The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Design
2.3. Data Sources and Searches
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Outcomes
2.8. Data Synthesis
3. Results
3.1. Search Results
3.2. Trial Characteristics
3.3. Risk of Bias
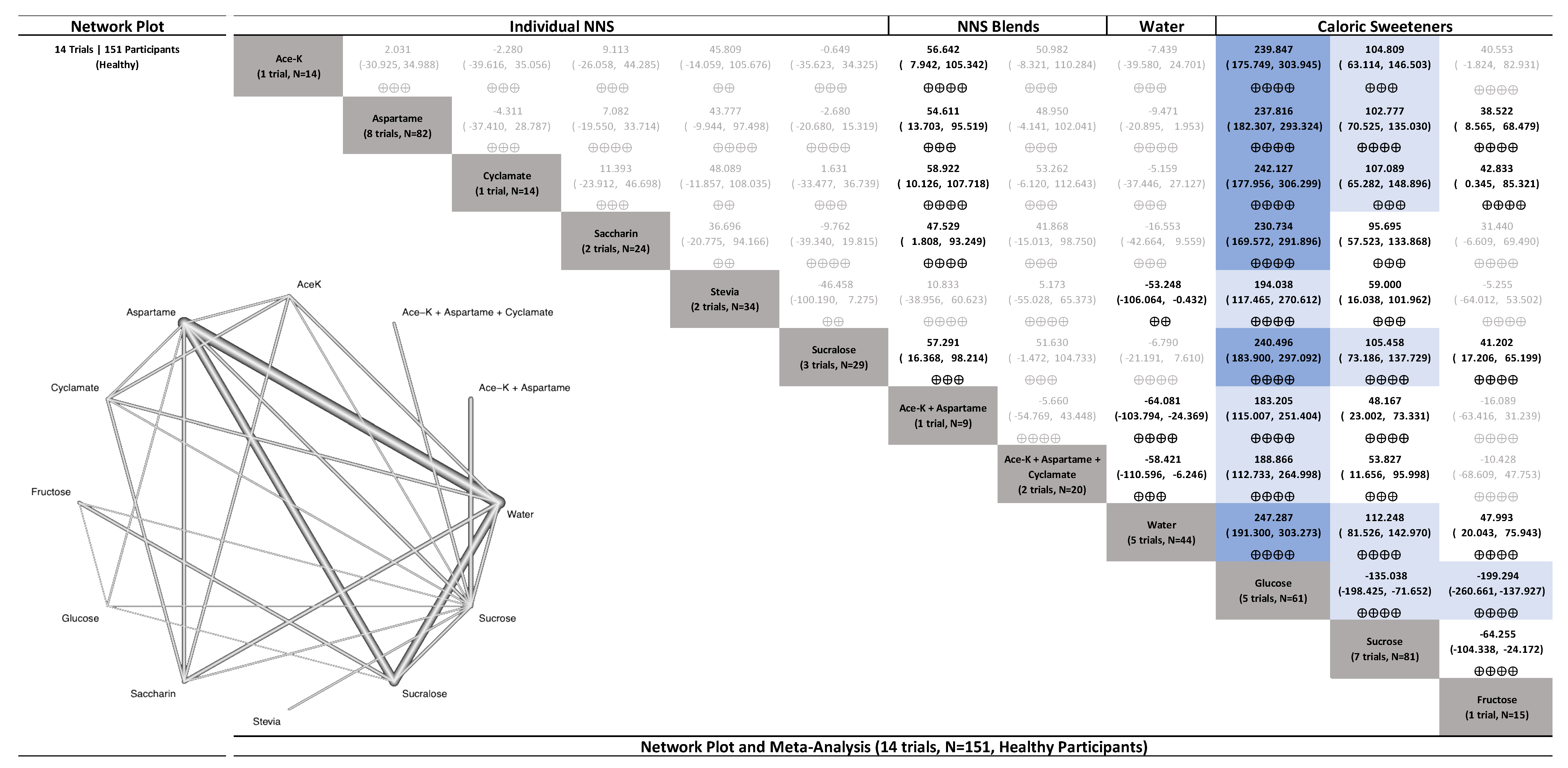
3.4. Primary Outcome (Glucose)
3.4.1. Uncoupling Interventions
3.4.2. Coupling Interventions
3.4.3. Delayed Coupling Interventions
3.5. Secondary Endocrine Outcomes (Insulin, GLP-1, PYY, GIP, Ghrelin, Leptin, and Glucagon)
3.5.1. Uncoupling Interventions
3.5.2. Coupling Interventions
3.5.3. Delayed Coupling Interventions
4. Discussion
4.1. Summary of Findings
4.2. Findings in the Context of Existing Studies
4.3. Potential Mechanisms
4.4. Strengths and Limitations
4.5. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sugars Intake for Adults and Children. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/149782/9789241549028_eng.pdf?sequence=1 (accessed on 21 September 2021).
