The Healthy Eating Assessment Tool (HEAT): A Simplified 10-Point Assessment of CHILD-2 Dietary Compliance for Children and Adolescents with Dyslipidemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Healthy Eating Assessment Tool (HEAT)
2.3. Data Collection
2.4. Dietary Analysis
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
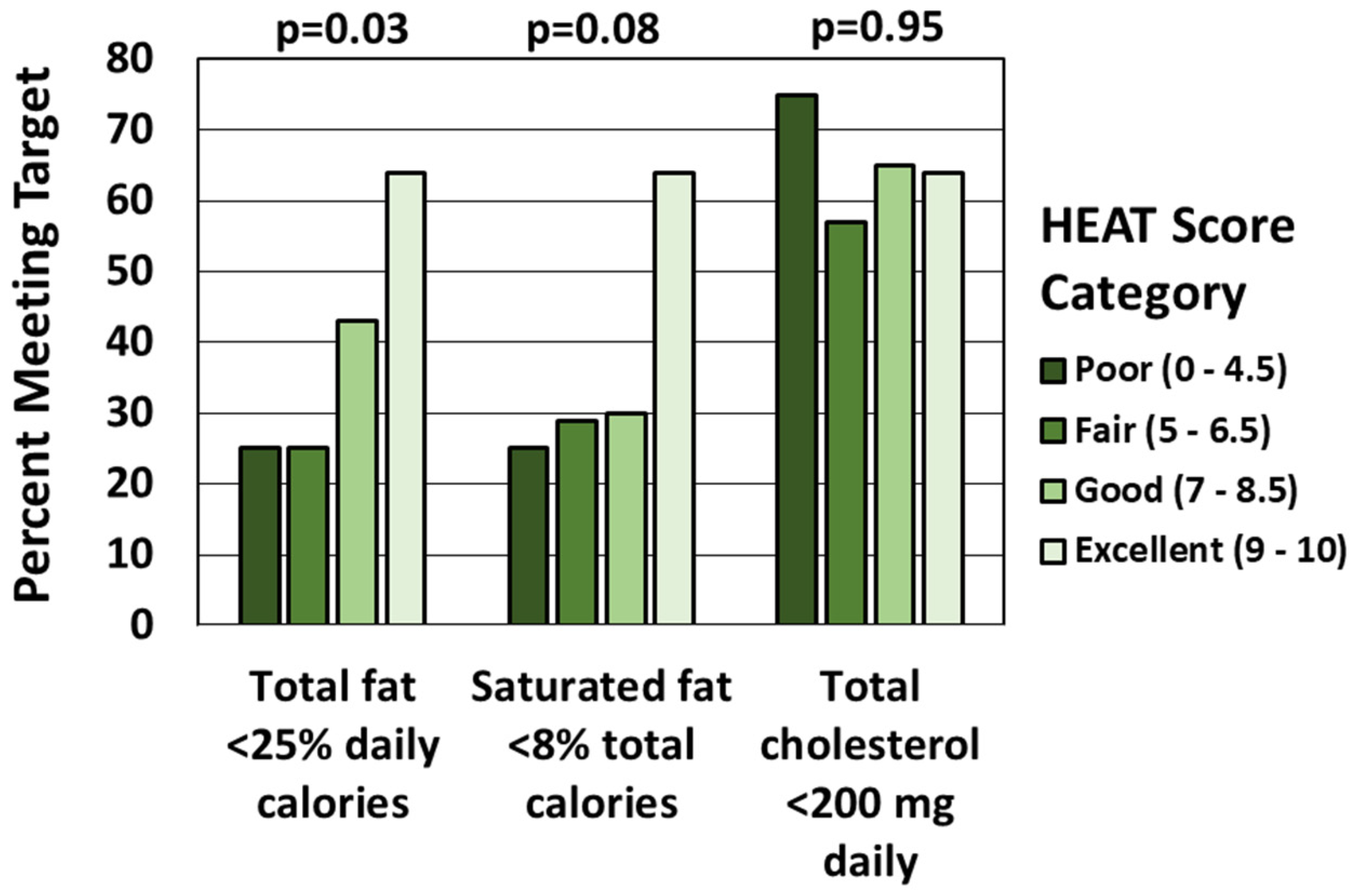
3.2. HEAT Score and Components of CHILD-2
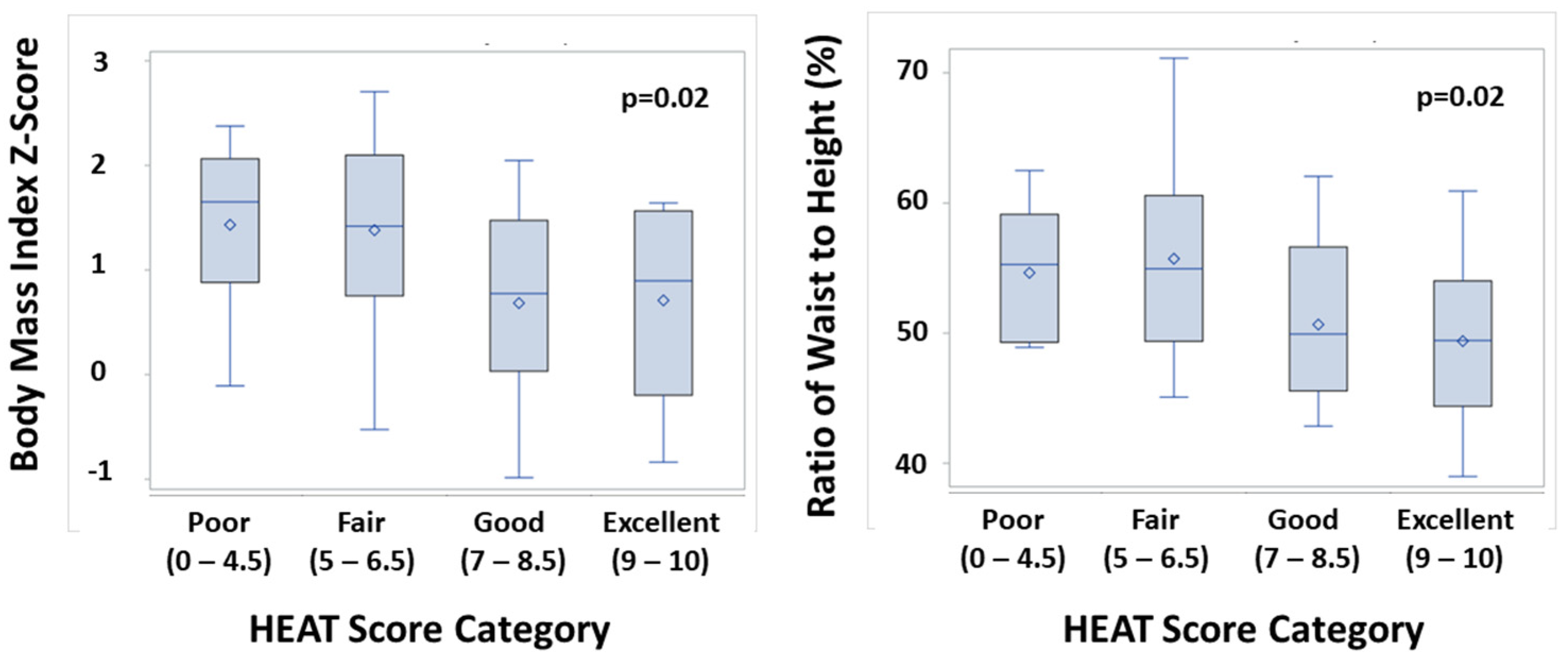
3.3. HEAT Score and Markers of Adiposity
3.4. HEAT Score and Lipid Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
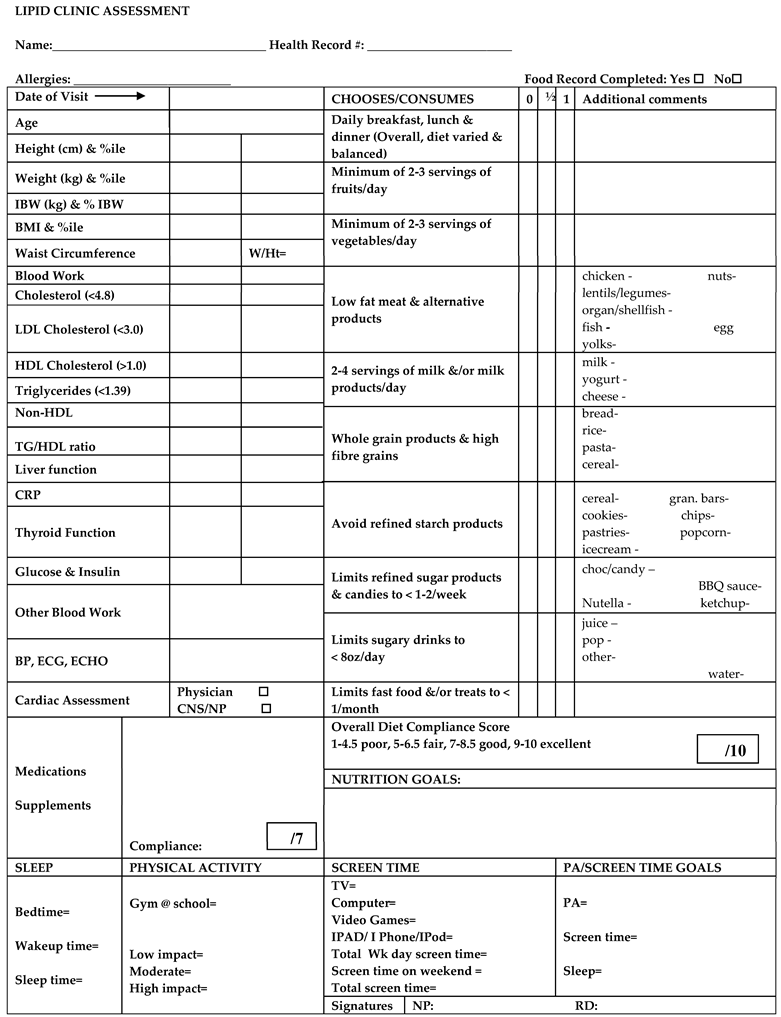
Appendix A. Lipid Clinic Assessment Form with the HEAT Tool Highlighted in Yellow
Appendix B. HEAT Tool Scoring
- Detailed description of how to score the 10-point Healthy Eating Assessment Tool (HEAT) during dietitian-led interview in Lipid Clinic at the Hospital for Sick Children
- During the dietitian led interview, patients and/or their families are asked specific questions on 10 different subcategories of their diet; (1) meal patterns and overall dietary variety and balance, (2) fruit consumption, (3) vegetable consumption, (4) meat and alternatives type and consumption, (5) milk and alternatives type and consumption, (6) grain products type and consumption, (7) refined starch and snack food product consumption, (8) refined sugar products, chocolate and candy consumption, (9) sweetened beverage consumption, and (10) frequency and type of meals/snacks prepared outside of the home (take-out, order-in and restaurant dining).
