The Influence of Nutritional Intervention in the Treatment of Hashimoto’s Thyroiditis—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
- original articles focusing on nutritional/dietary interventions (or nutritional/dietary interventions + supplementation) in patients diagnosed with Hashimoto’s disease;
- articles in English;
- articles to which full access has been granted.
- articles other than original (case study, review, meta-analysis, commentary, book chapter, post-conference materials, and so on);
- articles that do not include dietary interventions;
- nutritional interventions without a control group;
- non-human studies;
- articles not focused on Hashimoto’s disease;
- articles including patients with thyroidectomy;
- supplementation-only interventions;
- interventions that were designed to induce Hashimoto’s disease;
- articles to which full access has not been granted despite attempts to contact a corresponding author.
2.3. Construction of the Review
- Population: Patients diagnosed with Hashimoto’s disease at any stage of life and regardless of gender and levothyroxine treatment status.
- Intervention: Nutritional/dietary interventions (healthy eating, elimination diets) or nutrition + supplementation.
- Comparison: The control group with/no nutritional/dietary intervention and with/no supplementation.
- Outcome: Parameters’ evaluation including at least one marker from the following: thyroid hormones (fT3, fT4); TSH; antibodies: anti-TPO and anti-TG; ultrasound examination of the thyroid gland; quality of life; anthropometric measurements (body weight, waist circumference, hip circumference); or body composition analysis [30].
3. Results
3.1. Characteristics of Selected Articles
3.2. Characteristics of the Nutritional Intervention
3.3. Main Outcomes
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Attard, C.C.; Vella, S. Aetiology of thyroid autoimmunity. Malta Med. J. 2018, 30, 26–31. [Google Scholar]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- Uysal, H.B.; Ayhan, M. Autoimmunity affects health-related quality of life in patients with Hashimoto’s thyroiditis. Kaohsiung J. Med. Sci. 2016, 32, 427–433. [Google Scholar] [CrossRef]
- Patti, M.; Christian, R.; Palokas, M. Association between anti-thyroid antibodies and quality of life in patients with Hashimoto thyroiditis: A systematic review and meta-analysis. JBI Evid. Synth. 2021, 19, 2307–2338. [Google Scholar] [CrossRef]
- Groenewegen, K.L.; Mooij, C.F.; van Trotsenburg, A.S.P. Persisting symptoms in patients with Hashimoto’s disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J. Transl. Autoimmun. 2021, 4, 100101. [Google Scholar] [CrossRef]
- Danailova, Y.; Velikova, T.; Nikolaev, G.; Mitova, Z.; Shinkov, A.; Gagov, H.; Konakchieva, R. Nutritional Management of Thyroiditis of Hashimoto. Int. J. Mol. Sci. 2022, 23, 5144. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Drywien, M.; Wator, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Annals Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef]
- Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Anastasilakis, A.D.; Tzellos, T.G.; Goulis, D.G.; Kouvelas, D. Selenium supplementation in the treatment of Hashimoto’s thyroiditis: A systematic review and a meta-analysis. Thyroid 2010, 20, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Mandecka, A.; Regulska-Ilow, B. Metabolic disorders and nutritional status in autoimmune thyroid diseases. Postepy Hig. Med. Dosw. 2015, 69, 80–90. [Google Scholar]
- Ihnatowicz, P.; Wątor, P.; Drywień, M.E. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Annals Agric. Environ. Med. 2021, 28, 558–568. [Google Scholar] [CrossRef]
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar]
- Jiang, H.; Chen, X.; Qian, X.; Shao, S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis—A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 767–775. [Google Scholar] [CrossRef]
- Winther, K.H.; Wichman, J.E.M.; Bonnema, S.J.; Hegedüs, L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine 2017, 55, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Štefanić, M.; Tokić, S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: A systematic review, meta-analysis and meta-regression of observational studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Albusta, A.Y.; Fedorowicz, Z.; Carter, B.; Pijl, H. Selenium supplementation for Hashimoto’s thyroiditis. Cochrane Database Syst. Rev. 2013, 3, CD010223. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Camastra, S.; Bonofiglio, D.; Antonelli, A.; Fallahi, P. Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies. Nutrients 2020, 12, 1337. [Google Scholar] [CrossRef]
- Medical Subject Headings 2023. Available online: https://meshb.nlm.nih.gov/search (accessed on 10 September 2022).
