Abstract
Skeletal disabilities are a prominent burden on the present population with an increasing life span. Advances in osteopathy have provided various medical support for bone-related diseases, including pharmacological and prosthesis interventions. However, therapeutics and post-surgery complications are often reported due to side effects associated with modern-day therapies. Thus, therapies utilizing natural means with fewer toxic or other side effects are the key to acceptable interventions. Flavonoids constitute a class of bioactive compounds found in dietary supplements, and their pharmacological attributes have been well appreciated. Recently, flavonoids’ role is gaining renowned interest for its effect on bone remodeling. A wide range of flavonoids has been found to play a pivotal role in the major bone signaling pathways, such as wingless-related integration site (Wnt)/β-catenin, bone morphogenetic protein (BMP)/transforming growth factor (TGF)-β, mitogen-activated protein kinase (MAPK), etc. However, the reduced bioavailability and the absorption of flavonoids are the major limitations inhibiting their use against bone-related complications. Recent utilization of nanotechnological approaches and other delivery methods (biomaterial scaffolds, micelles) to target and control release can enhance the absorption and bioavailability of flavonoids. Thus, we have tried to recapitulate the understanding of the role of flavonoids in regulating signaling mechanisms affecting bone remodeling and various delivery methods utilized to enhance their therapeutical potential in treating bone loss.
1. Introduction
Skeletal disabilities are a prominent modern-day problem and have become a concern lately. An increase in life expectancy and a growth in the elderly population worldwide have substantially burdened the existing health systems [1,2]. Bone is a living organ, constituting 30% organic and 70% inorganic material with various functions such as protecting internal organs, making the body frame, and safe storage for some vital minerals in the body [3,4]. There are various classes of bone cells, such as osteoblasts, osteocytes, bone-lining cells, and osteoclasts [4]. All these cells are responsible for bone metabolism, characterized by a constant equilibrium of bone formation (by the osteoblasts) and bone resorption (mediated by osteoclasts). Despite that, the disruption between bone formation and bone resorption contributes to several metabolic bone disorders, namely osteoporosis, osteopetrosis, and Paget’s disease [5,6]. Osteoporosis is regarded as a health problem affecting over 200 million people globally, according to the World Health Organization [7]. The crucial risk factor for osteoporosis is age-associated bone loss, which occurs in people over 50 years of age, including approximately 25% of men and 50% of women [8]. Osteoporosis can be categorized into two main groups, primary and secondary. Primary osteoporosis is the most typical type and is mainly caused by loss of bone mass during the aging process or in postmenopausal women because of decreased estrogen levels. Secondary osteoporosis is associated with lifestyle, secondary systemic disorders such as diabetes, hypothyroidism, etc., and long-term use of drugs such as glucocorticoids [9]. The method of treating bone loss during osteoporosis is to stimulate bone growth. The understanding of why a difference in normal osteoblastogenesis regulation can result in bone disorders has improved due to recent advancements and expanded knowledge in the regulation of osteoblastic bone growth and maintenance of bone mass [10].
The current therapeutic approach to treat bone loss includes either inhibiting elevated bone resorption or stimulating suppressed bone formation. Drugs used for antiresorptive characteristics included bisphosphonates, hormone replacement therapy (HRT) (estrogen), Raloxifene, and monoclonal such as Denosumab and Romosozumab; while for inducing bone formation and increasing bone density, anabolic drugs such as Teriparatide (parathyroid hormone/PTH 1-34) are recommended [11]. However, estrogen replacement therapy has been found to be associated with heart attack, stroke, and risk of cancer. The use of Teriparatide as a drug for osteoporosis has highlighted the risk of osteosarcoma in rodent models [12]. Moreover, long-term bisphosphonates treatment could lead to skeletal lesions, developing into bisphosphonate-related osteonecrosis of the jaw [11,13]. Though the commercially available drugs have been remarkable in treating bone loss during osteoporosis, a few cases of side effects may make them consider before prescribing. Hence, finding a new, effective, safe therapeutic agent with no or fewer side effects is essential for bone loss pathologies.
Flavonoids are present in dietary supplements, including vegetables, grains, fruits, stems, bark, flowers, etc. [11,14] and have been well acknowledged for their diverse bioactivities, including anti-oxidant, anti-allergic, anti-inflammatory, anti-carcinogenic, and antiviral activities [15]. The flavonoids contain over 5000 polyphenol compounds and are divided into seven flavonoid groups: anthocyanidins, flavanols, flavanones, flavanonols, flavones, flavonols, and isoflavones, categorized on the presence of hydroxyl groups structure and their glycosylation or alkylation status [16,17]. Recent studies on flavonoids have shown their notable effect on bone cells, such as increasing osteoblast activity, suppressing osteoclast activity, and protecting against bone loss, in addition to decreasing calcium and phosphate urinary excretion [18]. Some in vivo studies proved that flavonoids, such as Daidzein, Quercetin, Kaempferol, and Genistein, could affect osteogenesis and bone formation, while some studies report inhibiting effect on osteoclastogenesis and bone resorption [19,20]. Some of the flavonoids, such as isoflavones, accelerate bone formation by inducing osteoblasts differentiation and cell proliferation along with the inhibition of adipogenesis through the nitric oxide and estrogen receptor pathways [21,22].
Low systemic bioavailability is a general problem for flavonoids [23]. Mostly, it is associated with the absorption of flavonoids. The majority of flavonoids reach the colon unabsorbed [24]. Even then, several studies have reported the efficacy of flavonoids for bone health, such as stimulating osteoblastogenesis in in vitro and in vivo models [25,26,27,28]. Overcoming the low absorption and bioavailability of flavonoids, recent advancements in delivery systems are offering flavonoids as potential therapeutics for bone loss. Delivery vehicles such as nanoparticles, micelles, biomaterials, and scaffolds are commonly used carriers of flavonoids for bone [14,29]. In this review, we summarize the potential effect of flavonoids on stimulating bone formation by regulating various signaling pathways. In addition, the variety of methods used for delivering the flavonoids is also discussed to assess their possibility as a next-generation therapeutic for bone loss.
2. Types of Flavonoids
There are seven classes of flavonoids, namely anthocyanidins, flavanols, flavanones, flavanonols, flavones, flavonols, and isoflavones (Figure 1A).
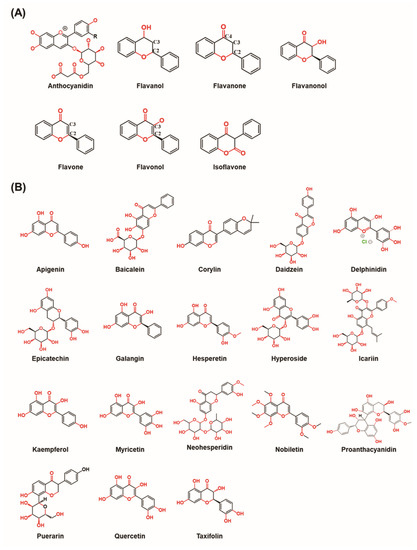
Figure 1.
The chemical structures of flavonoids. (A) The chemical structure of different types of flavonoids. (B) The chemical structure of flavonoids involved in the different bone–related signaling pathways. (Chemical structures source: PubChem (NCBI, NIH, USA; https://pubchem.ncbi.nlm.nih.gov/) accessed on 7 February 2023).
2.1. Anthocyanidins
Anthocyanidins are purple, blue, or red pigments in many foods, vegetables, and fruits, particularly in berries such as bilberries, blackberries, raspberries, blueberries, grapes, etc. [30,31]. Red or blue pigments of the anthocyanidins depend on their acid-base balance. Anthocyanin’s appearance varies in different conditions. For instance, it is red in acidic conditions, while blue pigment exists in alkaline conditions. It carries a positive charge in the C-ring oxygen called the flavylium or 2-phenylchromenylium ion. The stability of anthocyanin depends on its structure, pH, light, and temperature [30,32]. Anthocyanin is mainly synthesized with glucose, galactose, and rhamnose in natural products. According to the different substituent groups on the flavylium ring, anthocyanins can be differentiated into each other, such as Delphinidin, Petunidin, Pelargonidin, Cyanidin, Peonidin, and Malvidin [33].
2.2. Flavanols
Flavanol monomers known as procyanidins are abundant in the tea plant’s leaves, cocoas, apples, grapes, and wine. An (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-EC-gallate (ECG), and (−)-EGC-3-gallate (EGCG) are involved in the foremost flavanols [34,35]. Catechins are the most readily absorbable flavonoids because they are the only form not bound to sugars (flavonoid glycosides are more easily absorbed after transformation in a glycan form). The flavonol chemical structure has a hydroxyl group on C3 and no double bond between C2 and C3 [36,37].
2.3. Flavanones
Flavanones are capable of attaching to various kinds of receptors in the body. Owing to this potential, they are remarked as “privileged structures” and represent various biological reactions [38]. Citrus juices derived from blond or blood and sour oranges, limes, grapefruits, lemons, mandarins, and tangerines belong to the natural sources of flavanones [39]. Hesperidin and Naringin, such as citrus flavanone glycosides, are mostly found in Yuja peel [40]. The chemical structure is remarked by the absence of the ketone group in the C4 position and the presence of a double bond between C2 and C3.
2.4. Flavanonols
Flavanonols are the 3-hydroxy derivatives of flavanones and are also referred to as dihydroflavanonols [41]. Flavanonols are not abundant in plants and plant parts that are used for the human diet and are commonly found in wood as free aglycones [42].
2.5. Flavones
Flavones broadly exist as glucosides in leaves, flowers, and fruits. The common sources of flavones are celery, parsley, citrus fruits, chamomile, mint, and vegetables. This subclass of flavonoids comprises compounds such as Luteolin, Apigenin, and Tangeritin. Moreover, polymethoxylated flavones such as Tangeretin, Sinensetin, and Nobiletin are found mostly in the peels of citrus fruits [43]. The differentiation between other flavonoids and flavones is a double bond between C2 and C3, which have no alternative to the C3 in flavonoid chemical structure. The oxidation process takes place in the C4 position [44].
2.6. Flavonols
Flavonols are also one of the most well-known subclasses of flavonoids. There are two types of flavonols: aglycone form, which is not connected to sugar moieties, and flavonol glycosides, which connect to sugar moieties [45]. Flavonols are pale yellow or colorless compounds that have been shown to impact anthocyanin-mediated coloration by co-pigmentation effects [46,47]. Flavonols are mostly found in fruits and vegetables such as apples, grapes, berries, onions, lettuce, and tomatoes. In addition, flavonols are abundant in tea and red wine. Kaempferol, Quercetin, Myricetin, and Fisetin are the most commonly recognized flavonols [43]. Flavonols chemical structure is remarked by the hydroxyl group in the C3 position [48].
2.7. Isoflavones
Isoflavones belong to a subclass of flavonoids that contain phytoestrogen chemicals generated from plants with estrogenic activity [49]. Bioflavonoids, called soybean isoflavones, can interact with a variety of hormones, including estrogen, and they are found in some plants and soy products. It has a molecular structure comparable to estrogen and exists in nature as a molecule with a polyphenolic hydroxyl group called β-glucoside and can be induced by estrogen. Owing to the antiestrogenic and estrogenic activities, it is often referred to as a selective estrogen receptor modulator [50,51]. These compounds may be protective against osteoporosis due to their ability to exert osteogenic and antiresorptive actions on bone, particularly on bone turnover and growth [52,53].
Some of the flavonoids, along with their effect on the bone, are listed in Table 1.

Table 1.
Flavonoids and their effect on bone.
3. Bone Signaling Mechanism Affected by Flavonoids
Many studies have demonstrated that flavonoids can induce osteoblast differentiation/proliferation and inhibit osteoclast differentiation/proliferation. The mechanism of action includes the expressions of cytokines, transcription factors, bone-specific matrix proteins, bone signaling pathways, and receptor activators of nuclear factors κB ligand (RANKL)/osteoprotegerin (OPG) system hormone-like biological mechanisms [51]. The most important bone signaling pathways that can be targeted for stimulating bone formation are wingless-related integration site (Wnt)/β-catenin, bone morphogenetic protein (BMP)/transforming growth factor-beta (TGF-β) signaling, mitogen-activated protein kinase (MAPK) signaling, reactive oxygen species (ROS) signaling, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling and inflammatory NFκB/nuclear factor of activated T cell c1 (NFATc-1) signaling. The chemical structures of flavonoids involved in different bone–related signaling pathways are illustrated in Figure 1B.
3.1. Wnt/β-Catenin Signaling Pathway
Wnt/β-catenin signaling pathway regulates numerous physiological events in many organs, tissues and during growth and development, varying from functions of cell determination, polarity, migration, differentiation, and proliferation [96]. It can be segregated into two groups namely β-catenin (canonical or, β-catenin-dependent) and the non-canonical pathways (β-catenin-independent). The canonical pathway is one of the most important pathways responsible for fracture healing and bone homeostasis. [42].
The Wnt/β-catenin signaling pathway comprises a family of essential proteins for both embryonic development and homeostasis of adult tissues [97]. Wnt proteins, including Wnt3a, Wnt1, and Wnt5a, play a major role in transmitting extracellular signals. The Wnt receptors lipoprotein receptor-related protein (LRP) 5/6 and Frizzled (a unique sevenfold transmembrane receptor Frizzled protein: FZD) are primarily found embedded in the cell membrane. The glycogen synthase kinase-3 (GSK-3) complex, β-catenin, disheveled proteins (DVL), axis inhibition protein (AXIN), adenomatous polyposis coli (APC), and casein kinase 1 (CK1) make up most of the cytoplasmic cascade of Wnt signaling. T-cell factor/lymphoid enhancing factor (TCF/LEF) family members of the β-catenin downstream target gene family include matrix Metalloproteinases (MMPs) and c-Myc, and β-catenin (which translocate to the nucleus) are the key components of the nuclear cascade of Wnt signaling [98]. Once the Wnt ligand binds to its receptor, most DVL protein moves toward the plasma membrane. The clustering of LRP6 and FZD, including the phosphorylation of LRP6, is directed by activated DVL [99,100,101]. Additionally, AXIN and GSK-3β are attracted to the plasma membrane by activated DVL, where they are inhibited from functioning (ubiquitinated degradation of β-catenin) [100,102]. Stabilization of β-catenin in the cytoplasm leads to its nuclear translocation, and it then acts as a coactivator of TCF/LEF transcription factors leading to gene transduction [103,104].
