COVID-19 in Pregnancy: Influence of Body Weight and Nutritional Status on Maternal and Pregnancy Outcomes—A Review of Literature and Meta-Analysis
Abstract
:1. Introduction
2. Methods
Ethics Approval
3. Results
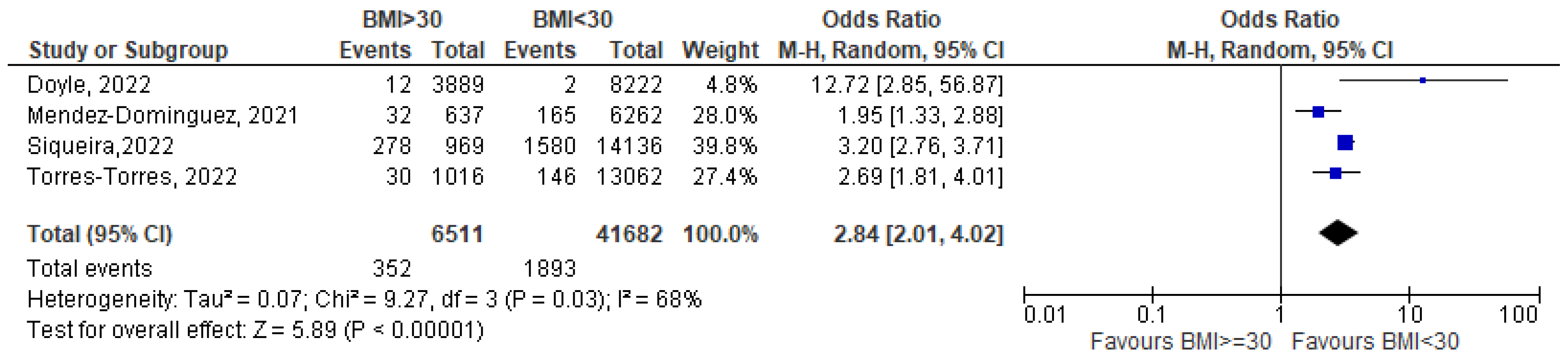
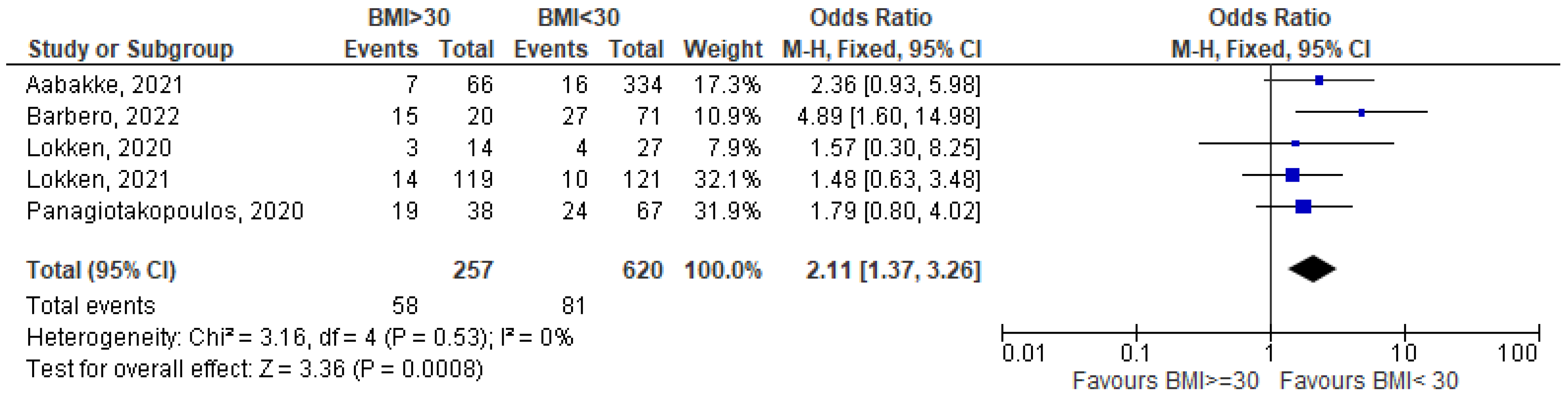
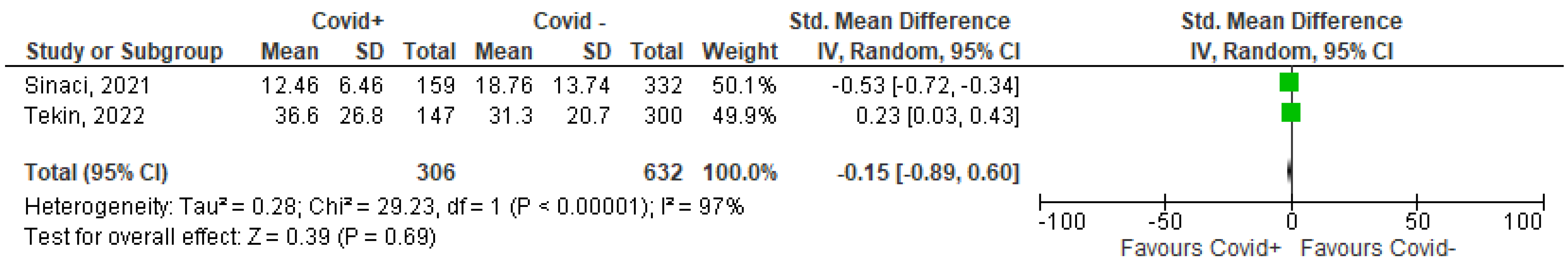
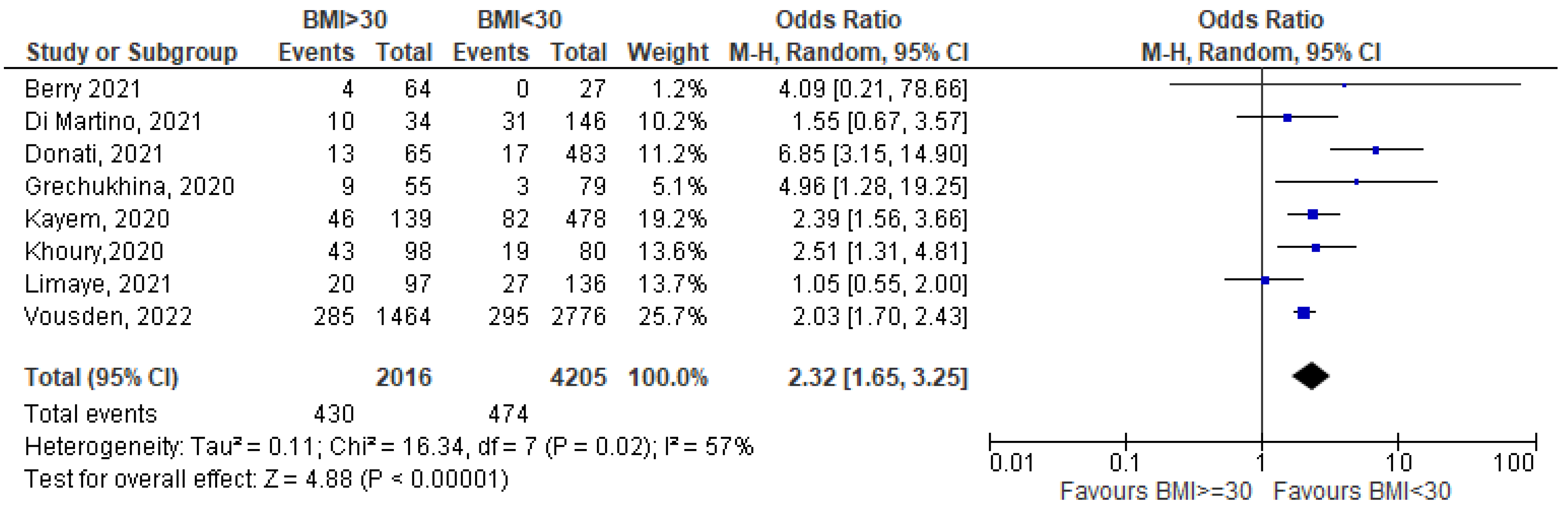
3.1. Meta-Analysis
3.2. Narrative Review
| First Author | Country | Objective(s) | Women with COVID-19 | Definition of Disease Severity | Disease Severity Status | Obese/Overweight Women | Mortality in Obese Women | Obstetric Outcome in Obese Women | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Results | Comments as Reported in the Paper | ||||||||||
| 1 | Babic 2022 [46] | Saudi Arabia | To analyze the relationship between BMI and symptoms | 209 | Symptomatic: fever, cough, dyspnea, headache, sore throat, diarrhea, anosmia and/or ageusia, nausea, vomit, dizziness, rhinorrhea, myalgia | 62 symptomatic | 53 overweight 112 obese | BMI and symptoms P = 0.973 for overweight P = 0.985 for obesity | Overweight or obese women had no higher incidence of symptoms | ||
| 2 | Donati 2022 [47] | Italy | To describe COVID-19 infection among pregnant women and the impact of virus variants on the severity of maternal and perinatal outcomes | 3306 | COVID-19 pneumonia | 424 COVID-19 pneumonia | 427 obese | Not reported | Not reported | Pneumonia and obesity OR 1.72 (1.29–2.27) | Obesity was associated with a higher occurrence of pneumonia due to COVID-19 |
| 3 | Galang 2021 [48] | USA | To determine risk factors for illness severity among pregnant women with COVID-19 | 7950 | Critical: mechanical ventilation/intubation, ECMO, ICU admission, ARDS, respiratory failure, septic shock, MOF, COVID-19 listed as cause of death Moderate-to-severe: not meeting criteria for critical; presence of dyspnea/shortness of breath and at least one among fever or cough Mild: symptomatic not meeting criteria of critical or moderate-to-severe | 512 with moderate-to severe or critical illness | 1974 obese | Not reported | Moderate to severe or critical disease and death RR 1.36 (1.23–1.51) | Pre-pregnancy obesity associated with moderate-to-severe or critical COVID-19 disease | |
| 4 | Eskenazi 2022 [49] | Multinational | To determine whether diabetes mellitus and high BMI are risk factors for COVID-19 in pregnancy | 672 | Symptomatic: any among chest pain, diarrhea or vomiting, limb or joint pain, sore throat, flu-like symptoms, runny nose, breathlessness, headache, tiredness or lethargy, loss of smell, fever, cough | 400 symptomatic | 328 with BMI > 28 208/328 symptomatic | Not reported | Not reported | BMI >28 and symptomatic disease: RR 1.6 (1.01–1.11) | Women overweight or obese more likely to develop symptomatic COVID-19 |
| 5 | Menezes 2020 [50] | Brazil | To evaluate clinical and social risk factors associated with negative outcomes of COVID-19 disease in pregnancy | 2475 | Adverse composite outcome: critical disease leading to death or admission to ICU or mechanical ventilation | 590 adverse outcome | 116 obese; 48/116 with adverse composite outcomes | Included in adverse composite outcome | Not reported | Obesity and adverse composite outcome: OR 2.124 (1.381–3.268) p = 0.0006 | Obesity associated with increased risk of adverse composite outcome |
| 6 | Overtoom 2022 [51] | Netherlands | To describe characteristics, risk factors and maternal, obstetric and neonatal outcomes of pregnant women with COVID-19 | 376 | Need for hospitalization | 74 hospitalized for COVID-19 6/62 admitted to ICU | 100/376 overweight, 67/376 obese 25/74 hospitalized were overweight, 19/74 hospitalized were obese | Not reported | Not reported | Severe illness and BMI > 28 OR 1.86 (1.51–3.20) | Having BMI >28 is a risk factor in severe COVID-19 (need for hospitalization) |
| 7 | Péju 2022 [52] | France | To assess the ventilatory management of pregnant women with COVID-19 admitted to the ICU and report on maternal and neonatal outcomes | 187 | Intubation | 114 intubated 73 non intubated | 76 obese 35/76 intubated 41/76 non intubated | Not reported | Not reported | Obesity and need for intubation: cause-specific hazard ratio (CSH) 2.00, 95% CI (1.05–3.80), p = 0.03 | Obesity associated with higher risk of intubation |
| 8 | Peter 2022 [53] | USA | To investigate the impact of maternal characteristics upon COVID-19 outcome, as well as whether disease severity impacted pregnancy outcomes | 34 | Symptomatic: fever, cough, myalgia, anosmia, congestion, headache, chills, dyspnea, nausea, vomiting, malaise | 19 symptomatic | 5 obese, all symptomatic | Not reported | Not reported | BMI of symptomatic vs. asymptomatic: 35.71 vs. 26.79, P = 0.004; | High BMI is associated with symptomatic COVID-19 |
