Comparative Analysis of Oligosaccharides in Breast Milk and Feces of Breast-Fed Infants by Using LC-QE-HF-MS: A Communication
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Subject Enrollment and Sample Collection
2.3. Sample Preparation for OS Analysis
2.4. OS Detection Using UPLC-QE-HF-MS
2.5. Statistical Analysis
3. Results
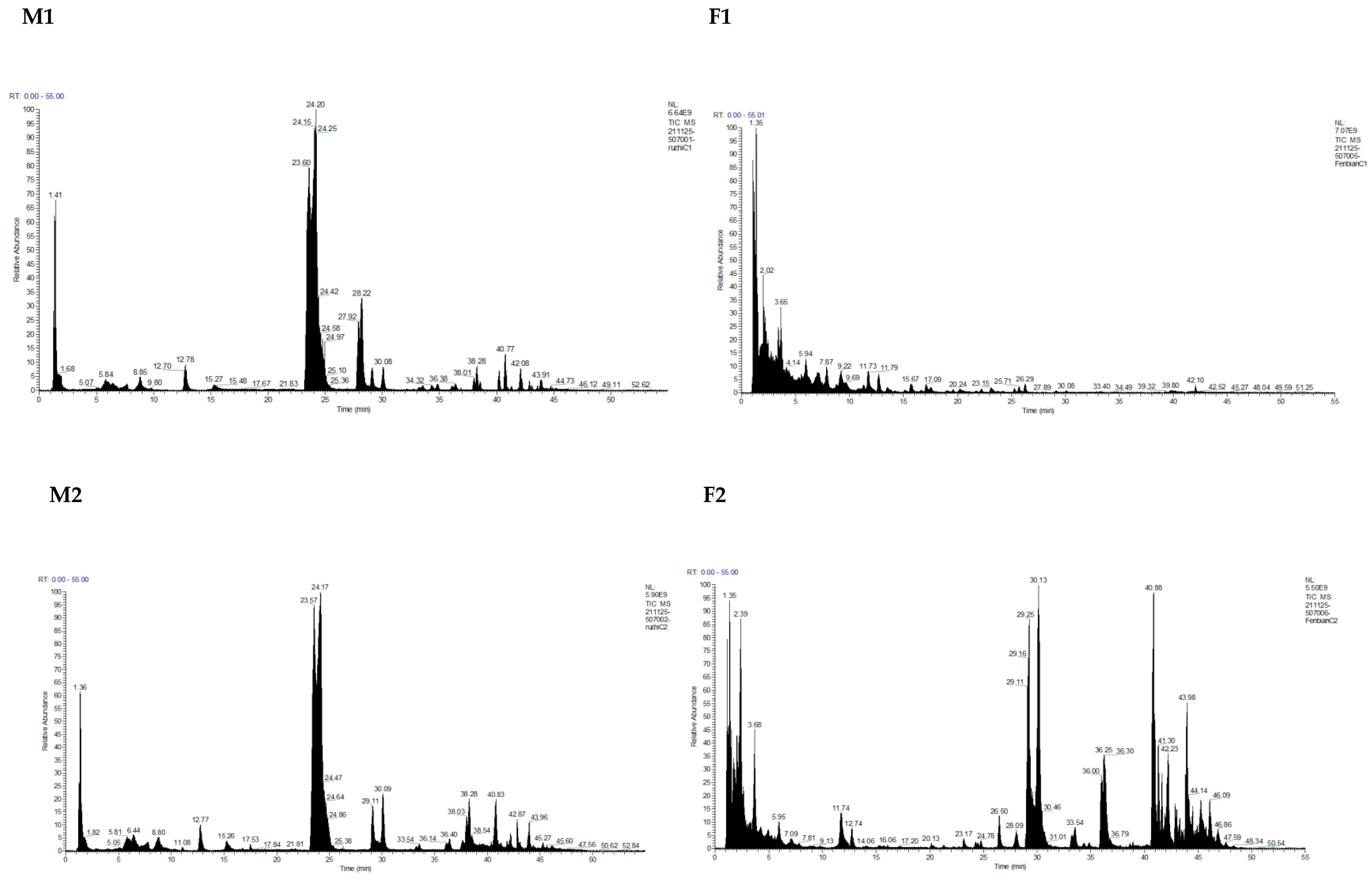
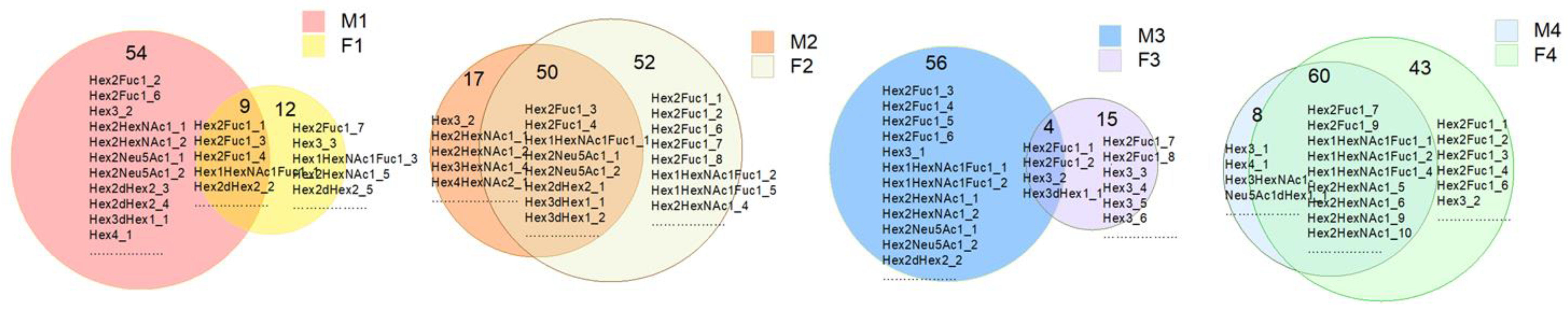
3.1. Identification and Analysis of OS in Breast Milk and Feces
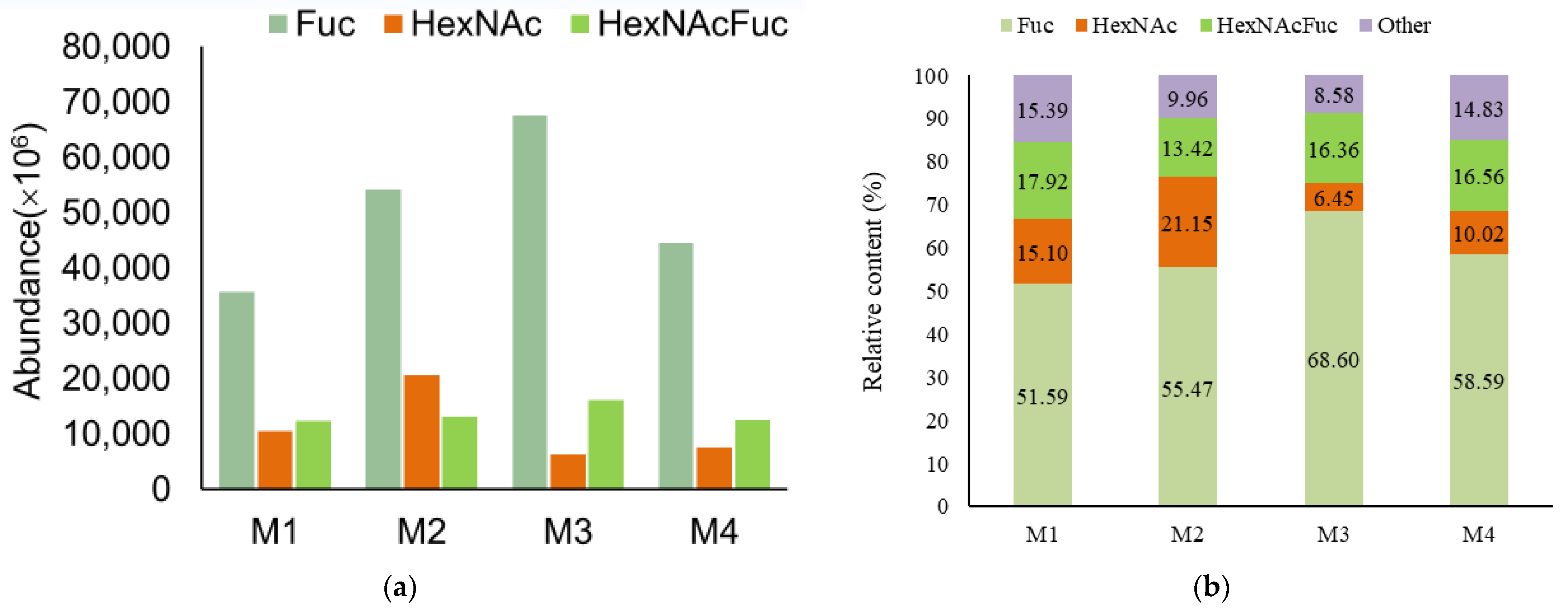
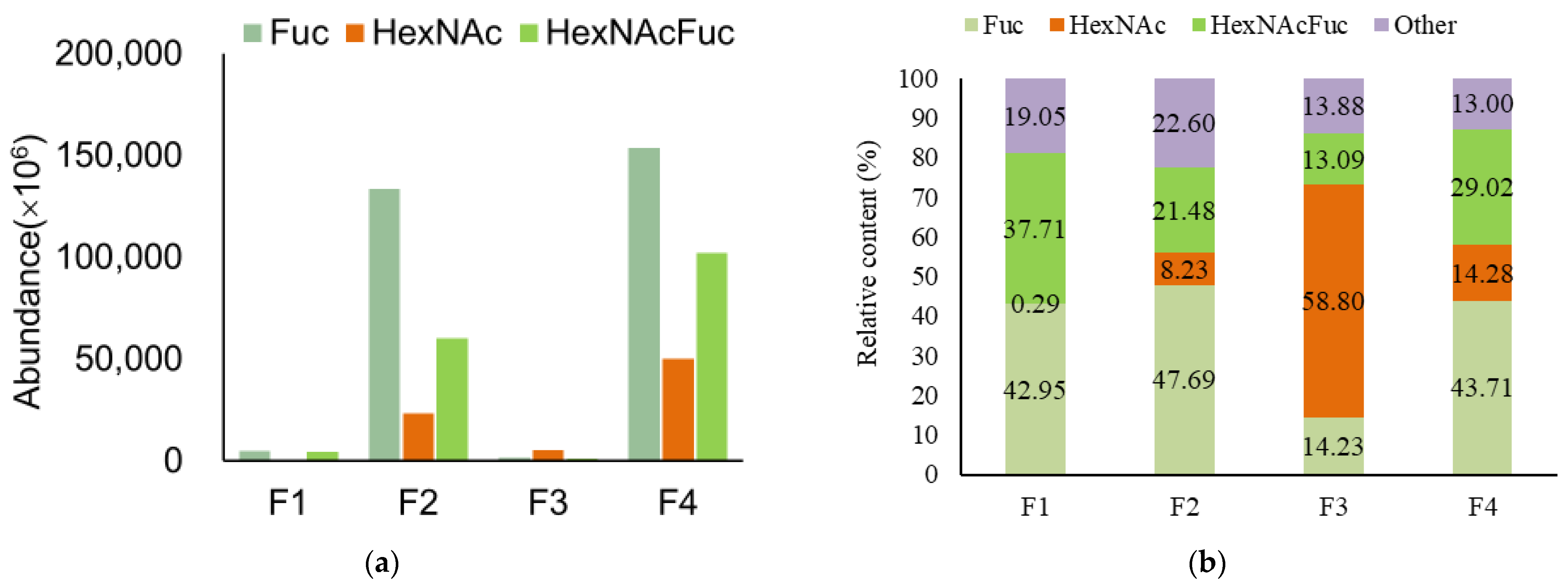
3.2. Analysis of Functional OS in Breast Milk and Feces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Zhang, Y.; Song, B.; Zhang, S.; Pang, X.; Sari, R.N.; Liu, L.; Wang, J.; Lv, J. Comparative analysis of oligosaccharides in Guanzhong and Saanen goat milk by using LC–MS/MS. Carbohydr. Polym. 2020, 235, 115965. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in Human Milk: Structural, Functional, and Metabolic Aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Gaerlan, S.C.; De Leoz, M.L.A.; Dimapasoc, L.M.; Kalanetra, K.M.; Lemay, D.G.; German, J.B.; Mills, D.A.; Lebrilla, C.B. Human milk oligosaccharides in premature infants: Absorption, excretion, and influence on the intestinal microbiota. Pediatr. Res. 2015, 78, 670–677. [Google Scholar]
- Tao, N.; DePeters, E.; Freeman, S.; German, J.; Grimm, R.; Lebrilla, C.B. Bovine milk glycome. J. Dairy Sci. 2008, 91, 3768–3778. [Google Scholar] [PubMed]
- Martín-Ortiz, A.; Salcedo, J.; Barile, D.; Bunyatratchata, A.; Moreno, F.; Martin-García, I.; Clemente, A.; Sanz, M.; Ruiz-Matute, A. Characterization of goat colostrum oligosaccharides by nano-liquid chromatography on chip quadrupole time-of-flight mass spectrometry and hydrophilic interaction liquid chromatography-quadrupole mass spectrometry. J. Chromatogr. A 2015, 1428, 143–153. [Google Scholar] [PubMed]