Various types of flavonoids have the ability to affect the Wnt signaling pathway to alter osteoblast differentiation/proliferation (Figure 2A). Tian X. et al. reported that flavonoid Baicalein could enhance the osteogenic differentiation of tendon-derived stem cells by the induction of the Wnt/β-catenin signaling pathway. The involvement of Wnt/β-catenin signaling was validated by the treatment of DKK-1 (Wnt signaling inhibitor), which reduced the effect of Baicalein on osteogenic differentiation [105]. Moreover, Baicalein was shown to enhance osteogenic differentiation in the pre-osteoblastic cell line, MC3T3-E1, by activating Wnt signaling through MEK/ERK signaling [106]. In another study, Icariin was found to stimulate human bone marrow mesenchymal stem cells (BMSCs) osteogenic differentiation via activation of Wnt signaling. Icariin increased the expression of low-density LRP5, TCF1, and β-catenin. Icariin-activated Wnt signaling and inhibited adipogenesis by regulating the expression of miR-23a [107]. In the rat femoral fracture model, Pan F.F. et al. showed that aApigenin stimulates the osteogenesis of mesenchymal stem cells (MSCs) by increasing the expression of LRP5 and FZD receptors, elevating the level of β-catenin. Apigenin restored the inhibition of osteogenesis when the expression of β-catenin was inhibited by small interfering RNA [108].
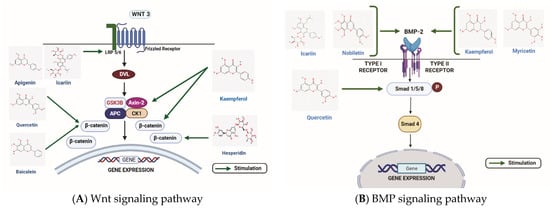
Figure 2.
The role of flavonoids in two major signaling pathways inducing bone formation. (A) The figure illustrates the stimulatory effects of quercetin, hesperidin, corylin, icariin, apigenin, baicalein on the Wnt/β-catenin signaling pathway. (B) Some of the flavonoids such as icariin, nobiletin, myricetin, and kaempferol stimulate osteogenic differentiation by stimulating the BMP2, whereas quercetin targets the Smad 1/5/8 molecule involved in the BMP signaling pathway for stimulating osteogenesis. WNT: Wingless-related integration site; DVL: Disheveled; LRP 5/6: Lipoprotein receptor-related protein 5/6; GSK3β: glycogen synthase kinase 3 beta ; Axin-2: Axis Inhibition protein-2; APC: Adenomatous polyposis coli; CK1: casein kinase 1; BMP: Bone morphogenetic protein; Smad: Suppressor of mothers against decapentaplegic. (Chemical structures source: PubChem (NCBI, NIH, USA; https://pubchem.ncbi.nlm.nih.gov/) accessed on 7 February 2023, Figures created with BioRender.com)).
Sharma A.R. et al. reported that Kaempferol activated Wnt signaling to induce osteogenesis in the human osteoblast cell line, SaOS-2. Involvement of Wnt signaling was confirmed by inhibiting the expression of β-catenin by its specific inhibitor, FH535. The effect of Kaempferol was further confirmed in primary human osteoblasts and drill-hole mice model. As observed in SaOS-2, both osteogenic models showed induction of β-catenin after Kaempferol treatment [20]. Similarly, Quercetin was shown to promote the protein expression levels of Wnt3 and β-catenin in osteoblasts. Pretreatment of Quercetin rescued the Lipopolysaccharide (LPS)-induced apoptosis and suppressive effect on the osteogenesis of MC3T3-E1 cells. The protective effect of Quercetin was abolished after the pretreatment of MAPK inhibitors or the Wnt/β-catenin inhibitor XAV939 [109]. Likewise, Quercetin was shown to protect TNFα induced inhibition of osteoblast differentiation by inactivation NFĸB and degradation of β-catenin in rat BMSCs [110].
Another flavonoid, Hesperidin, can promote differentiation of alveolar osteoblast cells via activation of Wnt signaling, and it was induced by increasing the expression of β-catenin and cyclin D1. After treating with a Wnt signaling inhibitor, DKK-1, Hesperidin-induced expression of β-catenin and cyclin D1 was decreased, proving Hesperidin’s role in activating Wnt signaling [111]. Chang Y.W. et al. studied the Neohesperidin effect on osteogenic differentiation in BMSCs. Neohesperidin stimulated the Wnt signaling by inducing the expression of β-catenin expression. The use of Wnt signaling inhibitors, DKK1 and XAV939, confirmed the effect of Neohesperidin. In treatment with Wnt signaling inhibitors, Neohesperidin-induced β-catenin expression was decreased [112].
Yu A.X. et al. reported that Corylin could induce osteoblast differentiation on primary osteoblast from calvaria of rats via Wnt signaling. Treatment of Corylin increased the rate of phosphorylation of GSK-3β, promoting Wnt signaling, while the treatment of antagonists such as DKK1 blocked its effect on osteogenesis [75]. In dexamethasone (DEX)-induced osteoporosis mouse model, the role of EGCG on osteogenesis was elucidated. Treatment of EGCG considerably increased the expression of cyclin D1 and β-catenin, stimulating Wnt signaling [113].
3.2. BMP/TGF-β Signaling Pathway
BMPs are multifunctional growth factors belonging to the TGF-β superfamily. These proteins represent their fundamental roles in bone repair and skeletal development by interacting with a tetrameric receptor complex leading to intracellular signal transduction with the help of the suppressor of mothers against decapentaplegic (Smad) proteins and expressing the osteoclastogenic genes with the help of transcription factor, runt-related transcription factor 2 (RUNX2) [27]. The noncanonical-Smad-independent pathway is another mechanism involved in TGF-β and BMP2-mediated osteogenesis, resulting in the phosphorylation and activity of RUNX2 [114].
It is well-known that the Smads proteins function as transcription factors and are essential intracellular effectors for BMP and TGF-β family members that influence osteoblast and osteoclast activities [115]. TGFβ or BMP ligands connect to particular type II receptors to attract the associated type I receptor and start a chain of events that phosphorylates their particular Smad receptor (R-Smads). Smad2 and Smad3 are typically required for TGFβ signaling, whereas Smad1, 5, and 8 are required for BMP signaling. The phosphorylated R-Smad and Smad4, the shared partner Smad, come together to form a heterocomplex (Co-Smad). The R-Smad/Co-Smad complex subsequently moves into the nucleus, where it attaches to target genes’ promoters to control the transcription of certain osteoblastic genes [116].
Various studies have highlighted the role of flavonoids in affecting the BMP/TGF- β signaling pathway (Figure 2B). Pang Y. et al. reported that Nobiletin could stimulate osteogenic differentiation by BMP signaling in MG-63 cells. Treatment of Nobiletin induced the expression of BMP2 in a dose and time-dependent manner, elevating the expression of RUNX2 and leading to induction in osteogenic differentiation [78]. Moreover, Icariin could reverse vancomycin-induced inhibition of osteogenesis of rat calvarial osteoblasts. After treatment with vancomycin BMP2 and RUNX2, mRNA expressions were reduced, but Icariin co-treatment with vancomycin was able to rescue BMP2 and RUNX expressions [117]. Adhikary S. et al. showed that Kaempferol could reverse the effect of glucocorticoid-induced bone loss on rat calvarial osteoblast cells in vitro and in vivo. The study concluded that glucocorticoid treatment reduced the expressions of RUNX2, BMP2, and BMP4, which was reversed after the treatment of Kaempferol. Similarly, DEX was used to confirm the effect of Kaempferol on BMP signaling. DEX reduced the expressions of RUNX2 and BMP2, but the treatment of kaempferol reversed their expression levels. In addition, Smad1/5/8 phosphorylation was decreased with DEX, followed by the suppressed stimulation of RUNX2 and osteoblast proliferation [118].
Furthermore, Quercetin which chemically resembles estrogen, could induce osteogenesis in BMSCs, as was evidenced by the increased expression of RUNX2, osterix, and osteopontin. The treatment of ICI1827280 (ER inhibitor) to BMSCs was used to validate the presence of estrogen signaling. BMP2, Smad1, Smad4, and p-Smad1 expressions were inhibited by ICI182780, highlighting Quercetin’s role in inducing BMSC differentiation through BMP and ER signaling [119]. Kim H.Y. et al.’s result elucidated that Myricetin could induce osteoblast differentiation in human periodontal ligament cells via BMP signaling along with Wnt/β-catenin and MAPK signaling pathways. BMP2, phosphorylation of Smad1/5/9, and BMP receptor IB levels were increased after treatment with Myricetin, which resulted in the stimulation of osteogenic-related proteins RUNX2 and osterix [120]. Moreover, evidence also suggests that Isoquercetin could induce cell proliferation of BMSCs via BMP signaling. BMP4 was stimulated after treatment with Isoquercetin, and Noggin (BMP antagonist) was able to inhibit the BMP signaling induced by Isoquercetin [121].
In brief, the flavonoids, namely Kaempferol, Nobiletin, Icariin, and Myricetin, target the BMP receptor, whereas Quercetin targets the Smad 1/5/8 molecule involved in the BMP signaling pathway.
3.3. MAPK Signaling Pathway
MAPKs are composed of a messenger’s family, which transports various signals from the cell surface to the nucleus, depending on different stimulants such as stress, hormones, and chemicals [122]. Cell migration, differentiation, and proliferation can be regulated by MAPK signaling. The extracellular-signal-regulated kinase (ERK), c-Jun N-amino-terminal kinase (JNK), and P38 are the key members of the MAPK signaling [123].
In multicellular organisms, controlling cell proliferation is a complicated process mostly mediated by external growth factors mediated by the neighboring cells [124]. In order to control cell proliferation, many protein kinases cascades, known as MAPK pathways, are essential [125,126,127]. In order to activate or deactivate their target, mitogen-activated protein kinases phosphorylate either their dual threonine and serine residues (autophosphorylation) or those present on their substrates. As a result, MAPKs control crucial cellular functions such as immune defense, stress reactions, and apoptosis. A MAP3K stimulates a MAP2K, which activates a MAPK in a MAPK module [128,129,130,131,132]. MAPK protein phosphatases (MKPs) dephosphorylate phosphotyrosine and phosphothreonine residues on MAPKs and can inhibit MAPK phosphorylation processes [126,128,133]. The ERK1/2, JNK1/2/3, and the p38 MAPK α, β, δ pathways are three well-known MAPK pathways in mammalian cells. According to their structure, activation motif, and functional forms, they are categorized as ERK, p38, and JNK isoforms [133,134,135]. Growth factors, proinflammatory stimuli, and hormones cause ERK1/2 to become active, while cellular and environmental stressors also cause JNK1/2/3 and p38 MAPK α, β, δ to become active [131,134].
Xing L.Z. et al. examined the effect of Quercitrin on an ovarian-ectomized rat model. After ovariectomization, expressions of osteoblast markers were decreased. Quercitrin increased the expression of the osteogenic marker alkaline phosphatase (ALP) as well as the osteogenic transcriptional factor RUNX2 in the treated ovariectomized rats. Administration of Quercitrin increased the phosphorylated forms of ERK, P38, and JNK in ovariectomized rats, implying that Quercitrin reversed the osteoporosis effect in ovariectomized mice model by employing MAPK signaling [136]. Similarly, Baicalein attenuated osteomyelitis by inhibiting Toll-like receptor 2 (TLR2) and MAPK signaling in Staphylococcus aureus-treated mice and MC3T3-E1 cells. Treatment with Staphylococcus aureus increased the expressions of TLR2 and MAPK signaling (p-ERK and p-JNK) in treated MC3T3-E1 cells. The knockdown of TLR2 with shRNA reversed the effect of Staphylococcus aureus on MC3T3-E1 cells. Baicalin utilized a similar mechanism of inhibition of TLR2 to induce osteogenesis in MC3T3-E1 cells [137].
Xu Q. et al. evaluated the effect of Icariin on RAW 264.7 cells treated with RANKL to induce osteoclastogenesis. Treatment of RAW 264.7 cells with RANKL increased NFĸB and MAPK signaling pathway. The phosphorylated forms of P38, ERK, and JNK were found to be elevated. Treating RAW 264.7 cells with Icariin reversed the RANKL effect of inducing osteoclastogenesis by inhibiting NFĸB and MAPK signaling [138].
Liu H. et al. studied the role of Hesperetin on LPS-induced osteoporosis. Hesperetin rescued the osteoclastogenesis-inducing effect of RANKL in RAW 264.7 cells. Hesperetin mediated effect was due to the inhibition of NFĸB and MAPK signaling pathways in RAW 264.7 cells. Similar to in vitro results, Hesperetin rescued LPS-induced bone loss, decreased osteoclast numbers, and suppressed the RANKL/OPG ratio in mice [139]. In addition, Kaempferol can also stimulate osteoblastogenesis in MC3T3-E1 cells treated with DEX by activating MAPK signaling. Treatment with DEX suppressed the expression of RUNX2, Osterix, and ALP activity. However, treatment of Kaempferol attenuates the DEX-induced inhibition of osteogenesis. In the MC3T3-E1 cells treated with DEX, p-P38 decreased significantly, but no significant changes were observed in p-JNK. However, after treatment with Kaempferol, both the phosphorylated forms were shown to increase [140].
3.4. Antioxidant/ROS
The ROS molecule contains unstable oxygen, which can affect other molecules in the cell. When ROS is less, these molecules are able to mediate several signals for cell differentiation, proliferation, and self-renewability. On the contrary, excess ROS will increase oxidative stress following an imbalance in normal tissue homeostasis, resulting in poor tissue management and wound healing. Oxidative stress can lead to cell death and damage to the proteins and nucleic acid [141,142]. In mitochondria, ROS are mainly produced in the electron transport chain as oxidative phosphorylation byproducts [143].
Enzymes produce intracellular ROS, generally as O2, hydrogen peroxide (H2O2), and OH [144,145,146]. H2O2 functions as a second messenger that can integrate environmental cues, swiftly diffuse through membranes, trigger downstream signal transduction cascades, and have a variety of downstream destinations [146,147]. Studies have also elucidated that the family of MAPK, such as p38 MAPK and ERK1/2, are some of the well-known downstream effector molecules of the ROS and usually play an important role in the differentiation of osteoblasts [148,149], probably by activating the p38 MAPK and ERK1/2 pathways. However, more detailed studies are required to ascertain these facts [150].
Flavonoids have high antioxidant activity against ROS and have been reported to rescue the negative effect of ROS on bone. The effect of different flavonoids on the ROS, namely iron, DEX, H2O2, and 2-deoxy-D-ribose (dRib), are shown in Figure 3. Icariin can suppress oxidative stress induced by iron on MC3T3-E1 cells. The viability of MC3T3-E1 cells was decreased after treatment with iron chloride. ROS production was induced by iron chloride. Nevertheless, cell viability was increased after treatment with Icariin, and the ROS production was inhibited dose-dependently. Furthermore, osteogenic differentiation markers such as RUNX2 and Osterix were significantly increased after Icariin treatment. Moreover, iron chloride stimulated osteoclast formation, but the treatment of Icariin inhibited it [151]. Similarly, the effect of Kaempferol was studied on MC3T3-E1 cells treated with dRib. dRib produces oxidative stress through autooxidation and glycosylation. The treatment of dRib notably decreased cell viability, collagen content, and mineralization of MC3T3-E1 cells, but Kaempferol significantly reversed the dRib-induced effects in osteoblasts. Moreover, the treatment of dRib to MC3T3-E1 cells increased the levels of Malondialdehyde (MDA), an indicator for ROS. However, the increased MDA level by dRib was reversed by Kaempferol [152].