- Page, M.J.; Moher, D.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Gluten-free diet attenuates the impact of exogenous vitamin D on thyroid autoimmunity in young women with autoimmune thyroiditis: A pilot study. Scand. J. Clin. Lab. Investig. 2022, 82, 518–524. [Google Scholar] [CrossRef]
- Ostrowska, L.; Gier, D.; Zyśk, B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Tajmiri, S. The effects of powdered black cumin seeds on markers of oxidative stress, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in patients with Hashimoto’s thyroiditis. Clin. Nutr. ESPEN 2020, 37, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Dehghan, P.; Tajmiri, S.; Abbasi, M.M. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF)-1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: A randomized controlled trial. BMC Complement. Altern. Med. 2016, 16, 471. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef]
- Esposito, T.; Lobaccaro, J.M.; Esposito, M.G.; Monda, V.; Messina, A.; Paolisso, G.; Varriale, B.; Monda, M.; Messina, G. Effects of low-carbohydrate diet therapy in overweight subject with autoimmune thyroiditis: Possible synergism with ChREBP. Drug Des. Devel. Ther. 2016, 10, 2939–2946. [Google Scholar]
- Asik, M.; Gunes, F.; Binnetoglu, E.; Eroglu, M.; Bozkurt, N.; Sen, H.; Akbal, E.; Bakar, C.; Beyazit, Y.; Ukinc, K. Decrease in TSH levels after lactose restriction in Hashimoto’s thyroiditis patients with lactose intolerance. Endocrine 2014, 46, 279–284. [Google Scholar] [CrossRef]
- Yoon, S.J.; Choi, S.R.; Kim, D.M.; Kim, J.U.; Kim, K.W.; Ahn, C.W.; Cha, B.S.; Lim, S.K.; Kim, K.R.; Lee, H.C.; et al. The Effect of Iodine Restriction on Thyroid Function in Patients with Hypothyroidism Due to Hashimoto’s Thyroiditis. Yonsei Med. J. 2003, 44, 227–235. [Google Scholar] [CrossRef]
- Ruchała, M.; Szczepanek-Parulska, E.; Zybek, A. The influence of lactose intolerance and other gastro-intestinal tract disorders on L-thyroxine absorption. Endokrynol. Pol. 2012, 63, 318–323. [Google Scholar]
- Cellini, M.; Santaguida, M.G.; Gatto, I.; Virili, C.; Del Duca, S.C.; Brusca, N.; Capriello, S.; Gargano, L.; Centanni, M. Systematic appraisal of lactose intolerance as cause of increased need for oral thyroxine. J. Clin. Endocrin. Metab. 2014, 99, E1454–E1458. [Google Scholar] [CrossRef]
- Marabotto, E.; Ferone, D.; Sheijani, A.D.; Vera, L.; Ziola, S.; Savarino, E.; Bodini, G.; Furnari, M.; Zentilin, P.; Savarino, V.; et al. Prevalence of Lactose Intolerance in Patients with Hashimoto Thyroiditis and Impact on LT4 Replacement Dose. Nutrients 2022, 14, 3017. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Dore, M.P.; Fanciulli, G.; Rouatbi, M.; Mereu, S.; Pes, G.M. Autoimmune Thyroid Disorders Are More Prevalent in Patients with Celiac Disease: A Retrospective Case-Control Study. J. Clin. Med. 2022, 11, 6027. [Google Scholar] [CrossRef]
