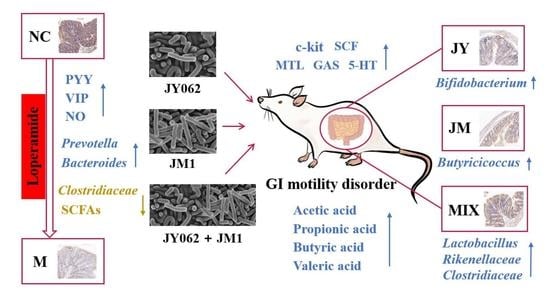
The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Mouse Model and Experimental Design
2.3. Physiological Indicators
2.4. Determination of GI Regulatory Peptides and Neurotransmitters
2.5. Reverse Transcription (RT) and Quantitative Real-Time PCR (qPCR)
2.6. Immunohistochemistry and TUNEL Staining Analysis
2.7. Western Blot (WB) Analysis
2.8. Gut Microbiota Sequencing and Analysis
2.9. Determination of SCFAs
2.10. Statistical Methods
3. Results
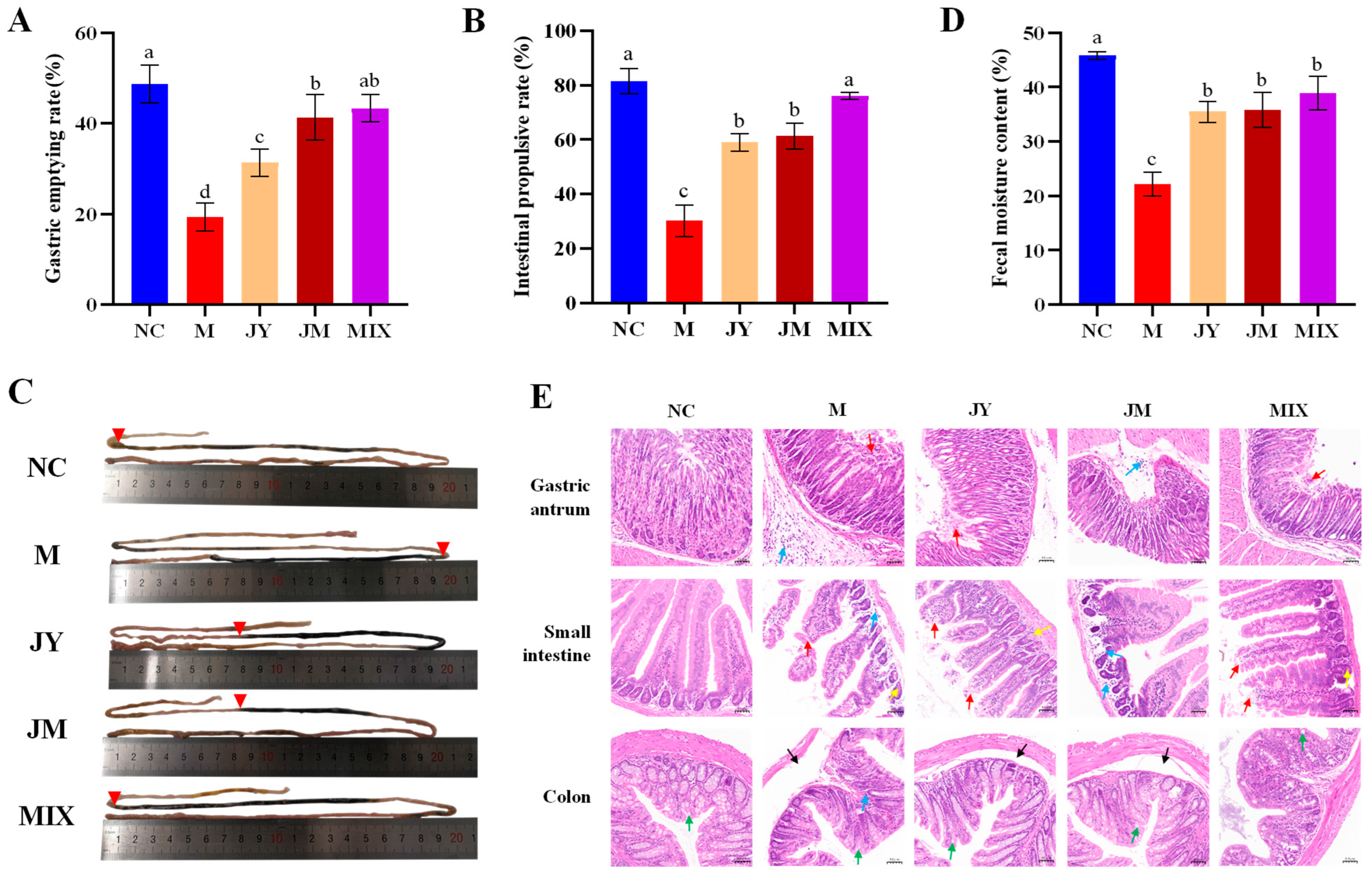
3.1. Effects on Physiological Indicators of Mice with GI Motility Disorder
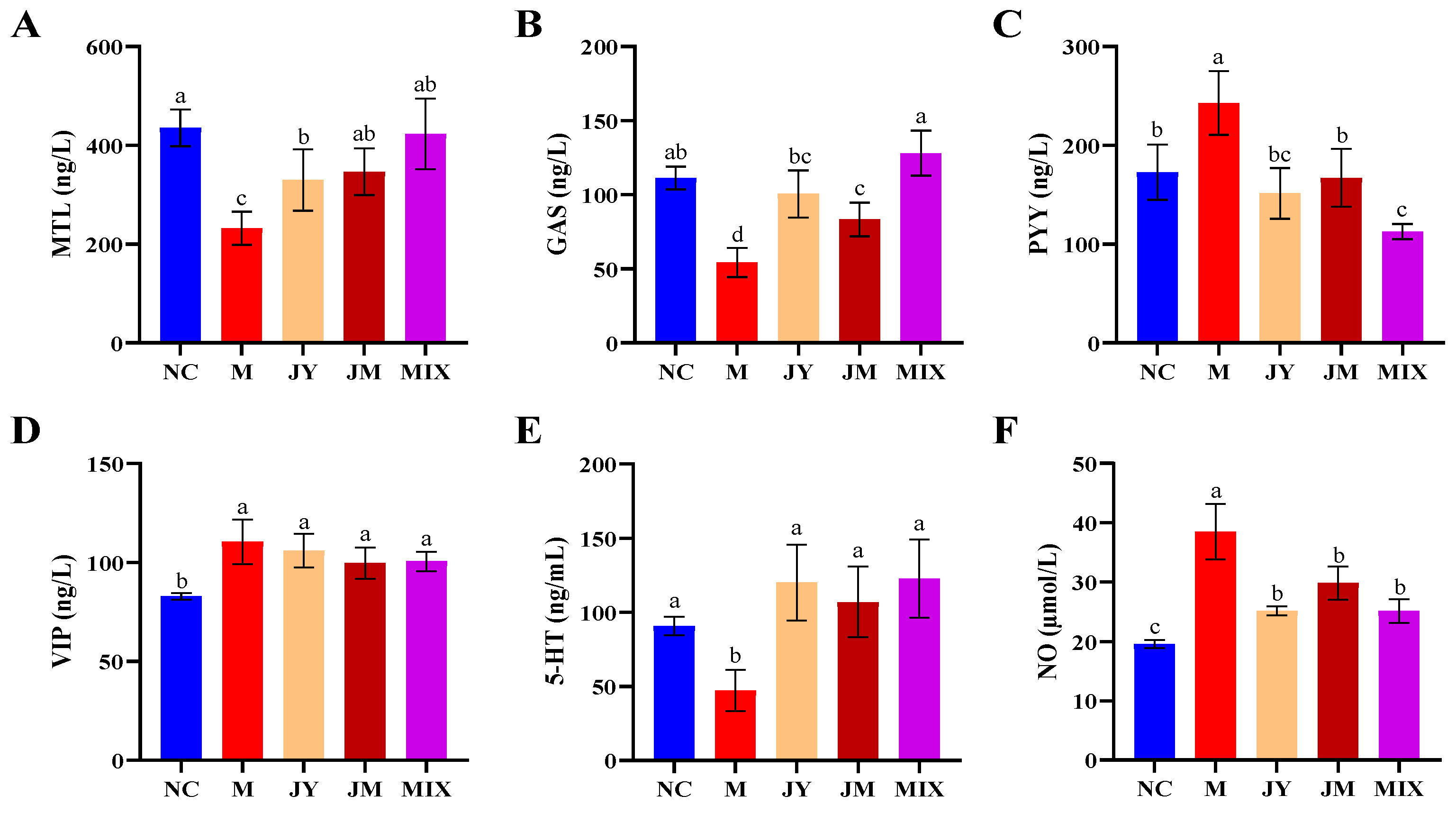
3.2. Effects on GI Regulatory Peptides and Neurotransmitters of Mice with GI Motility Disorder
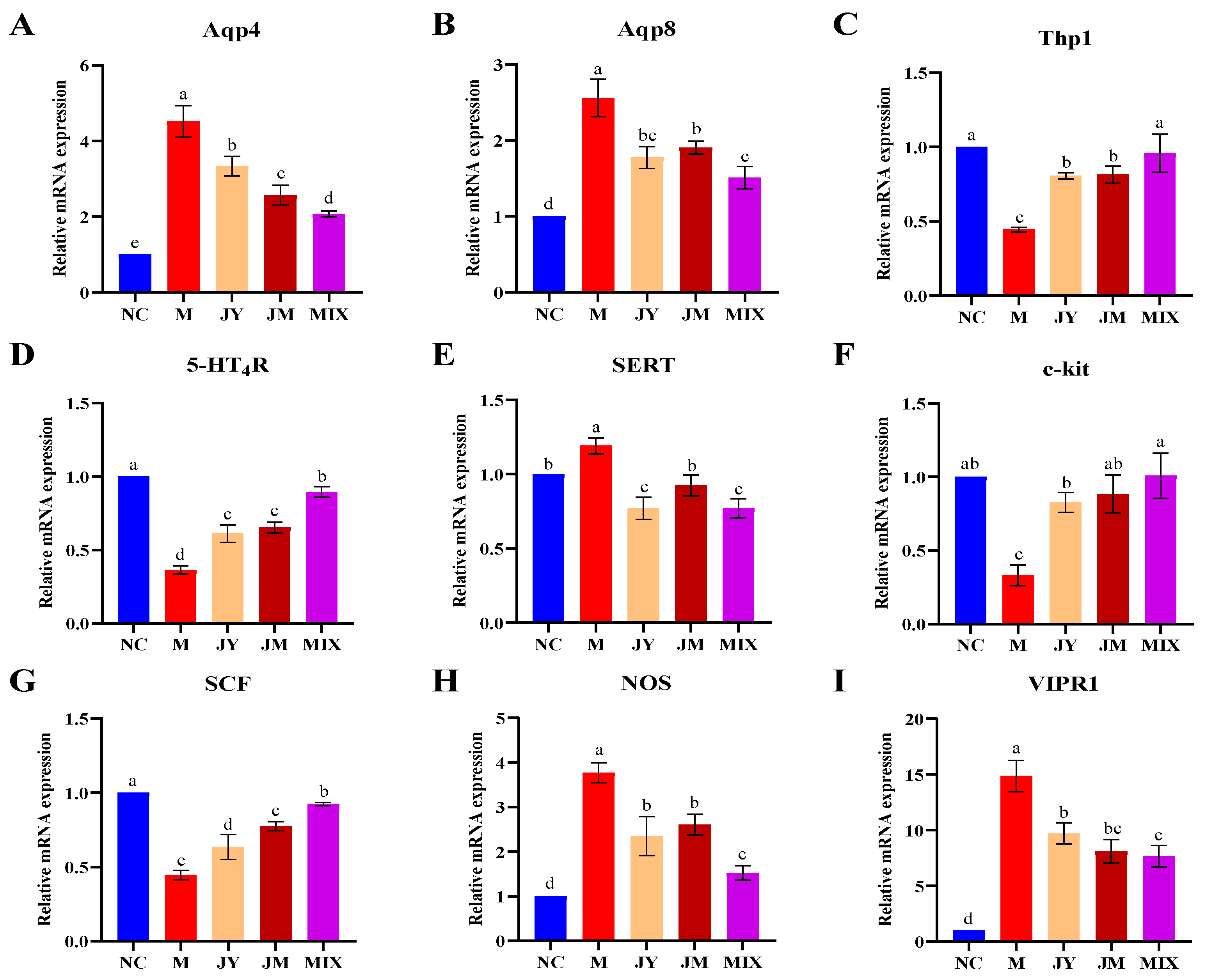
3.3. Effects on the Expression of Related Genes of Mice with GI Motility Disorder
3.4. Effects on ICC, c-kit Protein and SCF Protein of Mice with GI Motility Disorder
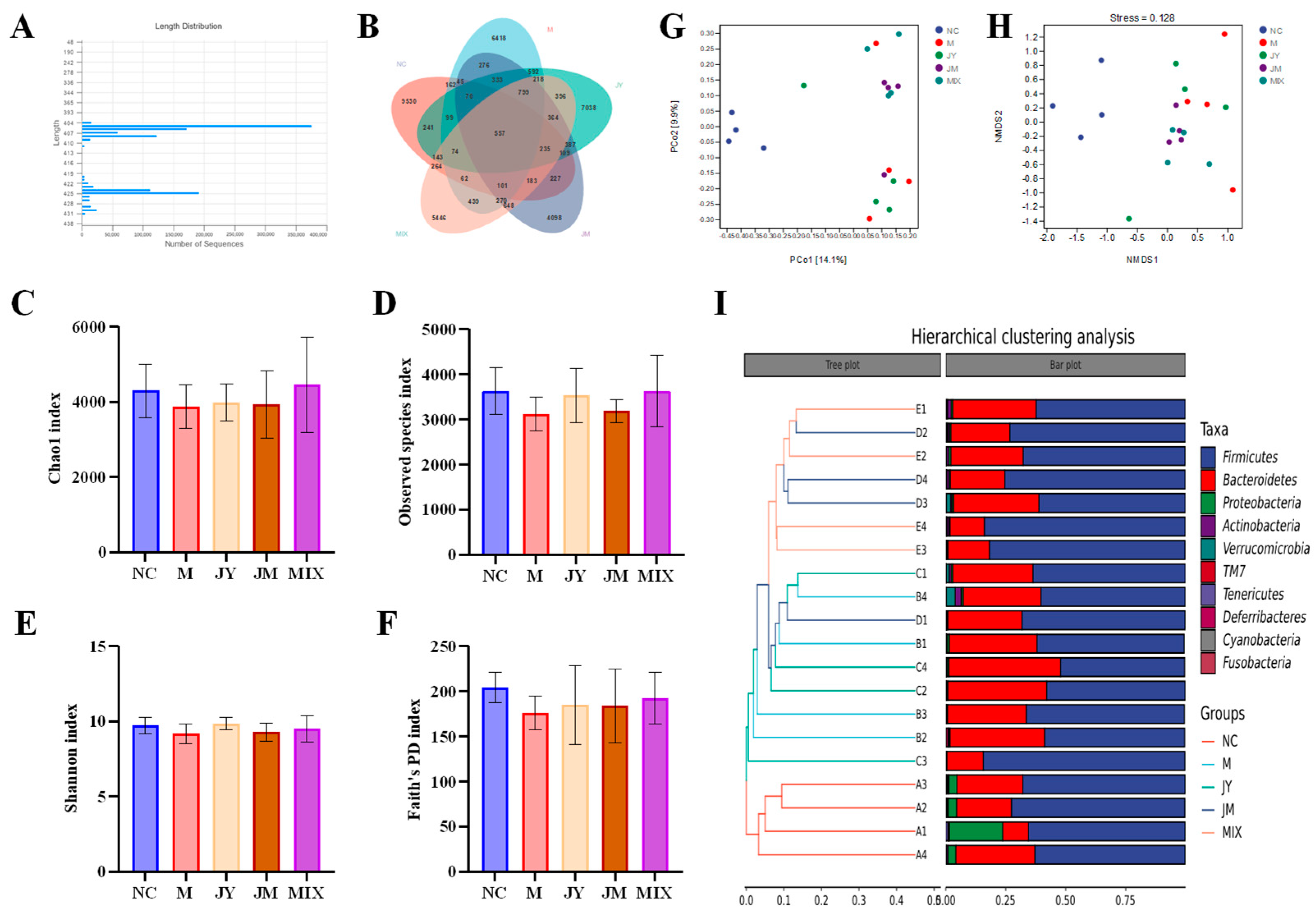
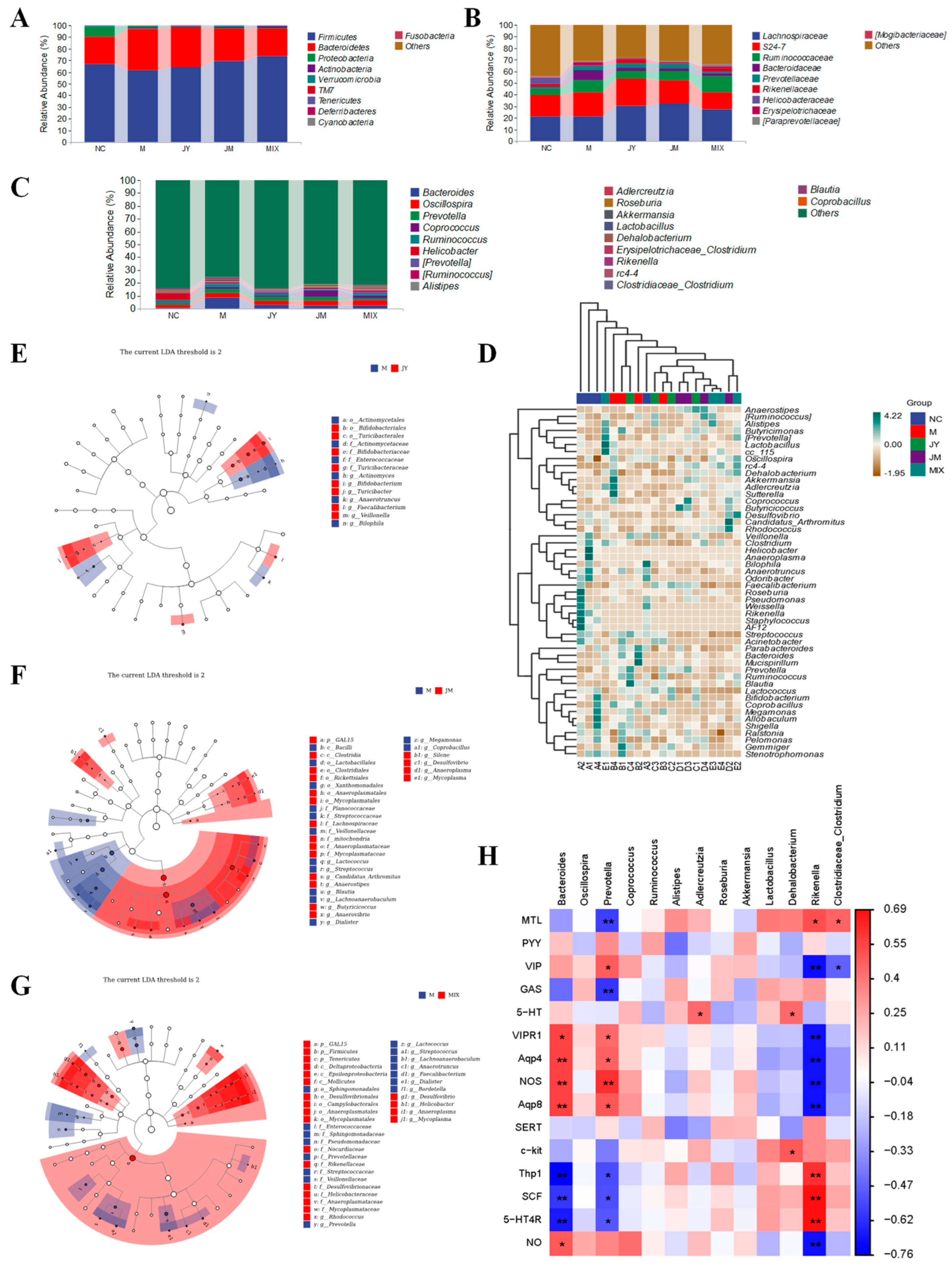
3.5. Effects on Gut Microbiota of Mice with GI Motility Disorder
3.6. Effects on SCFAs of Mice with GI Motility Disorder
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol. Motil. 2007, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Fragkos, K.C.; Scott, S.M.; Whelan, K. The effect of probiotics on functional constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Weiser, K. Gastrointestinal motility disorders: An update. Dig. Dis. 2006, 24, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Basilisco, G.; Coletta, M. Chronic constipation: A critical review. Dig. Liver Dis. 2013, 45, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Suares, N.C. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: Systematic review and meta-analysis. Gut 2011, 60, 209–218. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Tong, L.; Zhou, X.; Liang, X.; Yi, H.; Gong, P.; Liu, T.; Zhang, L.; Yang, L.; et al. Mechanisms underlying the promotion of 5-hydroxytryptamine secretion in enterochromaffin cells of constipation mice by Bifidobacterium and Lactobacillus. Neurogastroenterol. Motil. 2021, 33, e14082. [Google Scholar] [CrossRef]