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2015. Available online: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf (accessed on 21 September 2021).
- Scientific Advisory Committe on Nutrition. Carbohydrates and Health. Stationery Office. 2015. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 21 September 2021).
- Heart and Stroke Foundation of Canada, Sugar, Heart Disease and Stroke. Available online: https://www.heartandstroke.ca/-/media/pdf-files/canada/2017-position-statements/sugar-ps-eng.ashx (accessed on 23 September 2021).
- Diabetes Canada, Diabetes Canada’s Position on Sugars. Available online: https://www.diabetes.ca/DiabetesCanadaWebsite/media/Advocacy-and-Policy/Diabetes-Canada_Position-Statement_Sugar_ENGLISH_January-2020.pdf (accessed on 23 September 2021).
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef]
- Jayalath, V.H.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Huang, Y.; Reilly, K.H.; Li, S.; Zheng, R.; Barrio-Lopez, M.T.; Martinez-Gonzalez, M.A.; Zhou, D. Sugar-sweetened beverages and risk of hypertension and CVD: A dose-response meta-analysis. Br. J. Nutr. 2015, 113, 709–717. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. 2018. Available online: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states (accessed on 2 October 2021).
- European Food Safety Authority. Scientific Topic: Sweeteners. 2019. Available online: https://www.efsa.europa.eu/en/topics/topic/sweeteners (accessed on 7 October 2021).
- Mortensen, A. Sweeteners permitted in the European Union: Safety aspects. Scand. J. Food Nutr. 2006, 50, 104–116. [Google Scholar] [CrossRef]
- Health Canada. Sugar Substitutes. 2004. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/food-additives/sugar-substitutes.html (accessed on 5 October 2021).
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017, 189, E929–E939. [Google Scholar] [CrossRef]
- Khan, T.A.; Sievenpiper, J.L. Low-energy sweeteners and cardiometabolic health: Is there method in the madness? Am. J. Clin. Nutr. 2020, 112, 917–919. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Brown, R.J.; Blau, J.E.; Walter, M.; Rother, K.I. Hormonal responses to non-nutritive sweeteners in water and diet soda. Nutr. Metab. 2016, 13, 71. [Google Scholar] [CrossRef]
- Brown, R.J.; Rother, K.I. Non-nutritive sweeteners and their role in the gastrointestinal tract. J. Clin. Endocrinol. Metab. 2012, 97, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Dalenberg, J.R.; Patel, B.P.; Denis, R.; Veldhuizen, M.G.; Nakamura, Y.; Vinke, P.C.; Luquet, S.; Small, D.M. Short-Term Consumption of Sucralose with, but Not without, Carbohydrate Impairs Neural and Metabolic Sensitivity to Sugar in Humans. Cell Metab. 2020, 31, 493–502.e497. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zilberman-Schapira, G.; Segal, E.; Elinav, E. Non-caloric artificial sweeteners and the microbiome: Findings and challenges. Gut Microbes 2015, 6, 149–155. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Kendall, C.; Sievenpiper, J. Systematic Review and Network Meta-Analyses of Low-Calorie Sweeteners and Acute Glycemic Response. 2020. Available online: https://doi.org/10.17605/OSF.IO/QSZBP (accessed on 7 May 2020).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Cochrane: Chichester, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 5 March 2022).
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Brouns, F.; Bjorck, I.; Frayn, K.; Gibbs, A.L.; Lang, V.; Slama, G.; Wolever, T. Glycemic index methodology. Nutr. Res. Rev. 2005, 18, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Chapter 2.5.5. Propagation of Error Considerations. NIST/SEMATECH e-Handbook of Statistical Methods. 2003. Available online: https://www.itl.nist.gov/div898//handbook/mpc/section5/mpc55.htm#exactMay (accessed on 13 September 2021).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- CINeMA: Confidence in Network Meta-Analysis. Bern: Institute of Social and Preventive Medicine. 2017. Available online: https://cinema.ispm.unibe.ch/ (accessed on 3 November 2021).
- Rücker, G.; Krahn, U.; König, J.; Efthimiou, O.; Davies, A.; Papakonstantinou, T.; Schwarzer, G. Netmeta: Network Meta-Analysis Using Frequentist Methods. GitHub. 2020. Available online: https://github.com/guido-s/netmeta (accessed on 12 March 2020).