| 9 | Prasannan 2021 [54] | USA | To determine social determinants of health associated with severe acute respiratory syndrome due to COVID-19 | 544 | Mild disease: no shortness of breath, dyspnea, or abnormal chest imaging Moderate disease: lower respiratory disease and oxygen saturation of ≥94% on room air Severe disease: oxygen saturation <94% on room air, ratio of arterial oxygen partial pressure to fraction of inspired oxygen < 300 mm Hg, respiratory frequency >30 breaths/min, or lung infiltrates >50% Critical disease: respiratory failure, septic shock, or MOF | 115/544 with mild or moderate disease 70/544 with severe disease | 283/544 obese | Not reported | Not reported | Mean BMI: 32.7 (women with severe to critical) vs. 30.9 (asymptomatic; P < 0.04 | BMI associated with disease severity |
| 10 | Sakowicz 2020 [55] | USA | To compare clinical characteristics of pregnant women with and without severe acute COVID-19 disease | 101 | Symptomatic: fever, shortness of breath, cough, sore throat, body aches, chills, vomiting, diarrhea, loss of taste or smell, red or painful eyes | 77/101 symptomatic | 35/101 obese 19/35 symptomatic | Not reported | Not reported | Obesity and COVID-19 positivity P = 0.002 | Women positive for COVID-19 were more likely to be obese |
| Obesity and severity of symptoms P = 0.95 | No significant differences between women with and without symptoms as regards BMI and obesity | ||||||||||
| 11 | Savasi 2020 [56] | Italy | To investigate the clinical evolution of COVID-19 disease in hospitalized pregnant women and factors associated with severe maternal outcome | 77 | Severe: urgent delivery based on maternal respiratory function or ICU or sub intensive care admission or both | 14/77 with severe disease; 6/77 admitted to ICU | 7/14 with severe disease were obese | Not reported | Not reported | BMI non severe vs. severe: 30 (19.4–54.1) vs. 22.8 (17.5–54.1) p = 0.02 | High BMI associated with severe disease |
| 12 | Souza 2022 [57] | Brazil | To evaluate the effect of COVID-19 infection on obstetrical outcomes | 289 | SARS (severe acute respiratory syndrome) | 47 SARS 241 no SARS | 68 overweight (10/68 SARS) 72 obese (16/72 SARS) | Not reported | Not reported | RR of SARS 4.34 (1.04–19.01) for overweight, 6.55 (1.57–27.37) for obesity | Being overweight or obese is associated with higher risk of SARS |
| 13 | Torres-Torres 2022 [26] | Mexico | To evaluate the association of comorbidities and socioeconomic determinants with COVID-19-related mortality and severe disease in pregnant women in Mexico | 13,062 | Severe pneumonia: American Thoracic Society criteria ICU admission Intubation | 176 deaths due to COVID-19 322 were admitted to ICU 1191 were diagnosed with severe pneumonia 185 were intubated | 1016 obese; 30/176 deaths were obese | Reported | Not reported | Pneumonia RR 1.35 (1.14–1.59), p < 0.001 | Obesity is a risk factor for severe COVID-19 pneumonia |
| Intubation RR 1.37 (0.92–2.04) P = 0.122 | Obesity is not a risk factor for intubation | ||||||||||
| Severity of COVID-19 and BMI P = 0.17 | BMI is not associated with severity of COVID-19 disease | ||||||||||
| 14 | Vimercati 2022 [36] | Italy | To evaluate the maternal and perinatal outcomes of COVID-19 infection during pregnancy | 122 | Mild symptoms: requiring non-invasive respiratory support Severe symptoms: requiring ICU admission | 47 symptomatic 23/47 with mild to severe symptoms | 69 overweight, 25 obese | Not reported | Symptoms: OR 1.66 (1.19–2.31) p < 0.001 for overweight OR 1.72 (1.22–2.41) p < 0.001 for obese | Symptomatic women more frequent among overweight or obese | |
| 15 | Vousden 2021 [58] | UK | To compare incidence, characteristics, and outcomes of hospitalized pregnant women with symptomatic and asymptomatic COVID-19 vs. pregnant women without COVID-19 | 1148 | Symptomatic: any among fever, cough, sore throat, breathlessness, headache, fatigue, limb or joint pain, vomit, rhinorrhea,, diarrhea, anosmia, pneumonia | 722 symptomatic 63/722 required critical care 8/722 died | 237 overweight 235 obese | Not reported | Not reported | Symptoms: OR 1.66 (1.19–2.31) p < 0.001 for overweight OR 1.72 (1.22–2.41) p < 0.001 for obese | Symptomatic women more frequent among overweight or obese |
| First Author | Country | Objective(s) | Women with COVID-19 | Definition of Disease Severity | Disease Severity Status | Obese/Overweight Women | Mortality in Obese Women | Obstetric Outcome in Obese Women | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Results | Comments as Reported in the Paper | ||||||||||
