Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet
Abstract
1. Introduction
2. The Mediterranean Diet, Going over Its History
3. Mediterranean Diet and Kidney Disease
4. The Mediterranean Renal Diet
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Gallieni, M.; Avesani, C.M.; D’Alessandro, C.; Carrero, J.J.; Piccoli, G.B. Medical Nutritional Therapy for Patients with Chronic Kidney Disease not on Dialysis: The Low Protein Diet as a Medication. J. Clin. Med. 2020, 9, 3644. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Gallieni, M.; Cupisti, A. DASH and Mediterranean Diets as Nutritional Interventions for CKD Patients. Am. J. Kidney Dis. 2016, 68, 828–830. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Ajabshir, S.; Alizadeh, S. Dietary Approaches to Stop Hypertension and risk of chronic kidney disease: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2019, 39, 2035–2044. [Google Scholar] [CrossRef]
- Slagman, M.C.J.; Waanders, F.; Hemmelder, M.H.; Woittiez, A.-J.; Janssen, W.M.T.; Heerspink, H.J.L.; Navis, G.; Laverman, G.D.; for the HONEST (Holland Nephrology Study) Group. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 2011, 343, d4366. [Google Scholar] [CrossRef]
- Vogt, L.; Waanders, F.; Boomsma, F.; de Zeeuw, D.; Navis, G. Effects of Dietary Sodium and Hydrochlorothiazide on the Antiproteinuric Efficacy of Losartan. J. Am. Soc. Nephrol. 2008, 19, 999–1007. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Holtkamp, F.A.; Parving, H.-H.; Navis, G.J.; Lewis, J.B.; Ritz, E.; de Graeff, P.A.; de Zeeuw, D. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012, 82, 330–337. [Google Scholar] [CrossRef]
- McMahon, E.J.; Bauer, J.D.; Hawley, C.M.; Isbel, N.M.; Stowasser, M.; Johnson, D.W.; Campbell, K.L. A Randomized Trial of Dietary Sodium Restriction in CKD. J. Am. Soc. Nephrol. 2013, 24, 2096–2103. [Google Scholar] [CrossRef]
- Keys, A.; Aravanis, C.; Blackburn, H.W.; Van Buchem, F.S.P.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N. Epidemiological studies related to coronary heart disease. Characteristics of men aged 40–59 in Seven Countries. Acta. Med. Scand. 1967, 460, 1–392. [Google Scholar] [CrossRef]
- Keys, A.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S. Seven Countries Study. A Multivariate Analysis of Death and Coronary Heart Disease; Harvard Univ Press, Cambridge Mass: Cambridge, MA, USA, 1980; pp. 1–381. [Google Scholar]
- Urquiaga, I.; Echeverría, G.; Dussaillant, C.; Rigotti, A. Origen, componentes y posibles mecanismos de acción de la dieta mediterránea. Rev. Méd. Chile 2017, 145, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.; Echeverría, G.; Villarreal, G.; Martínez, X.; Ferreccio, C.; Rigotti, A. Introducing Plant-Based Mediterranean Diet as a Lifestyle Medicine Approach in Latin America: Opportunities Within the Chilean Context. Front. Nutr. 2021, 8, 680452. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Godos, J.; Platania, A.; Galvano, F.; Mistretta, A.; Grosso, G. Mediterranean diet adherence in the Mediterranean healthy eating, aging and lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2017, 69, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Divella, R.; Daniele, A.; Savino, E.; Paradiso, A. Anticancer Effects of Nutraceuticals in the Mediterranean Diet: An Epigenetic Diet Model. Cancer Genom. Proteom. 2020, 17, 335–350. [Google Scholar] [CrossRef]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2019, 2019, CD009825. [Google Scholar] [CrossRef]