- Meyrand, M.; Dallas, D.; Caillat, H.; Bouvier, F.; Martin, P.; Barile, D. Comparison of milk oligosaccharides between goats with and without the genetic ability to synthesize αs1-casein. Small Rumin. Res. 2013, 113, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Urashima, T.; Saito, T.; Nakamura, T.; Messer, M. Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 2001, 18, 357–371. [Google Scholar] [CrossRef]
- Bode, L.; Kunz, C.; Muhly-Reinholz, M.; Mayer, K.; Seeger, W.; Rudloff, S. Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb. Haemost. 2004, 92, 1402–1410. [Google Scholar] [CrossRef]
- Hickey, R.M. The role of oligosaccharides from human milk and other sources in prevention of pathogen adhesion. Int. Dairy J. 2012, 22, 141–146. [Google Scholar] [CrossRef]
- Lane, J.A.; Mariño, K.V.; Naughton, J.; Kavanaugh, D.; Clyne, M.; Carrington, S.D.; Hickey, R.M. Anti-infective bovine colostrum oligosaccharides: Campylobacter jejuni as a case study. Int. J. Food Microbiol. 2012, 157, 182–188. [Google Scholar] [CrossRef]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Zuurveld, M.; van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; Land, B.V.; Willemsen, L.E.M. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Marsaux, B.; Rodenas, C.L.G.; Rytz, A.; Vandevijver, G.; Marzorati, M.; Abbeele, P.V.D.; Calatayud, M.; Rochat, F. Human Milk Oligosaccharides and Lactose Differentially Affect Infant Gut Microbiota and Intestinal Barrier In Vitro. Nutrients 2022, 14, 2546. [Google Scholar] [CrossRef] [PubMed]
- Jantscher-Krenn, E.; Zherebtsov, M.; Nissan, C.; Goth, K.; Guner, Y.S.; Naidu, N.; Choudhury, B.; Grishin, A.V.; Ford, H.R.; Bode, L. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 2011, 61, 1417–1425. [Google Scholar] [CrossRef]