- Na, J.R.; Kim, E.; Na, C.S.; Kim, S. Citric acid-enriched extract of ripe prunus mume (Siebold) Siebold & Zucc. induces laxative effects by regulating the expression of aquaporin 3 and prostaglandin E2 in rats with loperamide-induced constipation. J. Med. Food 2022, 25, 12–23. [Google Scholar] [CrossRef]
- Gu, Y.; Qin, X.; Zhou, G.; Wang, C.; Mu, C.; Liu, X.; Zhong, W.; Xu, X.; Wang, B.; Jiang, K.; et al. Lactobacillus rhamnosus GG supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 2022, 13, 12144–12155. [Google Scholar] [CrossRef]
- Choi, N.R.; Kim, J.N.; Kwon, M.J.; Lee, J.R.; Kim, S.C.; Lee, M.J.; Choi, W.G.; Kim, B.J. Grape seed powder increases gastrointestinal motility. Int. J. Med. Sci. 2022, 19, 941–951. [Google Scholar] [CrossRef]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Sawada, D.; Sugawara, T.; Ishida, Y.; Aihara, K.; Aoki, Y.; Takehara, I.; Takano, K.; Fujiwara, S. Effect of continuous ingestion of a beverage prepared with Lactobacillus gasseri CP2305 inactivated by heat treatment on the regulation of intestinal function. Food Res. Int. 2016, 79, 33–39. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive effect and molecular mechanism of Lactobacillus rhamnosus JL1 on food-borne obesity in mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Dharmaprakash, V.; Nighot, P.; Guo, S.; Nighot, M.; Do, T.; Ma, T.Y. Bifidobacterium bifidum enhances the intestinal epithelial tight junction barrier and protects against intestinal inflammation by targeting the Toll-like receptor-2 pathway in an NF-kappa B-independent manner. Int. J. Mol. Sci. 2021, 22, 8070. [Google Scholar] [CrossRef]
- Li, F.; Zhou, H.; Zhou, X.; Yi, R.; Mu, J.; Zhao, X.; Liu, W. Lactobacillus plantarum CQPC05 isolated from pickled vegetables inhibits constipation in mice. Appl. Sci. 2019, 9, 159. [Google Scholar] [CrossRef]