| 1 | Galang 2021 [45] | USA | To determine risk factors for illness severity among pregnant women with COVID-19 | 7950 | Critical: mechanical ventilation/intubation, ECMO, ICU admission, ARDS, respiratory failure, septic shock, MOF, COVID-19 listed as cause of death Moderate-to-severe: not meeting criteria for critical; presence of dyspnea/shortness of breath and at least one among fever or cough Mild: symptomatic not meeting criteria of critical or moderate-to-severe | 512 with moderate-to severe or critical illness | 1974 obese | Not reported | Moderate to severe or critical disease and death RR 1.36 (1.23–1.51) | Pre-pregnancy obesity associated with moderate-to-severe or critical COVID-19 disease | |
| 2 | Leal 2021 [31] | Brazil | To analyze maternal morbidity and mortality due to severe acute respiratory infections, including COVID-19 | 5469 | Not reported | 362/5469 died | 264 obese: 44/264 died | 16.6% | Not reported | Obesity among women who died 12.1% vs. 4.4% | In women with COVID-19, obesity was more common among those who died than among survivors |
| 3 | Takemoto 2020 [34] | Brazil | To describe clinical characteristics of pregnant women with severe COVID-19 and to examine risk factors for mortality | 978 | Not reported | 978 symptomatic | 43 obese | Not reported | OR = 2.31; 95% (CI 1.10–4.84) for obesity as a risk factor for maternal death | Obesity was one of the main risk factors for maternal death by COVID-19 | |
| First Author | Country | Objective(s) | Women with COVID-19 | Definition of Disease Severity | Disease Severity Status | Obese/Overweight Women | Mortality in Obese Women | Obstetric Outcome in Obese Women | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Results | Comments as Reported in the Paper | ||||||||||
| 1 | Budhram 2021 [59] | South Africa | To describe the risk factors and outcomes of pregnant women infected with COVID-19 | 673 | Hospitalized for COVID-19 | 217 admitted to hospital for COVID-19 106 requiring critical medical care; 32 deaths | 108 overweight 253 obese | 14.7% | Not reported | BMI and hospital admission P = 0.16 | BMI is not a risk factor for admission to hospital due to COVID-19 |
| 2 | Doyle 2022 [23] | USA | To estimate the risk of COVID-19 infection in pregnancy and adverse maternal and perinatal outcomes | 12,976 | Need for ICU | Need for ICU 48/12,976 | 3455 overweight, 2079 Class I obesity 1048 Class II obesity 762 Class III obesity 12/14 maternal deaths involved obese patients | Not reported | Obesity and adverse composite outcome: OR 2.124 (1.381–3.268) p = 0.0006 | Obesity associated with increased risk of adverse composite outcome | |
| Obesity and ICU admission: OR 1.910 (1.227–2.974), p = 0.0041 | Obesity associated with increased risk of ICU admission | ||||||||||
| 3 | Mendez-Dominguez 2021 [24] | Mexico | To analyze the clinical course of pregnant women hospitalized for COVID-19 disease | 42,525 | Pneumonia Need for ICU | 7064 hospitalized 1586 pneumonia 254 needed ICU 197/7064 died | 637 obese 32/637 died | 5% | Not reported | Admission to ICU and obesity OR 1.17 (0.75–1.81) p = 0.01 | Obese COVID-19 patients -choose were significantly more prone/likely -choose to be admitted to the ICU |
| ICU admission: Overweight, 1.15 (.87–1.59) Obesity class 1, 1.16 (.79–1.70) Obesity class 2, 1.27 (.79–2.04) Obesity Class 3, 2.30 (1.49–3.55) | Risk of ICU admission increased with increasing levels of pre-pregnancy obesity | ||||||||||
| 4 | Menezes 2020 [50] | Brazil | To evaluate clinical and social risk factors associated with negative outcomes of COVID-19 disease in pregnancy | 2475 | Adverse composite outcome: critical disease leading to death or admission to ICU or mechanical ventilation | 590 adverse outcome | 116 obese; 48/116 with adverse composite outcomes | Included in adverse composite outcome | Not reported | Obesity and need for intubation: cause-specific hazard ratio (CSH) 2.00, 95% CI (1.05–3.80), p = 0.03 | Obesity associated with higher risk of intubation |
| 5 | Péju 2022 [52] | France | To assess the ventilatory management of pregnant women with COVID-19 admitted to the ICU and report on maternal and neonatal outcomes | 187 | Intubation | 114 intubated 73 non intubated | 76 obese 35/76 intubated 41/76 non intubated | Not reported | Not reported | ICU admission RR 1.17 (0.85–1.61), p = 0.321 | Obesity is not a risk factor for ICU admission |
