Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design
2.3. Antioxidant Testing of Black Chokeberry Extract
2.3.1. DPPH Antioxidant Test
2.3.2. ABTS Antioxidant Test
2.4. Participants
2.5. Supplementation
2.6. Exercise Protocol
2.7. Blood Collection and Analysis
2.8. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahri-Sahloul, R.; Ben Fredj, R.; Boughalleb, N.; Shriaa, J.; Saguem, S.; Hilbert, J.-L.; Trotin, F.; Ammar, S.; Bouzid, S.; Harzallah-Skhiri, F. Phenolic Composition and Antioxidant and Antimicrobial Activities of Extracts Obtained from Crataegus azarolus L. var. aronia (Willd.). Batt. Ovaries Calli. J. Bot. 2014, 2014, 623651. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef]
- Reeder, B.J.; Wilson, M.T. The effects of pH on the mechanism of hydrogen peroxide and lipid hydroperoxide consumption by myoglobin: A role for the protonated ferryl species. Free Radic. Biol. Med. 2001, 30, 1311–1318. [Google Scholar] [CrossRef]
- Halon-Golabek, M.; Borkowska, A.; Herman-Antosiewicz, A.; Antosiewicz, J. Iron Metabolism of the Skeletal Muscle and Neurodegeneration. Front. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Training surface and intensity: Inflammation, hemolysis, and hepcidin expression. Med. Sci. Sport Exerc. 2009, 41, 1138–1145. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Basta, P.; Sadowska, J.; Pilaczyńska-Szcześniak, L. Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers. J. Int. Soc. Sport Nutr. 2014, 11, 014–0048. [Google Scholar] [CrossRef] [PubMed]
- Skarpanska-Stejnborn, A.; Basta, P.; Trzeciak, J.; Michalska, A.; Kafkas, M.E.; Woitas-Slubowska, D. Effects of cranberry (Vaccinum macrocarpon) supplementation on iron status and inflammatory markers in rowers. J. Int. Soc. Sport Nutr. 2017, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Kortas, J.; Kuchta, A.; Prusik, K.; Prusik, K.; Ziemann, E.; Labudda, S.; Cwiklinska, A.; Wieczorek, E.; Jankowski, M.; Antosiewicz, J. Nordic walking training attenuation of oxidative stress in association with a drop in body iron stores in elderly women. Biogerontology 2017, 18, 517–524. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Kochanowicz, M.; Zychowska, M.; Kochanowicz, A.; Grzybkowska, A.; Anczykowska, K.; Sawicki, P.; Borkowska, A.; Niespodzinski, B.; Antosiewicz, J. Ferritin Genes Overexpression in PBMC and a Rise in Exercise Performance as an Adaptive Response to Ischaemic Preconditioning in Young Men. Biomed. Res. Int. 2019, 2019, 9576876. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Grünblatt, E.; Mandel, S. The pivotal role of iron in NF-kappa B activation and nigrostriatal dopaminergic neurodegeneration. Prospects for neuroprotection in Parkinson’s disease with iron chelators. Ann. N. Y. Acad. Sci. 1999, 890, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Léger, L.A.; Mercier, D.; Gadoury, C.; Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sport. Sci. 1988, 6, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The influence of chokeberry juice supplementation on the reduction of oxidative stress resulting from an incremental rowing ergometer exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, N.B.; Shearman, J.P.; Cooper, C.E. Exercise-induced oxidative stress:myths, realities and physiological relevance. Sports Med. 2005, 35, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, B.; Cieslicka, M.; Kujawski, S.; Piskorska, E.; Kowalik, T.; Korycka, J.; Skarpanska-Stejnborn, A. Effects of antioxidant supplementation on oxidative stress balance in young footballers- a randomized double-blind trial. J. Int. Soc. Sport Nutr. 2021, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Boersma, M.G.; Haan, L.; Spenkelink, B.; Awad, H.M.; Cnubben, N.H.; van Zanden, J.J.; Woude, H.; Alink, G.M.; Koeman, J.H. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Pharmacol. 2002, 11, 321–333. [Google Scholar] [CrossRef]