- Each diet subcategory is given either a score of 1 point, 0.5 points or 0 points depending on the patient’s consumption habits and quality of foods consumed (see below descriptions for detailed information on how points are awarded for each diet subcategory). The total dietary assessment score is tallied from addition of the points awarded for each of the 10 subcategory scores. A total dietary score of 1–4.5 out of 10 indicates a poor score, 5–6.5 indicates a fair score, 7–8.5 indicates a good score, and 9–10 indicates an excellent score.
- Scoring of Diet Subcategories
- (1)
- Meal patterns and overall dietary variety and balanceDoes the patient eat 3 meals a day—breakfast, lunch and dinner, −/+ snacks, all of which are appropriate portion sizes? Does the patient follow a high fibre, low fat, low added sugar diet? Does the patient meet Canada’s Food Guide to Healthy Eating (CFGHE) recommendations for all four food groups? How often does the patient eat foods or drink beverages high in added sugar? How often does the patient eat high fat, high sugar, high sodium foods prepared outside of the home? Rated the same for all ages and sexes.
- (a)
- 1 point—the patient consistently eats 3 meals a day, +/− snacks, all of appropriate portion size. Overall diet is well balanced and patient consistently follows a diet high in fibre, low in saturated and trans fat, and low in added sugar. Patient consistently meets, or comes very close to meeting, all of the CFGHE recommendations for fruit and vegetable intake, low fat milk and