Fatigue in Patients on Chronic Hemodialysis: The Role of Indoleamine 2,3-Dioxygenase (IDO) Activity, Interleukin-6, and Muscularity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient’s Characteristics
2.2. Evaluation of Fatigue, Body Composition, and Muscle Strength
2.3. Tryptophan and Kynurenine Assays by Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) Method
2.4. Serum Interleukin (IL)-6 Assay
2.5. Statistical Analyses
3. Results
3.1. Patient’s Characteristics
3.2. Fatigue and Indices of Muscle Mass and Function
3.3. Association between Fatigue and Kyn/Trp Ratio and IL-6 Serum Concentrations
3.4. Correlation between ICW, IDO Activity, and IL-6 Concentration
3.5. Regression Analysis of Factors Associated with Fatigue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregg, L.P.; Bossola, M.; Ostrosky-Frid, M.; Hedayati, S.S. Fatigue in CKD: Epidemiology, Pathophysiology, and Treatment. Clin. J. Am. Soc. Nephrol. 2021, 16, 1445–1455. [Google Scholar] [PubMed]
- Artom, M.; Moss-Morris, R.; Caskey, F.; Chilcot, J. Fatigue in advanced kidney disease. Kidney Int. 2014, 86, 497–505. [Google Scholar] [PubMed]
- Jhamb, M.; Pike, F.; Ramer, S.; Argyropoulos, C.; Steel, J.; Dew, M.A.; Weisbord, S.D.; Weissfeld, L.; Unruh, M. Impact of fatigue on outcomes in the hemodialysis (HEMO) study. Am. J. Nephrol. 2011, 33, 515–523. [Google Scholar] [PubMed]
- Muscaritoli, M.; Molfino, A.; Bollea, M.R.; Rossi Fanelli, F. Malnutrition and wasting in renal disease. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Pertosa, G.; Grandaliano, G.; Gesualdo, L.; Schena, F.P. Clinical relevance of cytokine production in hemodialysis. Kidney Int. Suppl. 2000, 76, S104–S111. [Google Scholar]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Kim, S.; Miller, B.J.; Stefanek, M.E.; Miller, A.H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer 2015, 121, 2129–2136. [Google Scholar]
- Schefold, J.C.; Zeden, J.P.; Fotopoulou, C.; von Haehling, S.; Pschowski, R.; Hasper, D.; Volk, H.D.; Schuett, C.; Reinke, P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: A possible link between chronic inflammation and uraemic symptoms. Nephrol. Dial. Transplant. 2009, 24, 1901–1908. [Google Scholar]
- Laviano, A.; Meguid, M.M.; Cascino, A.; Molfino, A.; Rossi Fanelli, F. Tryptophan in wasting diseases: At the crossing between immune function and behaviour. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 392–397. [Google Scholar] [CrossRef]
- Lanser, L.; Kink, P.; Egger, E.M.; Willenbacher, W.; Fuchs, D.; Weiss, G.; Kurz, K. Inflammation-Induced Tryptophan Breakdown is Related With Anemia, Fatigue, and Depression in Cancer. Front. Immunol. 2020, 11, 249. [Google Scholar] [CrossRef]