- Iwashima, T.; Kudome, Y.; Kishimoto, Y.; Saita, E.; Tanaka, M.; Taguchi, C.; Hirakawa, S.; Mitani, N.; Kondo, K.; Iida, K. Aronia berry extract inhibits TNF-α-induced vascular endothelial inflammation through the regulation of STAT3. Food Nutr. Res. 2019, 63, 3361. [Google Scholar] [CrossRef]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar] [CrossRef]
- Barak, V.; Birkenfeld, S.; Halperin, T.; Kalickman, I. The effect of herbal remedies on the production of human inflammatory and anti-inflammatory cytokines. Isr. Med. Assoc. J. IMAJ 2002, 4, 919–922. [Google Scholar]
- Aaseth, J.; Stoa Birketvedt, G. Hemolysis and rhabdomyolysis after marathon and long distance running. Immunol. Endocr. Metab. Agents Med. Chem. 2012, 12, 8–13. [Google Scholar] [CrossRef]
- Antosiewicz, J.; Kaczor, J.J.; Kasprowicz, K.; Laskowski, R.; Kujach, S.; Luszczyk, M.; Radziminski, L.; Ziemann, E. Repeated “all out” interval exercise causes an increase in serum hepcidin concentration in both trained and untrained men. Cell. Immunol. 2013, 283, 12–17. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
| Method | Group Division |
|---|---|
| Antioxidant Potential [μmol Trolox/cm3] | |
| DPPH | 172.4 ± 0.7 |
| ABTS | 211.1 ± 0.61 |
| Variable | Supplemented (n = 10) | Control (Placebo) (n = 12) | ||||
|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |
| Age (years) | 19.86 ± 0.61 | – | – | 20.05 ± 0.52 | – | – |
| Body height (cm) | 180.00 ± 4.65 * | – | – | 176.12 ± 4.21 | – | – |
| Body mass (kg) | 68.86 ± 6.49 | 67.54 ± 6.17 | 0.63 | 64.86 ± 5.46 | 65.12 ± 6.19 | 0.91 |
| Body mass index | 21.27 ± 1.75 | 21.04 ± 1.66 | 0.75 | 20.63 ± 1.38 | 20.85 ± 1.44 | 0.71 |
| Fat mass (%) | 10.80 ± 1.39 | 10.52 ± 1.34 | 0.64 | 9.55 ± 2.43 | 9.67 ± 2.53 | 0.90 |
| Variable | Supplemented (n = 12) | Control (Placebo) (n = 10) | Effect | p-Value | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| Distance (km) | 2.50 ± 0.20 | 2.82 ± 0.18 # | 2.47 ± 0.32 | 2.59 ± 0.19 | GR RM GR × RM | 0.21 0.01 ** 0.01 * |