- Kenanoglu, S.; Gokce, N.; Akalin, H.; Ergoren, M.C.; Beccari, T.; Bertelli, M.; Dundar, M. Implication of the Mediterrane-an diet on the human epigenome. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E44–E55. [Google Scholar]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), 1402S–1406S. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- B.Sc, V.V.; B.Sc, A.G.; Dolcetta, E.C. The New Modern Mediterranean Diet Italian Pyramid. Ann. Ig. Med. Prev. E Comunita 2016, 28, 179–186. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.; Pekarek, L.; Castellanos, A.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Pérez-Torres, A.; Caverni-Muñoz, A.; González García, E. Mediterranean Diet and Chronic Kidney Disease (CKD): A Practical Approach. Nutrients 2022, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Cumetti, A.; Pardini, E.; Mannucci, C.; Serio, P.; Morganti, R.; Cupisti, A. Prevalence and correlates of hyperkalemia in a renal nutrition clinic. Intern. Emerg. Med. 2020, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients 2017, 9, 444. [Google Scholar] [CrossRef]
- Guldris, S.C.; Catalá, J.A.L.; Amado, A.S.; Granados, N.M.; Varela, E.P. Fibre Intake in Chronic Kidney Disease: What Fibre Should We Recommend? Nutrients 2022, 14, 4419. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Podadera-Herreros, A.; Alcala-Diaz, J.F.; Gutierrez-Mariscal, F.M.; Jimenez-Torres, J.; de la Cruz-Ares, S.; Larriva, A.P.A.-D.; Cardelo, M.P.; Torres-Peña, J.D.; Luque, R.M.; Ordovas, J.M.; et al. Long-term consumption of a mediterranean diet or a low-fat diet on kidney function in coronary heart disease patients: The CORDIOPREV randomized controlled trial. Clin. Nutr. 2022, 41, 552–559. [Google Scholar] [CrossRef]
- Ajjarapu, A.S.; Hinkle, S.N.; Li, M.; Francis, E.C.; Zhang, C. Dietary Patterns and Renal Health Outcomes in the General Population: A Review Focusing on Prospective Studies. Nutrients 2019, 11, 1877. [Google Scholar] [CrossRef]
- Khatri, M.; Moon, Y.P.; Scarmeas, N.; Gu, Y.; Gardener, H.; Cheung, K.; Wright, C.B.; Sacco, R.L.; Nickolas, T.L.; Elkind, M.S. The Association between a Mediterranean-Style Diet and Kidney Function in the Northern Manhattan Study Cohort. Clin. J. Am. Soc. Nephrol. 2014, 9, 1868–1875. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Qiao, D.; Feng, D.; Wang, H. Vacuum tribological performance of phosphonium-based ionic liquids as lubricants and lubricant additives of multialkylatedcyclopentanes. Tribol. Int. 2013, 66, 289–295. [Google Scholar] [CrossRef]