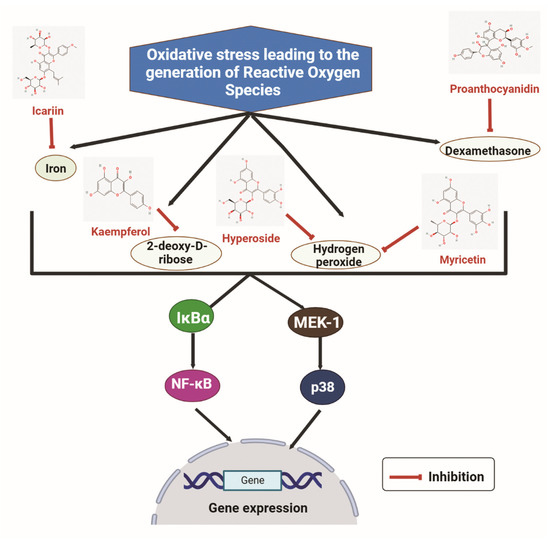
Figure 3.
The inhibitory effects of some flavonoids such as icariin, kaempferol, hyperoside, myricitrin and proanthocyanidin on some reactive oxygen species (iron, dexamethasone, hydrogen peroxide, and 2-deoxy-D-ribose). IκBα: Inhibitor of nuclear factor kappa B alpha; NF-κB: Nuclear factor kappa B; MEK-1: Mitogen-activated protein kinase-1. (Chemical structures source: PubChem (NCBI, NIH, USA; https://pubchem.ncbi.nlm.nih.gov/) accessed on 7 February 2023, Figures created with BioRender.com).
Qi X.C. et al. reported that another flavonoid Hyperoside (Quercetin 3-O-β-D-galactose) could protect against the oxidative stress induced by H2O2 in MC3T3-E1 cells. Hyperoside decreased apoptosis induced by H2O2 and rescued the osteogenesis-related markers collagen I and osteocalcin (OCN). Additionally, H2O2 treatment increased MAPK signaling (p-P38 and p-JNK) to inhibit the effect of H2O2, concluding that oxidative stress induced by H2O2 can be reversed by the antioxidant property of Hyperoside [153]. Huang Q. et al. showed the effect of Myricitrin on oxidative stress induced by H2O2 on human BMSCs and ovariectomy-induced osteoporosis model. Treatment of H2O2 results in decreased cell viability, mineralization, and expression of osteogenic markers ALP and OCN. However, the application of Myricitrin reversed the effect of H2O2 in human BMSCs. In the ovariectomized mice model, increased levels of MDA (end product of lipid peroxidation) and reduced glutathione (intracellular antioxidant) are observed. Treating Myricitrin to ovariectomized mice model attenuated the oxidative effect of H2O2 [154].
Chen L. et al. demonstrated that Proanthocyanidins (PAC) could suppress oxidative stress in primary osteoblasts induced by DEX. DEX-treatment induces cell apoptosis in osteoblasts by enhancing the expression of several apoptotic markers such as Caspase 3 and Bax. However, PAC reversed the DEX effect, stimulated Bcl2 expression, and inhibited Bax and Caspase 3. Moreover, DEX inhibited the Nrf2 transcription factor related to the regulation of oxidative stress. The effect of PAC was analyzed in the DEX-treated rat model. Nrf2 expression was higher in DEX-PAC treated model compared to the only DEX-treated one [155].
3.5. NFκB/NFATc-1 Signaling
NFκB is one of the most important regulators involved in bone remodeling and inflammation. The NFκB activity is promoted when the rate of bone resorption exceeds bone formation [156]. RelA (p65), p52, c-Rel, RelB, and p50 are the key members of NFκB signaling. The canonical p65/p50 and NFκB heterodimeric complex is a prevalent isoform of NFκB. Several pro-inflammatory stimulants such as Interleukin (IL)-1 and TNF can stimulate the NFκB pathway. However, the non-canonical NFκB pathway is induced by releasing a small subgroup of TNF family members [157].
The canonical NFκB pathway reacts to a variety of stimuli, such as ligands for pattern-recognition receptors (PRRs), various cytokine receptors, members of the TNF receptor (TNFR) superfamily, and T-cell and B-cell receptors [158]. The inducible degradation of inhibitor of nuclear factor kappa B alpha (IκBα), which is brought on by its site-specific phosphorylation by a multi-subunit IκB kinase (IKK) complex, is the main mechanism for canonical NFκB activation [159,160]. Two catalytic subunits, namely IKKα and IKKβ, as well as a regulatory component known as NFκB essential modulator (NEMO) or IKKγ, constitute the IKK molecule [161]. Various stimuli, such as cytokines, growth factors, mitogens, microbial components, and stressors, can activate IKK [162]. When IKK is activated, it phosphorylates IκBα at two N-terminal serines, which causes IκBα to be degraded in the proteasome in a ubiquitin-dependent manner. This causes the nuclear translocation of canonical NFκB members, primarily the p50/RelA and p50/c-Rel dimers, to occur very quickly and causes gene transduction [160,163,164].
Flavonoids have a role in effectively hindering the transcription of the NFATc-1 gene by targeting RANKL (Figure 4). Xiao L. et al. studied the effect of Puerarin on the differentiation of osteoclasts where RANKL was treated to RAW 264.7 cells. TRAF6 and ROS expressions were elevated in RANKL-stimulated RAW 264.7 cells, and the Puerarin treatment reversed the effect. Furthermore, NFκB and MAPK signaling were related to the effect of Peurarin on RANKL treatment. The phosphorylated form of p65 and IĸBα expressions were increased with RANKL treatment, followed by the increase in p-p38, p-JNK, and p-ERK. After treatment with puerarin, phosphorylated forms of NFĸb and MAPK signaling were significantly decreased. Puerarin inhibited osteoclastogenesis by suppressing NFĸb and MAPK signaling [165]. Alternatively, the flavonoid molecule Epicatechin 3-O-β-D-allopyranoside (ECAP) has an anti-osteoclastogenesis effect on RANKL-stimulated RAW 264.7 cells. RANKL treatment activates the NFATc-1 and NFκB, which is important for osteoclastogenesis. ECAP altered the effect of osteoclastogenesis induced by RANKL by downregulating the phosphorylation of p65, IĸBα, as well as NFATc-1 expression dose-dependently [65].
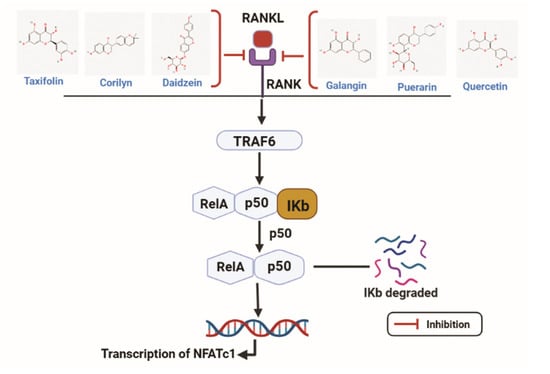
Figure 4.
Taxifolin, corilyn, daidzin, galangix, puerarin, and quercetin acts as antagonists by hindering the transcription of the NFATc-1 gene by targeting RANKL. RANKL: Receptor activator of nuclear factor kappa-Β ligand; RANK: Receptor activator of nuclear factor kappa-Β; TRAF6: Tumor necrosis factor receptor associated factor 6; NFATc1: Nuclear factor of activated T cells 1. (Chemical structures source: PubChem (NCBI, NIH, USA; https://pubchem.ncbi.nlm.nih.gov/) accessed on 7 February 2023, Figures created with BioRender.com).
Similarly, Hyperoside can protect against ovariectomy-induced bone loss. The bone resorption markers, namely C-terminal telopeptide of type I collagen (CTX) and tartrate-resistant acid phosphatase 5b (TRAP-5b) were increased after ovarian resection, but treatment with Hyperoside resulted in decreased bone resorption. In ovariectomized mice model p-p65, p-IĸBα and NFATc-1 expression levels were increased, which was further attenuated by Hyperoside. These results indicate that NFκB and NFATc-1 signaling pathways are related to the mechanism of Hyperoside in the ovariectomy-induced osteoporosis mice model [166]. EGCG can suppress osteoclastogenesis by inhibiting osteoclast-specific markers as well as NFκB and MAPK signaling in RANKL-stimulated RAW 264.7 cells [167].
Studies elucidated the effect of Daidzin on RANKL stimulation on the BMSCs. Daidzin can protect osteoclastogenesis on bone marrow-derived macrophages (BMMs) by inhibiting osteoclast-specific transcript factors such as NFATc-1, c-Fos, TRAP, CTSK, and the NFκB signaling pathway [168]. Lastly, Delphinidin-enriched maqui berry extract (MBE) was shown to improve the osteogenic activity of MC3T3-E1 cells. MBE inhibited the transnucleation of p65 induced by LPS. The osteoclastogenesis effect was evaluated by RANKL treatment on primary mouse bone marrow cells. The NFATc-1 and CTSK were increased with RANKL but time-dependently reversed with MBE treatment. Therefore, MBE can be associated with osteoblastogenesis by impeding the NFκB signaling pathway [169]. Zhang H.Q. et al. indicated that Taxifolin could suppress osteoclast differentiation induced by RANKL on BMMs. mRNA expressions of osteoclast markers such as TRAP, NFATc-1, and CTSK were increased with RANKL treatment, whereas treatment of Taxifolin reversed the expressions in a dose-dependent manner [170].
3.6. Other Signaling Pathways
Numerous studies reported the effects of flavonoids on osteoblastogenesis and osteoclastogenesis through many signaling pathways.
Zhou L. et al. examined Icariin’s effect in the ovariectomized rat model, MC3T3-E1 cells, and BMSCs. BMSCs isolated from the ovariectomized rat model shows a decrease in ALP and enhanced osteoclastogenesis. Icariin treatment reversed the effect similarly with a positive control E2 (phytoestrogen). Insulin-like growth factor I (IGF-I) signaling can activate estrogen receptors by stimulating phosphoinositide 3-kinase (PI3K). Icariin treatment stimulated the levels of ER and IGF-IR on BMSCs. In MC3T3-E1 cells, Icariin stimulated the expression of IGF-IR, but after blocking the IGF-IR with PPP (IGF-IR kinase inhibitor picropodophyllin), the Icariin effect was inhibited. Moreover, Icariin can stimulate bone formation by IGF-I1 and ER signaling in the ovariectomy-induced osteoporosis model [171]. Additionally, Icariin treatment elevated collagen 1-α1, OCN, and ALP levels. Notch signaling molecules were suppressed by Icariin treatment, such as Hes1 and Hey1. Moreover, inhibiting Notch signaling with DAPT (N-[N-(3, 5-difluorophenacetyl)-l-alanyl]-s-phenylglycinet-butyl ester), a γ-secretase inhibitor, enhanced the effect of Icariin. Ovariectomized mice model had increased expression levels of NFATc-1 and Notch 1, which was attenuated by Icariin, indicating its role in osteoblastogenesis by regulating Notch signaling [172]. Icariin also plays a major role in osteoblastogenesis by activating cAMP signaling in rat calvarial osteoblasts. Icariin treatment increased ALP activity and cAMP contents with an elevation in the level of p-PKA and p-CREB [173].
Another flavonoid, Luteoloside, is shown to inhibit osteoclastogenesis on RANKL-stimulated BMMs. Luteoloside inhibited the osteoclast markers NFATc-1, CTSK, and calcitonin dose-dependently. RANKL induced the Ca2+ oscillation and NFATc-1, but Luteoloside rescued the effect. Treatment of Luteoloside also reversed the effect of MAPK signaling. Activation of NFkB was significantly reduced with the Luteoloside treatment. Luteoloside also suppressed RANKL-induced osteoclastogenesis through NFATc-1 and Ca2+ signaling, as well as NFĸB and MAPK signaling [174]. Cai P. et al. reported the effect of Baicalein on MC3T3-E1 cells treated with glucocorticoid. Baicalein directly targeted genes were Ak strain transforming (AKT) 1, forkhead box protein O (FOXO) 1, and FOXO3. The glucocorticoid treatment decreased the levels of several osteoblastogenesis markers, namely ALP, RUNX2, and OCN. Treatment of Baicalein attenuated glucocorticoid’s effect, inhibited the p-AKT expression level, and stimulated the FOXO1 expression. Silencing of the AKT with siRNA-AKT increased FOXO1, suppressing OCN, ALP, and RUNX2. All evidence shows that Baicalein can inhibit glucocorticoid-induced osteoporosis by regulating AKT/FOXO1 signaling [175].
Similarly, Kaempferol can induce osteoblastogenesis by mTOR signaling on BMSCs from the ovariectomized mice model. BMSCs from ovariectomized mice have decreased osteoblastogenesis markers ALP, RUNX2, and osterix, but the treatment of Kaempferol increased their expressions. Rapamycin, an mTOR inhibitor, was used to assess the effect of Kaempferol. The downstream regulators of mTOR, 4E/BP1, and S6K1 concentrations were induced in the ovariectomized mice model, but Kaempferol reversed the effect indicating its potency to stimulate osteoblast differentiation on ovariectomized BMSCs by regulating mTOR signaling [176]. Zhou Y. et al. demonstrated that Puerarin stimulated osteoblastogenesis in BMSCs via p53/TNFα/STAT1 signaling. Treatment with Puerarin suppressed the pro-inflammatory cytokines such as IL-6, TNFα followed by the increased production of anti-inflammatory cytokines such as IL-2 and IL-10. Apoptotic markers such as caspase 3 and caspase 9 were decreased, followed by the upregulation in miR-155-3p level. Additionally, the levels of TNFα, STAT1, and p53 were decreased. Inhibiting miR-155 increased the TNFα, STAT1, and p53, showing that Puerarin can regulate osteoblastogenesis by inhibiting p53/TNFα/STAT1 signaling [177].
4. Absorption of Flavonoids
One of the most pivotal factors responsible for maintaining the normal physiological functions of flavonoid molecules in the body is its bioavailability [178]. In plant diets, flavonoids are primarily found as glycosides. Since they are bonded to sugars in the form of beta-glycosides, flavonoids found in food were formerly thought to be indigestible [43]. The aglycone part penetrates the epithelial cells of the intestine after undergoing the process of hydrolysis, where it is processed by the phase II enzymes to produce the corresponding conjugated metabolites [24]. Following the consumption of flavonoids, the conjugated metabolites are identified in the plasma, as the majority of flavonoids undergo methylation, glucuronidation, and sulfation in the liver and small intestine [178]. Most dietary nutrients are primarily absorbed in the small intestine [27]. The lacteals or portal veins receive the absorbed flavonoids after conjugation through the small intestine. Only the aglycone parts are generally absorbed since the glycosides were deemed extremely hydrophilic for passive diffusion via the small intestine. Thus, only a small amount of glycosylated flavonoids are absorbed. One of the theories for the tea flavonoids’ poor bioavailability and absorption is that they are unstable in the colon [24].