| 6 | Torres-Torres 2022 [26] | Mexico | To evaluate the association of comorbidities and socioeconomic determinants with COVID-19-related mortality and severe disease in pregnant women in Mexico | 13,062 | Severe pneumonia: American Thoracic Society criteria ICU admission Intubation | 176 deaths due to COVID-19 322 were admitted to ICU 1191 were diagnosed with severe pneumonia 185 were intubated | 1016 obese; 30/176 deaths were obese | Reported | Not reported | Intubation RR 1.37 (0.92–2.04) P = 0.122 | Obesity is not a risk factor for intubation |
| ICU admission RR 1.17 (0.85–1.61), p = 0.321 | Obesity is not a risk factor for ICU admission | ||||||||||
| First Author | Country | Objective(s) | Women with COVID-19 | Definition of Disease Severity | Disease Severity Status | Obese/Overweight Women | Mortality in Obese Women | Obstetric Outcome in Obese Women | Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Results | Comments as Reported in the Paper | ||||||||||
| 1 | Stenton 2022 [37] | UK | To assess pregnancy outcomes of patients with COVID-19 placentitis | 59 mothers, 61 newborns 47/59 positive at the time of labor | Placenta with positive immunohistochemical staining for COVID-19 spike protein in the syncytiotrophoblast | 59/59 with placentitis | 15/59 obese | Not reported | Pregnancy loss (miscarriage or stillbirth) | Pregnancy loss 67% (10/15) in obese versus 41% (14/34) In non-obese | Obesity associated with pregnancy loss |
| 2 | Vimercati 2022 [36] | Italy | To evaluate the maternal and perinatal outcomes of COVID-19 infection during pregnancy | 122 | Mild symptoms: requiring non-invasive respiratory support Severe symptoms: requiring ICU admission | 47 symptomatic 23/47 with mild to severe symptoms | 69 overweight, 25 obese | Not reported | Preterm birth and BMI P = 0.03 | High BMI associated with preterm birth | |
| First Author | Country | Objective | Population | Results | Details | Additional Comments | |
|---|---|---|---|---|---|---|---|
| 1 | Anuk 2020 [39] | Turkey | To evaluate the status of zinc, copper and magnesium in pregnant women diagnosed with COVID-19 infection | 100 COVID-19 positive 100 COVID-19 negative | p: 0.018 | Disease severity correlation with zinc/copper ratio in COVID-19 + | In the first and third trimesters serum zinc levels were lower, serum copper levels were higher, the Zn / Cu ratio decreased and serum magnesium levels were higher in the COVID-19 positive group In the second trimester COVID-19 patients had lower serum zinc and copper levels compared to negative controls Zn/Cu ratio showed correlation with inflammatory and acute phase markers including IL-6, CRP, ESR, procalcitonin |
| p < 0.0001 | Serum Magnesium level significantly higher in COVID-19 + | ||||||
| p: 0.004 | Serum zinc levels significantly lower in COVID-19 + | ||||||
| p: 0.006 | Serum copper levels higher in COVID-19 + | ||||||
| p: 0.0004 | In the second trimester copper levels decreased in COVID-19 + | ||||||
| p: 0.05 | In the second trimester serum zinc levels were lower in COVID-19 + | ||||||
| p: 0.07 | Disease severity correlated with serum zinc levels | ||||||
| 3 | Bahat 2020 [40] | Turkey | To measure serum Vit D, Vit B12, and zinc levels in COVID-19 positive pregnant women | 44 COVID-19 positive women | Mean serum Vit D, zinc, and Vit B12 levels p < 0.01 | Mean serum levels of Vit D, zinc and Vit B12 were significantly lower than the accepted cut-off values | Patients with low serum levels of Vit D, zinc and Vit B12 may be more susceptible to COVID-19 infection |
| 4 | Erol 2021 [43] | Turkey | To evaluate the maternal serum afamin and vitamin E levels in pregnant women with COVID-19 and to investigate their association with composite adverse perinatal outcomes | 60 COVID-19 positive 36 COVID-19 negative | p < 0.001, p < 0.001, and p = 0.004, respectively | Vitamin E levels were lower in COVID-19 + in all trimesters | Afamin levels were higher and vitamin E levels were lower in COVID-19 + pregnant women. This may support elevated oxidative stress and be related to composite adverse perinatal outcomes |
| p > 0.05 | Afamin levels were higher in COVID-19 + in all trimesters without reaching statistical significance | ||||||
| r = 0.264 | Positive significant correlation between afamin and C-reactive Protein levels | ||||||
| 5 | Erol 2021 [44] | Turkey | To assess the selenium status of pregnant women with COVID-19 and the effects of potential deficiency in serum selenium levels | 71 COVID-19 positive 70 COVID-19 negative | P = 0.0003 and P = 0.001, respectively | Serum selenium levels of pregnant women in the second and third trimesters were lower in COVID-19 + | Serum selenium levels gradually decreased during the pregnancy; this decrease was enhanced in COVID-19 + patients, possibly due to needs depending on the immune response against infection. The decrease in maternal selenium levels was related to IL-6 and D-dimer levels, which indicate selenium’s role in disease progression |