- Jafarzadeh Esfehani, A.; Dashti, S. Barriers to exercise participation among dialysis patients. Nephrol. Dial. Transplant. 2012, 27, 3964. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Amabile, M.I.; Ammann, T.; Farcomeni, A.; Lionetto, L.; Simmaco, M.; Lai, S.; Laviano, A.; Rossi Fanelli, F.; Chiappini, M.G.; et al. The metabolite beta-aminoisobutyric acid and physical inactivity among hemodialysis patients. Nutrition 2017, 34, 101–107. [Google Scholar]
- Jankowska, M.; Debska-Slizień, A.; Rutkowski, B. Bioelectrical impedance analysis before versus after a hemodialysis session in evaluation of nutritional status. J. Ren. Nutr. 2006, 16, 137–140. [Google Scholar] [PubMed]
- Delgado, C.; Chiang, J.M.; Kittiskulnam, P.; Sheshadri, A.; Grimes, B.; Segal, M.; Kaysen, G.A.; Johansen, K.L. Longitudinal Assessment of Body Composition and Its Association With Survival Among Participants of the ACTIVE/ADIPOSE Study. J. Ren. Nutr. 2022, 32, 396–404. [Google Scholar] [PubMed]
- Molfino, A.; Amabile, M.I.; Ammann, T.; Lai, S.; Grosso, A.; Lionetto, L.; Spagnoli, A.; Simmaco, M.; Monti, M.; Laviano, A.; et al. Longitudinal Physical Activity Change During Hemodialysis and Its Association With Body Composition and Plasma BAIBA Levels. Front. Physiol. 2019, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Cavallari, M.; Zappulla, C.; Ulivieri, M.; Napoletano, F.; Capi, M.; Corigliano, V.; et al. Xanthurenic Acid Activates mGlu2/3 Metabotropic Glutamate Receptors and is a Potential Trait Marker for Schizophrenia. Sci. Rep. 2015, 5, 17799. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, L.; Ulivieri, M.; Capi, M.; De Bernardini, D.; Fazio, F.; Petrucca, A.; Pomes, L.M.; De Luca, O.; Gentile, G.; Casolla, B.; et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166042. [Google Scholar]
- Fischer, M.J.; Kimmel, P.L.; Greene, T.; Gassman, J.J.; Wang, X.; Brooks, D.H.; Charleston, J.; Dowie, D.; Thornley-Brown, D.; Cooper, L.A.; et al. Sociodemographic factors contribute to the depressive affect among African Americans with chronic kidney disease. Kidney Int. 2010, 77, 1010–1019. [Google Scholar]
- Watnick, S.; Kirwin, P.; Mahnensmith, R.; Concato, J. The prevalence and treatment of depression among patients starting dialysis. Am. J. Kidney Dis. 2003, 41, 105–110. [Google Scholar]
- Farragher, J.F.; Polatajko, H.J.; Jassal, S.V. The Relationship Between Fatigue and Depression in Adults With End-Stage Renal Disease on Chronic In-Hospital Hemodialysis: A Scoping Review. J. Pain Symptom Manag. 2017, 53, 783–803.e1. [Google Scholar]
- Fürst, P. Amino acid metabolism in uremia. J. Am. Coll. Nutr. 1989, 8, 310–323. [Google Scholar] [CrossRef] [PubMed]
- Groven, N.; Reitan, S.K.; Fors, E.A.; Guzey, I.C. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology 2021, 131, 105287. [Google Scholar] [PubMed]
- Yamashita, M. Potential Role of Neuroactive Tryptophan Metabolites in Central Fatigue: Establishment of the Fatigue Circuit. Int. J. Tryptophan Res. 2020, 13, 1178646920936279. [Google Scholar]
