Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
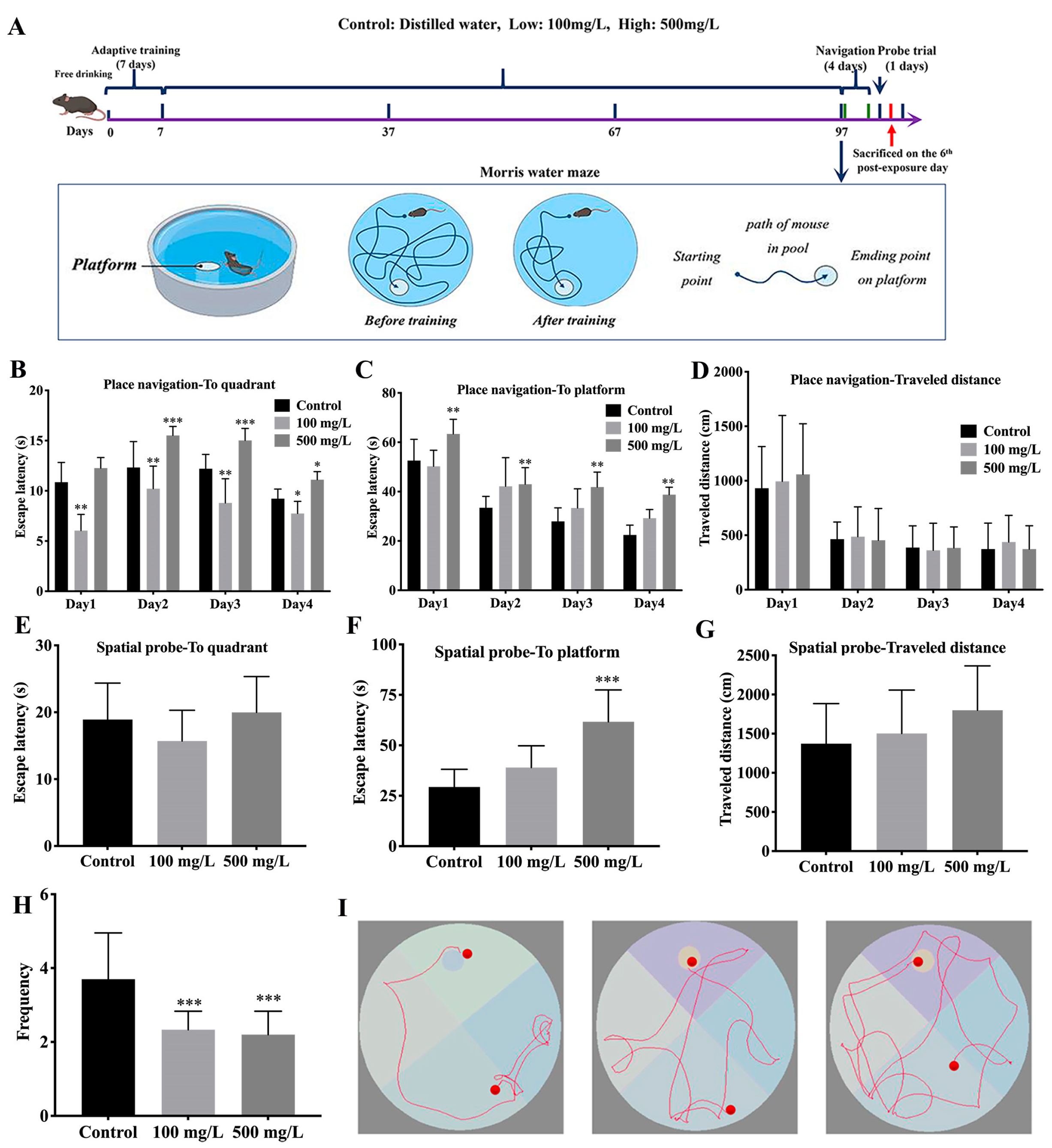
2.2. Experimental Design and Schedule
2.3. Morris Water Maze (MWM) Tests
2.4. ICP-MS Assay
2.4.1. Pre-Treatment of Urine Samples
2.4.2. Pre-Treatment of Blood Samples
2.4.3. Pre-Treatment of Brain Samples
2.4.4. ICP-MS Analysis
2.5. Hematoxylin-Eosin (HE) Staining
2.6. Immunohistochemical Staining
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Terminal Deoxynucleotidyl Transferase 2′-Deoxyuridine, 5′-Triphosphate Nick-End Labeling (TUNEL) Assay
2.9. Western Blotting
2.10. Cell Line and Treatment
2.11. Data Analysis
3. Results
3.1. Cu Induces Spatial Reference Memory Impairment Impair of C57BL/6J Mice