5. Delivery System of Flavonoids for Bone
The highly unsaturated structure of flavonoids causes reduced potential bioactive benefits by easy oxidation and degrading of flavonoids [179]. Flavonoids as therapeutic agents are more limited applications because of their low bioavailability and aqueous solubility. A strategy to overcome the limitations mentioned above is to apply the delivery system for flavonoids by carriers using chemical and biological methods [180,181]. For example, liposomes formed by lipid and water bilayers can catch both substances that have hydrophilic or hydrophobic properties, respectively. Some structures of flavonoids can indicate significant loading abilities in membranes of liposomes and can affect the properties of encapsulating and stabilizing liposomes by binding structures of loaded flavonoids and liposomes [179,180]. Recent advancements have elucidated the use of lipid nanoparticles, liposomes, micelles, scaffolds, and hydrogen nanocomposite to deliver different flavonoids (Figure 5).
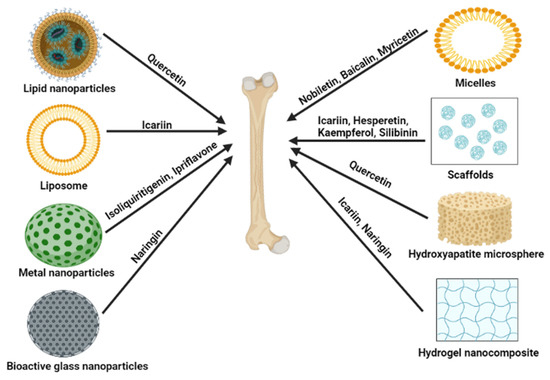
Figure 5.
Potential delivery systems for delivering flavonoids to the bone. Figures created with BioRender.com.
5.1. Lipid Nanoparticles
Quercetin-loaded solid lipid nanoparticles (oral administration, 5 mg/kg/day) have higher abilities to alleviate bone loss and enhance bone strength compared to only quercetin treatment in the ovariectomized rat model [182].
5.2. Liposome
For delivering the osteogenic phytomolecule to cells, Asp8-liposome can be an efficient delivery system. In an estrogen depletion-induced osteoporosis mice model by ovariectomy, Asp8-icaritin-liposome can prevent osteoporosis by inducing bone formation [183]. Asp8-liposome-Icaritin bone-targeting delivery system effectively prevents steroid-associated osteonecrosis in the rat model. In addition, the Asp8-liposome delivery system also can enhance osteogenesis and inhibits osteoclast activity [184]. Sun X. et al. have developed a novel formulation that is biomineral-binding liposomes (BBL) loaded with icariin as a new therapeutic candidate for osteoporosis. In BBL formulation, pyrophosphate acts as the bone-binding moiety, and drug-conjugated liposome rapidly strongly binds to hydroxyapatite (HA). BBL loaded with Icariin has good therapeutic efficacy on the ovariectomized + glucocorticoid group in the rat model [185].
5.3. Metal Nanoparticles
Isoliquiritigenin-encapsulated mesoporous silica nanoparticles (MSNs-ISL) suppress the remarkable RANKL-induced osteoclastogenesis and inhibit osteolysis by osteoclast in BMMs. Therefore, MSNs-ISL can protect against the destruction of bone inflammation [186]. SiO2-CaO systems with a hollow core surrounded by mesopores in a radial arrangement are called NanoMBGs. It induces the response of macrophages against stimulation of LPS and IL-4. Moreover, it does not induce the polarization of macrophages regarding the M1 pro-inflammatory phenotype. NanoMBGs loaded with Ipriflavone do not affect cell proliferation and cell viability of osteoblasts in coculture with osteoclasts while remarkably inhibiting the proliferation of osteoclasts and activating resorption [187].
5.4. Bioactive Glass Nanoparticles
Mesoporous bioactive glass nanoparticles suppress the inflammatory responses of macrophages. Bioactive glass nanoparticles was modified with β-cyclodextrin and was loaded with Naringin (Aladdin, Shanghai, China) (are called as NG@CD- bioactive glass nanoparticles). NG@CD-bioactive glass nanoparticles facilitates the transformation to the M2 phenotype in macrophages. In addition, NG@CD- bioactive glass nanoparticles synergistically promotes osteogenesis and suppresses osteoclast formation in the local immune microenvironment. In a rat model with a femoral defection, NG@CD-bioactive glass nanoparticles increases the expression level of osteogenesis-related genes and the formation of new bone [188].
5.5. Micelles
Nobiletin-PEG-PCL micelles developed by loading Nobiletin to poly(ethylene glycol)-block-poly(e-caprolactone) could increase its circulation time by preventing the rapid release of Nobiletin from micelles. Nobiletin-PEG-PCL restrains osteoclast differentiation in BMMs via the MAPK signal pathway by RANKL. In addition, Nobiletin-PEG-PCL reduces the loss of bone and enhances bone density in ovariectomized mice model [189]. Baicalein encapsulated D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) polymeric micelles (PMs) effectively reduced the damaged gingival fiber and destructed alveolar bone by direct injection in a rat model with periodontal disease [190]. The AL-P(LLA-CL)-PEG-P(LLA-CL)-Myricetin-loaded micelles are expected as a bone-targeting delivery system for the treatment of osteoporosis. In an ovariectomized rat model, AL-P(LLA-CL)-PEG-P(LLA-CL)-Myricetin-loaded micelles showed the improved oral bioavailability of Myricetin and excellent capability for bone-targeting [191]. Self-assembled nanomicelles could act as useful oral carriers to deliver therapeutics with low bioavailability for osteoporosis treatment. Circinal–Icaritin by self-assembled nanomicelles (CIT-SO-DOC) improved the bioavailability of CIT and increased the effect of preventing osteoporosis by reducing the size and enhancing the absorption of CIT [192].
5.6. Scaffolds
Porous composite scaffolds with Icariin-loaded HA/alginate (Icariin/HAA) stimulated cell proliferation in BMSCs, meanwhile no cytotoxicity. Especially porous composite scaffolds with icariin/HAA suppressed osteoclast activation in vivo. Moreover, it enhanced gene expression levels of osteogenic markers and the Wnt signaling-related genes. Further, bone regeneration of rabbits with radius bone defection was improved by porous composite scaffolds with Icariin/HAA [193].
Composite hierarchical porous scaffolds were developed for carrying the BMP2 and Icariin under a controlled drug delivery system (SFBMP2/SBA15IC) and composed of silk fibroin micropores and SBA15 mesopores (SF/SBA15). SFBMP2/SBA15IC has an excellent induction ability of osteogenesis than other BMP2 or Icariin-loaded groups by showing higher increased osteogenic differentiation gene expression and ALP staining and mineralization in co-cultured BMSCs [194].
Calcium phosphate cement (CPC) scaffolds loaded with Icariin were synthesized for successfully delivering Icariin. Icariin enhances the osteogenic and angiogenic effects in BMSCs and inhibits osteoclastogenesis. CPC scaffolds are a better delivery system for repairing bone defects because of their capability to improve osteogenesis and angiogenesis. Thereby, systemic administration of CPC scaffolds loaded with Icariin could promote the repair of bone defects and the prevention of osteoporosis in the ovariectomized rat model [194]. Dual drug release is achieved by incorporating Icariin and vancomycin into an injectable CPC. It is a notable potential therapeutic candidate for bone infection disease or contaminated bone injury because Icariin-VA-CPC scaffolds have antibiotic and osteoinductive effects. [195]. Icariin–small-intestine submucosa scaffolds (Icariin-SIS) elevated osteogenic markers such as ALP, bone sialoprotein (BSP), and OCN in MC3T3-E1 cells. The formation of new bone was accelerated after implanting IC-SIS in mice with calvarial defects [196]. Another study reported that icariin–extracellular matrix (ECM)-SIS induced higher expression levels of markers of osteogenic differentiation (such as ALP, BSP, and OCN) and BMP4 expression than ECM-SIS (same as Icariin-SIS). Moreover, Icariin-ECM-SIS implanted mice with defective calvaria have increased bone regeneration and a higher new bone formation ratio compared to ECM-SIS or Icariin-SIS implantation ground [197]. Hesperedin/gel sponge scaffolds successfully carried Hesperedin to human MSCs and induced osteogenic differentiation by activating the ERK and Smad signaling pathways. Hesperedin/gel sponge scaffolds significantly promoted fracture healing of rat tibia in the rat osteotomy model [198]. Layer-by-layer (LBL) nano-matrix, incorporated with Kaempferol characterized as stable, increased drug delivery ability and pharmacokinetics, and inhibited enzyme degradation. Therefore, the LBL nano-matrix incorporated with Kaempferol stimulated osteogenesis in the osteopenic rats [199]. Alginate/gelatin-Silibinin scaffolds could be usefully applicated to bone tissue engineering because they enable continuous release of Silibinin during an extended time and induction of bone formation in vitro [200].
5.7. HA Bioceramic Microspheres
For application in carrying Quercetin, nHA bioceramic microspheres developed having a micro–nano hybrid surface. nHA bioceramic microspheres sustained the release of Quercetin. Moreover, nHA bioceramic microspheres with Quercetin could induce the formation of bone and angiogenesis in femur defect rats with ovariectomy [201].
5.8. Hydrogel Nanocomposite
Improved alginate dialdehyde–gelatin hydrogel nanocomposite by incorporating mesoporous silica–calcium nanoparticles and Icariin loading assures enhanced osteoblast proliferation, adhesion, and differentiation in MC3T3-E1 cells [202]. The hydrogel loaded with Naringin indicated the rapid release of Naringin at pH 5.5 to 6.5. Cell viability increased at 0.85% after Naringin treatment. Especially, CHC-β-GP-glycerol hydrogel that carries Naringin inhibited loss of periodontal bone and infiltration by inflammation. Further, it meaningfully suppressed the expression of TLR2, RAGE, and TNFα in periodontitis [203].
5.9. Phase-Transited Lysozyme (PTL)-Primed Ti Surface
PTL-primed titanium surface can locally deliver Icariin through a layer-by-layer self-assembly system. PTL-primed titanium surface with Icariin-immobilized HA/chitosan multilayer enhances the osteogenesis on osteoblasts and improves early osseointegration in vivo by continuously releasing icariin [204].
5.10. Nano Coating
Flavonoids can be coated on the surface of osseo-integrable implants and can be made to release at the desired site of action. The Quercetin was grafted on titanium coins (between 64 ± 10 and 842 ± 361 nmol on 6.2 mm). Quercetin-nanocoated titanium surface promotes mineralization in human BMSCs and can accelerate osteointegration in bone implants [205].
6. Conclusions
Diseases associated with the skeletal system can limit an individual’s mobility and can often result in considerable morbidity. The increase in the activity of osteoclasts or decreased activity of osteoblasts is the underlying molecular cause of osteoporosis. This imbalance in the bone remodeling process causes accelerated bone resorption and suppressed bone formation [206]. Moreover, the pathophysiology of osteoporosis is now understood to include decreased bone density and skeletal fragility brought on by several factors, including (1) defects in the trabecular microarchitecture; (2) flaws in the intrinsic materials of the bone tissue; (3) imperfections in the repair of microdamage from routine daily activities; and (4) excessive rates of bone remodeling [207]. Numerous drugs and therapeutical approaches have been tested to establish an efficient cure for osteoporosis [208]. Patients with osteoporosis can employ pharmaceutical treatments to lower their fracture risk and boost bone mineral density. Still, their usage is constrained by the side effects, which depend on a variety of factors, including past medical history, genetics, and patient’s nutritional habits [209,210,211,212,213]. Commercially available drugs for osteoporosis, their signaling mechanism of action, and known side effects are listed in Table 2. The long-term use of commercially available bisphosphonates such as Alendronate, Zoledronate, and Risedronate has been reported to cause some substantial side-effects such as diarrhea, gastritis, nausea, and flatulence [214]. In addition, the implementation of RANKL inhibitors such as Denosumab has potential side effects such as urinary tract infection, polyoma (BK) viremia, and flu-like syndrome compared to the control subjects [215]. The prevention of osteoporosis by hormone-replacement therapy, including estrogen, has the chance of developing cardiovascular diseases, breast cancer, thromboembolic disorders, and stroke [216]. An anti-sclerostin antibody, namely Romosozumab, increases the Wnt signaling activity to maintain a homeostatic balance between bone resorption and bone formation. However, it has been shown to increase the chances of stroke and cardiovascular difficulties in seven patients in the first year of the trial [217]. New advancements in the field of therapeutics against bone diseases are urgently required to effectively treat osteoporosis while reducing side effects and regardless of changing patient-related circumstances [208].

Table 2.
Therapeutical options for the treatment of bone loss and their mechanisms of action and side effects. (Protelos® and Osseor® (Servier, France)).
Flavonoids have favorable and preventive effects on the pathological aspect of bone loss and the emergence of osteoporosis. Administering substances that have an impact on bone deposition and remodeling at the same time is one of the potential therapy options using purified chemicals. Dietary supplements consist of a range of natural bioflavonoids as essential components. Flavonoids have been shown to possess various therapeutic potentials such as antitumor, antimicrobial, anti-oxidant, anti-cancer, anti-inflammatory properties, stimulating osteogenic abilities, etc. Some flavonoids, including anthocyanins, flavonols, and isoflavones, have dual osteoclast and osteoblast stimulatory effects [11]. Compared to pharmaceutical treatments, these naturally occurring phytochemicals with powerful bone-conserving qualities beyond vitamin D and calcium have fewer or no adverse effects. In addition to their inherent chemical anti-oxidant abilities, flavonoids are also being researched for their potential anti-inflammatory properties. As discussed in previous sections, some flavonoids such as Cyanidin, Daidzein, Cladrin, Calycosin, Icariin, and Petunidin have been shown to function by activating osteoblasts and inhibiting osteoclasts [11]. Few clinical trials have been carried out, although several flavonoids have demonstrated to have largely preventive properties aimed at preventing pathological bone loss, highlighting their specific effects on osteoblast and osteoclast differentiation and activity through the same interactors. The preventive properties of flavonoids have been shown in a number of preclinical studies. Even though these chemicals definitely play epigenetic roles, only a few chemical studies have elaborated on their mechanism [244]. These bioactive substances are thought to stimulate bone formation and prevent bone resorption by controlling cell signaling pathways such as Wnt and BMP signaling that affects osteoblast and osteoclast development in tandem. For instance, with minimal or no carcinogenic side effects, the bioavailability of isoflavones possessing the selective estrogen receptor affinity can potentially prevent osteoporosis [245].
Additionally, data from a randomized, double-blind, placebo-controlled trial portrayed the beneficial effect of the flavonoid icariin in treating osteoporosis which has comparatively lower chances of causing cardiovascular ailments and breast cancer [246]. Nevertheless, the activities of flavonoids are not explained by a single mechanism but rather by a confluence of numerous routes. Studies currently underway point to the potential for including flavonoids in grafts and bone scaffolds to ensure local administration and continuous release of flavonoids that can speed up bone healing [247].