- Yamamoto, T.; Azechi, H.; Board, M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can. J. Neurol. Sci. 2012, 39, 40–47. [Google Scholar] [PubMed]
- Malhotra, R.; Persic, V.; Zhang, W.; Brown, J.; Tao, X.; Rosales, L.; Thijssen, S.; Finkelstein, F.O.; Unruh, M.L.; Ikizler, A.; et al. Tryptophan and Kynurenine Levels and Its Association With Sleep, Nonphysical Fatigue, and Depression in Chronic Hemodialysis Patients. J. Ren. Nutr. 2017, 27, 260–266. [Google Scholar]
- Riazati, N.; Kable, M.E.; Newman, J.W.; Adkins, Y.; Freytag, T.; Jiang, X.; Stephensen, C.B. Associations of microbial and indoleamine-2,3-dioxygenase-derived tryptophan metabolites with immune activation in healthy adults. Front. Immunol. 2022, 13, 917966. [Google Scholar] [CrossRef]
- Haverkamp, G.L.; Loosman, W.L.; Franssen, C.F.; Kema, I.P.; van Diepen, M.; Dekker, F.W.; Honig, A.; Siegert, C.E. The role of tryptophan degradation in the association between inflammatory markers and depressive symptoms in chronic dialysis patients. Nephrol. Dial. Transplant. 2017, 32, 1040–1047. [Google Scholar]
- Kaiser, H.; Yu, K.; Pandya, C.; Mendhe, B.; Isales, C.M.; McGee-Lawrence, M.E.; Johnson, M.; Fulzele, S.; Hamrick, M.W. Kynurenine, a Tryptophan Metabolite That Increases with Age, Induces Muscle Atrophy and Lipid Peroxidation. Oxid. Med. Cell. Longev. 2019, 2019, 9894238. [Google Scholar] [CrossRef]
- Shirai, N.; Inoue, T.; Ogawa, M.; Okamura, M.; Morishita, S.; Suguru, Y.; Tsubaki, A. Relationship between Nutrition-Related Problems and Falls in Hemodialysis Patients: A Narrative Review. Nutrients 2022, 14, 3225. [Google Scholar] [CrossRef]

| Parameter | Patients on Hemodialysis (N = 50) | p-Value | |
|---|---|---|---|
| Fatigue (N = 12) | No Fatigue (N = 38) | ||
| Male, n (%) | 8 (67) | 29 (76) | 0.506 |
| Age, years | 76.6 ± 11.7 | 63 ± 15.4 | 0.007 |
| Body mass index, kg/m2 | 24.58 ± 3.06 | 24.82 ± 6.06 | 0.899 |
| Time on dialysis, months | 57 (38; 92) | 26 (12; 79) | 0.116 |
| Protein catabolic rate, g/kg/d | 0.87 ± 0.19 | 0.98 ± 0.27 | 0.237 |
| Hemoglobin, g/dL | 10.7 ± 1.7 | 11.6 ± 1.3 | 0.072 |
| C-reactive protein, mg/dL | 0.92 (0.22; 1.70) | 0.32 (0.16; 0.67) | 0.103 |
| Albumin, g/dL | 3.6 ± 0.3 | 3.7 ± 0.3 | 0.263 |
| Creatinine, mg/dL | 8.5 ± 2.0 | 9.7 ± 2.6 | 0.165 |
| Diabetes, n (%) | 5 (42) | 8 (21) | 0.298 |
| Hypertension, n (%) | 9 (75) | 27 (71) | 0.918 |
| BIA parameters | |||
| ICW, L | 13.8 ± 2.0 | 18.4 ± 5.6 | <0.001 |
| ECW, L | 22.2 ± 7.0 | 18.0 ± 5.2 | 0.03 |
| ECW/ICW | 1.66 (1.27; 1.82) | 0.95 (0.84; 1.09) | <0.001 |
| ICW/h2, L/m2 | 4.7 ± 1.7 | 6.6 ± 1.7 | 0.001 |
| Hand grip strength, mmHg | 18.7 ± 5.0 | 26.3 ± 10.1 | 0.02 |
| Kynurenine, µg/mL | 0.52 ± 0.24 | 0.51 ± 0.15 | 0.806 |
| Tryptophane, µg/mL | 4.7 ± 2.1 | 5.6 ± 1.5 | 0.104 |
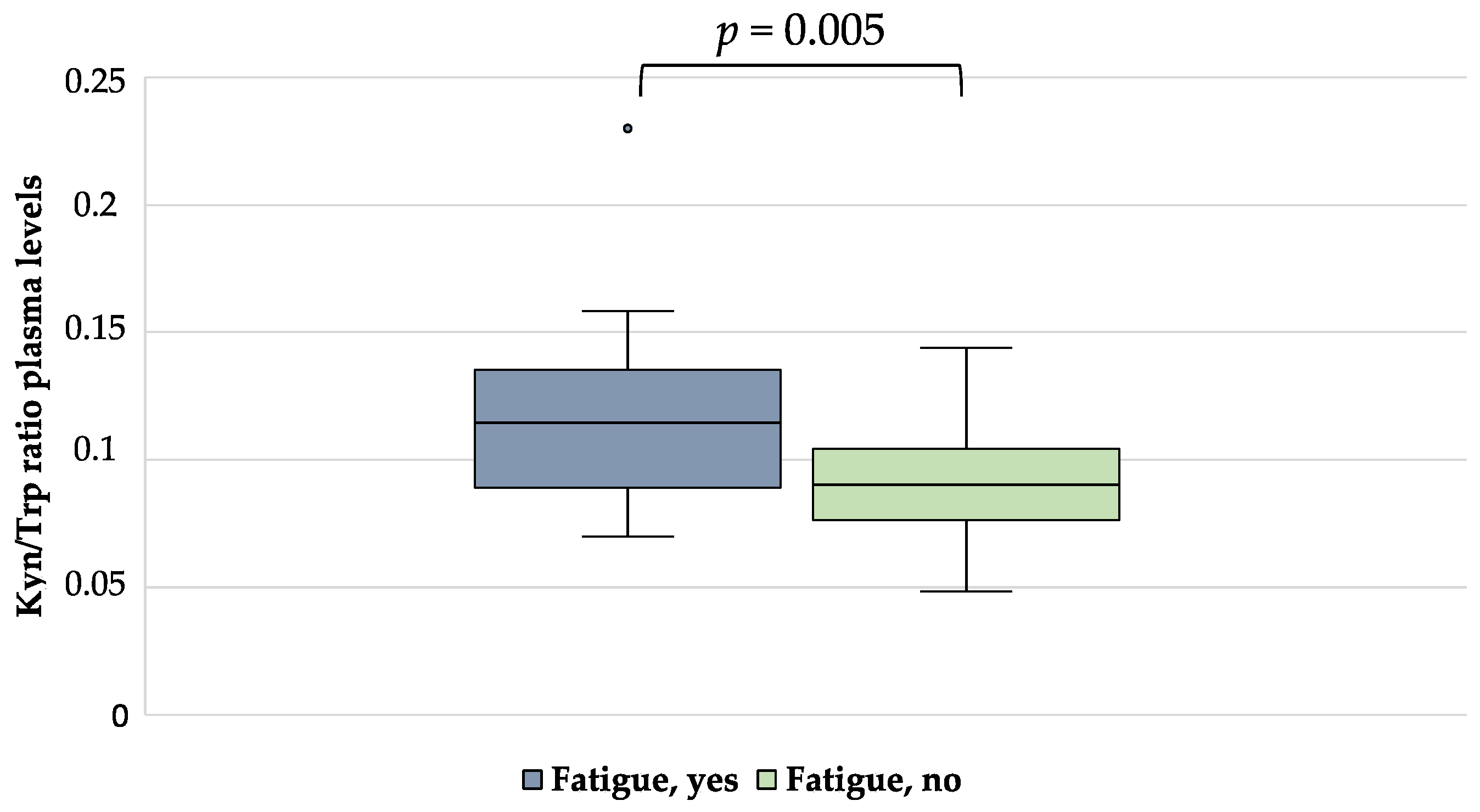