- Virili, C.; Bassotti, G.; Santaguida, M.G.; Iuorio, R.; Del Duca, S.C.; Mercuri, V.; Picarelli, A.; Gargiulo, P.; Gargano, L.; Centanni, M. Atypical celiac disease as cause of increased need for thyroxine: A systematic study. J. Clin. Endocrin. Metab. 2012, 97, E419–E422. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Malandrini, S.; Trimboli, P.; Guzzaloni, G.; Virili, C.; Lucchini, B. What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients 2022, 14, 1681. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; Savastano, S.; Tommaselli, A.P.; Dorato, M.; Scarpitta, M.T.; Gigante, M.; Micillo, M.; Paparo, F.; Petrone, E.; Lombardi, G.; et al. Prevalence of coeliac disease in patients with thyroid autoimmunity. Horm. Res. 1999, 51, 124–127. [Google Scholar] [CrossRef]
- Szostak-Wegierek, D.; Bednarczuk, T.; Respondek, W.; Traczyk, I.; Cukrowska, B.; Ostrowska, L.; Włodarek, D.; Jeznach-Steinhagen, A.; Bierła, J.; Lange, E.; et al. The validity of gluten-free diet in Hashimoto’s thyroiditis: Statement of the Expert Committee of the Section of Medical Dietetics of the Polish Society for Parenteral, Enteral Nutrition and Metabolism (POLSPEN). Adv. Clin. Nutr. 2018, 47, 33–47. [Google Scholar]
- Song, R.H.; Wang, B.; Yao, Q.M.; Li, Q.; Jia, X.; Zhang, J.A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Sheikhi, A.; Varkaneh, H.K.; Zarezadeh, M.; Rahmani, J.; Milajerdi, A. Effect of Nigella sativa supplementation on obesity indices: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2018, 38, 48–57. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Ayati, M.H.; Abdollahi, M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018, 219, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Khabbazi, A.; Javadivala, Z.; Seyedsadjadi, N.; Malek Mahdavi, A. A Systematic Review of the Potential Effects of Nigella sativa on Rheumatoid Arthritis. Planta Med. 2020, 86, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.A.; Fahmy, H.M.; Mohammed, F.F.; Elsayed, A.A.; Radwan, N.M. Nigella sativa amliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6269–6286. [Google Scholar] [PubMed]
- Nikkhah-Bodaghi, M.; Darabi, Z.; Agah, S.; Hekmatdoost, A. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytother. Res. 2019, 33, 1027–1032. [Google Scholar] [CrossRef]
- Ahmed, J.H.; Kadhim, S.N.; Al-Hamdi, K.I. The effectiveness of Nigella sativa, methotrexate and their combination in the treatment of moderate to severe psoriasis. J. Clin. Exp. Investig. 2014, 5, 521–528. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Møllehave, L.T.; Linneberg, A.; Skaaby, T.; Knudsen, N.; Jørgensen, T.; Thuesen, B.H. Trends in treatments of thyroid disease following iodine fortification in Denmark: A nationwide register-based study. Clin. Epidemiol. 2018, 10, 763–770. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Global Endocrinology: Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, 13–21. [Google Scholar] [CrossRef]
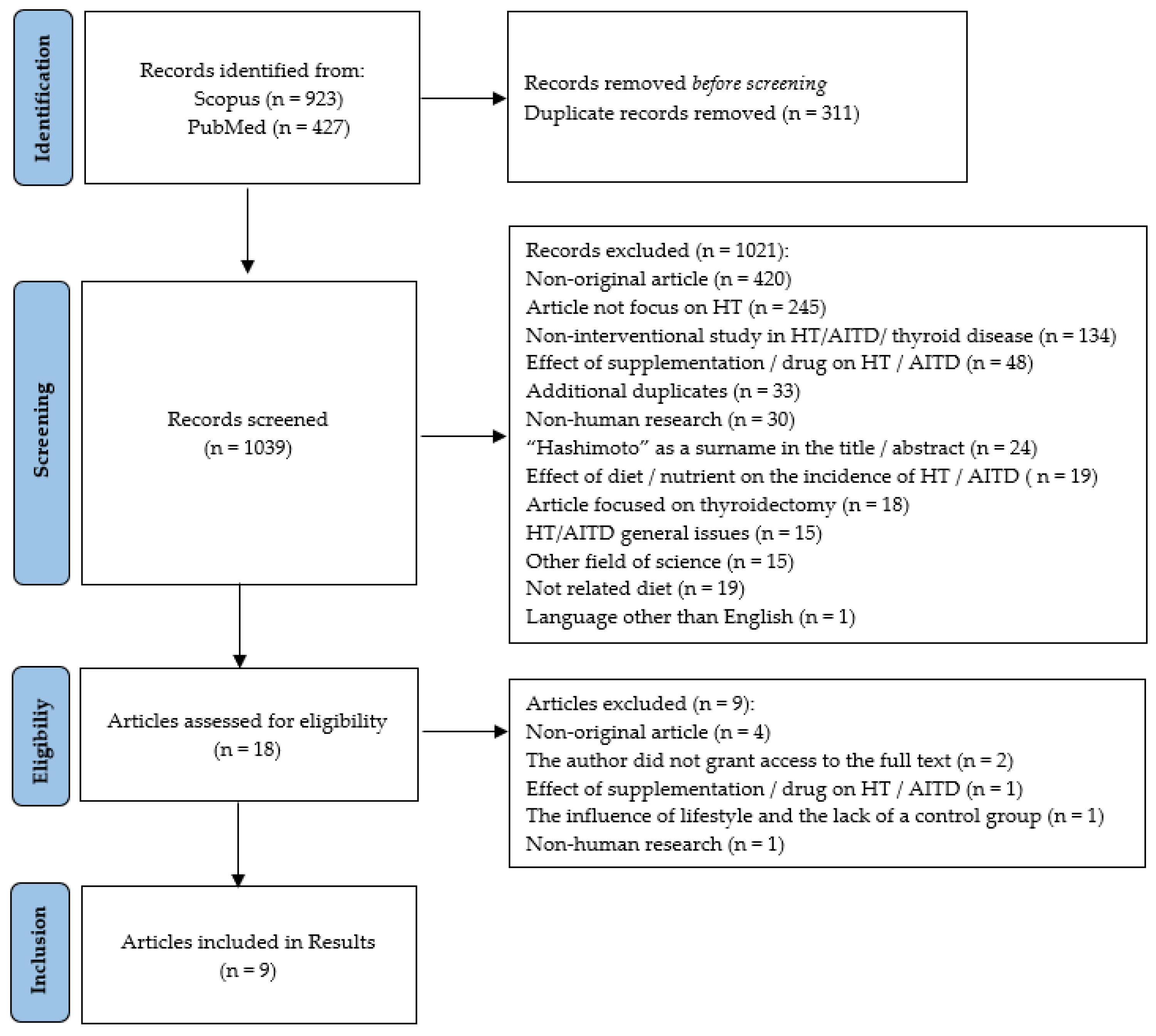
| Scopus (n = 923) |