- Tang, T.; Wang, J.; Jiang, Y.Y.; Zhu, X.; Zhang, Z.; Wang, Y.Y.; Shu, X.; Deng, Y.D.; Zhang, F. Bifidobacterium lactis TY-S01 prevents loperamide-induced constipation by modulating gut microbiota and its metabolites in mice. Front. Nutr. 2022, 9, 890314. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Sun, S.; Si, Q.; Wang, L.; Zhang, Q.; Wu, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus rhamnosus strains relieve loperamide-induced constipation via different pathways independent of short-chain fatty acids. Front. Cell Infect. Microbiol. 2020, 10, 423. [Google Scholar] [CrossRef]
- Liao, C.A.; Huang, C.H.; Ho, H.H.; Chen, J.F.; Kuo, Y.W.; Lin, J.H.; Tsai, S.Y.; Tsai, H.Y.; Yeh, Y.T. A combined supplement of probiotic strains AP-32, bv-77, and CP-9 increased Akkermansia mucinphila and reduced non-esterified fatty acids and energy metabolism in HFD-induced obese rats. Nutrients 2022, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of probiotic compounds in relieving constipation and their colonization in gut microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, L.; Gamallat, Y.; Zhang, B.; Xin, Y. Enteromorpha and polysaccharides from enteromorpha ameliorate loperamide-induced constipation in mice. Biomed. Pharmacother. 2017, 96, 1075–1081. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Y.; Tan, H.-L.; Wang, X.-J.; Xiao, L. The key ingredient acacetin in Weishu decoction alleviates gastrointestinal motility disorder based on network pharmacology analysis. Mediat. Inflamm. 2021, 2021, 5265444. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Huang, B.; Yu, Y.; Zhao, S.; Liu, J.; Jia, Z.; Xiao, H. Aster tataricus alleviates constipation by antagonizing the binding of acetylcholine to muscarinic receptor and inhibiting Ca2+ influx. Biomed. Pharmacother. 2021, 133, 111005. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.J.; Liu, S.; Hou, X.H. Electroacupuncture at ST36 ameliorates gastric emptying and rescues networks of interstitial cells of Cajal in the stomach of diabetic rats. PLoS ONE 2013, 8, e83904. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, H.Z.; Yu, Y.; Zhang, J.S.; Zeng, X.N.; Gong, F.T.; Duan, X.P.; Liu, Q.; Yang, B. Resveratrol improved the progression of chronic prostatitis via the downregulation of c-kit/SCF by activating Sirt1. J. Agric. Food Chem. 2017, 65, 5668–5673. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yan, Q.; Wen, Y.; Liu, J.; Sun, J.; Jiang, Z. Synbiotic yogurt containing konjac mannan oligosaccharides and Bifidobacterium animalis ssp. lactis BB12 alleviates constipation in mice by modulating the stem cell factor (SCF)/c-Kit pathway and gut microbiota. J. Dairy Sci. 2021, 104, 5239–5255. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef] [PubMed]
- Torihashi, S.; Yokoi, K.; Nagaya, H.; Aoki, K.; Fujimoto, T. New monoclonal antibody (AIC) identifies interstitial cells of Cajal in the musculature of the mouse gastrointestinal tract. Auton Neurosci. 2004, 113, 16–23. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Jiang, Y.; Wu, B.; Yu, Y. Aqueous extract of phyllanthus emblica L. alleviates functional dyspepsia through regulating gastrointestinal hormones and gut microbiome in vivo. Foods 2022, 11, 1491. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Zhao, Y.; Duan, S.; Liu, W.-H.; Zhang, C.; Sun, S.; Wang, T.; Wang, X.; Hung, W.-L.; et al. In vitro study of Bifidobacterium lactis BL-99 with fructooligosaccharide synbiotics effected on the intestinal microbiota. Front. Nutr. 2022, 9, 890316. [Google Scholar] [CrossRef]
- Chen, S.; Ou, Y.; Zhao, L.; Ll, Y.; Qiao, Z.; Hao, Y.; Ren, F. Differential effects of Lactobacillus casei strain Shirota on patients with constipation regarding stool consistency in China. J. Neurogastroenterol. Motil. 2019, 25, 148–158. [Google Scholar] [CrossRef]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.-K.; Zhao, J.; Zhan, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Ma, D.; Jin, H.; Lai-Yu, K.; Zhang, H. Effect of Lacticaseibacillus casei Zhang on iron status, immunity, and gut microbiota of mice fed with low-iron diet. J. Funct. Foods 2022, 88, 104906. [Google Scholar] [CrossRef]