Numerous studies have studied the interaction of flavonoids with various target proteins involved in osteogenesis. Jiang J. et al. performed molecular docking studies to predict and verify the roles of some flavonoids, Icariin, Baohuside-I, and Icartin, in reversing the inhibitory effect of the glucocorticoid-induced bone formation. These flavonoids were docked with some of the proteins such as RANKL, RUNX2, OPG, BMP2, and BMP4 [248]. Hu Y. et al.’s molecular docking studies also delineated the mechanism of using quercetin against osteoporosis. NFKB1, IL-1β, and RelA had an increased binding potency which mainly explains Quercetin’s anti-osteoporosis activity [249]. Additionally, the role of Kaempferol in curing senile osteoporosis was also confirmed by performing molecular docking studies. The study revealed that Kaempferol is a potent curative agent against this musculoskeletal disease as it can regulate oxidative stress, various inflammatory pathways, and bone homeostasis [250]. For the first time, molecular docking investigations performed by Yu X. et al. revealed that Naringin might treat osteoporotic fracture, presumably via modulating a variety of signaling pathways and targets associated with the development of osteoclasts and oxidative stress. The docking study was performed using 8 proteins, namely SERPINE1, CASP3, PPARG, ESR1, MMP1, TNF, CYP19A1 and ACE [251].
However, poor bioavailability and long-term stability issues have hindered their clinical impact. Recently, with the advent of new-age delivery methods, including polymer science and nanotechnology, the issues with flavonoid bioavailability and toxicity are being resolved. Thus, flavonoids with bone-sparing properties are being considered for bone tissue engineering and regenerative medicine for unmet needs for bone augmentation. Even the inclusion of certain biomaterials to deliver flavonoids can contribute to bone tissue engineering. A synergistic interaction between nanoscience and flavonoids might enable the formation of hybrid nanocomposites to enhance their epidemiological properties. However, researchers should consider each delivery system’s pros and cons before deciding the delivery method for flavonoids for therapeutic purpose (Table 3).

Table 3.
Advantages and Disadvantages of delivery systems for flavonoids.
In this review, we have discussed the role of flavonoids on the various osteogenic signaling pathways and how various studies have highlighted the potential of flavonoids on bone-forming ability. Natural flavonoids are often cost-effective and possess fewer side effects than their chemically synthesized counterparts (Figure 6). Though tons of research has been performed to decipher the mechanism of action of flavonoids on osteogenesis and osteoclastogenesis, no flavonoids have been approved as a drug for any bone diseases. However, in recent times, to validate the effect of flavonoids, few studies have been undertaken to assess their potential in human subjects (Table 4). Moreover, a few patents have been registered for flavonoids’ role in treating bone-related diseases (Table 5). More such clinical trials are required to tap the potential and ability of flavonoids to influence the bone remodeling process and project them as potential therapeutics.
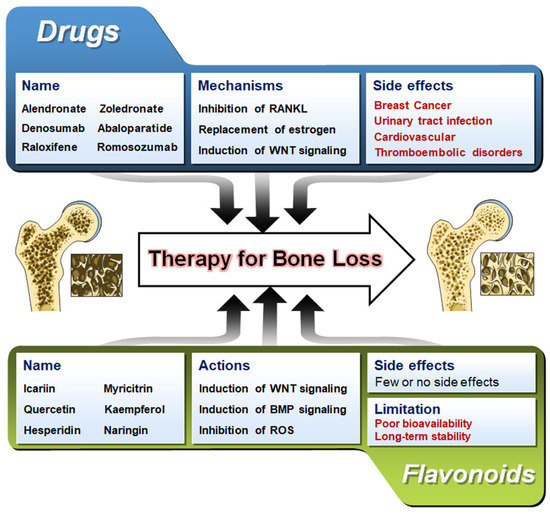
Figure 6.
A comparison of flavonoids and available pharmaceutical drugs in treating bone loss. Bone images are taken from Servier Medical Art (https://smart.servier.com accessed on 7 February 2023) which is licensed under a Creative Commons Attribution 3.0 Unported License (Suresnes, France).

Table 4.
Clinical trial studies of flavonoids for treating bone loss.

Table 5.
Patents of flavonoids for treating bone loss.
Despite the pharmacological potential of flavonoids, dietary flavonoids have a number of drawbacks. When consumed with other food ingredients, flavonoids may experience greater precipitation, complexation, and microbial degradation, significantly affecting their stability and bioavailability [261,262]. Even flavonoids in higher concentrations have the potential to be mutagens and pro-oxidants and can create free radicals and inhibitors of important enzymes involved in the metabolism of hormones. Because of this, flavonoids should only be consumed in reasonable amounts that are generally found in a regular vegetarian diet. High dosages may have more negative effects than positive ones. Increased flavonoid exposure from food or supplementation can potentially overwhelm the body, developing reactive oxygen species and ultimately causing DNA damage. Furthermore, due to the rapid cell proliferation that occurs during fetal development, adverse effects might be amplified and lead to increased sensitivity to phytochemical exposure [263]. When consumed at levels of 1000 mg per day, flavonoids frequently cause nausea, headaches, or tingling in the extremities in some persons. Similarly, a study found that some cancer patients may have liver damage from tea extract supplements. In addition, as the safety of flavonoid supplements in pregnancy and lactation has not been demonstrated, it is better to avoid them during these times [264].
The structural components present in flavonoids are also responsible for their potential side effects. When peroxidases oxidize phenol ring-containing flavonoids, they produce cytotoxic phenoxyl radicals, which co-oxidize the unsaturated lipids moieties such as NADH, GSH, nucleic acids, and ascorbate, generate ROS and induce mitochondrial toxicity [265,266,267,268]. The electrophilic quinone/quinone methides formed by flavonoids with catechol rings have been found to bind to GSH, protein, and DNA [267,269,270,271,272]. Because flavonoids have been demonstrated to both promote [273] and inhibit [274] drug-metabolizing enzymes, there are further reasons to be concerned about mega flavonoid supplementation. If flavonoids and other dietary phenolics are to be employed as therapeutic agents, it is clear that more investigation into their possible side effects is necessary [275].
Thus, a plethora of studies will be required to assess the benefits of the bioactivity of flavonoids and their reported drawbacks as therapeutics. Moreover, research must be continued to develop materials or delivery methods that can deliver controlled release kinetics and degradation and directly influence the rate of new bone formation. While developing such strategies, the researcher has to consider factors such as mimicking the bone microenvironment at the implantation site, promoting angiogenesis, stage of inflammation, and osteogenesis phases of new bone formation. Since flavonoid action is well acclaimed in improving bone health, the efficient delivery methods enabling them with easily absorbable and sustained release would benefit bone regeneration in vivo.
Author Contributions
Conceptualization, A.R.S.; investigation, A.R.S., Y.-H.L. and A.B.-U.; data curation, A.R.S. and Y.-H.L.; validation, M.B. and C.C.; writing—original draft preparation, A.R.S. and Y.-H.L.; visualization, A.B.-U., S.C. and C.C.; writing—review and editing, S.-S.L.; supervision, A.R.S. and S.-S.L.; project administration, A.R.S. and S.-S.L.; funding acquisition, A.R.S. and S.-S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Hallym University Research Fund and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1C1C1008694 & NRF-2020R1I1A3074575).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The Global Burden of Osteoporosis, Low Bone Mass, and Its Related Fracture in 204 Countries and Territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- de Villiers, T.J.; Goldstein, S.R. Bone health 2022: An update. Climacteric 2022, 25, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Molagoda, I.M.N.; Kang, C.H.; Lee, M.H.; Choi, Y.H.; Lee, C.M.; Lee, S.; Kim, G.Y. Fisetin promotes osteoblast differentiation and osteogenesis through GSK-3beta phosphorylation at Ser9 and consequent beta-catenin activation, inhibiting osteoporosis. Biochem. Pharmacol. 2021, 192, 114676. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Tarrant, S.M.; Balogh, Z.J. The Global Burden of Surgical Management of Osteoporotic Fractures. World J. Surg 2020, 44, 1009–1019. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Bellavia, D.; Caradonna, F.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Giavaresi, G. Non-flavonoid polyphenols in osteoporosis: Preclinical evidence. Trends Endocrinol. Metab. 2021, 32, 515–529. [Google Scholar] [CrossRef]
- Zuo, C.; Huang, Y.; Bajis, R.; Sahih, M.; Li, Y.P.; Dai, K.; Zhang, X. Osteoblastogenesis regulation signals in bone remodeling. Osteoporos. Int. 2012, 23, 1653–1663. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Khedgikar, V.; Ahmad, N.; Karvande, A.; Gautam, J.; Kumar, P.; Maurya, R.; Trivedi, R. A neoflavonoid dalsissooal isolated from heartwood of Dalbergia sissoo Roxb. has bone forming effects in mice model for osteoporosis. Eur. J. Pharmacol. 2016, 788, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, H.M.; Yu, S.B.; Song, J.M.; Kim, C.H.; Kim, B.J.; Park, B.S.; Shin, S.H.; Kim, I.R. Cytoprotective effect of flavonoid-induced autophagy on bisphosphonate mediated cell death in osteoblast. J. Cell. Biochem. 2018, 119, 5571–5580. [Google Scholar] [CrossRef] [PubMed]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rojas, B.; Gutierrez-Venegas, G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 2018, 209, 435–454. [Google Scholar] [CrossRef]
- Gutierrez-Venegas, G.; Sanchez-Carballido, M.A.; Delmas Suarez, C.; Gomez-Mora, J.A.; Bonneau, N. Effects of flavonoids on tongue squamous cell carcinoma. Cell Biol. Int. 2020, 44, 686–720. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Al Mamun, M.A.; Hosen, M.J.; Islam, K.; Khatun, A.; Alam, M.M.; Al-Bari, M.A. Tridax procumbens flavonoids promote osteoblast differentiation and bone formation. Biol. Res. 2015, 48, 65. [Google Scholar] [CrossRef]
- Chan, K.; Leung, H.C.M.; Tsoi, J.K. Predictive QSAR model confirms flavonoids in Chinese medicine can activate voltage-gated calcium (CaV) channel in osteogenesis. Chin. Med. 2020, 15, 31. [Google Scholar] [CrossRef]
- Sharma, A.R.; Nam, J.S. Kaempferol stimulates WNT/beta-catenin signaling pathway to induce differentiation of osteoblasts. J. Nutr. Biochem. 2019, 74, 108228. [Google Scholar] [CrossRef]
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteoblastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, H.; Jia, S. Puerarin enhances bone mass by promoting osteoblastogenesis and slightly lowering bone marrow adiposity in ovariectomized rats. Biol. Pharm. Bull. 2014, 37, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed]
- Murota, K. Absorption pathway of dietary flavonoids: The potential roles of the lymphatic transport in the intestine. Funct. Foods Health Dis. 2020, 10, 274–289. [Google Scholar] [CrossRef]
- Welch, A.A.; Hardcastle, A.C. The effects of flavonoids on bone. Curr. Osteoporos. Rep. 2014, 12, 205–210. [Google Scholar] [CrossRef]
- Shen, C.L.; Chyu, M.C. Tea flavonoids for bone health: From animals to humans. J. Investig. Med. 2016, 64, 1151–1157. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Jeppesen, P.B. Isoflavones and bone health in perimenopausal and postmenopausal women. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 475–480. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.G.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, e2007356. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Saulite, L.; Jekabsons, K.; Klavins, M.; Muceniece, R.; Riekstina, U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine 2019, 53, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Laleh, G.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare, S. The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pak. J. Nutr. 2006, 5, 90–92. [Google Scholar]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Bussy, U.; Ottaviani, J.I.; Kwik-Uribe, C. Evolution of cocoa flavanol analytics: Impact on reporting and cross-study comparison. Food Funct. 2021, 12, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.; Rivoira, M.; Picotto, G.; de Barboza, G.D.; Collin, A.; Tolosa de Talamoni, N. Analysis of the Molecular Mechanisms by Flavonoids with Potential Use for Osteoporosis Prevention or Therapy. Curr. Med. Chem. 2022, 29, 2913–2936. [Google Scholar] [CrossRef]
- Nibbs, A.E.; Scheidt, K.A. Asymmetric methods for the synthesis of flavanones, chromanones, and azaflavanones. Eur. J. Org. Chem. 2012, 2012, 449–462. [Google Scholar] [CrossRef]
- Iwashina, T. Flavonoid properties of five families newly incorporated into the order Caryophyllales. Bull. Natl. Mus. Nat. Sci. 2013, 39, 25–51. [Google Scholar]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Lagana, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. Biofactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Jeon, E.J.; Lee, D.H.; Kim, Y.J.; Ahn, J.; Kim, M.J.; Hwang, J.T.; Hur, J.; Kim, M.; Jang, Y.J.; Ha, T.Y.; et al. Effects of yuja peel extract and its flavanones on osteopenia in ovariectomized rats and osteoblast differentiation. Mol. Nutr. Food Res. 2016, 60, 2587–2601. [Google Scholar] [CrossRef]
- Ku, Y.S.; Ng, M.S.; Cheng, S.S.; Lo, A.W.; Xiao, Z.; Shin, T.S.; Chung, G.; Lam, H.M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef]
- Schupbach, D.; Comeau-Gauthier, M.; Harvey, E.; Merle, G. Wnt modulation in bone healing. Bone 2020, 138, 115491. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Xiao, J.; Sarker, S.D.; Asakawa, Y. Handbook of Dietary Phytochemicals; Springer Nature Singapore: Singapore, 2021. [Google Scholar]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Luo, L.; Pan, H.; Zheng, T.; Wang, J.; Cheng, T.; Zhang, Q. Determination of Flavonoids and Carotenoids and Their Contributions to Various Colors of Rose Cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Ning, G.; Wang, Z.; Shen, Y.; Jin, H.; Li, P.; Huang, S.; Zhao, J.; Bao, M. Disequilibrium of Flavonol Synthase and Dihydroflavonol-4-Reductase Expression Associated Tightly to White vs. Red Color Flower Formation in Plants. Front. Plant Sci. 2015, 6, 1257. [Google Scholar] [CrossRef]