3.2. Cu Exposure Increases Brain Cu Levels and Damages Hippocampal Tissue of C57BL/6J Mice
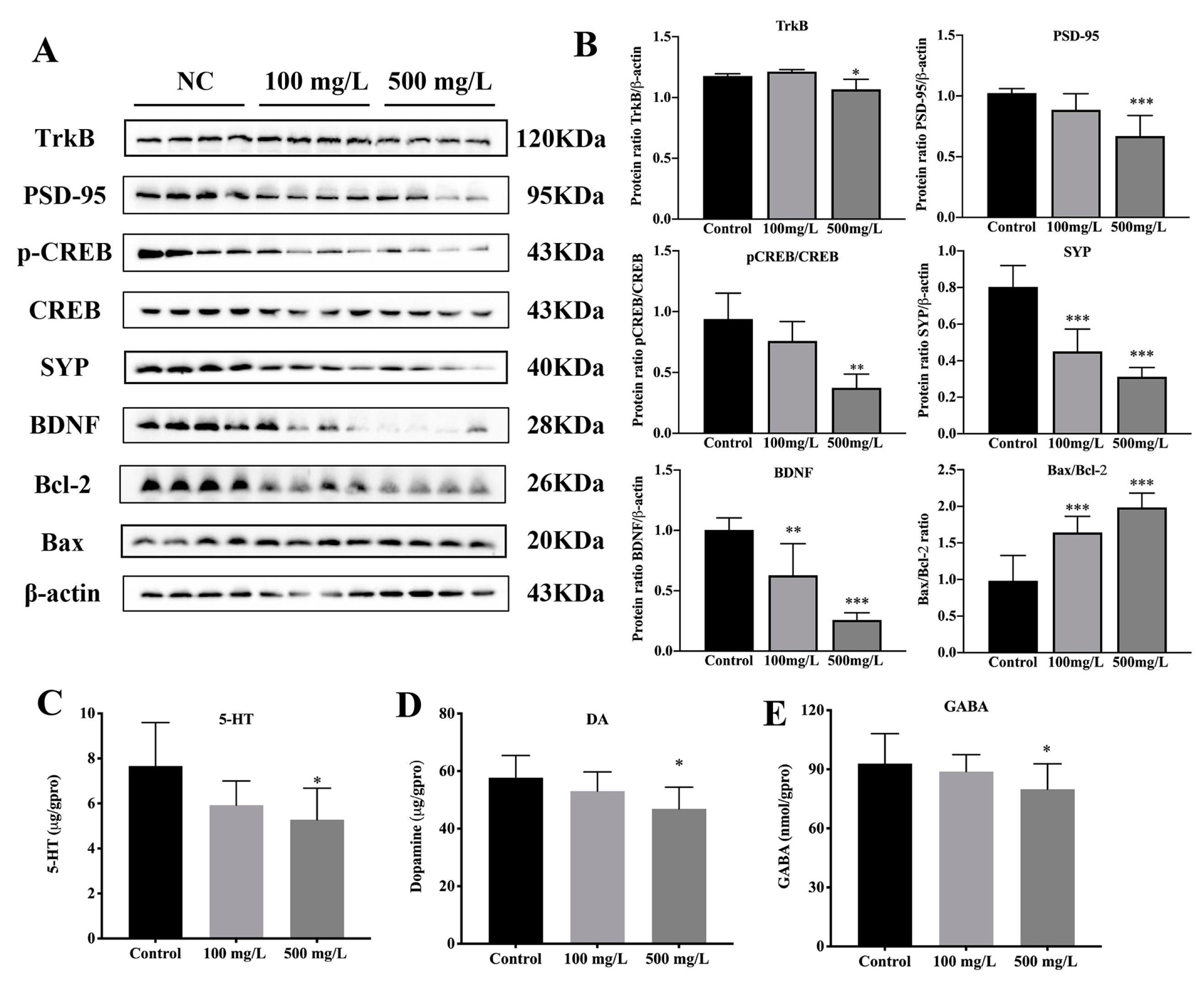
3.3. Cu Induces Oxidative Damage and Promotes Apoptosis in Hippocampal Tissue
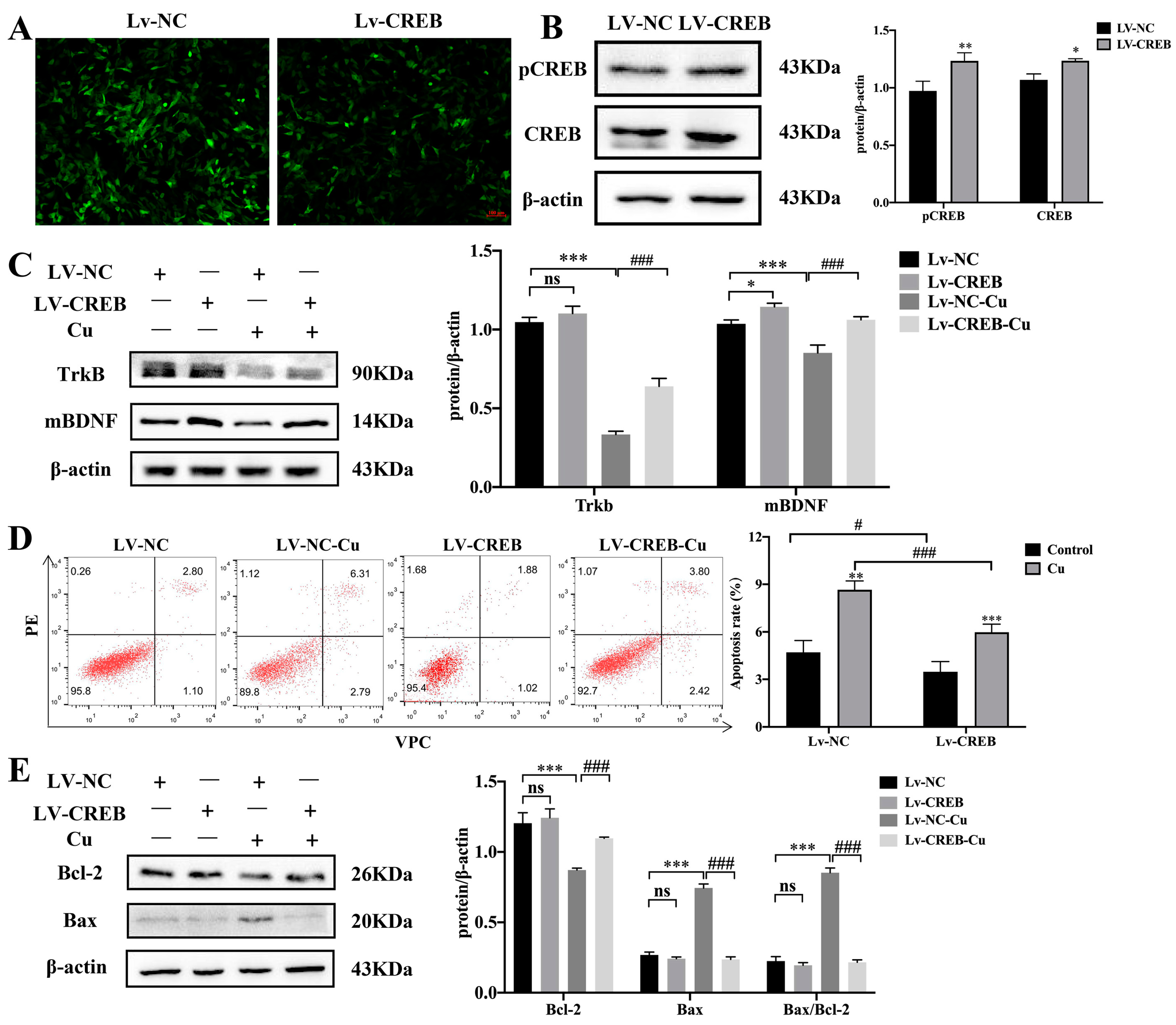
3.4. Cu Inhibits CREB/BDNF Signaling Pathway
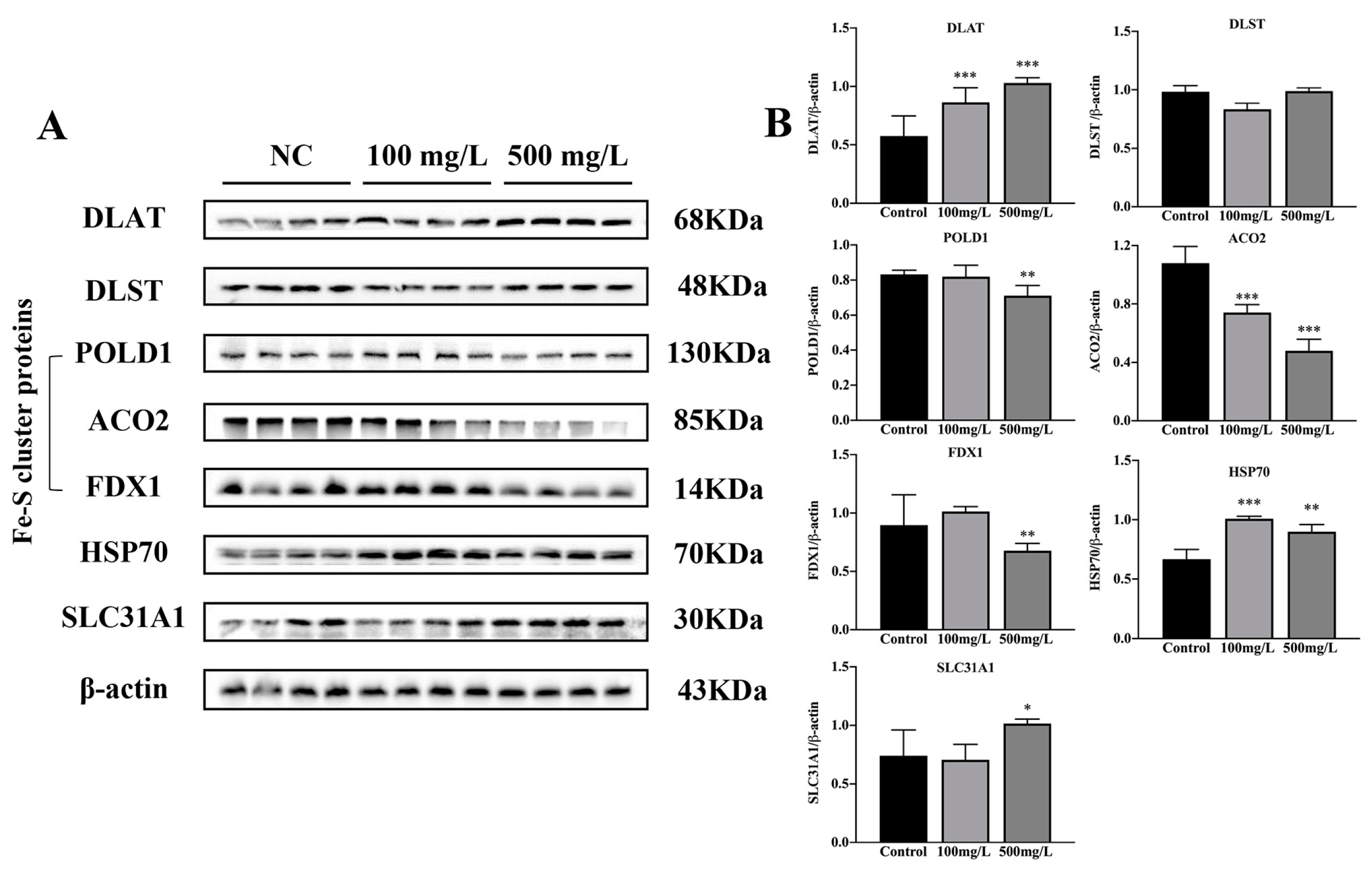
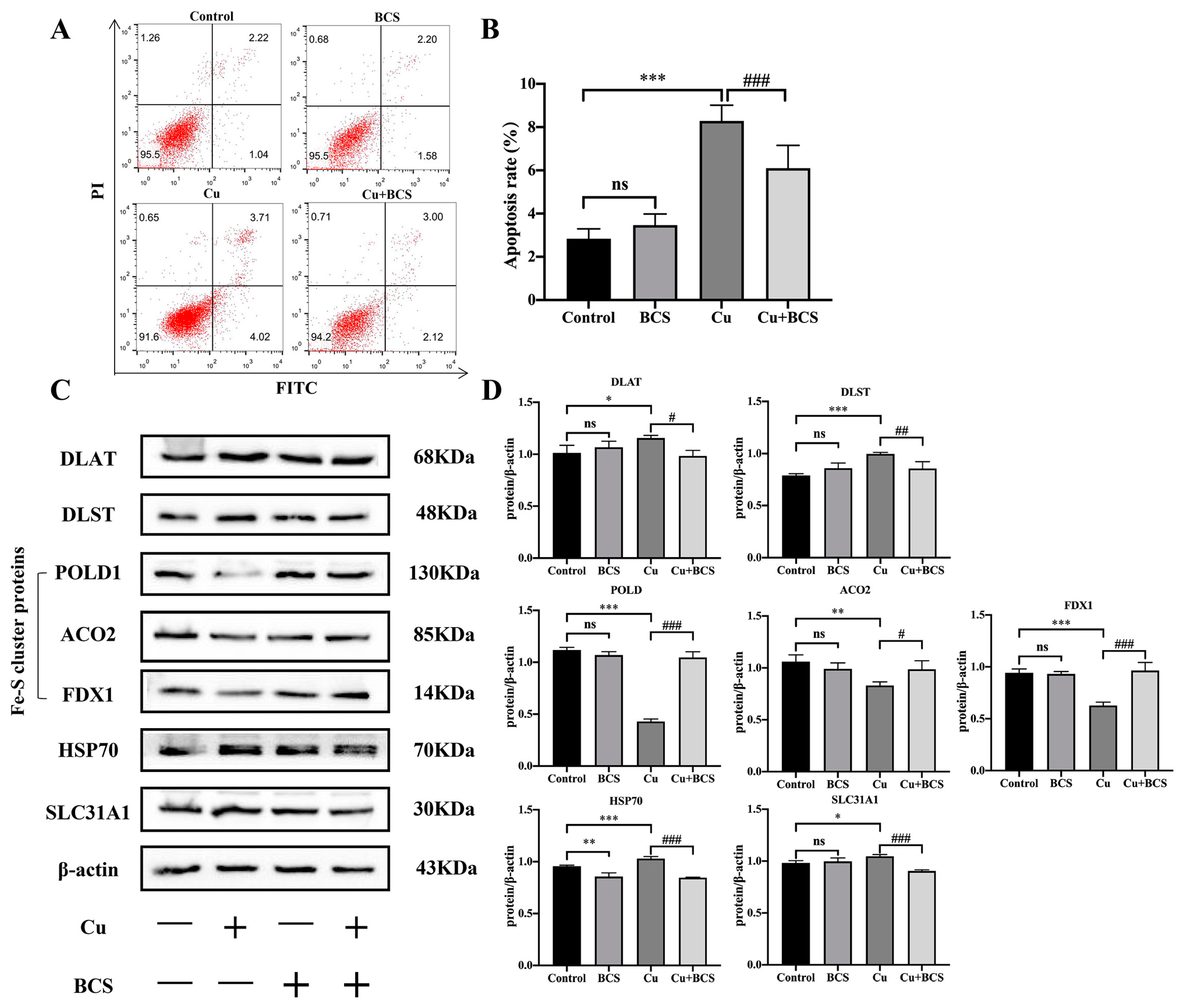
3.5. Cu Induces Cuproptosis
4. Discussion
4.1. Cu Might Induce Cognitive Impairment in Mice via Modulation of Cuproptosis
4.2. Cu Destroys Synaptic Plasticity and CREB-Mediated Memory Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Programme, U.E. Medium-Term Strategy 2022–2025. In The