- Nikolakopoulou, A.; Higgins, J.P.T.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef]
- Papakonstantinou, T.; Nikolakopoulou, A.; Higgins, J.P.T.; Egger, M.; Salanti, G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 2020, 16, e1080. [Google Scholar] [CrossRef]
- Shigeta, H.; Yoshida, T.; Nakai, M.; Mori, H.; Kano, Y.; Nishioka, H.; Kajiyama, S.; Kitagawa, Y.; Kanatsuna, T.; Kondo, M.; et al. Effects of aspartame on diabetic rats and diabetic patients. J. Nutr. Sci. Vitaminol. 1985, 31, 533–540. [Google Scholar] [CrossRef]
- Okuno, G.; Kawakami, F.; Tako, H.; Kashihara, T.; Shibamoto, S.; Yamazaki, T.; Yamamoto, K.; Saeki, M. Glucose tolerance, blood lipid, insulin and glucagon concentration after single or continuous administration of aspartame in diabetics. Diabetes Res. Clin. Pr. 1986, 2, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.L.; McLane, M.; Kobe, P. Response to single dose of aspartame or saccharin by NIDDM patients. Diabetes Care 1988, 11, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.E. Effect of aspartame and protein, administered in phenylalanine-equivalent doses, on plasma neutral amino acids, aspartate, insulin and glucose in man. Pharm. Toxicol. 1991, 68, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Härtel, B.; Graubaum, H.; Schneider, B. The Influence of Sweetener Solutions on the Secretion of Insulin and the Blood Glucose Level. Ernährungsumschau 1993, 40, 152–155. [Google Scholar]
- Nguyen, U.N.; Dumoulin, G.; Henriet, M.T.; Regnard, J. Aspartame ingestion increases urinary calcium, but not oxalate excretion, in healthy subjects. J. Clin. Endocrinol. Metab. 1998, 83, 165–168. [Google Scholar] [CrossRef]
- Coppola, L.; Coppola, A.; Grassia, A.; Mastrolorenzo, L.; Lettieri, B.; De Lucia, D.; De Nanzio, A.; Gombos, G. Acute hyperglycemia alters von Willebrand factor but not the fibrinolytic system in elderly subjects with normal or impaired glucose tolerance. Blood Coagul. Fibrinolysis 2004, 15, 629–635. [Google Scholar] [CrossRef]
- Berlin, I.; Vorspan, F.; Warot, D.; Manéglier, B.; Spreux-Varoquaux, O. Effect of glucose on tobacco craving. Is it mediated by tryptophan and serotonin? Psychopharmacology 2005, 178, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.E.; Peters, V.; Martin, N.M.; Sleeth, M.L.; Ghatei, M.A.; Frost, G.S.; Bloom, S.R. Effects of oral ingestion of sucralose on gut hormone response and appetite in healthy normal-weight subjects. Eur. J. Clin. Nutr. 2011, 65, 508–513. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Holst, J.J.; Fenger-Grøn, M.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Satiety scores and satiety hormone response after sucrose-sweetened soft drink compared with isocaloric semi-skimmed milk and with non-caloric soft drink: A controlled trial. Eur. J. Clin. Nutr. 2012, 66, 523–529. [Google Scholar] [CrossRef]
- Hazali, N.; Mohamed, A.; Ibrahim, M.; Masri, M.; Anuar, K.; Norazmir, M.N.; Ayob, M.; Fadzlan, F. Effect of Acute Stevia Consumption on Blood Glucose Response in Healthy Malay Young Adults. Sains Malays. 2014, 43, 649–654. [Google Scholar]
- Bloomer, R.; Peel, S.; Moran, R.; MacDonnchadh, J. Blood glucose and insulin response to artificially- and sugar-sweetened sodas in healthy men. Integr. Food Nutr. Metab. 2016, 3, 268–272. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M.; González-Cortés, J.J.; Corrales-Cuevas, M.; Rojas-Cots, J.A.; Segundo, C.; Schwarz, M. Synergic effects of sugar and caffeine on insulin-mediated metabolomic alterations after an acute consumption of soft drinks. Electrophoresis 2017, 38, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Goza, R.; Bunout, D.; Barrera, G.; de la Maza, M.; Hirsch, S. Effect of Acute Consumption of Artificially Sweetened Beverages on Blood Glucose and Insulin in Healthy Subjects. J. Nutr. Food Sci. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Eckstein, M.L.; Brockfeld, A.; Haupt, S.; Schierbauer, J.R.; Zimmer, R.T.; Wachsmuth, N.; Zunner, B.; Zimmermann, P.; Obermayer-Pietsch, B.; Moser, O. Acute Metabolic Responses to Glucose and Fructose Supplementation in Healthy Individuals: A Double-Blind Randomized Crossover Placebo-Controlled Trial. Nutrients 2021, 13, 4095. [Google Scholar] [CrossRef]