- Mouffouk, C.; Mouffouk, S.; Hambaba, L.; Haba, H. Flavonols as potential antiviral drugs targeting SARS-CoV-2 proteases (3CL(pro) and PL(pro)), spike protein, RNA-dependent RNA polymerase (RdRp) and angiotensin-converting enzyme II receptor (ACE2). Eur. J. Pharmacol. 2021, 891, 173759. [Google Scholar] [CrossRef]
- Messina, M. Soy and Health Update: Evaluation of the Clinical and Epidemiologic Literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xie, K.; Huang, K.; Lu, X.; Lu, L.; Shi, Y.; Tang, Y. Effects of soybean isoflavone on metabolism of rat osteoblasts and cytokines in vitro. J. Food Sci. 2020, 85, 1302–1306. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Sharan, K.; Siddiqui, J.A.; Swarnkar, G.; Maurya, R.; Chattopadhyay, N. Role of phytochemicals in the prevention of menopausal bone loss: Evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr. Med. Chem. 2009, 16, 1138–1157. [Google Scholar] [CrossRef]
- Jagga, S.; Sharma, A.R.; Kim, E.J.; Nam, J.S. Isoflavone-enriched whole soy milk powder stimulates osteoblast differentiation. J. Food Sci. Technol. 2021, 58, 595–603. [Google Scholar] [CrossRef]
- Sharma, A.; Choi, H.K.; Kim, Y.K.; Lee, H.J. Delphinidin and Its Glycosides’ War on Cancer: Preclinical Perspectives. Int. J. Mol. Sci. 2021, 22, 11500. [Google Scholar] [CrossRef]
- Imangali, N.; Phan, Q.T.; Mahady, G.; Winkler, C. The dietary anthocyanin delphinidin prevents bone resorption by inhibiting Rankl-induced differentiation of osteoclasts in a medaka (Oryzias latipes) model of osteoporosis. J. Fish Biol. 2021, 98, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Samarpita, S.; Ganesan, R.; Rasool, M. Cyanidin prevents the hyperproliferative potential of fibroblast-like synoviocytes and disease progression via targeting IL-17A cytokine signalling in rheumatoid arthritis. Toxicol. Appl. Pharmacol. 2020, 391, 114917. [Google Scholar] [CrossRef]
- Hu, B.; Chen, L.; Chen, Y.; Zhang, Z.; Wang, X.; Zhou, B. Cyanidin-3-glucoside Regulates Osteoblast Differentiation via the ERK1/2 Signaling Pathway. ACS Omega 2021, 6, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Huang, G.; Chen, H.; Xu, L.; Qin, S.; Li, A. Research Progress of the Role of Anthocyanins on Bone Regeneration. Front. Pharmacol. 2021, 12, 773660. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. Polyphenols in Human Health and Disease; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y.; Niida, S.; Kruger, M.C.; Suzuki, K. Petunidin, a B-ring 5’-O-Methylated Derivative of Delphinidin, Stimulates Osteoblastogenesis and Reduces sRANKL-Induced Bone Loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Raut, N.A.; Lawal, T.O.; Patel, S.R.; Lee, S.M.; Mahady, G.B. Peonidin-3-O-glucoside and cyanidin increase osteoblast differentiation and reduce RANKL-induced bone resorption in transgenic medaka. Phytother. Res. PTR 2021, 35, 6255–6269. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Hwang, J.K. Effects of (+)-catechin on the function of osteoblastic cells. Biol. Pharm. Bull. 2003, 26, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Q.; Wei, Y.; Hao, M.; Tan, W.S.; Cai, H. Sustained release of epigallocatechin-3-gallate from chitosan-based scaffolds to promote osteogenesis of mesenchymal stem cell. Int. J. Biol. Macromol. 2021, 176, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Cao, S.; Dong, X.; Law, M.C.; Chan, T.H.; Wong, M.S. (-)-Epiafzelechin Protects against Ovariectomy-induced Bone Loss in Adult Mice and Modulate Osteoblastic and Osteoclastic Functions In Vitro. Nutrients 2017, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.B.; Wu, J.B.; Lin, W.C. (-)-Epicatechin 3-O-beta-D-allopyranoside prevent ovariectomy-induced bone loss in mice by suppressing RANKL-induced NF-kappaB and NFATc-1 signaling pathways. BMC Complement. Altern. Med. 2017, 17, 245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, X.; Liu, Z.; Song, X.; Peng, D.; Zhu, W.; Ouyang, Z.; Wang, W. Hesperetin Prevents Bone Resorption by Inhibiting RANKL-Induced Osteoclastogenesis and Jnk Mediated Irf-3/c-Jun Activation. Front. Pharmacol. 2018, 9, 1028. [Google Scholar] [CrossRef]
- Miguez, P.A.; Tuin, S.A.; Robinson, A.G.; Belcher, J.; Jongwattanapisan, P.; Perley, K.; de Paiva Gonalves, V.; Hanifi, A.; Pleshko, N.; Barton, E.R. Hesperidin Promotes Osteogenesis and Modulates Collagen Matrix Organization and Mineralization In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 3223. [Google Scholar] [CrossRef]
- Swarnkar, G.; Sharan, K.; Siddiqui, J.A.; Mishra, J.S.; Khan, K.; Khan, M.P.; Gupta, V.; Rawat, P.; Maurya, R.; Dwivedi, A.K.; et al. A naturally occurring naringenin derivative exerts potent bone anabolic effects by mimicking oestrogen action on osteoblasts. Br. J. Pharmacol. 2012, 165, 1526–1542. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Luo, M.; Shen, M.; Xu, C.; Xu, G.; Chen, Y.; Xia, L. Naringenin inhibits osteoclastogenesis through modulation of helper T cells-secreted IL-4. J. Cell. Biochem. 2018, 119, 2084–2093. [Google Scholar] [CrossRef]
- Lee, J.; Noh, A.L.; Zheng, T.; Kang, J.H.; Yim, M. Eriodicyol inhibits osteoclast differentiation and ovariectomy-induced bone loss in vivo. Exp. Cell Res. 2015, 339, 380–388. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, H.Q.; Han, H.L.; Zou, Y.Y.; Gao, Q.L.; Yang, G.T. Taxifolin enhances osteogenic differentiation of human bone marrow mesenchymal stem cells partially via NF-kappaB pathway. Biochem. Biophys. Res. Commun. 2017, 490, 36–43. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Q.; Chen, K.; Xu, K.; Pan, H.; Chu, F.; Ye, Z.; Wang, Z.; Tickner, J.; Qiu, H.; et al. Astilbin prevents bone loss in ovariectomized mice through the inhibition of RANKL-induced osteoclastogenesis. J. Cell. Mol. Med. 2019, 23, 8355–8368. [Google Scholar] [CrossRef]
- Quan, H.; Dai, X.; Liu, M.; Wu, C.; Wang, D. Luteolin supports osteogenic differentiation of human periodontal ligament cells. BMC Oral Health 2019, 19, 229. [Google Scholar] [CrossRef]
- Tominari, T.; Hirata, M.; Matsumoto, C.; Inada, M.; Miyaura, C. Polymethoxy flavonoids, nobiletin and tangeretin, prevent lipopolysaccharide-induced inflammatory bone loss in an experimental model for periodontitis. J. Pharmacol. Sci. 2012, 119, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.X.; Xu, M.L.; Yao, P.; Kwan, K.K.; Liu, Y.X.; Duan, R.; Dong, T.T.; Ko, R.K.; Tsim, K.W. Corylin, a flavonoid derived from Psoralea Fructus, induces osteoblastic differentiation via estrogen and Wnt/beta-catenin signaling pathways. FASEB J. 2020, 34, 4311–4328. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Yan, Y.; Zhang, F.; Zhang, C.; Liang, W. Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein Cell 2013, 4, 539–547. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, L.; Mu, H.; Priya Veeraraghavan, V. Nobiletin promotes osteogenic differentiation of human osteoblastic cell line (MG-63) through activating the BMP-2/RUNX-2 signaling pathway. Saudi J. Biol. Sci. 2021, 28, 4916–4920. [Google Scholar] [CrossRef]
- Li, S.F.; Tang, J.J.; Chen, J.; Zhang, P.; Wang, T.; Chen, T.Y.; Yan, B.; Huang, B.; Wang, L.; Huang, M.J.; et al. Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des. Dev. Ther. 2015, 9, 5169–5183. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, B.; Zheng, G.; Du, S.; Li, X. Quercetin stimulates osteogenic differentiation of bone marrow stromal cells through miRNA-206/connexin 43 pathway. Am. J. Transl. Res. 2020, 12, 2062–2070. [Google Scholar]
- Li, Z.; Zhang, J.; Ren, X.; Liu, Q.; Yang, X. The mechanism of quercetin in regulating osteoclast activation and the PAR2/TRPV1 signaling pathway in the treatment of bone cancer pain. Int. J. Clin. Exp. Pathol. 2018, 11, 5149–5156. [Google Scholar]
- Huh, J.E.; Jung, I.T.; Choi, J.; Baek, Y.H.; Lee, J.D.; Park, D.S.; Choi, D.Y. The natural flavonoid galangin inhibits osteoclastic bone destruction and osteoclastogenesis by suppressing NF-kappaB in collagen-induced arthritis and bone marrow-derived macrophages. Eur. J. Pharmacol. 2013, 698, 57–66. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Wang, J.; Liu, X.; Wei, H.; Han, Y.; Yang, S.; Wang, X. Icariin regulates the osteoblast differentiation and cell proliferation of MC3T3-E1 cells through microRNA-153 by targeting Runt-related transcription factor 2. Exp. Ther. Med. 2018, 15, 5159–5166. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, Y. Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Ma, B.; Zi, Y.; Xiang, L.B.; Han, T.Y. Effects of rutin on osteoblast MC3T3-E1 differentiation, ALP activity and Runx2 protein expression. Eur. J. Histochem. EJH 2021, 65, 3195. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Gao, X.; Chen, P.; Li, X. Myricetin ameliorates glucocorticoid-induced osteoporosis through the ERK signaling pathway. Life Sci. 2018, 207, 205–211. [Google Scholar] [CrossRef]
- Zhou, F.; Mei, J.; Yuan, K.; Han, X.; Qiao, H.; Tang, T. Isorhamnetin attenuates osteoarthritis by inhibiting osteoclastogenesis and protecting chondrocytes through modulating reactive oxygen species homeostasis. J. Cell. Mol. Med. 2019, 23, 4395–4407. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Guan, T.; Fang, C.; Mo, Z.; Xiang, N.; Yang, J.; Zeng, N. Long-Term Outcomes of Hepatectomy for Bilateral Hepatolithiasis with Three-Dimensional Reconstruction: A Propensity Score Matching Analysis. J. Laparoendosc. Adv. Surg. Tech. Part A 2016, 26, 680–688. [Google Scholar] [CrossRef]
- Winzer, M.; Rauner, M.; Pietschmann, P. Glycitein decreases the generation of murine osteoclasts and increases apoptosis. Wien. Med. Wochenschr 2010, 160, 446–451. [Google Scholar] [CrossRef]
- Cao, J.; Qiu, X.; Gao, Y.; Cai, L. Puerarin promotes the osteogenic differentiation of rat dental follicle cells by promoting the activation of the nitric oxide pathway. Tissue Cell 2021, 73, 101601. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, B.; Shu, F.R.; Chen, K.; Mi, M.T. Equol promotes rat osteoblast proliferation and differentiation through activating estrogen receptor. Genet. Mol. Res. GMR 2014, 13, 5055–5063. [Google Scholar] [CrossRef]
- Khan, K.; Sharan, K.; Swarnkar, G.; Chakravarti, B.; Mittal, M.; Barbhuyan, T.K.; China, S.P.; Khan, M.P.; Nagar, G.K.; Yadav, D.; et al. Positive skeletal effects of cladrin, a naturally occurring dimethoxydaidzein, in osteopenic rats that were maintained after treatment discontinuation. Osteoporos. Int. 2013, 24, 1455–1470. [Google Scholar] [CrossRef]
- Quan, G.H.; Wang, H.; Cao, J.; Zhang, Y.; Wu, D.; Peng, Q.; Liu, N.; Sun, W.C. Calycosin Suppresses RANKL-Mediated Osteoclastogenesis through Inhibition of MAPKs and NF-kappaB. Int. J. Mol. Sci. 2015, 16, 29496–29507. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Lee, W.I.; Kang, J.W.; Nam, D.; Choi, D.Y.; Park, D.S.; Lee, S.H.; Lee, J.D. Formononetin attenuates osteoclastogenesis via suppressing the RANKL-induced activation of NF-kappaB, c-Fos, and nuclear factor of activated T-cells cytoplasmic 1 signaling pathway. J. Nat. Prod. 2014, 77, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Bonewald, L.F. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int. J. Biochem. Cell Biol. 2016, 77, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/beta-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Bilic, J.; Huang, Y.L.; Davidson, G.; Zimmermann, T.; Cruciat, C.M.; Bienz, M.; Niehrs, C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 2007, 316, 1619–1622. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, Y.G. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, H.; Tamai, K.; Zhang, X.; Harada, Y.; Yokota, C.; Almeida, K.; Wang, J.; Doble, B.; Woodgett, J.; et al. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 2008, 135, 367–375. [Google Scholar] [CrossRef]
- Seidensticker, M.J.; Behrens, J. Biochemical interactions in the wnt pathway. Biochim. Biophys. Acta 2000, 1495, 168–182. [Google Scholar] [CrossRef]
- Staal, F.J.; Clevers, H. Tcf/Lef transcription factors during T-cell development: Unique and overlapping functions. Hematol. J. 2000, 1, 3–6. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, H.; Chen, Y.; Ao, X.; Chen, C.; Zhang, W.; He, F.; Liao, X.; Jiang, X.; Li, T.; et al. Baicalein Accelerates Tendon-Bone Healing via Activation of Wnt/beta-Catenin Signaling Pathway in Rats. BioMed Res. Int. 2018, 2018, 3849760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, D.; Geng, Y.; Huang, Q.; Xiang, L. Baicalin augments the differentiation of osteoblasts via enhancement of microRNA-217. Mol. Cell. Biochem. 2020, 463, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Y.; Jia, B.; Wang, Y.; Li, T. Icariin stimulates osteogenesis and suppresses adipogenesis of human bone mesenchymal stem cells via miR-23a-mediated activation of the Wnt/beta-catenin signaling pathway. Phytomedicine 2021, 85, 153485. [Google Scholar] [CrossRef]
- Pan, F.F.; Shao, J.; Shi, C.J.; Li, Z.P.; Fu, W.M.; Zhang, J.F. Apigenin promotes osteogenic differentiation of mesenchymal stem cells and accelerates bone fracture healing via activating Wnt/beta-catenin signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E760–E771. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, R.J.; Jang, K.; Zhou, X.L.; Liu, Y.Z. Protective Effects of Pretreatment with Quercetin Against Lipopolysaccharide-Induced Apoptosis and the Inhibition of Osteoblast Differentiation via the MAPK and Wnt/beta-Catenin Pathways in MC3T3-E1 Cells. Cell. Physiol. Biochem. 2017, 43, 1547–1561. [Google Scholar] [CrossRef]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin rescued TNF-alpha-induced impairments in bone marrow-derived mesenchymal stem cell osteogenesis and improved osteoporosis in rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar]