- Huang, X.; Jiménez-Moleón, J.J.; Lindholm, B.; Cederholm, T.; Ärnlöv, J.; Risérus, U.; Sjögren, P.; Carrero, J.J. Mediterranean Diet, Kidney Function, and Mortality in Men with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Zeimbekis, A.; Kastorini, C.-M.; Stefanadis, C. Adherence to the Mediterranean Diet is Associated with Renal Function Among Healthy Adults: The ATTICA Study. J. Ren. Nutr. 2010, 20, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Aparicio, M.; Bellizzi, V.; Campbell, K.; Hong, X.; Johansson, L.; Kolko, A.; Molina, P.; Sezer, S.; Wanner, C.; et al. Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol. Dial. Transplant. 2018, 33, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Bach, K.E.; Kelly, J.T.; Palmer, S.C.; Khalesi, S.; Strippoli, G.F.M.; Campbell, K.L. Healthy Dietary Patterns and Incidence of CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2019, 7, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.T.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.L.; Strippoli, G.F.M. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin. J. Am. Soc. Nephrol. 2016, 12, 272–279. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Di Iorio, B.; Bottai, A.; Zullo, C.; Giannese, D.; Barsotti, M.; Egidi, M.F. Nutritional support in the tertiary care of patients affected by chronic renal insufficiency: Report of a step-wise, personalized, pragmatic approach. BMC Nephrol. 2016, 17, 124. [Google Scholar] [CrossRef]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Yu, D.; Liu, M. Plant or Animal-Based or PLADO Diets: Which Should Chronic Kidney Disease Patients Choose? J. Ren. Nutr. 2022, 6. [Google Scholar] [CrossRef]
- Cupisti, A.; Morelli, E.; Meola, M.; Barsotti, M.; Barsotti, G. Vegetarian diet alternated with conventional low-protein diet for patients with chronic renal failure. J. Ren. Nutr. 2002, 12, 32–37. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Piccoli, G.B.; Calella, P.; Brunori, G.; Pasticci, F.; Egidi, M.F.; Capizzi, I.; Bellizzi, V.; Cupisti, A. “Dietaly”: Practical issues for the nutritional management of CKD patients in Italy. BMC Nephrol. 2016, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, C.; Guidi, L.; D’Alessandro, C.; Cupisti, A. Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology. Toxins 2022, 14, 668. [Google Scholar] [CrossRef]
- Isakova, T.; Wolf, M.S. FGF23 or PTH: Which comes first in CKD? Kidney Int. 2010, 78, 947–949. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Piccoli, G.B.; Cupisti, A. The “phosphorus pyramid”: A visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Bottai, A.; Bellizzi, V.; Brunori, G.; Cianciaruso, B.; De Nicola, L.; Oldrizzi, L.; Quintaliani, G.; Santoro, D.; Di Iorio, B.R. Characteristics of patients with chronic kidney disease referred to a nephrology outpatient clinic:Results of Ne-frodata study. G Ital Nefrol. 2015, 32, gin/32.2.36. [Google Scholar]
- Aparicio, M.; Cano, N.J.; Cupisti, A.; Ecder, T.; Fouque, D.; Garneata, L.; Liou, H.H.; Lin, S.; Schober-Halstenberg, H.S.; Teplan, V.; et al. Keto-acid therapy in predialysis chronic kidney disease patients: Consensus statements. J. Ren. Nutrn. 2009, 9, S33–S35. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. S1), S1–S107, Erratum in Am. J. Kidney Dis. 2021, 77, 308. [Google Scholar] [CrossRef]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef]
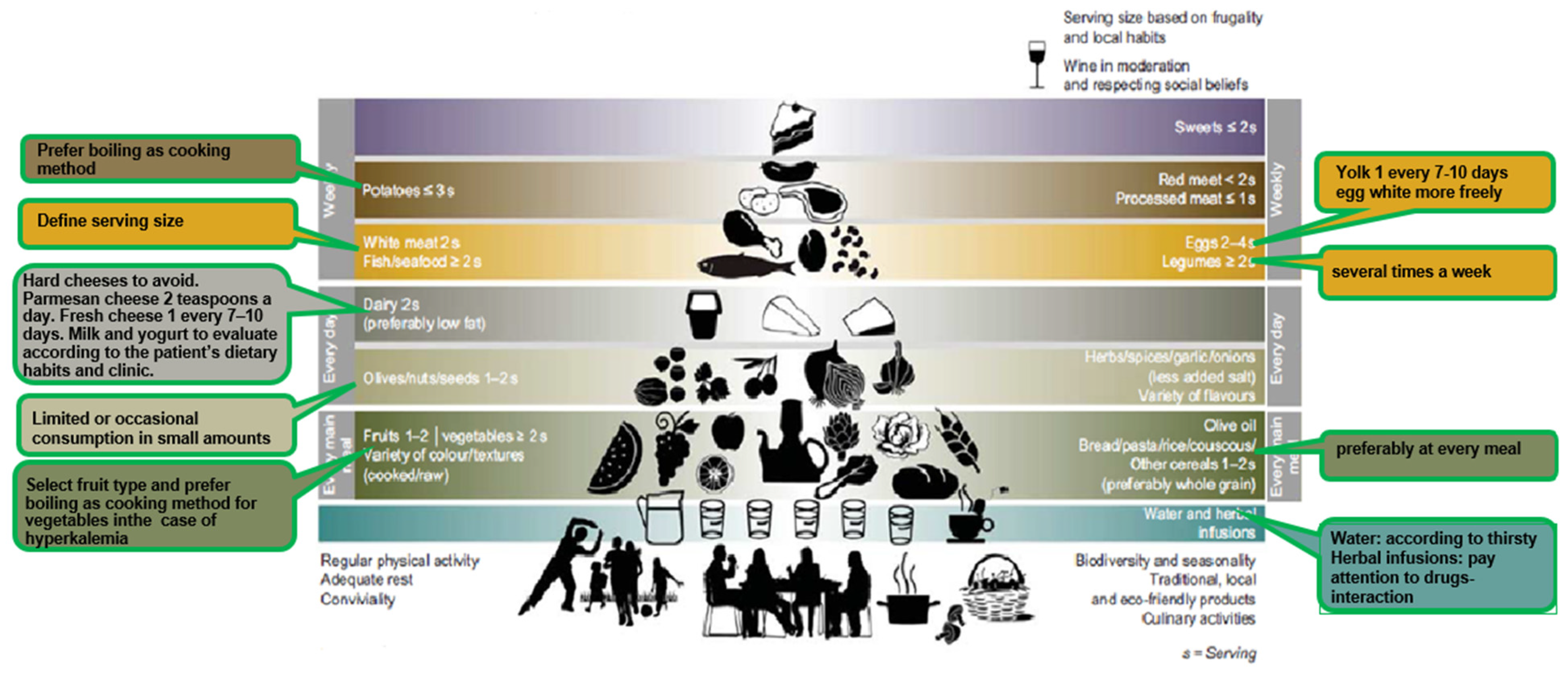
| Mediterranean Diet | Mediterranean Renal Diet | |
|---|---|---|
| Cereals (bread, wheat, corn, pasta, bread, rice, barley, etc.). | 1–2 times a day (preferably whole grain) | Daily use—should be consumed at every meal |