alternatives, lean meat and alternatives and whole grains. Patient limits egg yolk consumption to no more than 3 yolks in a week and limits high cholesterol-containing meat products such as organ meats, shrimp and shellfish, to no more than 2 times in a month. Patient is not drinking more than 8 oz a day of sweetened beverages nor eating an excessive amount of refined starch and snack foods, or refined sugar products.
- (b)
- 0.5 points—consistently eats 3 meals a day, +/− snacks, all of appropriate portion sizes. Overall diet is fairly well balanced and patient often follows a diet high in fibre, low in saturated and trans fat, and low in added sugar. Patient may be meeting, or come close to meeting all of the CFGHE recommendations for fruit and vegetable intake, low fat milk and alternatives, lean meat and alternatives and whole grains, but could make some improvements. Patient may or may not be drinking more than 8 oz a day of sweetened beverages or eating an excessive amount of refined starch and snack foods, or refined sugar products.
- (c)
- 0 points—patient misses or skips meal(s) on a regular basis. Portion sizes are inappropriate (too large or too small). Overall diet is not well-balanced and patient does not meet the majority of the CFGHE recommendations for fruit and vegetable intake, low fat milk and alternatives, lean meat and alternatives and whole grains. Patient may or may not be drinking more than 8 oz a day of sweetened beverages or eating an excessive amount of refined starch and snack foods, or refined sugar products.