- Albrecht, S.; Lane, J.A.; Mariño, K.V.; Al Busadah, K.A.; Carrington, S.D.; Hickey, R.M.; Rudd, P.M. A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 2014, 111, 1313–1328. [Google Scholar]
- Ninonuevo, M.R.; Park, Y.; Yin, H.; Zhang, J.; Ward, R.E.; Clowers, B.H.; German, J.B.; Freeman, S.L.; Killeen, K.; Grimm, R.; et al. A Strategy for Annotating the Human Milk Glycome. J. Agric. Food Chem. 2006, 54, 7471–7480. [Google Scholar]
- Urashima, T.; Katayama, T.; Fukuda, K.; Hirabayashi, J. Human Milk Oligosaccharides and Innate Immunity. Compr. Glycosci. 2021, 5, 389–439. [Google Scholar]
- Thongaram, T.; Hoeflinger, J.L.; Chow, J.; Miller, M.J. Human milk oligosaccharide consumption by probiotic and human-associated bifidobacteria and lactobacilli. J. Dairy Sci. 2017, 100, 7825–7833. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Gong, P.; Chen, Y.; Feng, Z.; Liu, P.; Zhang, P.; Wang, X.; Zhang, L.; Song, L. Comparative major oligosaccharides and lactose between Chinese human and animal milk. Int. Dairy J. 2020, 108, 104727. [Google Scholar]
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar] [PubMed]
- Bode, L. The functional biology of human milk oligosaccharides. Early Hum. Dev. 2015, 91, 619–622. [Google Scholar]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of Human Milk Oligosaccharides by Gut-Related Microbes. J. Agr. Food Chem. 2010, 58, 5334–5340. [Google Scholar]
- De Leoz, M.L.A.; Wu, S.; Strum, J.S.; Niñonuevo, M.R.; Gaerlan, S.C.; Mirmiran, M.; German, J.B.; Mills, D.A.; Lebrilla, C.B.; Underwood, M.A. A quantitative and comprehensive method to analyze human milk oligosaccharide structures in the urine and feces of infants. Anal. Bioanal. Chem. 2013, 405, 4089–4105. [Google Scholar]