- Wolf-Novak, L.C.; Stegink, L.D.; Brummel, M.C.; Persoon, T.J.; Filer, L.J., Jr.; Bell, E.F.; Ziegler, E.E.; Krause, W.L. Aspartame ingestion with and without carbohydrate in phenylketonuric and normal subjects: Effect on plasma concentrations of amino acids, glucose, and insulin. Metabolism 1990, 39, 391–396. [Google Scholar] [CrossRef]
- Melchior, J.C.; Rigaud, D.; Colas-Linhart, N.; Petiet, A.; Girard, A.; Apfelbaum, M. Immunoreactive beta-endorphin increases after an aspartame chocolate drink in healthy human subjects. Physiol. Behav. 1991, 50, 941–944. [Google Scholar] [CrossRef]
- Solomi, L.; Rees, G.A.; Redfern, K.M. The acute effects of the non-nutritive sweeteners aspartame and acesulfame-K in UK diet cola on glycaemic response. Int. J. Food Sci. Nutr. 2019, 70, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Walter, M.; Rother, K.I. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care 2012, 35, 959–964. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose affects glycemic and hormonal responses to an oral glucose load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef]
- Temizkan, S.; Deyneli, O.; Yasar, M.; Arpa, M.; Gunes, M.; Yazici, D.; Sirikci, O.; Haklar, G.; Imeryuz, N.; Yavuz, D.G. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2015, 69, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Karimian Azari, E.; Smith, K.R.; Yi, F.; Osborne, T.F.; Bizzotto, R.; Mari, A.; Pratley, R.E.; Kyriazis, G.A. Inhibition of sweet chemosensory receptors alters insulin responses during glucose ingestion in healthy adults: A randomized crossover interventional study. Am. J. Clin. Nutr. 2017, 105, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Nichol, A.D.; Salame, C.; Rother, K.I.; Pepino, M.Y. Effects of Sucralose Ingestion versus Sucralose Taste on Metabolic Responses to an Oral Glucose Tolerance Test in Participants with Normal Weight and Obesity: A Randomized Crossover Trial. Nutrients 2019, 12, 29. [Google Scholar] [CrossRef]
- Solomi, L. Diet cola and glycaemia: The acute effects of a preload containing the non-nutritive sweeteners aspartame and acesulfame-K on the glycaemic response to a glucose load. Plymouth Stud. Sci. 2020, 13, 97–111. [Google Scholar]
- Greyling, A.; Appleton, K.M.; Raben, A.; Mela, D.J. Acute glycemic and insulinemic effects of low-energy sweeteners: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Nichol, A.D.; Holle, M.J.; An, R. Glycemic impact of non-nutritive sweeteners: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2018, 72, 796–804. [Google Scholar] [CrossRef]
- Tucker, R.M.; Tan, S.Y. Do non-nutritive sweeteners influence acute glucose homeostasis in humans? A systematic review. Physiol. Behav. 2017, 182, 17–26. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Khan, T.A.; Ha, V.; Viguiliouk, E.; Auyeung, R. The importance of study design in the assessment of nonnutritive sweeteners and cardiometabolic health. CMAJ 2017, 189, E1424–E1425. [Google Scholar] [CrossRef]
- Mossavar-Rahmani, Y.; Kamensky, V.; Manson, J.E.; Silver, B.; Rapp, S.R.; Haring, B.; Beresford, S.A.A.; Snetselaar, L.; Wassertheil-Smoller, S. Artificially Sweetened Beverages and Stroke, Coronary Heart Disease, and All-Cause Mortality in the Women’s Health Initiative. Stroke 2019, 50, 555–562. [Google Scholar] [CrossRef]
- Malik, V.S. Non-sugar sweeteners and health. BMJ 2019, 364, k5005. [Google Scholar] [CrossRef]
- Lee, J.J.; Khan, T.A.; McGlynn, N.; Malik, V.S.; Hill, J.O.; Leiter, L.A.; Jeppesen, P.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J. Relation of Change or Substitution of Low-and No-Calorie Sweetened Beverages with Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, N.D.; Khan, T.A.; Wang, L.; Zhang, R.; Chiavaroli, L.; Au-Yeung, F.; Lee, J.J.; Noronha, J.C.; Comelli, E.M.; Mejia, S.B. Association of Low-and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages with Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis. JAMA Netw. 2022, 5, e222092. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Salleh, N.B.; Henry, J.; Forde, C.G. Effects of aspartame-, monk fruit-, stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int. J. Obes. 2017, 41, 450–457. [Google Scholar] [CrossRef]