- (2)
- Fruit consumptionDoes the patient consistently consume fruit on a daily basis? How many CFGHE servings (1 serving = 1 medium sized fruit or 125 mL or ½ cup) does the patient consume daily? Fruit juice of any kind is not considered a fruit serving in Lipid Clinic. Scoring varies per age based on the CFGHE recommendations. Goal is to consume 1/3 to ½ of CFGHE recommendations for vegetables and fruit through fruit daily.
- (a)
- 1 point—patient consistently consumes 1/3 to ½ of their recommended vegetable and fruit servings through fruit on a regular basis (1–2 servings per day for ages 2–3 years, 1.5–2.5 servings per day for ages 4–8 years, 2–3 servings per day for ages 9–13 years, 2.5–3.5 servings for females aged 14–18 years, and 2.5–4 servings for males aged 14–18 years).
- (b)
- 0.5 points—patient consumes less than 1/3 of their recommended vegetable and fruit servings through fruit on a regular basis, but has a minimum of 2 servings per day (ages 4 -18 years), or a minimum of 1 serving per day (ages 2–3 years)
- (c)
- 0 points—patient on average consumes 1 or less servings of fruit per day (ages 4–18 years), or does not consume fruit at all (ages 2–18 years)
- (3)
- Vegetable consumptionDoes the patient consistently consume vegetables on a daily basis? How many CFGHE servings (1 serving = 125 mL or ½ cup) does the patient consume daily? Tomato sauce and/or salsa (1/2 cup serving size) are considered a vegetable serving in Lipid Clinic. Scoring varies per age based on the CFGHE recommendations. Goal is to consume 1/3 to 1/2 of CFGHE recommendations for vegetables and fruit through vegetables.
- (a)
- 1 point—patient consistently consumes 1/3–½ of their recommended vegetable and fruit servings through vegetables on a daily basis (1–2 servings per day for ages 2–3 years, 1.5–2.5 servings per day for ages 4–8 years, 2–3 servings per day for ages 9–13 years, 2.5–3.5 servings for females aged 14–18 years, and 2.5–4 servings for males aged 14–18 years).
- (b)
- 0.5 points—patient consumes less than 1/3 of their recommended vegetable and fruit servings through vegetables on a regular basis, but has a minimum of 2 servings per day (ages 4–18 years), or minimum of 1 serving per day (ages 2–3 years)
- (c)
- 0 points—patient on average consumes 1 or less servings of vegetables per day (ages 4–18 years), or does not consume vegetables at all (ages 2–18 years)
- (4)
- Meat and alternatives type and consumptionHow often does the patient consume lean meat products such as skinless white poultry meat, lean cuts of red meat such as beef, pork, lamb, goat, etc.? How often does the patient consume fatty meats such as pork ribs, ribbed steak, etc., and fatty processed meats such as bacon, bologna, salami, mortadella, hot dogs, sausages, etc.? Which type of cooking method is used most often when preparing meat and alternatives? How often does the patient consume gravies or other high fat sauces? How many egg yolks does the patient consume weekly? How often does the patient consume high cholesterol-containing meat products such as organ meats, shrimp and shellfish? How often does the patient consume fish, especially oily fish high in omega 3 fatty acids such as salmon, trout, char, herring, mackerel, sardines? How often does the patient consume legumes such as baked beans, kidney beans, lentils, chickpeas, etc.,? How often does the patient eat nuts or nut butter products (ideally nuts are unsalted and unflavoured, and nut butters are natural nut butters with no additives such as sugar, oils, sodium or preservatives).
- (a)
- 1 point—patient consistently consumes lean meats and limits consumption of fatty meats and processed meats to no more than 1 time a week. Meat consumed is prepared using low-fat cooking methods such as baking, broiling, grilling, roasting or poaching. Patient limits the use of gravies or other high fat sauces. Patient limits egg yolk consumption to no more than 3 yolks per week and limits high cholesterol-containing meat products such as organ meats, shrimp and shellfish, to no more than 1 to 2 times in a month. Patient may or may not consume fish or legumes. Patient eats nuts or nut products in moderation (limits to ¼ cup/day of nuts or 2 tablespoons/day of nut butters).
- (b)
- 0.5 points—patient consumes both lean meats and fatty meats, but consumes lean meats more often. Patient may eat the skin on poultry and/or consume fatty and/or processed meats 1–2 times a week. Meat consumed is not always prepared using low-fat cooking methods. Patient may or may not use gravies or other high fat sauces. Patient may or may not consume more than 3 egg yolks per week. Patient may or may not consume high cholesterol-containing meat products such as organ meats, shrimp and shellfish, more than 2 times in a month. Patient may or may not consume fish or legumes. Patient may or may not eat nuts or nut products and may or may not limit portion sizes.