- Albrecht, S.; Schols, H.A.; van den Heuvel, E.G.H.M.; Voragen, A.G.J.; Gruppen, H. CE-LIF-MSn profiling of oligosaccharides in human milk and feces of breast-fed babies. Electrophoresis 2010, 31, 1264–1273. [Google Scholar] [CrossRef]
- Coppa, G.V.; Pierani, P.; Zampini, L.; Bruni, S.; Carloni, I.; Gabrielli, O. Characterization of Oligosaccharides in Milk and Feces of Breast-Fed Infants by High-Performance Anion-Exchange Chromatography. Adv. Exp. Med. Biol. 2001, 501, 307–314. [Google Scholar]
- Sabharwal, H.; Sjöblad, S.; Lundblad, A. Sialylated Oligosaccharides in Human Milk and Feces of Preterm, Full-Term, and Weaning Infants. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 480–484. [Google Scholar]
- Davis, J.C.C.; Totten, S.M.; Huang, J.O.; Nagshbandi, S.; Kirmiz, N.; Garrido, D.A.; Lewis, Z.T.; Wu, L.D.; Smilowitz, J.T.; German, J.B.; et al. Identification of Oligosaccharides in Feces of Breast-fed Infants and Their Correlation with the Gut Microbial Community. Mol. Cell. Proteom. 2016, 15, 2987–3002. [Google Scholar]
- Albrecht, S.; Schols, H.A.; van Den Heuvel, E.G.H.M.; Voragen, A.G.J.; Gruppen, H. Occurrence of oligosaccharides in feces of breast-fed babies in their first six months of life and the corresponding breast milk. Carbohydr. Res. 2011, 346, 2540–2550. [Google Scholar] [PubMed]
- Albrecht, S.; Schols, H.A.; van Zoeren, D.; van Lingen, R.A.; Jebbink, L.J.G.; Heuvel, E.G.V.D.; Voragen, A.G.; Gruppen, H. Oligosaccharides in feces of breast- and formula-fed babies. Carbohydr. Res. 2011, 346, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Tao, J.; Zhou, J.; Fan, Q.; Liu, M.; Hu, Y.; Xu, Y.; Zhang, L.; Yuan, J.; Li, W.; et al. Fucosylated Human Milk Oligosaccharides and N-Glycans in the Milk of Chinese Mothers Regulate the Gut Microbiome of Their Breast-Fed Infants during Different Lactation Stages. Msystems 2018, 3, e00206-18. [Google Scholar] [CrossRef]