| P = 0.0002 for correlation with D-dimer, P = 0.02 for correlation with IL-6 | Maternal selenium levels negatively correlated with D-dimer and interleukin-6 (IL-6) | ||||||
| P = 0.03 | In the third trimester, maternal selenium negatively correlated with C-reactive protein levels | ||||||
| 6 | Tekin 2022 [33] | Turkey | To investigate the association between Vit D and the clinical severity of COVID-19 in pregnant women | 147 COVID-19 positive 300 COVID-19 negative | RR = 0.568, 95% CI [0.311–1.036]; p = 0.065; After excluding patients on vitamin supplementation: RR = 0.625, 95% CI [0.275–1.419]; p = 0.261 | The clinical severity of COVID-19 disease was not affected by Vit D deficiency | The clinical severity of COVID-19 does not appear to be associated with vitamin D status in pregnant women |
| RR 0.767 (95% CI [0.570–1.030]; p = 0.078 | Testing positive for COVID-19 was not related to Vit D status | ||||||
| RR = 0.954; 95% CI [0.863–1.055]: p = 0.357 | Pulmonary involvement of COVID-19 was similar between patients with Vit D deficiency and adequate Vit D levels | ||||||
| Vit D levels in COVID-19 + 10.35 [8.27] ng/mL vs. 19.02 [8.35] ng/mL in COVID-19 -; p < 0.05 | Serum Vit D levels were significantly lower in COVID-19 + pregnant women | ||||||
| 7 | Schmitt 2022 [45] | France | To evaluate the serum oxidative stress status of pregnant women with and without COVID-19, their inflammatory status, and their serum Vit D levels | 15 COVID-19 positive (7 asymptomatic, 8 symptomatic) 20 COVID-19 negative | p > 0.05 | No significant differences between asymptomatic COVID-19 + and COVID-19 - | Vit D deficiency during the third trimester of pregnancy was more marked in COVID-19 + |
| p = 0.05 | Significantly decreased Vit D levels in COVID-19+ | ||||||
| p = 0.003 | Low magnesium intake (<450 mg) was an independent risk factor for a weak immune response | ||||||
| 8 | Citu 2022 [38] | Romania | To determine the effect of magnesium and magnesium-containing nutritional supplements on the immune response following COVID-19 infection in pregnant women, as well as to observe differences in pregnancy outcomes based on the supplements taken during pregnancy | 448 COVID-19 positive 61/448 took magnesium-only supplements 74/448 took a combination of calcium, magnesium, and zinc 313/448 had no supplementation | p = 0.868 | COVID-19 severity was similar in the three study groups | Pregnant women who supplemented their diet with calcium, zinc, and magnesium, or magnesium only did not have a different clinical course of COVID-19 disease, but no supplementation led to a weaker immune status |
| 14.4% vs. 6.6% vs. 5.4%, p = 0.038 | Significantly higher proportion of premature births in the group of COVID-19 pregnant women who did not supplement their diet compared with those who took magnesium supplements | ||||||
| Zinc: 0.97 (95% CI: 0.87–1.08), P = 0.55 Copper: 1.07 (95% CI: 1.00–1.14), P = 0.06 | Circulating zinc and copper levels show limited evidence of association with COVID-19 infection | ||||||
| 9 | Sobczyk 2022 [41] | UK | To test whether genetically predicted Zn, Se, Cu or vitamin K1 levels have a causal effect on COVID-19-related outcomes, including risk of infection, hospitalization and critical illness | Hospitalization and: Vitamin K1: 0.98 (95% CI: 0.87–1.09), p = 0.66 Copper: 1.07 (95% CI: 0.88–1.29), P = 0.49 Critical Illness and: Vitamin K1: 0.93 (95% CI: 0.72–1.19), p = 0.55 Zinc: 1.21 (95% CI: 0.79–1.86), P = 0.39 | Hospitalization and critical illness outcome are poorly related with circulating levels of vitamin K1, copper and zinc | No evidence that supplementation with zinc, copper or vitamin K1 can prevent COVID-19 infection, critical illness or hospitalization | |
| 73/82 (89%) COVID-19 + had vitamin D deficiency vs. 131/174 (75.3%) in COVID-19-P = 0.01 | Vitamin D deficiency is more frequent in COVID-19 + pregnant women | ||||||
| 10 | Ferrer-Sanchez 2022 [42] | Spain | To establish a relationship between serum Vit D levels and COVID-19 in pregnant women | 82 COVID-19 positive (75 mild symptoms, 7 moderate, severe or critical symptoms) 174 COVID-19 negative | Relationship between vitamin D deficiency in pregnant women and COVID-19 infection |
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


Appendix B
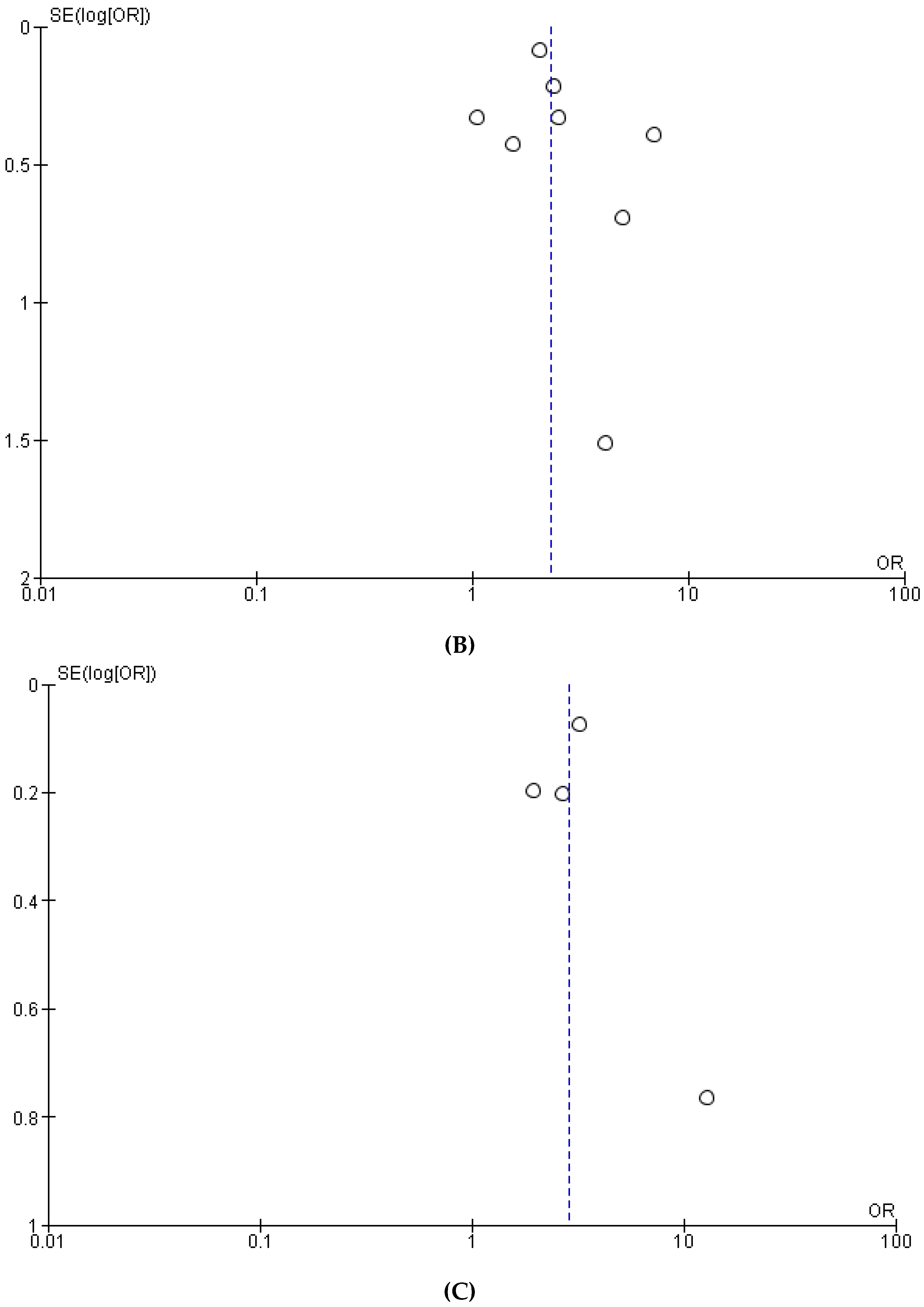
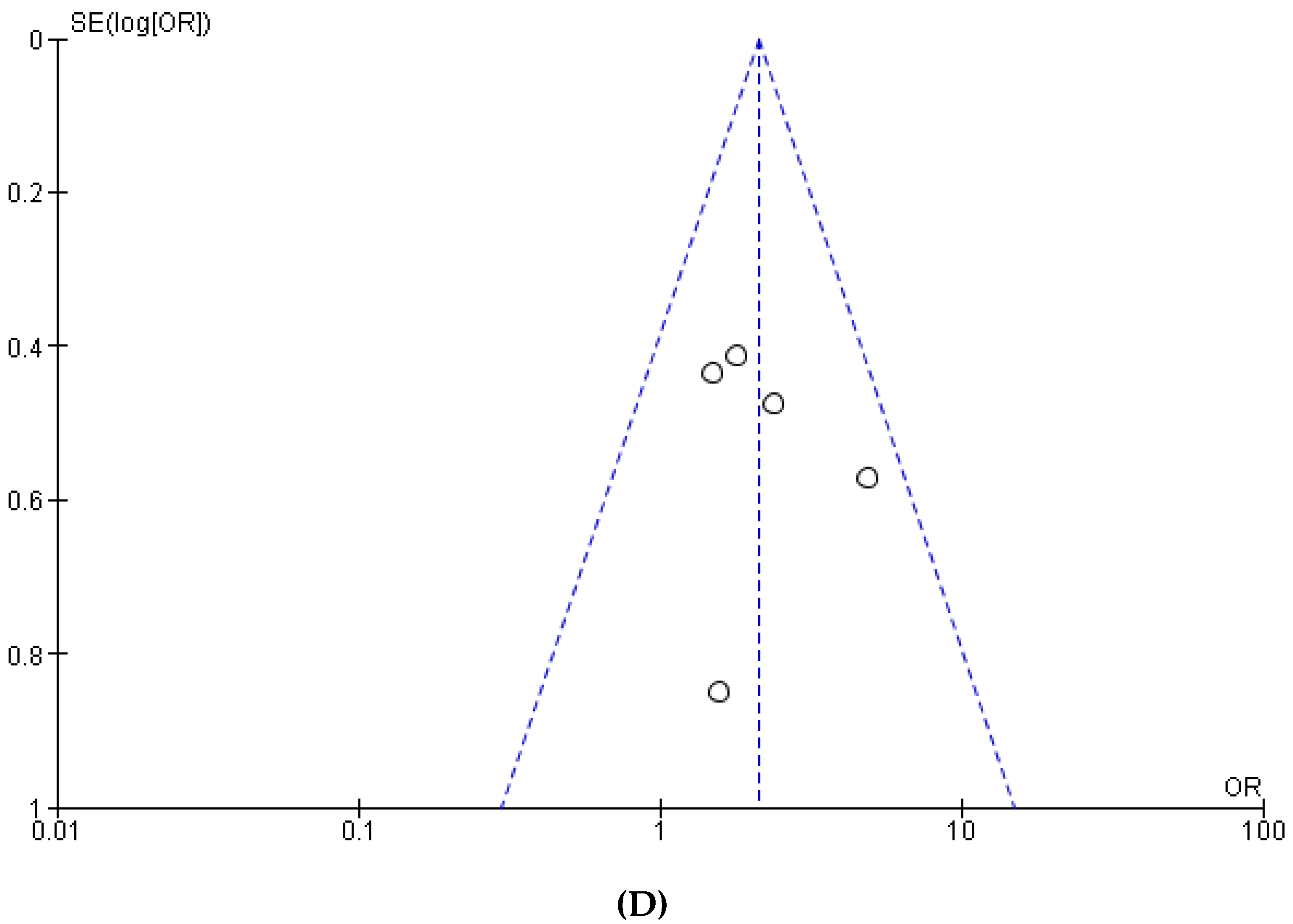
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 21 December 2022).