- Bai, M.; Huang, T.; Guo, S.; Wang, Y.; Wang, J.; Kwok, L.-Y.; Dan, T.; Zhang, H.; Bilige, M. Probiotic Lactobacillus casei Zhang improved the properties of stirred yogurt. Food Biosci. 2020, 37, 100718. [Google Scholar] [CrossRef]
- Merritt, J.E.; Brown, B.L.; Tomlinson, S. Loperamide and calmodulin. Lancet 1982, 1, 283. [Google Scholar] [CrossRef]
- Eor, J.Y.; Tan, P.L.; Lim, S.M.; Choi, D.H.; Yoon, S.M.; Yang, S.Y.; Kim, S.H. Laxative effect of probiotic chocolate on loperamide-induced constipation in rats. Food Res. Int. 2019, 116, 1173–1182. [Google Scholar] [CrossRef]
- Yde, J.; Keely, S.; Wu, Q.; Borg, J.F.; Lajczak, N.; O’Dwyer, A.; Dalsgaard, P.; Fenton, R.A.; Moeller, H.B. Characterization of AQPs in mouse, rat, and human colon and their selective regulation by bile acids. Front. Nutr. 2016, 3, 46. [Google Scholar] [CrossRef]
- Clevers, H.C.; Bevins, C.L. Paneth Cells: Maestros of the small intestinal crypts. In Annu Rev of Physiol; Julius, D., Ed.; American Physiological Society: Rockville, MD, USA, 2013; Volume 75, pp. 289–311. [Google Scholar] [CrossRef]
- Bevins, C.L. The Paneth cell and the innate immune response. Curr. Opin. Gastroenterol. 2004, 20, 572–580. [Google Scholar] [CrossRef]
- Ohno, T.; Mochiki, E.; Kuwano, H. The roles of motilin and ghrelin in gastrointestinal motility. Int. J. Pept. 2010, 2010, 820794. [Google Scholar] [CrossRef]
- Fourmy, D.; Gigoux, V.; Reubi, J.C. Gastrin in gastrointestinal diseases. Gastroenterology 2011, 141, 814–818.e3. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Salinas, C.B.G.; Rehfeld, J.F.; Secher, A.; Raun, K.; Wulff, B.S. PYY(3-36) and exendin-4 reduce food intake and activate neuronal circuits in a synergistic manner in mice. Neuropeptides 2019, 73, 89–95. [Google Scholar] [CrossRef]
- Hu, T.-G.; Wen, P.; Fu, H.-Z.; Lin, G.-Y.; Liao, S.-T.; Zou, Y.-X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef]