| (TITLE-ABS(“Hashimoto*”) OR KEY(“Hashimoto disease”)) AND (KEY(“diet”) OR KEY(“nutrition therapy”) OR KEY(“minerals”) OR KEY(“vitamins”) OR KEY(“nutrients”) OR TITLE-ABS(“diet*”) OR (TITLE-ABS(“nutrit*”) AND TI-TLE-ABS(“therap*”)) OR TITLE-ABS(“mineral*”) OR TITLE-ABS(“vitamin*”) OR TI-TLE-ABS(“nutrient*”)) |
| PubMed (n = 427) |
| (“diet”[MeSH Terms] OR “nutrition therapy”[MeSH Terms] OR “minerals”[MeSH Terms] OR “vitamins”[MeSH Terms] OR “nutrients”[MeSH Terms] OR “di-et*”[Title/Abstract] OR (“nutrit*”[Title/Abstract] AND “therap*”[Title/Abstract]) OR “mineral*”[Title/Abstract] OR “vitamin*”[Title/Abstract] OR “nutrient*” [Ti-tle/Abstract]) AND (“Hashimoto*”[Title/Abstract] OR “Hashimoto Disease”[MeSH Terms]) |
| Authors, Year, Country | N, % Women, Age (Mean ± SD), BMI (Mean ± SD), % of Euthyroid Patients, % of Patients Taking LT4 | Duration | Description of the Nutritional Intervention | Results |
|---|---|---|---|---|
| Krysiak et al., 2022, Poland [31] | EG (with non-celiac gluten sensitivity): 31, 100%, 35 ± 7, 22.6 ± 3, 100%, 0% CG (without non-celiac gluten sensitivity): 31, 100%, 36 ± 7, 23.5 ± 3.5, 100%, 0%, 0% | 12 months | EG: Gluten-free diet + vitamin D (100 µg [4000 IU]/day) CG: Gluten diet + vitamin D (100 µg [4000 IU]/day) | ↑ Anti-TPO (p < 0.0017), anti-TG (p < 0.0056) <-> TSH, fT4, fT3, fT3/fT4 ratio |
| Ostrowska et al., 2021, Poland [32] | EG: 45, 100%, 42.74 ± 10.51, 35.63 ± 4.06, nd, 100% CG: 40, 100%, 41.02 ± 11.96, 35.87 ± 5.59, nd, 100% | 6 months | EG (reducing + elimination): Deficit at the level of 1400–1600 kcal + dietary elimination based on the IgG test + 200 mcg of 1-selenomethionine/day and 30 mg of zinc gluconate/day CG (reducing): Deficit at the level of 1400–1600 kcal + 200 mcg of 1-selenomethionine/day and 30 mg of zinc gluconate/day | ↓ Weight (p < 0.001), BMI (p < 0.002), % body fat (p = 0.026), TSH (p < 0.001), anti-TPO (p < 0.001), anti-TG (p < 0.048) ↑ fT3 (p < 0.001) and fT4 (p < 0.001) |
| Pobłocki et al., 2021, Poland [33] | EG: 31, 100%, 36.64 ± nd, 26.27 ± nd, 100%, 100% CG: 31, 100%, 37.07 ± nd, 24.53 ± nd, 100%, 100% | 12 months | EG: Gluten-free diet CG: Gluten diet | ↓ TSH (p = 0.044) <-> fT3, fT4, anti-TPO, anti-TG |
| Farhangi et al., 2020 [34]; Farhangi et al., 2016, Iran [35] | EG: 20, 85%, 35.70 ± 8.18, 27.10 ± 4.63, nd, 100% CG: 20, 85%, 33.95 ± 8.72, 25.93 ± 4.07, nd, 100% | 8 weeks | EG: Nigella sativa powder 2 g/day (1 g before lunch and 1 g before dinner) CG: Placebo-starch powder 2 g/day (1 g before lunch and 1 g before dinner) | ↓ TSH (p = 0.02), anti-TPO (p = 0.01) ↑ T4 (p = 0.04) <-> weight, BMI, WHR, T3 |
| Krysiak et al., 2018, Poland [36] | EG: 16, 100%, 30 ± 5, 22.9 ± 2.3, 100%, 0% CG: 18, 100%, 31 ± 6, 23.1 ± 2.1, 100%, 0% | 6 months | EG: Gluten-free diet CG: Gluten diet | ↓ Anti-TPO, anti-TG (p < 0.05) <->TSH, fT3, fT4 |