- Coronavirus (COVID-19), Pregnancy and Women’s Health | RCOG. Available online: https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/ (accessed on 21 December 2022).
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Pazos, M.; Sperling, R.S.; Moran, T.M.; Kraus, T.A. The influence of pregnancy on systemic immunity. Immunol. Res. 2012, 54, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tu, N.; Patterson, P.H. Maternal influenza infection is likely to alter fetal brain development indirectly: The virus is not detected in the fetus. Int. J. Dev. Neurosci. 2005, 23, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yang, W.; Wei, X.; Wu, K.; Huang, D. The Unique Microbiome and Innate Immunity During Pregnancy. Front. Immunol. 2019, 10, 2886. [Google Scholar] [CrossRef]
- Wilson, R.L.; Gummow, J.A.; McAninch, D.; Bianco-Miotto, T.; Roberts, C.T. Vitamin and mineral supplementation in pregnancy: Evidence to practice. J. Pharm. Pract. Res. 2018, 48, 186–192. [Google Scholar] [CrossRef]
- Alpert, P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017, 29, 199–202. [Google Scholar] [CrossRef]
- McCartney, S.A.; Kachikis, A.; Huebner, E.M.; Walker, C.L.; Chandrasekaran, S.; Adams Waldorf, K.M. Obesity as a contributor to immunopathology in pregnant and non-pregnant adults with COVID-19. Am. J. Reprod. Immunol. 2020, 84, e13320. [Google Scholar] [CrossRef]
- Kikut, J.; Komorniak, N.; Ziętek, M.; Palma, J.; Szczuko, M. Inflammation with the participation of arachidonic (AA) and linoleic acid (LA) derivatives (HETEs and HODEs) is necessary in the course of a normal reproductive cycle and pregnancy. J. Reprod. Immunol. 2020, 141, 103177. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The role of arachidonic and linoleic acid derivatives in pathological pregnancies and the human reproduction process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef]
- Tanacan, A.; Oluklu, D.; Koc, B.L.; Sinaci, S.; Beser, D.M.; Hendem, D.U.; Yildirim, M.; Sakcak, B.; Besimoglu, B.; Ersak, D.T.; et al. The utility of systemic immune-inflammation index and systemic immune-response index in the prediction of adverse outcomes in pregnant women with coronavirus disease 2019: Analysis of 2649 cases. J. Obstet. Gynaecol. Res. 2022; Published online. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: The Ottawa Hospital Research Institute. Available online: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 6 February 2023).
- Higgins, J.P.T. (Ed.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: 2022. Available online: www.Training.Cochrane.Org/Handbook (accessed on 18 January 2023).
- Berry, M.; Wang, A.; Clark, S.M.; Harirah, H.M.; Jain, S.; Olson, G.L.; Pacheco, L.D.; Saade, G.R.; Saad, A.F. Clinical Stratification of Pregnant COVID-19 Patients based on Severity: A Single Academic Center Experience. Am. J. Perinatol. 2021, 38, 515–522. [Google Scholar] [CrossRef]
- Di Martino, D.; Chiaffarino, F.; Patanè, L.; Prefumo, F.; Vergani, P.; Ornaghi, S.; Savasi, V.; Spinillo, A.; Cromi, A.; D’Ambrosi, F.; et al. Assessing risk factors for severe forms of COVID-19 in a pregnant population: A clinical series from Lombardy, Italy. Int. J. Gynecol. Obstet. 2021, 152, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Grechukhina, O.; Greenberg, V.; Lundsberg, L.S.; Deshmukh, U.; Cate, J.; Lipkind, H.S.; Campbell, K.H.; Pettker, C.M.; Kohari, K.S.; Reddy, U.M. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am. J. Obstet. Gynecol. MFM 2020, 2, 100246. [Google Scholar] [CrossRef] [PubMed]
- Kayem, G.; Lecarpentier, E.; Deruelle, P.; Bretelle, F.; Azria, E.; Blanc, J.; Bohec, C.; Bornes, M.; Ceccaldi, P.-F.; Chalet, Y.; et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J. Gynecol. Obstet Hum. Reprod. 2020, 49, 101826. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.M.; Bernstein, P.S.; Debolt, C.; Stone, J.; Sutton, D.M.; Simpson, L.L.; Limaye, M.A.; Roman, A.S.; Fazzari, M.; Penfield, C.A.; et al. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at Five New York City Medical Centers. Obstet. Gynecol. 2020, 136, 273–282. [Google Scholar] [CrossRef]
- Limaye, M.A.; Roman, A.S.; Trostle, M.E.; Venkatesh, P.; Martinez, M.L.; Brubaker, S.G.; Chervenak, J.; Wei, L.S.; Sahani, P.; Grossman, T.B.; et al. Predictors of severe and critical disease in pregnant women with SARS-CoV-2. J. Matern.-Fetal Neonatal Med. 2021, 35, 7536–7540. [Google Scholar] [CrossRef]
- Vousden, N.; Ramakrishnan, R.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Management and implications of severe COVID-19 in pregnancy in the UK: Data from the UK Obstetric Surveillance System national cohort. Acta Obs. Gynecol. Scand. 2022, 101, 461–470. [Google Scholar] [CrossRef]
- Donati, S.; Corsi, E.; Maraschini, A.; Salvatore, M.A.; Baltaro, F.; Boldrini, R.; Bonassisa, S.; Brunelli, R.; Cagnacci, A.; Casucci, P.; et al. The first SARS-CoV-2 wave among pregnant women in Italy: Results from a prospective population-based study. Ann. Ist. Super Sanita. 2021, 57, 272–285. [Google Scholar] [CrossRef]
- Doyle, T.J.; Kiros G egziabhe Schmitt-Matzen, E.N.; Propper, R.; Thompson, A.; Phillips-Bell, G.S. Maternal and perinatal outcomes associated with SARS-CoV-2 infection during pregnancy, Florida, 2020–2021: A retrospective cohort study. Clin. Infect. Dis. 2022, 2 (Suppl. 2), 2020–2021. [Google Scholar] [CrossRef]
- Mendez-Dominguez, N.; Santos-Zaldívar, K.; Gomez-Carro, S.; Datta-Banik, S.; Carrillo, G. Maternal mortality during the COVID-19 pandemic in Mexico: A preliminary analysis during the first year. BMC Public Health 2021, 21, 1297. [Google Scholar] [CrossRef]
- Siqueira, T.S.; de Souza, E.K.G.; Martins-Filho, P.R.; Silva, J.R.S.; Gurgel, R.Q.; Cuevas, L.E.; Santos, V.S. Clinical characteristics and risk factors for maternal deaths due to COVID-19 in Brazil: A nationwide population-based cohort study. J. Travel Med. 2022, 29, taab199. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Martinez-Portilla, R.J.; Espino-Y-Sosa, S.; Estrada-Gutierrez, G.; Solis-Paredes, J.M.; Villafan-Bernal, J.R.; Medina-Jimenez, V.; Rodriguez-Morales, A.J.; Rojas-Zepeda, L.; Poon, L.C. Comorbidity, poverty and social vulnerability as risk factors for mortality in pregnant women with confirmed SARS-CoV-2 infection: Analysis of 13,062 positive pregnancies including 176 maternal deaths in Mexico. Ultrasound Obstet. Gynecol. 2022, 59, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Aabakke, A.J.M.; Krebs, L.; Petersen, T.G.; Kjeldsen, F.S.; Corn, G.; Wøjdemann, K.; Ibsen, M.H.; Jonsdottir, F.; Rønneberg, E.; Andersen, C.S.; et al. SARS-CoV-2 infection in pregnancy in Denmark—Characteristics and outcomes after confirmed infection in pregnancy: A nationwide, prospective, population-based cohort study. Acta Obstet. Gynecol. Scand. 2021, 100, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Barbero, P.; Mugüerza, L.; Herraiz, I.; Burguillo, A.G.; Juan, R.S.; Forcén, L.; Mejía, I.; Batllori, E.; Montañez, M.D.; Vallejo, P.; et al. SARS-CoV-2 in pregnancy: Characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. J. Matern.-Fetal Neonatal Med. 2020, 35, 2648–2654. [Google Scholar] [CrossRef]