- (c)
- 0 points—patient may consume both lean meats and fatty meats, but consumes fatty meats more often. Patient may eat the skin on poultry and/or consume fatty and/or processed meats more than 2 times per week. Meat consumed may often be prepared using high-fat cooking methods, such as pan-frying or deep-frying. Patient may or may not use gravies or other high fat sauces Patient may or may not consume more than 3 egg yolks per week. Patient may or may not consume high cholesterol-containing meat products such as organ meats, shrimp and shellfish, more than 2 times in a month. Patient may or may not consume fish or legumes. Patient may or may not eat nuts or nut products but may or may not limit portion sizes.
- (5)
- Milk and alternatives type and consumptionDoes the patient consistently consume the CFGHE recommended servings of milk and alternatives for their age daily? How many CFGHE servings (1 serving = 1 cup of milk or ¾ cup yogurt, 50 g of cheese) does the patient consume daily? Are milk and alternative products consumed low-fat or fat-free (skim or 1% milk, yogurt < 1.5% milk fat, cheese < 20% milk fat)? How often does the patient consume cheese? Scoring varies per age based on the CFGHE recommendations for age.
- (a)
- 1 point—patient consistently consumes their recommended servings of dairy and alternatives based on CFGHE recommendations for age on a daily basis (2 servings a day for ages 2–8 years, 3 to 4 servings a day for ages 9–18 years). The patient consistently consumes low-fat or non-fat dairy products and patient limits their consumption of cheese to no more than 2 to 3 times per week.
- (b)
- 0.5 points—patient consumes ½ of their recommended servings of dairy and alternatives based on CFGHE recommendations for age on a daily basis (1 serving a day for ages 2–8 years, 1 to 2 servings a day for ages 9–18 years). The patient may consume a combination of low-fat, non-fat and/or regular fat dairy products. Patient may or may not consume cheese more frequently than the recommended 2 to 3 times per week.
- (c)
- 0 points—patient does not consume dairy or alternatives on a daily basis.
- (6)
- Grain products type and consumptionDoes the patient consume whole grain products, or refined grains more often? What type of bread, rice and/or other grains, pasta, cereal, and crackers does the patient consume most often? Are appropriate portion sizes of grain products being consumed?
- (a)
- 1 point—patient consumes whole grain products more often than refined grain products. Patient consumes whole grain bread, brown rice and/or other whole grains such as quinoa, whole wheat couscous, bulgar, barely, oats, etc., whole grain pasta, whole grain low-sugar cereal, and whole grain crackers. Portion sizes are appropriate for meals and/or snacks.
- (b)
- 0.5 points—patient consumes a combination of whole grain and refined grain products, but has whole grain products slightly more often. Bread may be whole grain and/or white, rice may be brown and/or white, other whole grains such as quinoa, whole wheat or regular couscous, bulgar, barely and oats, may or may not be consumed, pasta may be whole grain or regular, cereal may be whole grain or refined grain, and crackers may or may not be whole grain. Portions sizes are appropriate for meals and/or snacks.
- (c)
- 0 points—patient does not consume whole grain products, or does so infrequently. Patient consumes refined grain products such as white bread, white rice, regular pasta, refined grain cereals and refined grain crackers on a regular basis. Portion sizes may or may not be appropriate for meals and/or snacks.
- (7)
- Refined starch and snack food product consumptionWhat type of foods does the patient regularly consume for snacks? How often does the patient consume refined starch products and snack foods that are high in sugar and/or fat such as cookies, granola bars, sugary cereals, ice cream, popsicles, chips, buttered popcorn, and refined grain crackers? Does the patient make healthy choices when choosing snack foods?
- (a)
- 1 point—patient limits high sugar and/or high fat snack foods to 1–2 x week. Patient makes healthy choices when choosing snack foods, and snacks on low sugar, low fat, high fibre foods like fruit and vegetables and whole grain products more often.
- (b)
- 0.5 points—patient consumes high sugar and/or high fat snack foods 2–4 x week but will also consume healthy snack foods that are low in sugar and/or low in fat, and high in fibre.
- (c)
- 0 points—patient consume high sugar and/or high fat snack foods on a regular basis (>2 times a week) and rarely makes healthy choices when choosing snack foods.
- (8)
- Refined sugar products, chocolate and candy consumptionHow often does the patient consume chocolate and candy? How often does the patient consume refined sugar products such as ketchup, barbeque sauce, nutella, chocolate syrup, etc.?
- (a)
- 1 point—patient limits consumption of refined sugar products, chocolate and/or candy to no more than 1 to 2 times per week. Patient may or may not consume refined sugar products, but if does consume, will use in moderation.