United Nations Environment Programme Strategy for Tackling Climate Change, Biodiversity and Nature Loss, and Pollution and Waste from 2022–2025; UN Environment Programme: Nairobi, Kenya, 2021; p. 56. [Google Scholar]
- Cosselman, K.E.; Navas-Acien, A.; Kaufman, J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, E.M.; Komarova, T.; Comino, E.; Sumner, R.; Whitfield, K.M.; Shaw, P.N. Effects of storage conditions on the stability and distribution of clinical trace elements in whole blood and plasma: Application of ICP-MS. J. Trace Elem. Med. Biol. 2021, 68, 126804. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, G.; Mikula, K.; Skrzypczak, D.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Potential environmental pollution from copper metallurgy and methods of management. Environ. Res. 2021, 197, 111050. [Google Scholar] [CrossRef] [PubMed]
- Flemming, C.A.; Trevors, J.T. Copper Toxicity and Chemistry in the Environment—A review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Davis, A.; Ashenberg, D. The aqueous geochemistry of the Berkeley Pit, Butte, Montana, USA. Appl. Geochem. 1989, 4, 23–36. [Google Scholar] [CrossRef]
- Wei, X.; Gao, B.; Wang, P.; Zhou, H.; Lu, J. Pollution characteristics and health risk assessment of heavy metals in street dusts from different functional areas in Beijing, China. Ecotoxicol. Environ. Saf. 2015, 112, 186–192. [Google Scholar] [CrossRef]
