferrantelli, V.; Amato, A. Pistachio Consumption Alleviates Inflammation and Improves Gut Microbiota Composition in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2020, 21, 365. [Google Scholar] [CrossRef]
- Lei, H.; Wang, Y.; Liang, F.; Su, W.; Feng, Y.; Guo, X.; Wang, N. Composition and Variability of Essential Oils of Platycladus Orientalis Growing in China. Biochem. Syst. Ecol. 2010, 38, 1000–1006. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Argentieri, M.P.; Laquale, S.; Candido, V.; Avato, P. Relationship between Chemical Composition and Nematicidal Activity of Different Essential Oils. Plants 2020, 9, 1546. [Google Scholar] [CrossRef]
- FDA. Cedryl Acetate. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=FoodSubstances&id=CEDRYLACETATE (accessed on 28 September 2022).
- Sultan, S.; Choudhary, M.I.; Khan, S.N.; Fatima, U.; Atif, M.; Ali, R.A.; Rahman, A.U.; Fatmi, M.Q. Fungal Transformation of Cedryl Acetate and A-Glucosidase Inhibition Assay, Quantum Mechanical Calculations and Molecular Docking Studies of Its Metabolites. Eur. J. Med. Chem. 2013, 62, 764–770. [Google Scholar] [CrossRef]
- Wang, S.; Liu, F.; Zhou, Q.; Xu, S.; Huang, S.; Luo, J. Preparation of Cedrol and Cedryl Acetate and Their Antifungal Activities. J. For. Res. 2021, 6, 74–80. (In Chinese) [Google Scholar]
- Tong, T.; Yu, R.; Park, T. A-Cedrene Protects Rodents from High-Fat Diet-Induced Adiposity Via Adenylyl Cyclase 3. Int. J. Obes. 2019, 43, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kang, S.-G.; Huang, K.; Tong, T. Dietary Supplementation of Methyl Cedryl Ether Ameliorates Adiposity in High-Fat Diet-Fed Mice. Nutrients 2023, 15, 788. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, T. Genes Are Differentially Expressed in the Epididymal Fat of Rats Rendered Obese by a High-Fat Diet. Nutr. Res. 2008, 28, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2007, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food Flavourings and Compounds of Related Structure I. Acute Oral Toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. [Google Scholar] [CrossRef]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of High-Fat Diet on Gut Microbiota: A Driving Force for Chronic Disease Risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial Dysbiosis-Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Moossavi, S.; Bishehsari, F. Microbes: Possible Link between Modern Lifestyle Transition and the Rise of Metabolic Syndrome. Obes. Rev. 2019, 20, 407–419. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.t.