| Kyn/Trp ratio | 0.12 ± 0.04 | 0.09 ± 0.02 | 0.005 |
| Interleukin-6, pg/mL | 30.9 (19.1; 80.9) | 18.6 (14.0; 25.1) | 0.011 |
| Parameters | OR (95% CI) | p-Value |
|---|---|---|
| IL-6 (pg/mL) | 1.048 (0.991; 1.108) | 0.101 |
| Kyn/Trp ratio | 1.317 (0.818; 2.120) | 0.257 |
| ICW/h2 (L/m2) | 0.100 (0.014; 0.731) | 0.023 |
| Instrument Used to Diagnose Fatigue | Number of Items Included | Scoring System | Time of Administration | Notes |
|---|---|---|---|---|
| FACIT-Fatigue | 13 questions | 5-point Likert-type scale for each item with a minimum score of 0 and maximum score of 52. Lower is the score, higher is the grade of fatigue | Less than 5 min to complete the entire questionnaire | The scale represents a quantitative measure of fatigue, and a cut-off of ≤44 was utilized to diagnose the presence of fatigue The scale was used in several chronic conditions, including cancer and chronic kidney disease |
| POSs | 1 out of 17 questions identifying fatigue as “weakness or lack of energy” | 5-point Likert-type scale (absent, mild, moderate, severe and overwhelming) | Less than 5 min to complete the entire questionnaire | The questionnaire was originally designed for patients on palliative care and then adapted for patients with chronic kidney disease |
| DSI | 1 out of 17 questions identifying fatigue as “feeling tired or lack of energy” | 5-point Likert-type scale (not at all, a little bit, some-what, quite a bit, and very much) ranging from 0 to 4 | Less than 5 min to complete the entire questionnaire | The questionnaire was designed to investigate 30 symptoms among patients on dialysis |
| MSAS-SF | 1 out of 32 items identifying fatigue as “lack of energy” | 5-point Likert-type scale; 0 (not at all) to 4 (very much) | Less than 5 min to complete the entire questionnaire | The MSAS-SF include global distress index, the physical symptom distress score, the psychologic symptom distress score comprehending the evaluation of 6 psychological symptoms |
| ESAS-r | 1 out of 10 items identifying fatigue as tiredness | Symptoms rated from 0 to 10 | Less than 5 min to complete the entire questionnaire | It is a quantitative score used originally in palliative care settings and more recently to assess physical and psychological symptoms in patients with end-stage renal disease |
| QIDS-SR16 | 1 out of 16 items identifying fatigue as grade of energy/fatigability | Likert scale of 0 (no change in usual level of energy) to 3 (unable to carry out most of usual daily activities due to lack of energy) | From 5 to 10 min to complete the entire questionnaire | The QIDS-SR16 has 16 items based on the 9 symptom domains of major depressive disorder. The score ranges between 0 to 27 |