- Nose, Y.; Kim, B.E.; Thiele, D.J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Chandan, V.S.; Shah, S.S.; Mounajjed, T.; Torbenson, M.S.; Wu, T.-T. Copper deposition in focal nodular hyperplasia and inflammatory hepatocellular adenoma. J. Clin. Pathol. 2017, 71, 504–507. [Google Scholar] [CrossRef]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases—Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. North Am. 2019, 103, 263–293. [Google Scholar] [CrossRef]
- Goldman, J.G.; Sieg, E. Cognitive Impairment and Dementia in Parkinson Disease. Clin. Geriatr. Med. 2020, 36, 365–377. [Google Scholar] [CrossRef]
- Cui, M.Y.; Lin, Y.; Sheng, J.Y.; Zhang, X.; Cui, R.J. Exercise Intervention Associated with Cognitive Improvement in Alzheimer’s Disease. Neural Plast. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J. Cognitive decline and quality of life in incident Parkinson’s disease: The role of attention. Park. Relat. Disord. 2016, 27, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F. Copper: Effects of deficiency and overload. Met. Ions. Life Sci. 2013, 13, 359–387. [Google Scholar]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- USEPA. Aquatic Life Ambient Freshwater Quality Criteria-Copper; Office of Water United States Environmental Protection Agency: Washington, DC, USA, 2007; pp. 1–204.