| Olive oil | Every meal | Rich in oleic acid with antioxidant properties. Important source of vegetable fats. Animal fats, like butter or cream, should only be allowed in patients with poor appetite, to increase energy density and to improve food palatability |
| Dairy | 2 times a day (preferably low fat) | Source of animal protein but also rich in phosphate and salt (hard cheeses in particular). Prefer fresh cheese once every 7–10 days and limit/avoid hard cheese. As regards milk and yogurt, dietitians will evaluate their introduction on the basis of patient’s dietary habits and clinic. Consider replacing milk with plant-based drink such as rice, oats or almond drink. Parmesan cheese is a hard cheese rich in phosphorus and salt, so just small amounts over pasta should be allowed |
| Nuts/seeds | 1–2 times a day | Rich in potassium and phosphorus: limited or occasional consumption |
| Red meat White meat | <2 times a week 2 times a week | Protein content of “white” or “red” meat is more or less the same. Pay attention to the serving defined by the dietician and limit its consumption to 1–2 times a week |
| Processed meat | ≤Once a week | Processed meat (sausages, cold cuts) is rich in salt and may potentially contain phosphorus-based preservatives; for these reasons, its consumption should not be recommended |
| Fish and seafood | ≥2 a week | Fish is preferable to meat for the good quality of fats but the serving size should be respected to avoid exceeding protein intake. Fish and seafood can be consumed 2–3 times a week |
| Eggs | 2–4 a week | Yolk is rich in phosphorus; thus, the occasional consumption of whole egg should be considered. Conversely, egg white has very little phosphorus and a large amount of proteins of high biologicalvalue, so more frequent consumption could be suggested |
| Legumes (beans, chickpeas, peas, lentils, etc.). | ≥2 a week | Consumption several times a week should be suggested. Legumes contain protein of good biological value and should be used as a substitute for meat, fish, etc., together with cereals to guarantee the introduction of all essential amino acids. Recommendations are given regarding their preparation, that is to use legumes after boiling and discarding cooking water. |
| Fruits Vegetables | 1–2 a day ≥2 a day | Fruits and vegetables are rich in vitamins, fiber and minerals. Given the high potassium content their consumption may require some precautions (e.g., using boiling as cooking method) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, C.; Giannese, D.; Panichi, V.; Cupisti, A. Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet. Nutrients 2023, 15, 1256. https://doi.org/10.3390/nu15051256
D’Alessandro C, Giannese D, Panichi V, Cupisti A. Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet. Nutrients. 2023; 15(5):1256. https://doi.org/10.3390/nu15051256
Chicago/Turabian StyleD’Alessandro, Claudia, Domenico Giannese, Vincenzo Panichi, and Adamasco Cupisti. 2023. "Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet" Nutrients 15, no. 5: 1256. https://doi.org/10.3390/nu15051256
APA StyleD’Alessandro, C., Giannese, D., Panichi, V., & Cupisti, A. (2023). Mediterranean Dietary Pattern Adjusted for CKD Patients: The MedRen Diet. Nutrients, 15(5), 1256. https://doi.org/10.3390/nu15051256