- Lokken, E.M.; Walker, C.L.; Delaney, S.; Kachikis, A.; Kretzer, N.M.; Erickson, A.; Resnick, R.; Vanderhoeven, J.; Hwang, J.K.; Barnhart, N.; et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2020, 223, e1–e911. [Google Scholar] [CrossRef] [PubMed]
- Lokken, E.M.; Huebner, E.M.; Taylor, G.G.; Hendrickson, S.; Vanderhoeven, J.; Kachikis, A.; Coler, B.; Walker, C.L.; Sheng, J.S.; Al-Haddad, B.J.; et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2021, 225, e1–e77. [Google Scholar] [CrossRef]
- Panagiotakopoulos, L.; Myers, T.R.; Gee, J.; Lipkind, H.S.; Kharbanda, E.O.; Ryan, D.S.; Williams, J.T.; Naleway, A.L.; Klein, N.P.; Hambidge, S.J.; et al. SARS-CoV-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics—Eight U.S. Health Care Centers, 1 March–30 May 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1355–1359. [Google Scholar] [CrossRef]
- Sinaci, S.; Ocal, D.F.; Yetiskin, D.F.Y.; Hendem, D.U.; Buyuk, G.N.; Ayhan, S.G.; Tanacan, A.; Ozgu-Erdinc, A.S.; Tekin, O.M.; Sahin, D. Impact of vitamin D on the course of COVID-19 during pregnancy: A case control study. J. Steroid Biochem. Mol. Biol. 2021, 213, 105964. [Google Scholar] [CrossRef]
- Tekin, A.B.; Yassa, M.; Birol, P.; Unlu, S.N.; Sahin, T.; Buran, A.M.; Ayanoglu, E.; Tug, N. Vitamin D status is not associated with clinical severity of COVID-19 in pregnant women. Eur. J. Nutr. 2022, 61, 1035–1041. [Google Scholar] [CrossRef]
- Takemoto, M.L.; Menezes, M.O.; Andreucci, C.B.; Knobel, R.; Sousa, L.A.; Katz, L.; Fonseca, E.B.; Nakamura-Pereira, M.; Magalhães, C.G.; Diniz, C.S.; et al. Clinical characteristics and risk factors for mortality in obstetric patients with severe COVID-19 in Brazil: A surveillance database analysis. BJOG 2020, 127, 1618–1626. [Google Scholar] [CrossRef]
- Leal, L.F.; Merckx, J.; Fell, D.B.; Kuchenbecker, R.; Miranda, A.E.; de Oliveira, W.K.; Platt, R.W.; Antunes, L.; Silveira, M.F.; Barbieri, N.B. Characteristics and outcomes of pregnant women with SARS-CoV-2 infection and other severe acute respiratory infections (SARI) in Brazil from January to November 2020. Braz. J. Infect. Dis. 2021, 25, 101620. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, A.; De Nola, R.; Trerotoli, P.; Metta, M.E.; Cazzato, G.; Resta, L.; Malvasi, A.; Lepera, A.; Ricci, I.; Capozza, M.; et al. COVID-19 Infection in Pregnancy: Obstetrical Risk Factors and Neonatal Outcomes—A Monocentric, Single-Cohort Study. Vaccines 2022, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Stenton, S.; McPartland, J.; Shukla, R.; Turner, K.; Marton, T.; Hargitai, B.; Bamber, A.; Pryce, J.; Peres, C.L.; Burguess, N.; et al. SARS-COV2 placentitis and pregnancy outcome: A multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine 2022, 47, 101389. [Google Scholar] [CrossRef] [PubMed]
- Citu, I.M.; Citu, C.; Margan, M.-M.; Craina, M.; Neamtu, R.; Gorun, O.M.; Burlea, B.; Bratosin, F.; Rosca, O.; Grigoras, M.L.; et al. Calcium, Magnesium, and Zinc Supplementation during Pregnancy: The Additive Value of Micronutrients on Maternal Immune Response after SARS-CoV-2 Infection. Nutrients 2022, 14, 1445. [Google Scholar] [CrossRef]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Tekin, O.M.; Yazihan, N.; Sahin, D. The Relation Between Trace Element Status (Zinc, Copper, Magnesium) and Clinical Outcomes in COVID-19 Infection During Pregnancy. Biol. Trace Elem. Res. 2021, 199, 3608–3617. [Google Scholar] [CrossRef]
- Yalcin Bahat, P.; Aldikactioglu Talmac, M.; Bestel, A.; Topbas Selcuki, N.F.; Aydın, Z.; Polat, İ. Micronutrients in COVID-19 Positive Pregnancies. Cureus 2020, 12, 10–14. [Google Scholar] [CrossRef]
- Sobczyk, M.K.; Gaunt, T.R. The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study. Nutrients 2022, 14, 233. [Google Scholar] [CrossRef]
- Ferrer-Sánchez, N.; Díaz-Goicoechea, M.; Mayoral-Cesar, V.; García-Solbas, S.; Nievas-Soriano, B.J.; Parrón-Carreño, T.; Fernández-Alonso, A.M. Serum 25(OH) Vitamin D Levels in Pregnant Women with Coronavirus Disease 2019 (COVID-19): A Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 3965. [Google Scholar] [CrossRef]
- Erol, S.A.; Polat, N.; Akdas, S.; Ayral, P.A.; Anuk, A.T.; Tokalioglu, E.O.; Ayhan, G.; Kesikli, B.; Ceylan, M.N.; Tanacan, A.; et al. Maternal selenium status plays a crucial role on clinical outcomes of pregnant women with COVID-19 infection. J. Med. Virol. 2021, 93, 5438–5445. [Google Scholar] [CrossRef]
- Erol, S.A.; Tanacan, A.; Anuk, A.T.; Tokalioglu, E.O.; Biriken, D.; Keskin, H.L.; Moraloglu, O.T.; Yazihan, N.; Sahin, D. Evaluation of maternal serum afamin and vitamin E levels in pregnant women with COVID-19 and its association with composite adverse perinatal outcomes. J. Med. Virol. 2021, 93, 2350–2358. [Google Scholar] [CrossRef]
- Schmitt, G.; Labdouni, S.; Soulimani, R.; Delamare, C.; Bouayed, J. Oxidative stress status and vitamin D levels of asymptomatic to mild symptomatic COVID-19 infections during the third trimester of pregnancy: A retrospective study in Metz, France. J. Med. Virol. 2022, 94, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Babic, I.; Alsomali, F.; Aljuhani, S.; Baeissa, S.; Alhabib, I.; AlAhmari, E.; Omer, M.; Alkhalifa, K. COVID-19 Pandemic and Its Impact on Perinatal Outcomes between Symptomatic and Asymptomatic Women. Obstet. Gynecol. Int. 2022, 2022, 1756266. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Corsi, E.; Maraschini, A.; Salvatore, M.A.; Arena, M.G.; Boldrini, R.; Brunelli, R.; Cagnacci, A.; Casucci, P.; Cetin, I.; et al. SARS-CoV-2 infection among hospitalised pregnant women and impact of different viral strains on COVID-19 severity in Italy: A national prospective population-based cohort study. BJOG 2022, 129, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Galang, R.R.; Newton, S.M.; Woodworth, K.R.; Griffin, I.; Oduyebo, T.; Sancken, C.L.; Olsen, E.O.; Aveni, K.; Wingate, H.; Shephard, H.; et al. Risk Factors for Illness Severity Among Pregnant Women With Confirmed Severe Acute Respiratory Syndrome Coronavirus 2 Infection-Surveillance for Emerging Threats to Mothers and Babies Network, 22 State, Local, and Territorial Health Departments, 29 March. Clin. Infect. Dis. 2021, 73, S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Rauch, S.; Iurlaro, E.; Gunier, R.B.; Rego, A.; Gravett, M.G.; Cavoretto, P.I.; Deruelle, P.; García-May, P.K.; Mhatre, M.; et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: The INTERCOVID study. Am. J. Obstet. Gynecol. 2022, 227, e1–e74. [Google Scholar] [CrossRef]