- (b)
- 0.5 points—patient consumes chocolate and/or candy or refined sugar products 2 to 3 times in a week, but limits portion sizes.
- (c)
- 0 points –patient consumes chocolate and/or candy or refined sugar products more than 4 times in a week, and may or may not limit portion sizes.
- (9)
- Sweetened beverage consumptionHow often does the patient consume sweetened beverages and naturally sweet beverages, such as fruit juice, fruit punch, ice tea, pop, flavoured milks, flavoured water, specialty coffees and teas, hot chocolate, sports drinks, energy drinks, etc.? Does the patient add sugar to beverages, such as coffee or tea? Does the patient consume artificially sweetened beverages, such as diet pop or Crystal Light? Does the patient consume water on a daily basis?
- (a)
- 1 point—patient may or may not consume sweetened beverages. If patient consumes sweetened beverages, patient limits consumption to under 4 to 6 oz per day (ages 1 to 6 years), and under 8 oz per day (ages 7 to 18 years). Patient regularly consumes water for hydration.
- (b)
- 0.5 points—patient consumes sweetened beverages and often limits to 4 to 6 oz per day (ages 1 to 6 years), and under 8 oz per day (ages 7 to 18 years), but may exceed this volumes on a weekly basis. Patient may or may not consume water for hydration.
- (c)
- 0 points—patient consumes greater than 8 oz of sweetened beverages in a day regardless of age. Patient may or may not consume water for hydration.
- (10)
- Frequency and type of meals/snacks prepared outside of the homeHow frequently does the patient consume foods prepared outside of the home, such as dining at a family style restaurant or fast-food restaurant, ordering take-out/delivery, or ordering foods during school lunchtime (purchases from cafeteria or pizza lunches)? Does the patient make healthy choices and consume appropriate portion sizes when eating foods prepared outside of the home?
- (a)
- 1 point—patient eats foods prepared outside of the home once a month or less. Patient may eat foods prepared outside of the home more than once per month, up to 2 times per month, but makes healthy choices which include low fat, low sugar, high fibre options and consuming appropriate portion sizes.
- (b)
- 0.5 points—patient eats foods prepared outside of the home up to 2 to 4 times per month, but makes healthy choices which include low fat, low sugar, high fibre options and consuming appropriate portion sizes.
- (c)
- 0 points—patient eats food prepared outside of the home regularly, greater than 4 times per month, and rarely makes healthy choices and/or does not control portion sizes.
References
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- McGill, H.C., Jr.; McMahan, C.A.; Zieske, A.W.; Sloop, G.D.; Walcott, J.V.; Troxclair, D.A.; Malcom, G.T.; Tracy, R.E.; Oalmann, M.C.; Strong, J.P.; et al. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Katamay, S.W.; Esslinger, K.A.; Vigneault, M.; Johnston, J.L.; Junkins, B.A.; Robbins, L.G.; Sirois, I.V.; Jones-Mclean, E.M.; Kennedy, A.F.; Bush, M.A.; et al. Eating well with Canada’s Food Guide (2007): Development of the food intake pattern. Nutr. Rev. 2007, 65, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Niinikoski, H.; Viikari, J.; Ronnemaa, T.; Lapinleimu, H.; Jokinen, E.; Salo, P.; Seppanen, R.; Leino, A.; Tuominen, J.; Valimaki, I.; et al. Prospective randomized trial of low-saturated-fat, low-cholesterol diet during the first 3 years of life. The STRIP baby project. Circulation 1996, 94, 1386–1393. [Google Scholar] [CrossRef]
- Kaitosaari, T.; Ronnemaa, T.; Raitakari, O.; Talvia, S.; Kallio, K.; Volanen, I.; Leino, A.; Jokinen, E.; Valimaki, I.; Viikari, J.; et al. Effect of 7-year infancy-onset dietary intervention on serum lipoproteins and lipoprotein subclasses in healthy children in the prospective, randomized Special Turku Coronary Risk Factor Intervention Project for Children (STRIP) study. Circulation 2003, 108, 672–677. [Google Scholar] [CrossRef]