| BDI | 1 out of 21 items identifying fatigue as tiredness | Likert scale of 0 (no fatigue) to 3 (severe fatigue) | From 5 to 10 min to complete the entire questionnaire | The BDI accounts for cognitive/affective features of depression and somatic aspects such as sleep disturbance and health concerns) |
| SF-12 vitality scale | 1 out of 12 items identifying fatigue as grade of energy | Six-point Likert scale for the question “did you have a lot of energy”? | Less than 5 min to complete the entire questionnaire | The SF-12 is a self-reported outcome measure which is often used for the evaluation of quality of life in different settings. The SF-12 is a short version of the SF-36 |
| DPEBBS | 2 out of 24 items evaluating tiredness and muscle fatigue | Binary variable (i.e., yes or no) | Less than 5 min to complete the entire questionnaire | It is a 24-item questionnaire used to evaluate the perceived benefits and barriers to exercise of the patients. The scale includes 24 items (12 items of exercise benefits and 12 items of exercise barriers) and 2 open questions |
| Questions regarding barriers to physical activity | 2 out of 23 barriers are represented by fatigue on non-dialysis days and fatigue on dialysis days | Patients were asked if “never”, “sometimes”, “often”, or “always” experienced that barrier. ”never” classified as not having the barrier | Less than 5 min to complete the entire questionnaire | These questions investigate broad the following barriers to exercise: psychological barriers, physical barriers, lack of time, and presence of comorbidities. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfino, A.; Imbimbo, G.; Amabile, M.I.; Ammann, T.; Lionetto, L.; Salerno, G.; Simmaco, M.; Chiappini, M.G.; Muscaritoli, M. Fatigue in Patients on Chronic Hemodialysis: The Role of Indoleamine 2,3-Dioxygenase (IDO) Activity, Interleukin-6, and Muscularity. Nutrients 2023, 15, 876. https://doi.org/10.3390/nu15040876
Molfino A, Imbimbo G, Amabile MI, Ammann T, Lionetto L, Salerno G, Simmaco M, Chiappini MG, Muscaritoli M. Fatigue in Patients on Chronic Hemodialysis: The Role of Indoleamine 2,3-Dioxygenase (IDO) Activity, Interleukin-6, and Muscularity. Nutrients. 2023; 15(4):876. https://doi.org/10.3390/nu15040876
Chicago/Turabian StyleMolfino, Alessio, Giovanni Imbimbo, Maria Ida Amabile, Thomas Ammann, Luana Lionetto, Gerardo Salerno, Maurizio Simmaco, Maria Grazia Chiappini, and Maurizio Muscaritoli. 2023. "Fatigue in Patients on Chronic Hemodialysis: The Role of Indoleamine 2,3-Dioxygenase (IDO) Activity, Interleukin-6, and Muscularity" Nutrients 15, no. 4: 876. https://doi.org/10.3390/nu15040876
APA StyleMolfino, A., Imbimbo, G., Amabile, M. I., Ammann, T., Lionetto, L., Salerno, G., Simmaco, M., Chiappini, M. G., & Muscaritoli, M. (2023). Fatigue in Patients on Chronic Hemodialysis: The Role of Indoleamine 2,3-Dioxygenase (IDO) Activity, Interleukin-6, and Muscularity. Nutrients, 15(4), 876. https://doi.org/10.3390/nu15040876