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.R. Obese-Type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhanel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-Chain Fatty Acids and Inulin, but Not Guar Gum, Prevent Diet-Induced Obesity and Insulin Resistance through Differential Mechanisms in Mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The Role of Gut Microbiota in the Resistance to Obesity in Mice Fed a High Fat Diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Chang, W.T.; Li, S.; Wu, J.C.; Badmeav, V.; Ho, C.T.; Pan, M.H. Citrus Peel Extracts Attenuated Obesity and Modulated Gut Microbiota in Mice with High-Fat Diet-Induced Obesity. Food Funct. 2018, 9, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, T.; Li, Y.; Tang, Y.; Huang, Z. Gut Microbiota Are Associated with Sex and Age of Host: Evidence from Semi-Provisioned Rhesus Macaques in Southwest Guangxi, China. Ecol. Evol. 2021, 11, 8096–8122. [Google Scholar] [CrossRef] [PubMed]
- Valeri, F.; Endres, K. How Biological Sex of the Host Shapes Its Gut Microbiota. Front. Neuroendocrinol. 2021, 61, 100912. [Google Scholar] [CrossRef] [PubMed]
- Allali, I.; Arnold, J.W.; Roach, J.; Cadenas, M.B.; Butz, N.; Hassan, H.M.; Koci, M.; Ballou, A.; Mendoza, M.; Ali, R.; et al. A Comparison of Sequencing Platforms and Bioinformatics Pipelines for Compositional Analysis of the Gut Microbiome. BMC Microbiol. 2017, 17, 194. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping Faecal Gut Microbiota Composition by the Intake of Trans-Resveratrol and Quercetin in High-Fat Sucrose Diet-Fed Rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Zou, J.; Tran, H.Q.; Chassaing, B.; Gewirtz, A.T. Amelioration of Metabolic Syndrome by Metformin Associates with Reduced Indices of Low-Grade Inflammation Independently of the Gut Microbiota. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1121–E1130. [Google Scholar] [CrossRef]
- Miranda, C.L.; Johnson, L.A.; de Montgolfier, O.; Elias, V.D.; Ullrich, L.S.; Hay, J.J.; Paraiso, I.L.; Choi, J.; Reed, R.L.; Revel, J.S.; et al. Non-Estrogenic Xanthohumol Derivatives Mitigate Insulin Resistance and Cognitive Impairment in High-Fat Diet-Induced Obese Mice. Sci. Rep. 2018, 8, 613. [Google Scholar] [CrossRef]
- Zhang, Y.; Bobe, G.; Revel, J.S.; Rodrigues, R.R.; Sharpton, T.J.; Fantacone, M.L.; Raslan, K.; Miranda, C.L.; Lowry, M.B.; Blakemore, P.R.; et al. Improvements in Metabolic Syndrome by Xanthohumol Derivatives Are Linked to Altered Gut Microbiota and Bile Acid Metabolism. Mol. Nutr. Food Res. 2020, 64, e1900789. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green Tea Polyphenol (Epigallocatechin-3-Gallate) Improves Gut Dysbiosis and Serum Bile Acids Dysregulation in High-Fat Diet-Fed Mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Larose, L. Harnessing Adipogenesis to Prevent Obesity. Adipocyte 2019, 8, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miskic, B.; Miksic, S.; Juranic, B.; Cosic, V.; Schwarz, D.; Vcev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of Srebp by a Small Molecule, Betulin, Improves Hyperlipidemia and Insulin Resistance and Reduces Atherosclerotic Plaques. Cell Metab. 