- Hong, W.; Zhang, W. Hesperidin promotes differentiation of alveolar osteoblasts via Wnt/beta-Catenin signaling pathway. J. Recept. Signal Transduct. Res. 2020, 40, 442–448. [Google Scholar] [CrossRef]
- Chang, Y.W.; Zhu, W.J.; Gu, W.; Sun, J.; Li, Z.Q.; Wei, X.E. Neohesperidin promotes the osteogenic differentiation of bone mesenchymal stem cells by activating the Wnt/beta-catenin signaling pathway. J. Orthop. Surg. Res. 2021, 16, 334. [Google Scholar] [CrossRef]
- Xi, J.; Li, Q.; Luo, X.; Li, J.; Guo, L.; Xue, H.; Wu, G. Epigallocatechin3gallate protects against secondary osteoporosis in a mouse model via the Wnt/betacatenin signaling pathway. Mol. Med. Rep. 2018, 18, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-beta and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, L.; Mao, Z.; Wang, N.; Wang, X.; Huang, X.; Hu, Y.; Shou, D.; Wen, C. Icariin Enhances Bone Repair in Rabbits with Bone Infection during Post-infection Treatment and Prevents Inhibition of Osteoblasts by Vancomycin. Front. Pharmacol. 2017, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Choudhary, D.; Ahmad, N.; Karvande, A.; Kumar, A.; Banala, V.T.; Mishra, P.R.; Trivedi, R. Dietary flavonoid kaempferol inhibits glucocorticoid-induced bone loss by promoting osteoblast survival. Nutrition 2018, 53, 64–76. [Google Scholar] [CrossRef]
- Pang, X.G.; Cong, Y.; Bao, N.R.; Li, Y.G.; Zhao, J.N. Quercetin Stimulates Bone Marrow Mesenchymal Stem Cell Differentiation through an Estrogen Receptor-Mediated Pathway. BioMed Res. Int. 2018, 2018, 4178021. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, S.Y.; Choung, S.Y. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP-2/Smad and ERK/JNK/p38 mitogen-activated protein kinase signaling pathway. Eur. J. Pharmacol. 2018, 834, 84–91. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Li, X.; Lv, Z.; Chen, Y.; Zhao, J. Isoquercitrin promotes the osteogenic differentiation of osteoblasts and BMSCs via the RUNX2 or BMP pathway. Connect. Tissue Res. 2019, 60, 189–199. [Google Scholar] [CrossRef]
- Wu, Y.; Xia, L.; Zhou, Y.; Xu, Y.; Jiang, X. Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner. Cell Prolif. 2015, 48, 375–384. [Google Scholar] [CrossRef]
- Liu, M.; Fan, F.; Shi, P.; Tu, M.; Yu, C.; Du, M. Lactoferrin promotes MC3T3-E1 osteoblast cells proliferation via MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 107, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Davis, R.J.; Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 2002, 20, 55–72. [Google Scholar] [CrossRef]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases--regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Pimienta, G.; Pascual, J. Canonical and alternative MAPK signaling. Cell Cycle 2007, 6, 2628–2632. [Google Scholar] [CrossRef]
- Turjanski, A.G.; Vaque, J.P.; Gutkind, J.S. MAP kinases and the control of nuclear events. Oncogene 2007, 26, 3240–3253. [Google Scholar] [CrossRef]
- Johnson, G.L. Defining MAPK interactomes. ACS Chem. Biol. 2011, 6, 18–20. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, C. Regulatory mechanisms of mitogen-activated kinase signaling. Cell. Mol. Life Sci. 2007, 64, 2771–2789. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.M.; Keyse, S.M. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 2007, 26, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Chen, W.; Cobb, M.H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.Z.; Ni, H.J.; Wang, Y.L. Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharmacother. = Biomed. Pharmacother. 2017, 89, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Weng, Z.; Xu, J.; Wen, G.; Yu, Y.; Chai, Y. Baicalin alleviates osteomyelitis by regulating TLR2 in the murine model. Pathog. Dis. 2018, 76, ftx123. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, G.; Liu, X.; Dai, M.; Zhang, B. Icariin inhibits RANKL-induced osteoclastogenesis via modulation of the NF-kappaB and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2019, 508, 902–906. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Gao, Y.; Zhao, L.; Cai, C.; Qi, D.; Zhu, M.; Liu, C.; Guo, F.; Xiao, J.; et al. Hesperetin suppresses RANKL-induced osteoclastogenesis and ameliorates lipopolysaccharide-induced bone loss. J. Cell. Physiol. 2019, 234, 11009–11022. [Google Scholar] [CrossRef]
- Xie, B.; Zeng, Z.; Liao, S.; Zhou, C.; Wu, L.; Xu, D. Kaempferol Ameliorates the Inhibitory Activity of Dexamethasone in the Osteogenesis of MC3T3-E1 Cells by JNK and p38-MAPK Pathways. Front. Pharmacol. 2021, 12, 739326. [Google Scholar] [CrossRef]
- Badila, A.E.; Radulescu, D.M.; Ilie, A.; Niculescu, A.G.; Grumezescu, A.M.; Radulescu, A.R. Bone Regeneration and Oxidative Stress: An Updated Overview. Antioxidants 2022, 11, 318. [Google Scholar] [CrossRef]
- Abdulhameed, E.A.; Al-Rawi, N.H.; Omar, M.; Khalifa, N.; Samsudin, A.B.R. Titanium dioxide dental implants surfaces related oxidative stress in bone remodeling: A systematic review. PeerJ 2022, 10, e12951. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu. Rev. Pathol. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Kobayashi, C.I.; Suda, T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J. Cell. Physiol. 2012, 227, 421–430. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Shim, J.H.; Zou, W.; Sitara, D.; Schweitzer, M.; Hu, D.; Lotinun, S.; Sano, Y.; Baron, R.; Park, J.M.; et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J. Clin. Investig. 2010, 120, 2457–2473. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Igarashi, Y.; Ogawa, T. N-acetyl cysteine protects osteoblastic function from oxidative stress. J. Biomed. Mater. Res. A 2011, 99, 523–531. [Google Scholar] [CrossRef]
- Arakaki, N.; Yamashita, A.; Niimi, S.; Yamazaki, T. Involvement of reactive oxygen species in osteoblastic differentiation of MC3T3-E1 cells accompanied by mitochondrial morphological dynamics. Biomed. Res. 2013, 34, 161–166. [Google Scholar] [CrossRef]
- Jing, X.; Du, T.; Chen, K.; Guo, J.; Xiang, W.; Yao, X.; Sun, K.; Ye, Y.; Guo, F. Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J. Cell. Physiol. 2019, 234, 10123–10137. [Google Scholar] [CrossRef]
- Suh, K.S.; Choi, E.M.; Kwon, M.; Chon, S.; Oh, S.; Woo, J.T.; Kim, S.W.; Kim, J.W.; Kim, Y.S. Kaempferol attenuates 2-deoxy-d-ribose-induced oxidative cell damage in MC3T3-E1 osteoblastic cells. Biol. Pharm. Bull. 2009, 32, 746–749. [Google Scholar] [CrossRef]
- Qi, X.C.; Li, B.; Wu, W.L.; Liu, H.C.; Jiang, Y.P. Protective effect of hyperoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 377–383. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, B.; Wang, L.; Hu, Y.Q.; Lu, W.G.; Yang, L.; Luo, Z.J.; Liu, J. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol. Appl. Pharmacol. 2014, 280, 550–560. [Google Scholar] [CrossRef]
- Chen, L.; Hu, S.L.; Xie, J.; Yan, D.Y.; Weng, S.J.; Tang, J.H.; Wang, B.Z.; Xie, Z.J.; Wu, Z.Y.; Yang, L. Proanthocyanidins-Mediated Nrf2 Activation Ameliorates Glucocorticoid-Induced Oxidative Stress and Mitochondrial Dysfunction in Osteoblasts. Oxidative Med. Cell. Longev. 2020, 2020, 9102012. [Google Scholar] [CrossRef]
- Lin, T.H.; Gibon, E.; Loi, F.; Pajarinen, J.; Cordova, L.A.; Nabeshima, A.; Lu, L.; Yao, Z.; Goodman, S.B. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. J. Orthop. Res. 2017, 35, 281–288. [Google Scholar] [CrossRef]
- Jeon, H.H.; Yang, C.Y.; Shin, M.K.; Wang, J.; Patel, J.H.; Chung, C.H.; Graves, D.T. Osteoblast lineage cells and periodontal ligament fibroblasts regulate orthodontic tooth movement that is dependent on Nuclear Factor-kappa B (NF-kB) activation. Angle Orthod. 2021, 91, 664–671. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-kappaB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Karin, M.; Delhase, M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Semin. Immunol. 2000, 12, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Ley, S.C. New insights into NF-kappaB regulation and function. Trends Immunol. 2008, 29, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Israel, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [PubMed]
- Beinke, S.; Ley, S.C. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 2004, 382, 393–409. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Xiao, L.; Zhong, M.; Huang, Y.; Zhu, J.; Tang, W.; Li, D.; Shi, J.; Lu, A.; Yang, H.; Geng, D.; et al. Puerarin alleviates osteoporosis in the ovariectomy-induced mice by suppressing osteoclastogenesis via inhibition of TRAF6/ROS-dependent MAPK/NF-kappaB signaling pathways. Aging 2020, 12, 21706–21729. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, F.; He, Y.; Chen, Q.; Xia, Q.; Cheng, G.; Lu, Y.; Zhang, Q. Beneficial effects of hyperoside on bone metabolism in ovariectomized mice. Biomed. Pharmacother. = Biomed. Pharmacother. 2018, 107, 1175–1182. [Google Scholar] [CrossRef]
- Xu, H.; Liu, T.; Jia, Y.; Li, J.; Jiang, L.; Hu, C.; Wang, X.; Sheng, J. (-)-Epigallocatechin-3-gallate inhibits osteoclastogenesis by blocking RANKL-RANK interaction and suppressing NF-kappaB and MAPK signaling pathways. Int. Immunopharmacol. 2021, 95, 107464. [Google Scholar] [CrossRef]
- Wei, G.; Liang, T.; Wei, C.; Nong, X.; Lu, Q.; Zhao, J. Daidzin inhibits RANKL-induced osteoclastogenesis in vitro and prevents LPS-induced bone loss in vivo. J. Cell. Biochem. 2019, 120, 5304–5314. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Maeda, T.; Chatani, M.; Handa, K.; Yamakawa, T.; Kiyohara, S.; Negishi-Koga, T.; Kato, Y.; Takami, M.; Niida, S.; et al. A Delphinidin-Enriched Maqui Berry Extract Improves Bone Metabolism and Protects against Bone Loss in Osteopenic Mouse Models. Antioxidants 2019, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Wang, Y.J.; Yang, G.T.; Gao, Q.L.; Tang, M.X. Taxifolin Inhibits Receptor Activator of NF-kappaB Ligand-Induced Osteoclastogenesis of Human Bone Marrow-Derived Macrophages in vitro and Prevents Lipopolysaccharide-Induced Bone Loss in vivo. Pharmacology 2019, 103, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Poon, C.C.; Wong, K.Y.; Cao, S.; Dong, X.; Zhang, Y.; Wong, M.S. Icariin ameliorates estrogen-deficiency induced bone loss by enhancing IGF-I signaling via its crosstalk with non-genomic ERalpha signaling. Phytomedicine 2021, 82, 153413. [Google Scholar] [CrossRef]
- Xu, Y.; Li, L.; Tang, Y.; Yang, J.; Jin, Y.; Ma, C. Icariin promotes osteogenic differentiation by suppressing Notch signaling. Eur. J. Pharmacol. 2019, 865, 172794. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Gao, Y.; Wang, Y.; Zhou, J.; Wei, Z.; Ma, X.; Ma, H.; Xian, C.J.; Wang, J.; Chen, K. The flavonol glycoside icariin promotes bone formation in growing rats by activating the cAMP signaling pathway in primary cilia of osteoblasts. J. Biol. Chem. 2017, 292, 20883–20896. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wei, C.; Zhou, L.; Qin, A.; Yang, M.; Tickner, J.; Huang, Y.; Zhao, J.; Xu, J. Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades. J. Cell. Physiol. 2018, 233, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Lu, Y.; Yin, Z.; Wang, X.; Zhou, X.; Li, Z. Baicalein ameliorates osteoporosis via AKT/FOXO1 signaling. Aging 2021, 13, 17370–17379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, J.; Xu, B.; Yuan, Z.; Leng, Y.; Min, J.; Lan, X.; Luo, J. Kaempferol promotes bone formation in part via the mTOR signaling pathway. Mol. Med. Rep. 2019, 20, 5197–5207. [Google Scholar] [CrossRef]
- Zhou, Y.; Lian, H.; Liu, K.; Wang, D.; Xiu, X.; Sun, Z. Puerarin improves graft bone defect through microRNA1553pmediated p53/TNFalpha/STAT1 signaling pathway. Int. J. Mol. Med. 2020, 46, 239–251. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, D.; Sousa, A. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197. [Google Scholar] [CrossRef]
- Ahmad, N.; Banala, V.T.; Kushwaha, P.; Karvande, A.; Sharma, S.; Tripathi, A.K.; Verma, A.; Trivedi, R.; Mishra, P.R. Quercetin-loaded solid lipid nanoparticles improve osteoprotective activity in an ovariectomized rat model: A preventive strategy for post-menopausal osteoporosis. RSC Adv. 2016, 6, 97613–97628. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Cao, H.; Li, L.; Chow, D.H.; Tian, L.; Wu, H.; Zhang, J.; Wang, N.; Zheng, L.; et al. A bone-targeting delivery system carrying osteogenic phytomolecule icaritin prevents osteoporosis in mice. Biomaterials 2018, 182, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zheng, L.; Zhang, J.; Wu, H.; Wang, N.; Tong, W.; Xu, J.; Huang, L.; Zhang, Y.; Yang, Z.; et al. A novel bone targeting delivery system carrying phytomolecule icaritin for prevention of steroid-associated osteonecrosis in rats. Bone 2018, 106, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wei, J.; Lyu, J.; Bian, T.; Liu, Z.; Huang, J.; Pi, F.; Li, C.; Zhong, Z. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J. Nanobiotechnol. 2019, 17, 10. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Wang, Z.; Liu, B.; Zhu, S.; Zhu, L.; Peng, B. Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention. Theranostics 2019, 9, 5183–5199. [Google Scholar] [CrossRef] [PubMed]