- Fung, C.; Unterweger, P.; Parry, L.J.; Bornstein, J.C.; Foong, J.P.P. VPAC(1) receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in guinea pig jejunum. Am. J. Physiol. Gastrointestinal. Liver Physiol. 2014, 306, G748–G758. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Tyler, K.; MacEachern, S.J.; Balemba, O.B.; Johnson, A.; Brooks, E.M.; Zhao, H.; Swain, G.; Moses, P.L.; Galligan, J.J.; et al. Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 2012, 142, 844–854.e4. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Chen, J.J.; Li, Z.; Pan, H.; Murphy, D.L.; Tamir, H.; Koepsell, H.; Gershon, M.D. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J. Neurosci. 2001, 21, 6348–6361. [Google Scholar] [CrossRef]
- Hara, T.; Mihara, T.; Ishibashi, M.; Kumagai, T.; Joh, T. Heat-killed Lactobacillus casei subsp casei 327 promotes colonic serotonin synthesis in mice. J. Funct. Foods 2018, 47, 585–589. [Google Scholar] [CrossRef]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric oxide: From gastric motility to gastric dysmotility. Int. J. Mol. Sci. 2021, 22, 9990. [Google Scholar] [CrossRef]
- Mourad, F.H.; Barada, K.A.; Abdel-Malak, N.; Rached, N.A.B.; Khoury, C.I.; Saadé, N.E.; Nassar, C.F. Interplay between nitric oxide and vasoactive intestinal polypeptide in inducing fluid secretion in rat jejunum. J. Physiol. 2003, 550, 863–871. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Suo, H.; Wang, W.; Wang, H.; Zhang, Y.; Hu, Q.; Zhao, X.; Li, J. Preventive effects of different fermentation times of Shuidouchi on diphenoxylate-induced constipation in mice. Foods 2019, 8, 86. [Google Scholar] [CrossRef]
- Liu, L.W.; Thuneberg, L.; Huizinga, J.D. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am. J. Physiol. 1994, 266, G485–G496. [Google Scholar] [CrossRef]
- Ronnstrand, L. Signal transduction via the stem cell factor receptor/c-Kit. Cell. Mol. Life Sci. 2004, 61, 2535–2548. [Google Scholar] [CrossRef]
- Tong, W.; Jia, H.; Zhang, L.; Li, C.; Ridolfi, T.J.; Liu, B. Exogenous stem cell factor improves interstitial cells of Cajal restoration after blockade of c-kit signaling pathway. Scand. J. Gastroenterol. 2010, 45, 844–851. [Google Scholar] [CrossRef]