2011, 13, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Park, K.W.; Mitro, N.; Pei, L.; Damoiseaux, R.; Wilpitz, D.C.; Reue, K.; Saez, E.; Tontonoz, P. The Small Molecule Harmine Is an Antidiabetic Cell-Type-Specific Regulator of Ppargamma Expression. Cell Metab. 2007, 5, 357–370. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Tong, T.; Ryu, S.E.; Min, Y.; de March, C.A.; Bushdid, C.; Golebiowski, J.; Moon, C.; Park, T. Olfactory Receptor 10j5 Responding to A-Cedrene Regulates Hepatic Steatosis Via the Camp–Pka Pathway. Sci. Rep. 2017, 7, 9471. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and Insulin Resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of Nonalcoholic Hepatic Steatosis, Hepatic Insulin Resistance, and Hyperglycemia by Moderate Weight Reduction in Patients with Type 2 Diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef]
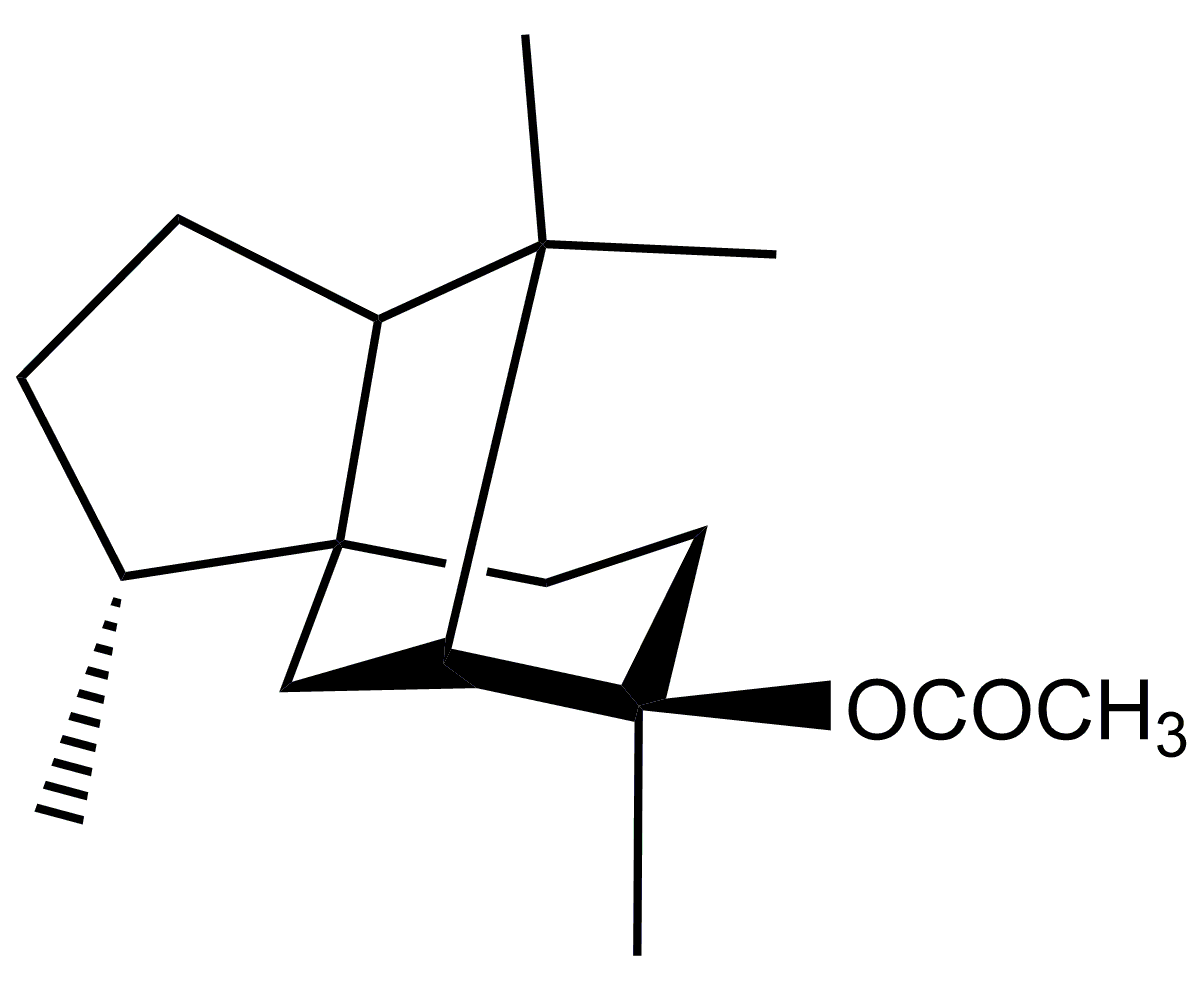






| Ingredients | HFD Group | CA Group |
|---|---|---|
| Casein | 200 | 200 |
| DL-Methionine | 3 | 3 |
| Corn starch | 111 | 110 |
| Sucrose | 370 | 370 |
| Cellulose | 50 | 50 |
| Corn oil | 30 | 30 |
| Lard | 170 | 170 |
| AIN-76A Mineral mixture | 42 | 42 |
| AIN-76A Vitamin mixture | 12 | 12 |
| Choline bitartrate | 2 | 2 |
| Cholesterol | 10 | 10 |
| Cedryl acetate | - | 1 |
| tert-Butylhydroquinone | 0.04 | 0.04 |
| Fat, % kJ | 40 | 40 |
| Target Genes | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|
| C/EBPα | TCAGCTTACAACAGGCCAGG | ACACAAGGCTAATGGTCCCC |
| Cidea | GGAATCTGCTGAGGTTTATG | ATCCCACAGCCTATAACAGA |
| COX4 | GTACCGCATCCAGTTTAACGA | CCATACACATAGCTCTTCTCCCA |
| Cytc | ACACTGTGGAAAAGGGAGGC | GCACTGGTTAACCCAAGCAA |
| FABP4 | CATGCGACAAAGGCAGAAAT | GTTACAAGGCAAGGAAGGGC |
| FAS | TTGCTGGCACTACAGAATGC | AACAGCCTCAGAGCGACAAT |
| Fbp1 | GTGTCAACTGCTTCATGCTG | GAGATACTCATTGATGGCAGGG |
| G6pase | CGACTCGCTATCTCCAAGTGA | GTTGAACCAGTCTCCGACCA |
| Pepck | CCATCACCTCCTGGAAGAACA | ACCCTCAATGGGTACTCCTTCTG |
| PGC-1α | AAATCTGCGGGATGATGGA | GTTTCGTTCGACCTGCGTAA |
| PPARγ | TTCGGAATCAGCTCTGTGGA | CCATTGGGTCAGCTCTTGTG |
| PRDM16 | CCACCAGCGAGGACTTCAC | GGAGGACTCTCGTAGCTCGAA |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| Chow | HFD | CA | |
|---|---|---|---|
| Initial body weight (g) | 23.85 ± 0.39 | 24.93 ± 0.34 | 24.75 ± 0.39 |
| Final body weight (g) | 29.84 ± 0.54 | 46.61 ± 1.39 **** | 33.68 ± 0.92 #### |
| Body weight gain (g) | 5.99 ± 0.41 | 21.69 ± 1.41 **** | 8.93 ± 0.90 #### |
| Food intake (g/day) | 4.09 ± 0.02 | 3.32 ± 0.10 ** | 3.20 ± 0.10 |
| Chow | HFD | CA | |
|---|---|---|---|