- Pearson, R.C.; Green, E.S.; Olenick, A.A.; Jenkins, N.T. Comparison of aspartame- and sugar-sweetened soft drinks on postprandial metabolism. Nutr. Health 2021, 02601060211057415. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W.; Bohan Brown, M.M.; Onken, K.L.; Beitz, D.C. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr. Res. 2011, 31, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P. Low-calorie sweetener use and energy balance: Results from experimental studies in animals, and large-scale prospective studies in humans. Physiol. Behav. 2016, 164, 517–523. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef]
- Hunter, S.R.; Reister, E.J.; Cheon, E.; Mattes, R.D. Low calorie sweeteners differ in their physiological effects in humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Redruello-Requejo, M.; González-Rodríguez, M.; Samaniego-Vaesken, M.D.L.; Montero-Bravo, A.; Partearroyo, T.; Varela-Moreiras, G. Low-and no-calorie sweetener (LNCS) consumption patterns amongst the Spanish adult population. Nutrients 2021, 13, 1845. [Google Scholar] [CrossRef]
- Nunn, R.; Young, L.; Ni Mhurchu, C. Prevalence and Types of Non-Nutritive Sweeteners in the New Zealand Food Supply, 2013 and 2019. Nutrients 2021, 13, 3228. [Google Scholar] [CrossRef]
- Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch. Summary of Health Canada’s Assessment of a Health Claim about a Polysaccharide Complex (Glucomannan, Xanthan Gum, Sodium Alginate) and a Reduction of Post-Prandial Blood Glucose Response. 2016. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/label-etiquet/claims-reclam/assess-evalu/glucose-complex-polysaccharides-complexe-glycemique-eng.pdf (accessed on 20 June 2021).
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: Systematic review and meta-analyses of clinical studies. Diabetologia 2013, 56, 965–972. [Google Scholar] [CrossRef]
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: Systematic review and meta-analysis of clinical studies. Diabetes Care 2013, 36, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Jenkins, D.J.; Josse, R.G.; Vuksan, V. Dilution of the 75-g oral glucose tolerance test increases postprandial glycemia: Implications for diagnostic criteria. Can. Med. Assoc. J. 2000, 162, 993–996. [Google Scholar]
- Braunstein, C.R.; Noronha, J.C.; Glenn, A.J.; Viguiliouk, E.; Noseworthy, R.; Khan, T.A.; Au-Yeung, F.; Mejia, S.B.; Wolever, T.M.; Josse, R.G.; et al. A Double-Blind, Randomized Controlled, Acute Feeding Equivalence Trial of Small, Catalytic Doses of Fructose and Allulose on Postprandial Blood Glucose Metabolism in Healthy Participants: The Fructose and Allulose Catalytic Effects (FACE) Trial. Nutrients 2018, 10, 750. [Google Scholar] [CrossRef]
- Noronha, J.C.; Braunstein, C.R.; Glenn, A.J.; Khan, T.A.; Viguiliouk, E.; Noseworthy, R.; Mejia, S.B.; Kendall, C.W.C.; Wolever, T.M.S.; Leiter, L.A.; et al. The effect of small doses of fructose and allulose on postprandial glucose metabolism in type 2 diabetes: A double-blind, randomized, controlled, acute feeding, equivalence trial. Diabetes Obes. Metab. 2018, 20, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group. 2013. Available online: www.guidelinedevelopment.org/handbook (accessed on 20 November 2022).
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Noronha, J.C.; Khan, T.A.; McGlynn, N.; Back, S.; Grant, S.M.; Kendall, C.W.C.; Sievenpiper, J.L. The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis. Nutrients 2023, 15, 1050. https://doi.org/10.3390/nu15041050
Zhang R, Noronha JC, Khan TA, McGlynn N, Back S, Grant SM, Kendall CWC, Sievenpiper JL. The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis. Nutrients. 2023; 15(4):1050. https://doi.org/10.3390/nu15041050
Chicago/Turabian StyleZhang, Roselyn, Jarvis C. Noronha, Tauseef A. Khan, Néma McGlynn, Songhee Back, Shannan M. Grant, Cyril W. C. Kendall, and John L. Sievenpiper. 2023. "The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis" Nutrients 15, no. 4: 1050. https://doi.org/10.3390/nu15041050
APA StyleZhang, R., Noronha, J. C., Khan, T. A., McGlynn, N., Back, S., Grant, S. M., Kendall, C. W. C., & Sievenpiper, J. L. (2023). The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis. Nutrients, 15(4), 1050. https://doi.org/10.3390/nu15041050