- Lehtovirta, M.; Matthews, L.; Laitinen, T.; Nuotio, J.; Niinikoski, H.; Rovio, S.; Lagström, H.; Viikari, J.; Rönnemaa, T.; Jula, A.; et al. Achievement of the Targets of the 20-Year Infancy-Onset Dietary Intervention-Association with Metabolic Profile from Childhood to Adulthood. Nutrients 2021, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Obarzanek, E.; Kimm, S.Y.S.; Barton, B.A.; Van Horn, L.; Kwiterovich, P.O.; Simons-Morton, D.G.; Hunsberger, S.A.; Lasser, N.L.; Robson, A.M.; Franklin, F.A.; et al. Long-term safety and efficacy of a cholesterol-lowering diet in children with elevated low-density lipoprotein cholesterol: Seven-year results of the Dietary Intervention Study in Children (DISC). Pediatrics 2001, 107, 256–264. [Google Scholar] [CrossRef]
- Lehtovirta, M.; Pahkala, K.; Niinikoski, H.; Kangas, A.; Soininen, P.; Lagström, H.; Viikari, J.S.; Rönnemaa, T.; Jula, A.; Ala-Korpela, M.; et al. Effect of Dietary Counseling on a Comprehensive Metabolic Profile from Childhood to Adulthood. J. Pediatr. 2018, 195, 190–198.e3. [Google Scholar] [CrossRef] [PubMed]
- Lauer, R.M.; Obarzanek, E.; Hunsberger, A.S.; Van Horn, L.; Hartmuller, V.W.; Barton, A.B.; Stevens, V.J.; Kwiterovich, O.P.; Franklin, A.F.; Kimm, S.Y.; et al. Efficacy and safety of lowering dietary intake of total fat, saturated fat, and cholesterol in children with elevated LDL cholesterol: The Dietary Intervention Study in Children. Am. J. Clin. Nutr. 2000, 72 (Suppl. 5), 1332S–1342S. [Google Scholar] [CrossRef] [PubMed]
- The Dietary Intervention Study in Children (DISC). The Writing Group for the DISC Collaborative Research Group. Efficacy and safety of lowering dietary intake of fat and cholesterol in children with elevated low-density lipoprotein cholesterol. JAMA 1995, 273, 1429–1435. [Google Scholar] [CrossRef] [PubMed]

| Dietary Components of the Cardiovascular Health Integrated Lifestyle Diet (CHILD)-2 |
|---|
| (1) Total fat 25–30% of daily kcal intake |
| (2) Saturated fat ≤ 7% daily kcal intake |
| (3) Avoid trans fat |
| (4) Monounsaturated fat ~10% daily kcal intake |
| (5) Cholesterol < 200 mg/day (6) Reduce sugar 1 (7) Replace simple carbohydrates with complex carbohydrates 1 (8) Avoid sugar-sweetened beverages 1 (9) Increase dietary fish to increase omega-3 fatty acid intake 1 |
| Mean (±SD) or Total (%) | |
|---|---|
| Age (years) | 12.6 (±3.8) |
| Female gender | 29 (41%) |
| HEAT score | 6.7 (±2.0) |
| Poor (score 0–4.5) | 8 (11%) |
| Fair (score 5–6.5) | 28 (40%) |
| Good (score 7–8.5) | 23 (33%) |
| Excellent (score 9–10) | 11 (16%) |
| BMI (kg/m2) | 23.5 (±5.9) |
| BMI z-score | +1.05 (±0.92) |
| BMI percentile (%) | 78 (±24) |
| Waist-to-height ratio (%) | 53 (±7) |
| Fasting blood glucose (mmol/L) | 5.1 (±1.9) |
| Fasting lipid biomarkers | |
| Total cholesterol (mmol/L) | 5.08 (±1.26) |
| HDL-C (mmol/L) | 1.21 (±0.30) |
| LDL-C (mmol/L) | 3.29 (±1.22) |
| Triglycerides (mmol/L) Non-HDL-C (mmol/L) | 1.34 (±0.94) 3.87 (±1.22) |
| Average Components of CHILD-2 | Mean (SD) | Pearson’s Correlation with HEAT Score (r) | p-Value |
|---|---|---|---|
| Total fat percent of total daily calories (%) | 29.2 (±11.8) | −0.27 | 0.02 |
| Saturated fat percent of total daily calories (%) | 9.9 (±5.0) | −0.17 | 0.14 |
| Trans fat percent of total daily calories (%) | 1.0 (±1.4) | −0.09 | 0.44 |
| Total dietary cholesterol (mg) | 218 (±182) | −0.03 | 0.78 |
| Dietary fiber intake | |||
| Average daily dietary fiber (g) | 18.6 (±9.3) | 0.46 | <0.01 |
| Average daily vegetables intake (servings) | 1.8 (±2.4) | 0.32 | <0.01 |