- Morris, R.G.M.; Garrud, P.; Rawlins, J.N.P.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. [Google Scholar] [CrossRef]
- Li, Y.; Trush, M.A. DNA damage resulting from the oxidation of hydroquinone by copper: Role for a Cu(II)/Cu(I) redox cycle and reactive oxygen generation. Carcinogenesis 1993, 14, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Y.; Zhao, C.; Zhang, H.; Pu, Y.; Yin, L. Copper induces oxidative stress and apoptosis of hippocampal neuron via pCREB/BDNF/ and Nrf2/HO-1/NQO1 pathway. J. Appl. Toxicol. 2021, 42, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elements Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.; Leong, W.; Lees, G.J. Comparative effects of metal chelating agents on the neuronal cytotoxicity induced by copper (Cu+2), iron (Fe+3) and zinc in the hippocampus. Brain. Res. 2001, 892, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Q.; Gao, W.; Liu, S.; Wu, R.; Shen, Z.; Liu, W.; Chen, Y. Copper chloride dose-dependently alters spatial learning and memory, and glutamate levels, in the hippocampus of rats. Mol. Med. Rep. 2017, 17, 4074–4082. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzym. 1990, 186, 1–85. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2020, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Turgut, G.; Akdogan, I.; Adiguzel, E.; Genç, O. Effect of Copper Overload Together with Ethanol Uptake on Hippocampal Neurons. Tohoku J. Exp. Med. 2003, 199, 239–245. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.-C.; Nakanishi, I.; Matsumoto, K.-I.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.-C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Fan, J.; Zhang, X.; Song, H.; Wan, R.; Wang, W.; Yin, Y. Decreased neuronal synaptosome associated protein 29 contributes to poststroke cognitive impairment by disrupting presynaptic maintenance. Theranostics 2021, 11, 4616–4636. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Zhang, Y.-P.; Hou, Z.; Huang, C.; Zhu, H.; Zhang, C.Q.; Yin, Q. Novel Insights into NeuN: From Neuronal Marker to Splicing Regulator. Mol. Neurobiol. 2016, 53, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Rowland, E.A.; Snowden, C.K.; Cristea, I.M. Protein lipoylation: An evolutionarily conserved metabolic regulator of health and disease. Curr. Opin. Chem. Biol. 2018, 42, 76–85. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, I.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. Role of GirK Channels in Long-Term Potentiation of Synaptic Inhibition in an In Vivo Mouse Model of Early Amyloid-β Pathology. Int. J. Mol. Sci. 2019, 20, 1168. [Google Scholar] [CrossRef]
- Taoufik, E.; Kouroupi, G.; Zygogianni, O.; Matsas, R. Synaptic dysfunction in neurodegenerative and neurodevelopmental diseases: An overview of induced pluripotent stem-cell-based disease models. Open Biol. 2018, 8, 180138. [Google Scholar] [CrossRef]
- Ilic, K.; Mlinac-Jerkovic, K.; Sedmak, G.; Rosenzweig, I.; Kalanj-Bognar, S. Neuroplastin in human cognition: Review of literature and future perspectives. Transl. Psychiatry 2021, 11, 394. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.X.; Xu, X.X.; Gao, J.; Zhang, T. Enriched Environment and Social Isolation Affect Cognition Ability via Altering Excitatory and Inhibitory Synaptic Density in Mice Hippocampus. Neurochem. Res. 2020, 45, 2417–2432. [Google Scholar] [CrossRef]