- Casarrubios, L.; Gomez-Cerezo, N.; Feito, M.J.; Vallet-Regi, M.; Arcos, D.; Portoles, M.T. Incorporation and effects of mesoporous SiO2-CaO nanospheres loaded with ipriflavone on osteoblast/osteoclast cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhao, F.; Lin, Z.; Cao, X.; Chen, D.; Chen, X. Local delivery of naringin in beta-cyclodextrin modified mesoporous bioactive glass promotes bone regeneration: From anti-inflammatory to synergistic osteogenesis and osteoclastogenesis. Biomater. Sci. 2022, 10, 1697–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, J.; Ai, Z.; Su, J. Nobiletin-loaded micelles reduce ovariectomy-induced bone loss by suppressing osteoclastogenesis. Int. J. Nanomed. 2019, 14, 7839–7849. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Chen, X.; Su, J.; Huang, C. Enhanced efficacy of baicalin-loaded TPGS polymeric micelles against periodontitis. Mater. Sci Eng. C Mater. Biol. Appl. 2019, 101, 387–395. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, W.; Xu, N.; Shi, C.; Xu, G.; Sun, J.; He, H.; Jiang, T. Myricetin Loaded Nano-micelles Delivery System Reduces Bone Loss Induced by Ovariectomy in Rats Through Inhibition of Osteoclast Formation. J. Pharm. Sci. 2022, 111, 2341–2352. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Zhang, Z.; Sun, E.; Feng, L.; Jia, X. Mechanism of enhanced antiosteoporosis effect of circinal-icaritin by self-assembled nanomicelles in vivo with suet oil and sodium deoxycholate. Int. J. Nanomed. 2015, 10, 2377–2389. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, W.; Yan, F.; Liu, H.; Deng, Z.; Cai, L. Icariin-loaded porous scaffolds for bone regeneration through the regulation of the coupling process of osteogenesis and osteoclastic activity. Int. J. Nanomed. 2019, 14, 6019–6033. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Xia, L.; Wu, Q.; Wang, J.; Wang, X.; Xu, L.; Zhou, Y.; Xu, Y.; Jiang, X. Evaluation of Osteogenesis and Angiogenesis of Icariin in Local Controlled Release and Systemic Delivery for Calvarial Defect in Ovariectomized Rats. Sci. Rep. 2017, 7, 5077. [Google Scholar] [CrossRef]
- Huang, J.G.; Pang, L.; Chen, Z.R.; Tan, X.P. Dual-delivery of vancomycin and icariin from an injectable calcium phosphate cement-release system for controlling infection and improving bone healing. Mol. Med. Rep. 2013, 8, 1221–1227. [Google Scholar] [CrossRef]
- Li, M.; Gu, Q.; Chen, M.; Zhang, C.; Chen, S.; Zhao, J. Controlled delivery of icariin on small intestine submucosa for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Zhong, Y.; Zhao, J. A Novel Approach to Utilize Icariin as Icariin-Derived ECM on Small Intestinal Submucosa Scaffold for Bone Repair. Ann. Biomed. Eng. 2017, 45, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Chen, E.; Zhang, W.; Gao, X.; Wang, S.; Zheng, Q.; Pan, Z.; Li, H.; Liu, L. The role of hesperetin on osteogenesis of human mesenchymal stem cells and its function in bone regeneration. Oncotarget 2017, 8, 21031–21043. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, G.K.; Khedgikar, V.; Gautam, J.; Kushwaha, P.; Changkija, B.; Nagar, G.K.; Gupta, V.; Verma, A.; Dwivedi, A.K.; et al. In vivo efficacy studies of layer-by-layer nano-matrix bearing kaempferol for the conditions of osteoporosis: A study in ovariectomized rat model. Eur. J. Pharm. Biopharm. 2012, 82, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Leena, R.S.; Vairamani, M.; Selvamurugan, N. Alginate/Gelatin scaffolds incorporated with Silibinin-loaded Chitosan nanoparticles for bone formation in vitro. Colloids Surf. B Biointerfaces 2017, 158, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Y.; Ma, W.; Jiang, X.; Takemra, A.; Uemura, M.; Xia, L.; Lin, K.; Xu, Y. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater. Chem. B 2017, 5, 612–625. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandia, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112470. [Google Scholar] [CrossRef]
- Chang, P.C.; Chao, Y.C.; Hsiao, M.H.; Chou, H.S.; Jheng, Y.H.; Yu, X.H.; Lee, N.; Yang, C.; Liu, D.M. Inhibition of Periodontitis Induction Using a Stimuli-Responsive Hydrogel Carrying Naringin. J. Periodontol. 2017, 88, 190–196. [Google Scholar] [CrossRef]
- Song, Y.; Ma, A.; Ning, J.; Zhong, X.; Zhang, Q.; Zhang, X.; Hong, G.; Li, Y.; Sasaki, K.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int. J. Nanomed. 2018, 13, 6751–6767. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, A.; Monjo, M.; Hierro-Oliva, M.; Gonzalez-Martin, M.L.; Ramis, J.M. Bioinspired Quercitrin Nanocoatings: A Fluorescence-Based Method for Their Surface Quantification, and Their Effect on Stem Cell Adhesion and Differentiation to the Osteoblastic Lineage. ACS Appl. Mater. Interfaces 2015, 7, 16857–16864. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Burley, G.; Lin, S.; Shi, Y.C. Osteoporosis pathogenesis and treatment: Existing and emerging avenues. Cell. Mol. Biol. Lett. 2022, 27, 72. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. North Am. 2012, 41, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, Y.J.; Lim, W. Osteoporosis therapies and their mechanisms of action (Review). Exp. Ther. Med. 2021, 22, 1379. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Harslof, T. Medical treatment of osteoporotic vertebral fractures. Ther. Adv. Musculoskelet. Dis. 2011, 3, 17–29. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2016. Endocr. Pr. 2016, 22, 1111–1118. [Google Scholar] [CrossRef]
- Akkawi, I.; Zmerly, H. Osteoporosis: Current Concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef]
- Schuiling, K.D.; Robinia, K.; Nye, R. Osteoporosis update. J. Midwifery Women′s Health 2011, 56, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Pazianas, M.; Abrahamsen, B. Osteoporosis treatment: Bisphosphonates reign to continue for a few more years, at least? Ann. N. Y. Acad. Sci. 2016, 1376, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bonani, M.; Frey, D.; de Rougemont, O.; Mueller, N.J.; Mueller, T.F.; Graf, N.; Wuthrich, R.P. Infections in De Novo Kidney Transplant Recipients Treated With the RANKL Inhibitor Denosumab. Transplantation 2017, 101, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. and on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Szulc, P.; Delmas, P.D. Biochemical markers of bone turnover: Potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos. Int. 2008, 19, 1683–1704. [Google Scholar] [CrossRef]
- Colucci, S.; Minielli, V.; Zambonin, G.; Cirulli, N.; Mori, G.; Serra, M.; Patella, V.; Zambonin Zallone, A.; Grano, M. Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated surfaces. Calcif. Tissue Int. 1998, 63, 230–235. [Google Scholar] [CrossRef]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef]
- Kim, S.Y.; Zhang, M.; Bockman, R. Bone Mineral Density Response from Teriparatide in Patients with Osteoporosis. HSS J. 2017, 13, 171–177. [Google Scholar] [CrossRef]
- Rogers, M.J. From molds and macrophages to mevalonate: A decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif. Tissue Int. 2004, 75, 451–461. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Noble, B.S. The osteocyte lineage. Arch. Biochem. Biophys. 2008, 473, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.Y.; Lee, T.M.; Jordan, V.C. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr. Clin. Pharm. 2013, 8, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 2014, 5, 5215. [Google Scholar] [CrossRef] [PubMed]
- Gooi, J.H.; Pompolo, S.; Karsdal, M.A.; Kulkarni, N.H.; Kalajzic, I.; McAhren, S.H.; Han, B.; Onyia, J.E.; Ho, P.W.; Gillespie, M.T.; et al. Calcitonin impairs the anabolic effect of PTH in young rats and stimulates expression of sclerostin by osteocytes. Bone 2010, 46, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, L.; Lewiecki, E.M.; Bilezikian, J.P. Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis. Expert Opin. Drug Metab. Toxicol. 2016, 12, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Oursler, M.J.; Khosla, S. Cathepsin K Inhibitors for Osteoporosis: Biology, Potential Clinical Utility, and Lessons Learned. Endocr. Rev. 2017, 38, 325–350. [Google Scholar] [CrossRef]
- Khan, B.; Ahmed, Z.; Ahmad, W. A novel missense mutation in cathepsin K (CTSK) gene in a consanguineous Pakistani family with pycnodysostosis. J. Investig. Med. 2010, 58, 720–724. [Google Scholar] [CrossRef]
- Mullard, A. Merck &Co. drops osteoporosis drug odanacatib. Nat. Rev. Drug Discov 2016, 15, 669. [Google Scholar] [CrossRef]
- Delany, A.M.; Amling, M.; Priemel, M.; Howe, C.; Baron, R.; Canalis, E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Investig. 2000, 105, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Benkusky, N.A.; Pike, J.W. The RUNX2 cistrome in osteoblasts: Characterization, down-regulation following differentiation, and relationship to gene expression. J. Biol. Chem. 2014, 289, 16016–16031. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Borba, V.Z.; Manas, N.C. The use of PTH in the treatment of osteoporosis. Arq. Bras. Endocrinol. Metab. 2010, 54, 213–219. [Google Scholar] [CrossRef]
- Cheloha, R.W.; Gellman, S.H.; Vilardaga, J.P.; Gardella, T.J. PTH receptor-1 signalling-mechanistic insights and therapeutic prospects. Nat. Rev. Endocrinol. 2015, 11, 712–724. [Google Scholar] [CrossRef]
- Hattersley, G.; Dean, T.; Corbin, B.A.; Bahar, H.; Gardella, T.J. Binding Selectivity of Abaloparatide for PTH-Type-1-Receptor Conformations and Effects on Downstream Signaling. Endocrinology 2016, 157, 141–149. [Google Scholar] [CrossRef]
- Boyce, E.G.; Mai, Y.; Pham, C. Abaloparatide: Review of a Next-Generation Parathyroid Hormone Agonist. Ann. Pharm. 2018, 52, 462–472. [Google Scholar] [CrossRef]
- Wysolmerski, J.J. Parathyroid hormone-related protein: An update. J. Clin. Endocrinol. Metab. 2012, 97, 2947–2956. [Google Scholar] [CrossRef]
- Pietrzyk, B.; Smertka, M.; Chudek, J. Sclerostin: Intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv. Clin. Exp. Med. 2017, 26, 1283–1291. [Google Scholar] [CrossRef]
- Lerner, U.H.; Ohlsson, C. The WNT system: Background and its role in bone. J. Intern. Med. 2015, 277, 630–649. [Google Scholar] [CrossRef]
- Canalis, E. MANAGEMENT OF ENDOCRINE DISEASE: Novel anabolic treatments for osteoporosis. Eur. J. Endocrinol. 2018, 178, R33–R44. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, J.; He, J.; Cai, Z.; Chen, J.; Yin, J. Prediction and Verification of Epimedium Flavonoids With Different Glycosylation Numbers in Reversing Glucocorticoid-Induced Bone Formation Inhibition by Molecular Docking and Zebrafish. Front. Environ. Sci. 2022, 9, 793527. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, W.; Cai, N.; Jia, K.; Meng, Y.; Wang, F.; Ge, Y.; Lu, H. Exploring Quercetin Anti-Osteoporosis Pharmacological Mechanisms with In Silico and In Vivo Models. Life 2022, 12, 980. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, P.; Zhao, W.; Zhu, G.; Shen, G.; Chen, H.; Yu, X.; Zhang, Z.; Shang, Q.; Liang, D.; et al. Research on the Mechanism of Kaempferol for Treating Senile Osteoporosis by Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2022, 2022, 6741995. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, P.; Tang, K.; Shen, G.; Chen, H.; Zhang, Z.; Zhao, W.; Shang, Q.; Zhu, G.; Tan, R.; et al. Network Pharmacology Integrated with Molecular Docking Explores the Mechanisms of Naringin against Osteoporotic Fracture by Regulating Oxidative Stress. Evid. Based Complement. Altern. Med. 2021, 2021, 6421122. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Klebowski, B.; Depciuch, J.; Parlinska-Wojtan, M.; Baran, J. Applications of Noble Metal-Based Nanoparticles in Medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Majumdar, S.; Krishnamurthy, S. Bioactive glass: A multifunctional delivery system. J. Control. Release 2021, 335, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Brien, F. Biomaterials & scaffolds Every day thousands of surgical procedures are performed to replace. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Zhong, X.; Song, Y.; Yang, P.; Wang, Y.; Jiang, S.; Zhang, X.; Li, C. Titanium Surface Priming with Phase-Transited Lysozyme to Establish a Silver Nanoparticle-Loaded Chitosan/Hyaluronic Acid Antibacterial Multilayer via Layer-by-Layer Self-Assembly. PLoS ONE 2016, 11, e0146957. [Google Scholar] [CrossRef]
- Keeney, M.; Jiang, X.Y.; Yamane, M.; Lee, M.; Goodman, S.; Yang, F. Nanocoating for biomolecule delivery using layer-by-layer self-assembly. J. Mater. Chem. B 2015, 3, 8757–8770. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr. Med. Chem. 2010, 17, 74–95. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000, 29, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M. Health Benefits of Flavonoids. Available online: https://www.medindia.net/patients/lifestyleandwellness/health-benefits-of-flavonoids (accessed on 20 January 2023).
- Galati, G.; Teng, S.; Moridani, M.Y.; Chan, T.S.; O’Brien, P.J. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metab. Drug Interact. 2000, 17, 311–349. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Chan, T.; Wu, B.; O’Brien, P.J. Glutathione-dependent generation of reactive oxygen species by the peroxidase-catalyzed redox cycling of flavonoids. Chem. Res. Toxicol. 1999, 12, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Moridani, M.Y.; Chan, T.S.; O’Brien, P.J. Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: Glutathione oxidation and conjugation. Free Radic. Biol. Med. 2001, 30, 370–382. [Google Scholar] [CrossRef]
- Chan, T.; Galati, G.; O’Brien, P.J. Oxygen activation during peroxidase catalysed metabolism of flavones or flavanones. Chem. Biol. Interact. 1999, 122, 15–25. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M. Structure-activity study on the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol. 2001, 14, 398–408. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Peroxidase-catalyzed formation of quercetin quinone methide-glutathione adducts. Arch. Biochem. Biophys. 2000, 378, 224–233. [Google Scholar] [CrossRef]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; van der Woude, H.; van Zanden, J.; van Bladeren, P.J.; Vervoort, J.; Rietjens, I.M. Identification of o-quinone/quinone methide metabolites of quercetin in a cellular in vitro system. FEBS Lett. 2002, 520, 30–34. [Google Scholar] [CrossRef]
- Walle, T.; Vincent, T.S.; Walle, U.K. Evidence of covalent binding of the dietary flavonoid quercetin to DNA and protein in human intestinal and hepatic cells. Biochem. Pharmacol. 2003, 65, 1603–1610. [Google Scholar] [CrossRef]
- Ciolino, H.P.; Wang, T.T.; Yeh, G.C. Diosmin and diosmetin are agonists of the aryl hydrocarbon receptor that differentially affect cytochrome P450 1A1 activity. Cancer Res. 1998, 58, 2754–2760. [Google Scholar]
- Kang, Z.C.; Tsai, S.J.; Lee, H. Quercetin inhibits benzo[a]pyrene-induced DNA adducts in human Hep G2 cells by altering cytochrome P-450 1A1 gene expression. Nutr. Cancer 1999, 35, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).