- Porfirio, S.; Archer-Hartmann, S.; Moreau, G.B.; Ramakrishnan, G.; Haque, R.; Kirkpatrick, B.D.; Petri, W.A.; Azadi, P. New strategies for profiling and characterization of human milk oligosaccharides. Glycobiology 2020, 30, 774–786. [Google Scholar] [CrossRef]
- György, P.; Jeanloz, R.W.; von Nicolai, H.; Zilliken, F. Undialyzable growth factors for Lactobacillus bifidus var. pennsylvanicus: Protective effect of sialic acid bound to glycoproteins and oligosaccharides against bacterial degradation. Eur. J. Biochem. 1974, 43, 29–33. [Google Scholar]
- Simon, P.M.; Goode, P.L.; Mobasseri, A.; Zopf, D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect. Immun. 1997, 65, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Stepans, M.B.; Wilhelm, S.L.; Hertzog, M.; Rodehorst, T.K.; Blaney, S.; Clemens, B.; Polak, J.J., III; Newburg, D.S. Early consumption of human milk oligosaccharides is inversely related to subsequent risk of respiratory and enteric disease in infants. Breastfeed. Med. 2006, 1, 207–215. [Google Scholar] [CrossRef]
- Moreno, F.J.; Sanz, M.L. Food Oligosaccharides: Production, Analysis and Bioactivity; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Katayama, T.; Sakuma, A.; Kimura, T.; Makimura, Y.; Hiratake, J.; Sakata, K.; Yamanoi, T.; Kumagai, H.; Yamamoto, K. Molecular cloning and characterization of Bifidobacterium bifidum 1, 2-α-L-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J Bacteriol. 2004, 186, 4885–4893. [Google Scholar] [CrossRef]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef]
- Strecker, G.; Hondi-Assah, T.; Fournet, B.; Spik, G.; Montreuil, J.; Maroteaux, P.; Durand, P.; Farriaux, J.-P. Structure of the three major sialyl-oligosaccharides excreted in the urine of five patients with three distinct inborn diseases: “I cell disease” and two new types of mucolipidosis. BBA-Gen Subj. 1976, 444, 349–358. [Google Scholar] [CrossRef]