| Average daily fruit intake (servings) | 1.7 (±1.8) | 0.31 | <0.01 |
| Mono and polyunsaturated fats | |||
| Monounsaturated fat percent of total daily calories (%) | 6.9 (±4.6) | −0.18 | 0.13 |
| Polyunsaturated fat percent of total daily calories (%) | 3.3 (±2.2) | −0.19 | 0.10 |
| Weekly total of moderate-to-vigorous physical activity (hours) | 8.2 (±5.4) | 0.01 | 0.90 |
| Low Density Lipoprotein-Cholesterol (LDL-C, mmol/L; model R2 = 0.33): | ||
| PE (SE) | p value | |
| Intercept | 7.14 (2.26) | |
| HEAT score | −0.065 (0.078) | 0.42 |
| Age (years) | −0.200 (0.043) | <0.001 |
| Females | 0.268 (0.302) | 0.38 |
| Body mass index Z-score | 0.279 (0.339) | 0.42 |
| Waist to height ratio as percent | −0.023 (0.044) | 0.61 |
| Weekly moderate-vigorous physical activity (hours) | −0.015 (0.026) | 0.58 |
| Weekly screen time (hours) | 0.009 (0.011) | 0.42 |
| Taking lipid lowering medication | −0.286 (0.284) | 0.32 |
| High Density Lipoprotein-Cholesterol (HDL-C, mmol/L; model R2 = 0.36): | ||
| PE (SE) | p value | |
| Intercept | 1.930 (0.552) | |
| HEAT score | −0.023 (0.018) | 0.22 |
| Age (years) | −0.012 (0.010) | 0.27 |
| Females | −0.027 (0.073) | 0.72 |
| Body mass index Z-score | −0.164 (0.083) | 0.06 |
| Waist to height ratio as percent | −0.002 (0.011) | 0.84 |
| Weekly moderate-vigorous physical activity (hours) | −0.005 (0.006) | 0.41 |
| Weekly screen time (hours) | 0.001 (0.003) | 0.69 |
| Taking lipid lowering medication | −0.177 (0.069) | 0.02 |
| Non-High Density Lipoprotein-Cholesterol (Non-HDL-C, mmol/L; model R2 = 0.33): | ||
| PE (SE) | p value | |
| Intercept | 6.491 (2.271) | |
| HEAT score | −0.069 (0.075) | 0.37 |
| Age (years) | −0.199 (0.043) | <0.001 |
| Females | 0.166 (0.302) | 0.59 |
| Body mass index Z-score | 0.246 (0.342) | 0.48 |
| Waist to height ratio as percent | 0.000 (0.045) | 0.99 |
| Weekly moderate-vigorous physical activity (hours) | −0.026 (0.026) | 0.32 |
| Weekly screen time (hours) | 0.016 (0.011) | 0.15 |
| Taking lipid lowering medication | −0.228 (0.283) | 0.43 |
| Triglycerides (mmol/L; model R2 = 0.28): | ||
| PE (SE) | p value | |
| Intercept | −0.242 (1.801) | |
| HEAT score | −0.078 (0.060) | 0.20 |
| Age (years) | −0.006 (0.034) | 0.86 |
| Females | 0.026 (0.240) | 0.92 |
| Body mass index Z-score | −0.057 (0.271) | 0.84 |
| Waist to height ratio as percent | 0.037 (0.035) | 0.31 |
| Weekly moderate-vigorous physical activity (hours) | −0.021 (0.020) | 0.30 |
| Weekly screen time (hours) | 0.024 (0.009) | 0.01 |
| Taking lipid lowering medication | −0.049 (0.225) | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiLauro, S.; Wong, J.P.; Collins, T.; Chahal, N.; McCrindle, B.W. The Healthy Eating Assessment Tool (HEAT): A Simplified 10-Point Assessment of CHILD-2 Dietary Compliance for Children and Adolescents with Dyslipidemia. Nutrients 2023, 15, 1062. https://doi.org/10.3390/nu15041062
DiLauro S, Wong JP, Collins T, Chahal N, McCrindle BW. The Healthy Eating Assessment Tool (HEAT): A Simplified 10-Point Assessment of CHILD-2 Dietary Compliance for Children and Adolescents with Dyslipidemia. Nutrients. 2023; 15(4):1062. https://doi.org/10.3390/nu15041062
Chicago/Turabian StyleDiLauro, Sara, Jonathan P. Wong, Tanveer Collins, Nita Chahal, and Brian W. McCrindle. 2023. "The Healthy Eating Assessment Tool (HEAT): A Simplified 10-Point Assessment of CHILD-2 Dietary Compliance for Children and Adolescents with Dyslipidemia" Nutrients 15, no. 4: 1062. https://doi.org/10.3390/nu15041062
APA StyleDiLauro, S., Wong, J. P., Collins, T., Chahal, N., & McCrindle, B. W. (2023). The Healthy Eating Assessment Tool (HEAT): A Simplified 10-Point Assessment of CHILD-2 Dietary Compliance for Children and Adolescents with Dyslipidemia. Nutrients, 15(4), 1062. https://doi.org/10.3390/nu15041062