- Zhu, X.K.; Wang, P.; Liu, H.J.; Zhan, J.; Wang, J.; Li, M.; Zeng, L.; Xu, P. Changes and Significance of SYP and GAP-43 Expression in the Hippocampus of CIH Rats. Int. J. Med. Sci. 2019, 16, 394–402. [Google Scholar] [CrossRef]
- Pollak, D.D.; Herkner, K.; Hoeger, H.; Lubec, G. Behavioral testing upregulates pCaMKII, BDNF, PSD-95 and egr-1 in hippocampus of FVB/N mice. Behav. Brain Res. 2005, 163, 128–135. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, B.; Wu, Y.P.; Li, B. The Effects of Maternal Atrazine Exposure and Swimming Training on Spatial Learning Memory and Hippocampal Morphology in Offspring Male Rats via PSD95/NR2B Signaling Pathway. Cell. Mol. Neurobiol. 2019, 39, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Yan, D.D.; Wang, Y.; Wang, N.; Liu, Y.; Tan, A.; Chen, X.; Yan, H. Chronic acrylamide exposure induced glia cell activation, NLRP3 inflammasome upregulation and cognitive impairment. Toxicol. Appl. Pharmacol. 2020, 393, 20. [Google Scholar] [CrossRef] [PubMed]
- Marinesco, S.; Carew, T.J. Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: Characterization and relationship to heterosynaptic plasticity. J. Neurosci. 2002, 22, 2299–2312. [Google Scholar] [CrossRef]
- Ben-Ari, Y.; Gaiarsa, J.L.; Tyzio, R.; Khazipov, R. GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 2007, 87, 1215–1284. [Google Scholar] [CrossRef]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Smolen, P.; Alberini, C.M.; Baxter, D.A.; Byrne, J.H. Computational model of a positive BDNF feedback loop in hippocampal neurons following inhibitory avoidance training. Learn Mem. 2016, 23, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Kaldun, J.C.; Sprecher, S.G. Initiated by CREB: Resolving Gene Regulatory Programs in Learning and Memory: Switch in Cofactors and Transcription Regulators between Memory Consolidation and Maintenance Network. Bioessays 2019, 41, e1900045. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 23. [Google Scholar] [CrossRef]
- Lisman, J.; Cooper, K.; Sehgal, M.; Silva, A.J. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat. Neurosci. 2018, 21, 309–314. [Google Scholar] [CrossRef]
- Kandel, E.R.; Dudai, Y.; Mayford, M.R. The Molecular and Systems Biology of Memory. Cell 2014, 157, 163–186. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]


| Group | Control | 100 mg/L | 500 mg/L |
|---|---|---|---|
| Water intake (mL) | 5.112 ± 0.712 | 4.072 ± 0.882 a | 2.817 ± 0.836 ab |
| Daily Cu intake (mg/mouse) | 0 | 0.407 ± 0.083 a | 1.409 ± 0.392 ab |
| Cu intake of unit weight (mg/g) | 0 | 0.015 ± 0.003 a | 0.055 ± 0.016 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, Q.; Lu, L.; Su, Y.; Shi, W.; Zhang, H.; Liu, R.; Pu, Y.; Yin, L. Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling. Nutrients 2023, 15, 972. https://doi.org/10.3390/nu15040972
Zhang Y, Zhou Q, Lu L, Su Y, Shi W, Zhang H, Liu R, Pu Y, Yin L. Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling. Nutrients. 2023; 15(4):972. https://doi.org/10.3390/nu15040972
Chicago/Turabian StyleZhang, Ying, Qian Zhou, Lu Lu, Yu Su, Wei Shi, Hu Zhang, Ran Liu, Yuepu Pu, and Lihong Yin. 2023. "Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling" Nutrients 15, no. 4: 972. https://doi.org/10.3390/nu15040972
APA StyleZhang, Y., Zhou, Q., Lu, L., Su, Y., Shi, W., Zhang, H., Liu, R., Pu, Y., & Yin, L. (2023). Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling. Nutrients, 15(4), 972. https://doi.org/10.3390/nu15040972