| Milk | Total OSs | Neutral OSs | Acidic OSs | |||||
|---|---|---|---|---|---|---|---|---|
| Abundance (×106) | Number | Abundance (×106) | Content (%) | Number | Abundance (×106) | Content (%) | Number | |
| M1 | 69,218.8355 | 64 | 62,978.1215 | 90.98 | 46 | 6240.714 | 9.02 | 18 |
| F1 | 11,510.129 | 25 | 11,488.215 | 99.81 | 24 | 21.914 | 0.19 | 1 |
| M2 | 97,732.49 | 67 | 85,318.297 | 87.30 | 45 | 12,414.193 | 12.70 | 22 |
| F2 | 279,884.703 | 183 | 190,641.31 | 68.11 | 121 | 89,243.393 | 31.89 | 62 |
| M3 | 98,446.8111 | 61 | 93,316.3521 | 94.79 | 45 | 5130.459 | 5.21 | 16 |
| F3 | 9040.734 | 36 | 8975.228 | 99.28 | 32 | 65.506 | 0.72 | 4 |
| M4 | 76,029.096 | 69 | 67,880.439 | 89.28 | 50 | 8148.657 | 10.72 | 19 |
| F4 | 351,025.401 | 163 | 289,776.038 | 82.55 | 108 | 61,249.363 | 17.45 | 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zhou, Y.; Xu, Y. Comparative Analysis of Oligosaccharides in Breast Milk and Feces of Breast-Fed Infants by Using LC-QE-HF-MS: A Communication. Nutrients 2023, 15, 888. https://doi.org/10.3390/nu15040888
Li R, Zhou Y, Xu Y. Comparative Analysis of Oligosaccharides in Breast Milk and Feces of Breast-Fed Infants by Using LC-QE-HF-MS: A Communication. Nutrients. 2023; 15(4):888. https://doi.org/10.3390/nu15040888
Chicago/Turabian StyleLi, Rui, Yalin Zhou, and Yajun Xu. 2023. "Comparative Analysis of Oligosaccharides in Breast Milk and Feces of Breast-Fed Infants by Using LC-QE-HF-MS: A Communication" Nutrients 15, no. 4: 888. https://doi.org/10.3390/nu15040888
APA StyleLi, R., Zhou, Y., & Xu, Y. (2023). Comparative Analysis of Oligosaccharides in Breast Milk and Feces of Breast-Fed Infants by Using LC-QE-HF-MS: A Communication. Nutrients, 15(4), 888. https://doi.org/10.3390/nu15040888