- Menezes, M.O.; Takemoto, M.L.S.; Nakamura-Pereira, M.; Katz, L.; Amorim, M.M.R.; Salgado, H.O.; Melo, A.; Diniz, C.S.G.; De Sousa, L.A.; Magalhaes, C.G.; et al. Risk factors for adverse outcomes among pregnant and postpartum women with acute respiratory distress syndrome due to COVID-19 in Brazil. Int. J. Gynecol. Obstet. 2020, 151, 415–423. [Google Scholar] [CrossRef]
- Overtoom, E.M.; Rosman, A.N.; Zwart, J.J.; Vogelvang, T.E.; Schaap, T.P.; Akker, T.V.D.; Bloemenkamp, K.W. SARS-CoV-2 infection in pregnancy during the first wave of COVID-19 in the Netherlands: A prospective nationwide population-based cohort study (NethOSS). BJOG 2022, 129, 91–100. [Google Scholar] [CrossRef]
- Péju, E.; Belicard, F.; Silva, S.; Hraiech, S.; Painvin, B.; Kamel, T.; Thille, A.W.; Goury, A.; Grimaldi, D.; Jung, B.; et al. Management and outcomes of pregnant women admitted to intensive care unit for severe pneumonia related to SARS-CoV-2 infection: The multicenter and international COVIDPREG study. Intensiv. Care Med. 2022, 48, 1185–1196. [Google Scholar] [CrossRef]
- Peter, B.; Ree, N.; Ferrer, K.; Younes, L.; Lepe, B.; Manhal, K.; Mydam, J. Risk Factors Associated With COVID-19 Symptoms and Potential Vertical Transmission During Pregnancy: A Retrospective Cohort Study. Cureus 2022, 14, e22900. [Google Scholar] [CrossRef]
- Prasannan, L.; Rochelson, B.; Shan, W.; Nicholson, K.; Solmonovich, R.; Kulkarni, A.; Lewis, D.; Greenberg, M.; Nimaroff, M.; Blitz, M.J. Social determinants of health and coronavirus disease 2019 in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100349. [Google Scholar] [CrossRef]
- Sakowicz, A.; Ayala, A.E.; Ukeje, C.C.; Witting, C.S.; William, A.G.; Miller, E.S. Risk factors for severe acute respiratory syndrome. Am. J. Obstet. Gynaecol. MFM 2020, 2, 100198. [Google Scholar] [CrossRef]
- Savasi, V.M.; Parisi, F.; Patanè, L.; Ferrazzi, E.; Frigerio, L.; Pellegrino, A.; Spinillo, A.; Tateo, S.; Ottoboni, M.; Veronese, P.; et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.T.; Cecatti, J.G.; Pacagnella, R.C.; Ribeiro-Do-Valle, C.C.; Luz, A.G.; Lajos, G.J.; Nobrega, G.M.; Griggio, T.B.; Charles, C.M.; Bento, S.F.; et al. The COVID-19 pandemic in Brazilian pregnant and postpartum women: Results from the REBRACO prospective cohort study. Sci. Rep. 2022, 12, 11758. [Google Scholar] [CrossRef] [PubMed]
- Vousden, N.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; Knight, M. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS ONE 2021, 16, e0251123. [Google Scholar] [CrossRef] [PubMed]
- Budhram, S.; Vannevel, V.; Botha, T.; Chauke, L.; Bhoora, S.; Balie, G.M.; Odell, N.; Lombaard, H.; Wise, A.; Georgiou, C.; et al. Maternal characteristics and pregnancy outcomes of hospitalized pregnant women with SARS-CoV-2 infection in South Africa: An International Network of Obstetric Survey Systems-based cohort study. Int. J. Gynecol. Obstet. 2021, 155, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Sinopoli, A.; Caminada, S.; Isonne, C.; Santoro, M.M.; Baccolini, V. What Are the Effects of Vitamin A Oral Supplementation in the Prevention and Management of Viral Infections? A Systematic Review of Randomized Clinical Trials. Nutrients 2022, 14, 4081. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, A.J.M.; Luttikhuis, M.A.M.O.; Wellen, A.C.; Müller, C.; Calkhoven, C.F. Obesity and its impact on COVID-19 The obesity pandemic. Published online 2021. [CrossRef]
- Masturzo, B.; Franzè, V.; Germano, C.; Attini, R.; Gennarelli, G.; Lezo, A.; Rolfo, A.; Plazzotta, C.; Brunelli, E.; Youssef, A.; et al. Risk of adverse pregnancy outcomes by pre-pregnancy Body Mass Index among Italian population: A retrospective population-based cohort study on 27,807 deliveries. Arch. Gynecol. Obstet. 2019, 299, 983–991. [Google Scholar] [CrossRef]
- Frey, H.A.; Ashmead, R.; Farmer, A.; Kim, Y.H.; Shellhaas, C.; Oza-Frank, R.; Jackson, R.D.; Costantine, M.M.; Lynch, C.D. Association of Prepregnancy Body Mass Index with Risk of Severe Maternal Morbidity and Mortality among Medicaid Beneficiaries. JAMA Netw. Open 2022, 5, e2218986. [Google Scholar] [CrossRef]
- Saucedo, M.; Esteves-Pereira, A.P.; Pencolé, L.; Rigouzzo, A.; Proust, A.; Bouvier-Colle, M.-H.; Chassard, D.; Cohen, H.; Dreyfus, M.; Ducloy, J.-C.; et al. Understanding maternal mortality in women with obesity and the role of care they receive: A national case-control study. Int. J. Obes. 2021, 45, 258–265. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attini, R.; Laudani, M.E.; Versino, E.; Massaro, A.; Pagano, A.; Petey, F.; Revelli, A.; Masturzo, B. COVID-19 in Pregnancy: Influence of Body Weight and Nutritional Status on Maternal and Pregnancy Outcomes—A Review of Literature and Meta-Analysis. Nutrients 2023, 15, 1052. https://doi.org/10.3390/nu15041052
Attini R, Laudani ME, Versino E, Massaro A, Pagano A, Petey F, Revelli A, Masturzo B. COVID-19 in Pregnancy: Influence of Body Weight and Nutritional Status on Maternal and Pregnancy Outcomes—A Review of Literature and Meta-Analysis. Nutrients. 2023; 15(4):1052. https://doi.org/10.3390/nu15041052
Chicago/Turabian StyleAttini, Rossella, Maria Elena Laudani, Elisabetta Versino, Alessio Massaro, Arianna Pagano, Francesca Petey, Alberto Revelli, and Bianca Masturzo. 2023. "COVID-19 in Pregnancy: Influence of Body Weight and Nutritional Status on Maternal and Pregnancy Outcomes—A Review of Literature and Meta-Analysis" Nutrients 15, no. 4: 1052. https://doi.org/10.3390/nu15041052
APA StyleAttini, R., Laudani, M. E., Versino, E., Massaro, A., Pagano, A., Petey, F., Revelli, A., & Masturzo, B. (2023). COVID-19 in Pregnancy: Influence of Body Weight and Nutritional Status on Maternal and Pregnancy Outcomes—A Review of Literature and Meta-Analysis. Nutrients, 15(4), 1052. https://doi.org/10.3390/nu15041052