- Ley, R.E. Prevotella in the gut: Choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W.; Mao, Y.; Zhang, X.; Pang, X.; Wei, C.; et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008, 3, 213–223. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial role of human gut lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A six-day, lifestyle-based immersion program mitigates cardiovascular risk factors and induces shifts in gut microbiota, specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium prausnitzii: A pilot study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef]
- Tavella, T.; Rampelli, S.; Guidarelli, G.; Bazzocchi, A.; Gasperini, C.; Pujos-Guillot, E.; Comte, B.; Barone, M.; Biagi, E.; Candela, M.; et al. Elevated gut microbiome abundance of Christensenellaceae, Porphyromonadaceae and Rikenellaceae is associated with reduced visceral adipose tissue and healthier metabolic profile in Italian elderly. Gut Microbes. 2021, 13, 1880221. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Xu, L.; Liu, B.; Huang, L.; Li, Z.; Cheng, Y.; Tian, Y.; Pan, G.; Li, H.; Xu, Y.; Wu, W.; et al. Probiotic consortia and their metabolites ameliorate the symptoms of inflammatory bowel diseases in a colitis mouse model. Microbiol. Spectr. 2022, 10, e00657-22. [Google Scholar] [CrossRef]
- Ou, J.H.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J.D. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, D.; Sun, R.; Zhang, Y.; Pegg, R.B.; Zhong, G. Prevention of loperamide induced constipation in mice by KGM and the mechanisms of different gastrointestinal tract microbiota regulation. Carbohydr. Polym. 2021, 256, 117418. [Google Scholar] [CrossRef]
- In, Y.-W.; Kim, J.-J.; Kim, H.-J.; Oh, S.-W. Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J. Food Safety. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Park, Y.T.; Kim, T.; Ham, J.; Choi, J.; Lee, H.S.; Yeon, Y.J.; Choi, S.I.; Kim, N.; Kim, Y.R.; Seok, Y.J. Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 2022, 15, 832–843. [Google Scholar] [CrossRef]