| Esposito et al., 2016, Italy [37] | EG: 108, 50%, nd, nd, nd, nd CG: 72, 44%, nd, nd, nd, nd | 3 weeks | EG: Diet based on the proportions of macronutrients: proteins 50–60%, fats 25–30%, carbohydrates 12–15%. Additional mandatory recommendations: eat vegetables, including large leafy vegetables, and only lean parts of white and red meat. Products such as eggs, dairy products, legumes, fruit, bread, pasta, goitre food, and rice were excluded CG: Low-energy diet with no exclusions as to the type of food consumed, but the patient should follow the recommended diet, according to the assumptions of the National Food and Nutrition Research Institute | ↓ Weight (p < 0.05) and body fat mass (p < 0.05) (in the groups) |
| Asik et al., 2014, Turkey [38] | EG: 38 of LI, 97%, Age: E 45.67 ± 10.28, SCH 35.5 ± 9.87, 79%, 100% BMI: E 27.54 ± 5.77, SCH 30.34 ± 4.59 CG: 12 E without LI, 92%, 47.9 ± 8.73, 29.27 ± 3.67, 100%, 100% | 8 weeks | EG: Lactose-free diet CG: Lactose-free diet | ↓ TSH (p < 0.05) <-> fT4 |
| Yoon et al., 2003, South Korea [39] | EG: 23, 100%, 40.70 ± 10.49, nd, 0%, 0% CG: 22, 86%, 43.50 ± 11.88, nd, 0%, 0% | 3 months | EG: Limiting iodine intake with the diet to 100 mcg per day in a region with excessive iodine intakes CG: No restriction of iodine intake with the diet in a region with excessive iodine intakes | No investigated correlations between groups |
| Authors, Year, Country | Description of the Implemented Nutritional Support | |
|---|---|---|
| Experimental Group | Control Group | |
| Krysiak et al., 2022, Poland [31] | Avoiding cereal products containing gluten. As gluten-free substitutes, the researchers recommended eating foods produced by certified gluten-free food producers; Supplementation: vitamin D 100 µg [4000 IU] (once a day in the morning) | Remained on a gluten diet typical for the examined women; Supplementation: vitamin D 100 µg [4000 IU] (once a day in the morning) |
| Ostrowska et al., 2021, Poland [32] | Individual elimination of products, in accordance with the results obtained from the laboratory tests of food sensitivity type III in the immunoglobulin class G 1–3 by ELISA method; Macronutrient content in the diet—25% protein, 30% fat, 45% carbohydrates; The diet was balanced in terms of the standards for the demand for micro and macro elements for a given age group; Energy supply was at the level of 1400–1600 kcal (deficit of about 1000 kcal/day, depending on the individual resting metabolism and energy expenditure); Supplementation: 200 mcg of 1-selenomethionine/day and 30 mg of zinc gluconate/day. |
Macronutrient content in the diet—25% protein, 30% fat, 45% carbohydrates; The diet covered the daily requirement for micro and macro elements for a given age group; Energy supply of 1400–1600 kcal (deficit of about 1000 kcal/day, depending on the individual resting metabolic rate and energy expenditure); Supplementation: 200 mcg of 1-selenomethionine/day and 30 mg of zinc gluconate/day. |