| LDL-C (mmol/L) | 0.17 ± 0.01 | 1.49 ± 0.25 **** | 0.72 ± 0.11 ## |
| HDL-C (mmol/L) | 3.46 ± 0.18 | 6.57 ± 0.24 **** | 4.83 ± 0.51 ## |
| TC (mmol/L) | 3.06 ± 0.18 | 8.57 ± 0.74 **** | 4.70 ± 0.35 #### |
| ALT (U/L) | 43.1 ± 4.0 | 237.3 ± 60.4 ** | 61.4 ± 11.8 ## |
| AST (mmol/L) | 369 ± 28 | 568 ± 67 * | 429 ± 50 |
| Chow | HFD | CA | |
|---|---|---|---|
| Phylum | |||
| Firmicutes | 32.67% | 73.61% **** | 78.82% |
| Bacteroidota | 52.75% | 11.08% **** | 7.49% |
| Deferribacteres | 0.63% | 2.54% | 3.56% |
| Desulfobacterota | 1.81% | 2.58% | 0.72% |
| Proteobacteria | 0.76% | 1.09% | 1.49% |
| Actinobacteriota | 0.84% | 1.54% | 1.00% |
| Acidobacteriota | 0.35% | 0.53% | 0.41% |
| Campylobacterota | 0.08% | 0.10% | 0.21% |
| Chloroflexi | 0.12% | 0.2% | 0.18% |
| Ratio of F/B | 0.64 | 9.50 * | 13.92 |
| Family | |||
| Erysipelotrichaceae | 1.55% | 40.92% ** | 46.17% |
| Muribaculaceae | 44.91% | 9.22% **** | 4.79% |
| Lachnospiraceae | 19.34% | 21.39% | 19.25% |
| Oscillospiraceae | 5.29% | 5.11% | 4.88% |
| Lactobacillaceae | 2.1% | 2.99% | 4.42% |
| Deferribacteraceae | 0.63% | 2.54% | 3.56% |
| Streptococcaceae | 0.38% | 2.62% * | 1.68% |
| Bacteroidaceae | 0.45% | 0.26% | 1.77% |
| Desulfovibrionaceae | 1.78% | 2.52% | 0.66% |
| Rikenellaceae | 3.42% | 0.96% ** | 0.55% |
| Prevotellaceae | 2.24% | 0.26% * | 0.15% |
| Saccharimonadaceae | 2.08% | 0.04% * | 0.01% |
| Erysipelatoclostridiaceae | 0.08% | 0.12% | 1.01% |
| Marinifilaceae | 1.47% | 0.03% ** | 0.01% |
| Ruminococcaceae | 1.88% | 1.08% | 1.15% |
| Atopobiaceae | 0.08% | 0.54% | 0.11% |
| Bifidobacteriaceae | 0.04% | 0.32% | 0.50% |
| Sutterellaceae | 0.18% | 0.07% | 0.64% ## |
| Eggerthellaceae | 0.43% | 0.41% | 0.17% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, M.; Zhao, Y.; Kang, S.-G.; Huang, K.; Tong, T. Dietary Supplementation of Cedryl Acetate Ameliorates Adiposity and Improves Glucose Homeostasis in High-Fat Diet-Fed Mice. Nutrients 2023, 15, 980. https://doi.org/10.3390/nu15040980
Guo J, Li M, Zhao Y, Kang S-G, Huang K, Tong T. Dietary Supplementation of Cedryl Acetate Ameliorates Adiposity and Improves Glucose Homeostasis in High-Fat Diet-Fed Mice. Nutrients. 2023; 15(4):980. https://doi.org/10.3390/nu15040980
Chicago/Turabian StyleGuo, Jingya, Mengjie Li, Yuhan Zhao, Seong-Gook Kang, Kunlun Huang, and Tao Tong. 2023. "Dietary Supplementation of Cedryl Acetate Ameliorates Adiposity and Improves Glucose Homeostasis in High-Fat Diet-Fed Mice" Nutrients 15, no. 4: 980. https://doi.org/10.3390/nu15040980
APA StyleGuo, J., Li, M., Zhao, Y., Kang, S.-G., Huang, K., & Tong, T. (2023). Dietary Supplementation of Cedryl Acetate Ameliorates Adiposity and Improves Glucose Homeostasis in High-Fat Diet-Fed Mice. Nutrients, 15(4), 980. https://doi.org/10.3390/nu15040980