- Han, R.; Nusbaum, O.; Chen, X.; Zhu, Y. Valeric Acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Mol. Ther. Oncolytics 2020, 19, 8–18. [Google Scholar] [CrossRef]


| Gene | Primer Sequences (5′→3′) | |
|---|---|---|
| Forward | Reverse | |
| GAPDH | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
| Aqp4 | CAGCATCGCTAAGTCCGTCTTCTAC | ACCGTGGTGACTCCCAATCCTC |
| Aqp8 | GGAACATCAGCGGTGGACACTTC | GGGAATTAGCAGCATGGTCTTGAGG |
| Thp1 | ATCCGTCCTGTGGCTGGTTACC | AGGTGTCTGGCTCTGGAGTGTAG |
| 5-HT4R | AAGGCTGGAACAACATCGGCATAG | ACCACAGAGCAGGTGATAGCATAGG |
| SERT | CGTCGTCGTGTCTTGGTTCTATGG | GAACAGGAGAAACAGAGGGCTGATG |
| c-kit | GATCTGCTCTGCGTCCTGTTGG | AACTCTGATTGTGCTGGATGGATGG |
| SCF | TGCGGGAATCCTGTGACTGATAATG | CCGGCGACATAGTTGAGGGTTATC |
| VIPR1 | AAGAAGGCTGGTCACAACTGGAAC | AGAACTCAGTCTGTTGCTGCTCATC |
| NOS | GTCAGAAGATGTCCGCACCAAGG | TGTTCACCTCCTCCAGCCTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Li, H.; Ding, Y.; Huo, J.; Zheng, Y.; Jiang, Y.; Zhang, Y.; Man, C. The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota. Nutrients 2023, 15, 839. https://doi.org/10.3390/nu15040839
Cheng S, Li H, Ding Y, Huo J, Zheng Y, Jiang Y, Zhang Y, Man C. The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota. Nutrients. 2023; 15(4):839. https://doi.org/10.3390/nu15040839
Chicago/Turabian StyleCheng, Shasha, Hongxuan Li, Yixin Ding, Jiacheng Huo, Yaping Zheng, Yujun Jiang, Yu Zhang, and Chaoxin Man. 2023. "The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota" Nutrients 15, no. 4: 839. https://doi.org/10.3390/nu15040839
APA StyleCheng, S., Li, H., Ding, Y., Huo, J., Zheng, Y., Jiang, Y., Zhang, Y., & Man, C. (2023). The Probiotic Combination of Lacticaseibacillus paracasei JY062 and Lactobacillus gasseri JM1 Alleviates Gastrointestinal Motility Disorder via Improving Gut Microbiota. Nutrients, 15(4), 839. https://doi.org/10.3390/nu15040839