| Lactate (mM) delta change | 8.24 ± 1.71 | 9.47 ± 1.85 # | 9.00 ± 1.72 | 7.79 ± 1.53 | GR RM GR × RM | 0.49 0.98 0.01 ** |
| Variable | Effect | F | df | p-Value | Effect Size (η2) | Post-hoc Outcome |
|---|---|---|---|---|---|---|
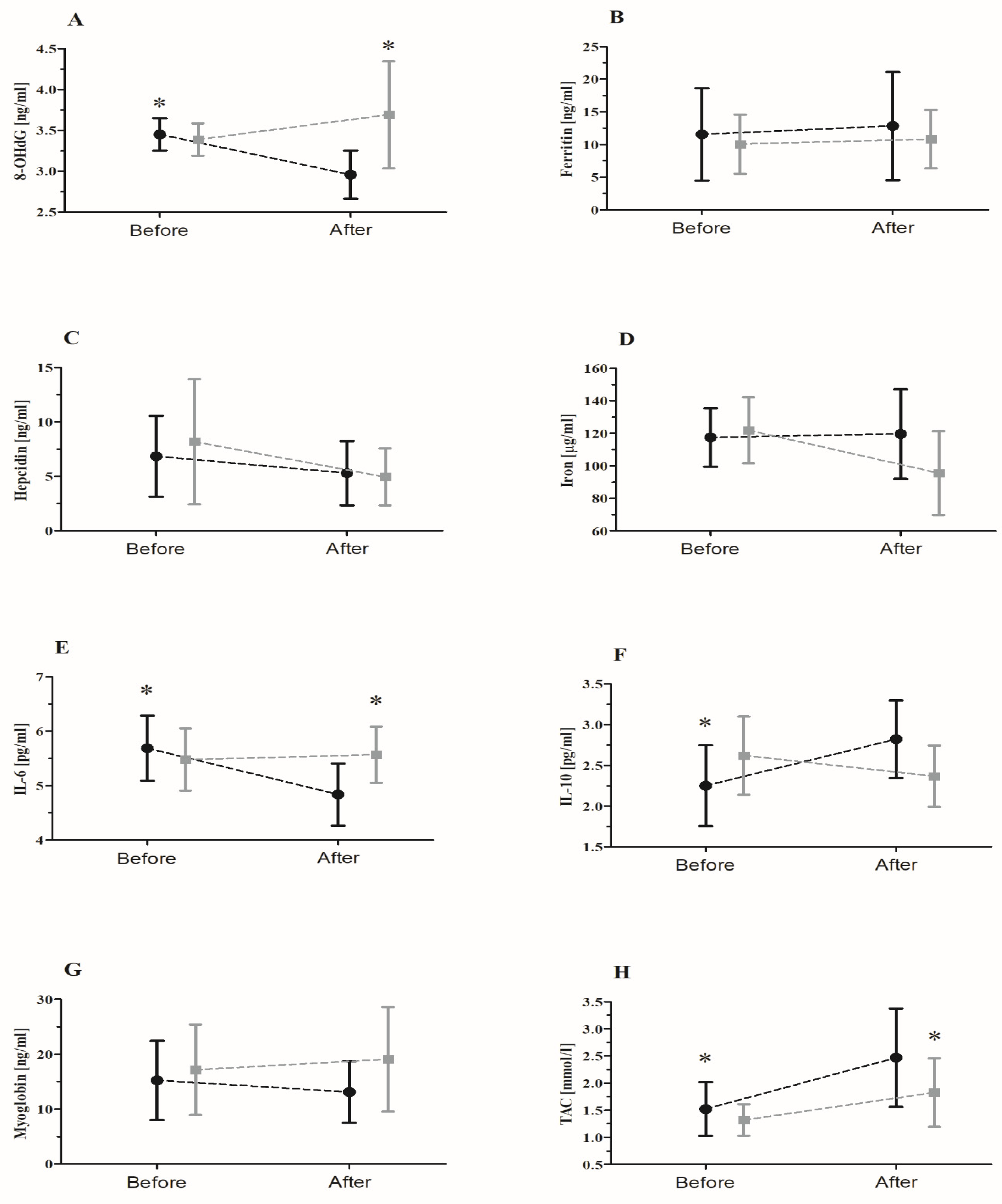
| 8-OHdG | GR RM GR × RM | 11.80 0.55 1.73 | 1, 20 1, 20 1, 20 | <0.01 ** 0.46 <0.01 ** | 0.37 0.02 0.32 | S < P Sbefore > Safter Safter < Pafter |
| Ferritin | GR RM GR × RM | 0.47 0.97 0.06 | 1, 20 1, 20 1, 20 | 0.50 0.33 0.80 | 0.02 0.04 < 0.01 | |
| Hepcidin | GR RM GR × RM | 0.02 0.01 2.92 | 1, 20 1, 20 1, 20 | 0.86 0.90 0.10 | <0.01 <0.01 0.12 | |
| Iron | GR RM GR × RM | 0.37 <0.01 0.94 | 1, 20 1, 20 1, 20 | 0.55 0.99 0.34 | 0.02 <0.01 0.06 | |
| IL-6 | GR RM GR × RM | 1.36 15.08 23.06 | 1, 20 1, 20 1, 20 | 0.25 <0.01 ** <0.01 ** | 0.06 0.43 0.53 | Before > After Sbefore > Safter Safter < Pafter |
| IL-10 | GR RM GR × RM | 0.21 1.89 21.41 | 1, 20 1, 20 1, 20 | 0.64 0.18 <0.01 ** | 0.01 0.08 0.51 | Sbefore < Safter |
| Myoglobin | GR RM GR × RM | 1.84 0.01 1.77 | 1, 20 1, 20 1, 20 | 0.18 0.94 0.19 | 0.08 <0.01 0.08 | |
| TAC | GR RM GR × RM | 6.88 28.87 7.24 | 1, 20 1, 20 1, 20 | 0.02 * <0.01 0.01 * | 0.25 0.59 0.26 | S > P Before > After Sbefore < Safter Safter > Pafter |
| Variable | Effect | F | df | p-Value | Effect Size (η2) | Post-Hoc Outcome |
|---|---|---|---|---|---|---|
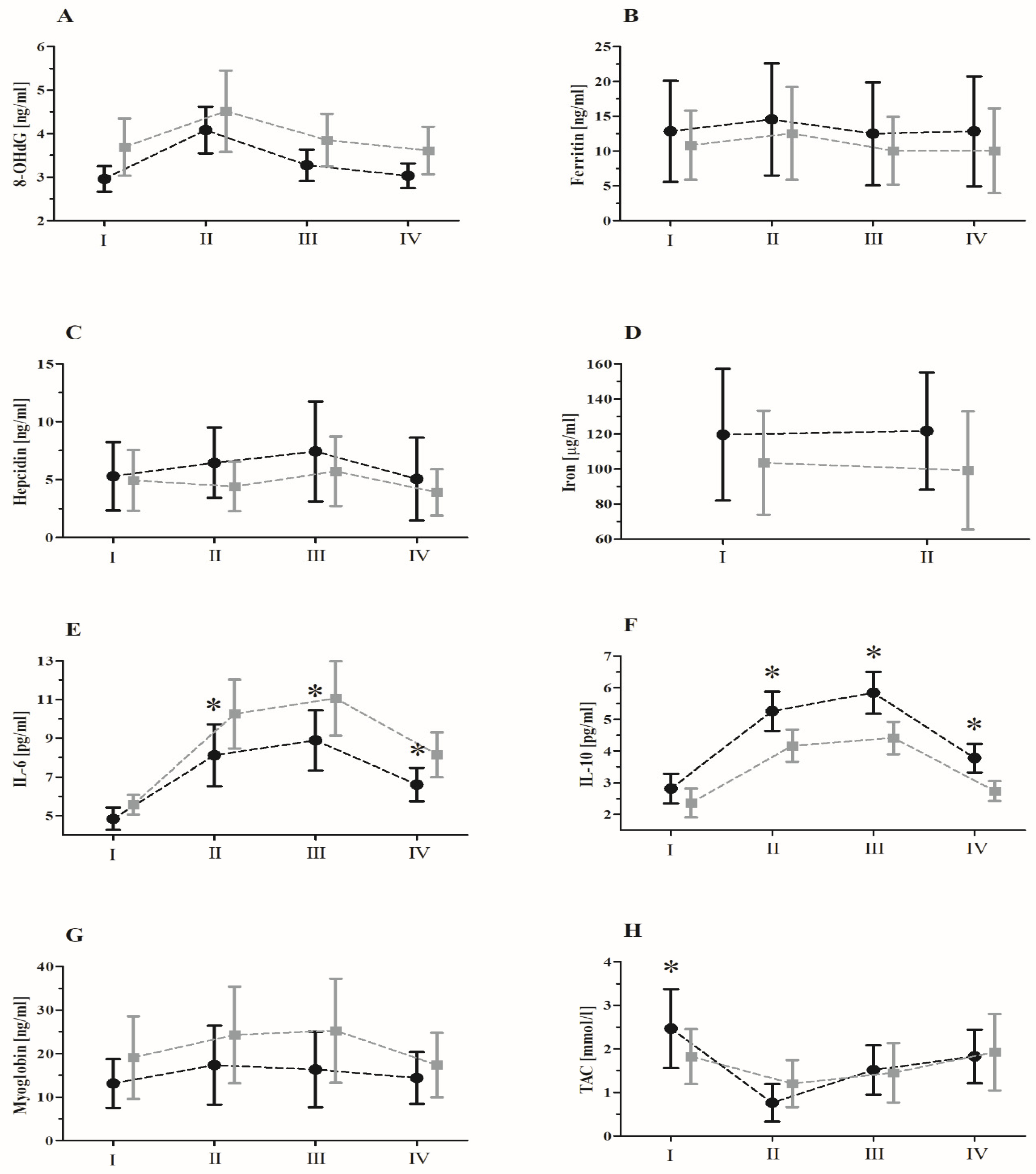
| 8-OHdG | GR RM GR × RM | 7.25 72.54 1.26 | 1, 20 3, 60 3, 60 | 0.01 <0.01 * 0.29 | 0.26 0.78 0.05 | S < P II, III > I, IV; II > III |
| Ferritin | GR RM GR × RM | 0.55 3.32 0.26 | 1, 20 3, 60 3, 60 | 0.46 0.06 0.85 | 0.02 0.11 0.01 | |
| Hepcidin | GR RM GR × RM | 2.74 0.38 0.55 | 1, 20 3, 60 3, 60 | 0.11 0.76 0.64 | 0.12 0.02 0.02 | |
| Iron | GR RM GR × RM | 1.84 0.10 0.52 | 1, 20 3, 60 3, 60 | 0.19 0.75 0.47 | 0.09 0.01 0.02 | |