| Pobłocki et al., 2021, Poland [33] | The group received a freely available brochure on the gluten-free diet; Gluten-free diet was defined as the consumption of gluten-free natural and processed products containing ≤20 mg gluten/1 kg; All participants received a sample of a gluten-free diet menu; Education on the following: proper distribution of meals, energy during the day and the correct structure of fatty acid consumption, increased supply of omega-3 fatty acids and reduced consumption of saturated and trans fatty acids, products with a low glycemic index, a reduction in the supply of simple carbohydrates, an increased consumption of fiber. | A diet containing gluten, typical for the Polish population. |
| Farhangi et al., 2020 [34]; Farhangi et al., 2016, Iran [35] | Capsules with Nigella sativa powder 2 g/day (1 g before lunch and 1 g before dinner). | Capsules with placebo-starch powder 2 g/day (1 g before lunch and 1 g before dinner). |
| Krysiak et al., 2018, Poland [36] | Education by a medical doctor and dietitian; gluten-free diet leaflets. | A diet containing gluten, with no additional dietary recommendations. |
| Esposito et al., 2016, Italy [37] |
Diet based on the proportions of macronutrients: proteins 50–60%, fats 25–30%, carbohydrates 12–15%; Eat vegetables, including large leafy vegetables and only lean parts of white and red meat; Products such as eggs, dairy products, legumes, fruit, bread, pasta, goitrogens food, and rice are excluded. | Low-energy diet with no exclusions as to the type of food consumed, but the patient should follow the recommended diet, in accordance with the assumptions of the National Research Institute on Food and Nutrition. |
| Asik et al., 2014, Turkey [38] |
Reduction in amount of lactose in the diet (avoiding milk and dairy products, including skimmed milk powder and whey powder); Avoiding, especially in the morning, beverages (grapefruit juice, coffee) and foods rich in fiber and soybean meal. |
Reduction of the consumption of these foods as the study group, especially in the morning; Avoiding, especially in the morning, beverages (grapefruit juice, coffee) and foods rich in fiber and soybean meal. |
| Yoon et al., 2003, South Korea [39] | Limiting iodine intake in the diet to 100 mcg per day. | No restriction of iodine intake in the diet. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osowiecka, K.; Myszkowska-Ryciak, J. The Influence of Nutritional Intervention in the Treatment of Hashimoto’s Thyroiditis—A Systematic Review. Nutrients 2023, 15, 1041. https://doi.org/10.3390/nu15041041
Osowiecka K, Myszkowska-Ryciak J. The Influence of Nutritional Intervention in the Treatment of Hashimoto’s Thyroiditis—A Systematic Review. Nutrients. 2023; 15(4):1041. https://doi.org/10.3390/nu15041041
Chicago/Turabian StyleOsowiecka, Karolina, and Joanna Myszkowska-Ryciak. 2023. "The Influence of Nutritional Intervention in the Treatment of Hashimoto’s Thyroiditis—A Systematic Review" Nutrients 15, no. 4: 1041. https://doi.org/10.3390/nu15041041
APA StyleOsowiecka, K., & Myszkowska-Ryciak, J. (2023). The Influence of Nutritional Intervention in the Treatment of Hashimoto’s Thyroiditis—A Systematic Review. Nutrients, 15(4), 1041. https://doi.org/10.3390/nu15041041