| IL-6 | GR RM GR × RM | 24.27 190.65 10.32 | 1, 20 3, 60 3, 60 | <0.01 * <0.01 * <0.01 * | 0.54 0.90 0.34 | S < P I < II, III, IV; II < III; III > IV S-II < P-II; S-III < P-III; |
| IL-10 | GR RM GR × RM | 57.57 572.43 16.43 | 1, 20 3, 60 3, 60 | <0.01 * <0.01 * <0.01 * | 0.74 0.96 0.45 | S > P I < II, III, IV; II < III; III > IV S-II > P-II; S-III > P-III; S-IV > P-IV |
| Myoglobin | GR RM GR × RM | 3.92 8.56 1.71 | 1, 20 3, 60 3, 60 | 0.06 <0.01 * 0.17 | 0.16 0.29 0.07 | II, III > I, IV |
| TAC | GR RM GR × RM | 6.42 26.09 4.39 | 1, 20 3, 60 3, 60 | 0.02 <0.01 * <0.01 * | 0.24 0.56 0.18 | S > P I > III, IV > II, S-I > P-I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankiewicz, B.; Cieślicka, M.; Mieszkowski, J.; Kochanowicz, A.; Niespodziński, B.; Szwarc, A.; Waldziński, T.; Reczkowicz, J.; Piskorska, E.; Petr, M.; et al. Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial. Nutrients 2023, 15, 975. https://doi.org/10.3390/nu15040975
Stankiewicz B, Cieślicka M, Mieszkowski J, Kochanowicz A, Niespodziński B, Szwarc A, Waldziński T, Reczkowicz J, Piskorska E, Petr M, et al. Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial. Nutrients. 2023; 15(4):975. https://doi.org/10.3390/nu15040975
Chicago/Turabian StyleStankiewicz, Błażej, Mirosława Cieślicka, Jan Mieszkowski, Andrzej Kochanowicz, Bartłomiej Niespodziński, Andrzej Szwarc, Tomasz Waldziński, Joanna Reczkowicz, Elżbieta Piskorska, Miroslav Petr, and et al. 2023. "Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial" Nutrients 15, no. 4: 975. https://doi.org/10.3390/nu15040975
APA StyleStankiewicz, B., Cieślicka, M., Mieszkowski, J., Kochanowicz, A., Niespodziński, B., Szwarc, A., Waldziński, T., Reczkowicz, J., Piskorska, E., Petr, M., Skarpańska-Stejnborn, A., & Antosiewicz, J. (2023). Effect of Supplementation with Black Chokeberry (Aronia melanocarpa) Extract on Inflammatory Status and Selected Markers of Iron Metabolism in Young Football Players: A Randomized Double-Blind Trial. Nutrients, 15(4), 975. https://doi.org/10.3390/nu15040975










