Neither Trimethylamine-N-Oxide nor Trimethyllysine Is Associated with Atherosclerosis: A Cross-Sectional Study in Older Japanese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics
2.3. Data Collection
2.4. Measurements of L-Carnitine-Related Metabolites
2.5. Statistics
3. Results
3.1. Demographic Data of the Studied Population
3.2. Simple and Multiple Regression Analyses of All Participants
3.3. Multiple Regression Analyses in Women and Men
3.4. Plasma Levels of TMAO and Other Metabolites Were Associated with the Plasma Concentrations of EPA and DHA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoshino, A.; Nakamura, T.; Enomoto, S.; Kawahito, H.; Kurata, H.; Nakahara, Y.; Ijichi, T. Prevalence of coronary artery disease in Japanese patients with cerebral infarction impact of metabolic syndrome and intracranial large artery atherosclerosis. Circ. J. 2008, 72, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Cenko, E.; Badimon, L.; Bugiardini, R.; Claeys, M.J.; De Luca, G.; de Wit, C.; Derumeaux, G.; Dorobantu, M.; Duncker, D.J.; Eringa, E.C.; et al. Cardiovascular disease and COVID-19: A consensus paper from the ESC Working Group on Coronary Pathophysiology & Microcirculation, ESC Working Group on Thrombosis and the Association for Acute CardioVascular Care (ACVC), in collaboration with the European Heart Rhythm Association (EHRA). Cardiovasc. Res. 2021, 117, 2705–2729. [Google Scholar] [PubMed]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; Chalcraft, K.; Ak, H.; Davis, A.D.; Lonn, E.; Miller, R.; Potter, M.A.; Yusuf, S.; Anand, S.S.; McQueen, M.J. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multiethnic population living in Canada. Can. J. Cardiol. 2015, 31, 1189–1194. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Lang, D.; Yeung, C.; Peter, R.; Ibarra, C.; Gasser, R.; Itagaki, K.; Philpot, R.; Rettie, A. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: Selective catalysis by FMO3. Biochem. Pharmacol. 1998, 56, 1005–1012. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Loscalzo, J. Gut microbiota, the genome, and diet in atherogenesis. N. Engl. J. Med. 2013, 368, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.; Rader, D.J. The diet–microbe morbid union. Nature 2011, 472, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.L.; Brown, J.M. Eggs as a Dietary Source for Gut Microbial Production of Trimethylamine-N-Oxide; Oxford University Press: Oxford, UK, 2014; Volume 100, pp. 741–743. [Google Scholar]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef]
- Yazdekhasti, N.; Brandsch, C.; Schmidt, N.; Schloesser, A.; Huebbe, P.; Rimbach, G.; Stangl, G.I. Fish protein increases circulating levels of trimethylamine-N-oxide and accelerates aortic lesion formation in apoE null mice. Mol. Nutr. Food Res. 2016, 60, 358–368. [Google Scholar] [CrossRef]
- Papandreou, C.; Moré, M.; Bellamine, A. Trimethylamine N-oxide in relation to cardiometabolic health—Cause or effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef]
- Skagen, K.; Trøseid, M.; Ueland, T.; Holm, S.; Abbas, A.; Gregersen, I.; Kummen, M.; Bjerkeli, V.; Reier-Nilsen, F.; Russell, D.; et al. The Carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016, 247, 64–69. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Z.; Cajka, T.; Buffa, J.A.; Nemet, I.; Hurd, A.G.; Gu, X.; Skye, S.M.; Roberts, A.B.; Wu, Y.; et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018, 3, e99096. [Google Scholar] [CrossRef]
- Li, X.S.; Obeid, S.; Wang, Z.; Hazen, B.J.; Li, L.; Wu, Y.; Hurd, A.G.; Gu, X.; Pratt, A.; Levison, B.S.; et al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near-and long-term prognostic value in patients presenting with acute coronary syndromes. Eur. Heart J. 2019, 40, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis 2015, 239, 61–66. [Google Scholar] [CrossRef]
- Yano, S.; Nagai, A.; Isomura, M.; Yamasaki, M.; Kijima, T.; Takeda, M.; Hamano, T.; Nabika, T. Relationship between blood myostatin levels and kidney function: Shimane CoHRE Study. PLoS ONE 2015, 10, e0141035. [Google Scholar] [CrossRef]
- Notsu, Y.; Yano, S.; Takeda, M.; Yamasaki, M.; Isomura, M.; Nabika, T.; Nagai, A. Association of high-density lipoprotein subclasses with carotid intima-media thickness: Shimane CoHRE Study. J. Atheroscler. Thromb. 2018, 25, 42–54. [Google Scholar] [CrossRef]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R., Jr.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in young adults study (CARDIA). J. Am. Heart Assoc. 2016, 5, e003970. [Google Scholar] [CrossRef]
- Stubbs, J.R.; Stedman, M.R.; Liu, S.; Long, J.; Franchetti, Y.; West, R.E.; Prokopienko, A.J.; Mahnken, J.D.; Chertow, G.M.; Nolin, T.D. Trimethylamine N-oxide and cardiovascular outcomes in patients with ESKD receiving maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Haissman, J.M.; Knudsen, A.; Hoel, H.; Kjær, A.; Kristoffersen, U.S.; Berge, R.K.; Katzenstein, T.L.; Svardal, A.; Ueland, T.; Aukrust, P.; et al. Microbiota-dependent marker TMAO is elevated in silent ischemia but is not associated with first-time myocardial infarction in HIV infection. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 71, 130–136. [Google Scholar] [CrossRef]
- Sinha, A.; Ma, Y.; Scherzer, R.; Rahalkar, S.; Neilan, B.D.; Crane, H.; Drozd, D.; Martin, J.; Deeks, S.G.; Hunt, P.; et al. Carnitine Is Associated with Atherosclerotic Risk and Myocardial Infarction in HIV-Infected Adults. J. Am. Heart Assoc. 2019, 8, e011037. [Google Scholar] [CrossRef]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Gibson, R.; Lau, C.-H.E.; Loo, R.L.; Ebbels, T.M.; Chekmeneva, E.; Dyer, A.R.; Miura, K.; Ueshima, H.; Zhao, L.; Daviglus, M.L.; et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am. J. Clin. Nutr. 2020, 111, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Aulisa, G.; Marcon, D.; Rizzo, G.; Tarsisano, M.G.; Di Renzo, L.; Federici, M.; Caprio, M.; De Lorenzo, A. Association of Urinary and Plasma Levels of Trimethylamine N-Oxide (TMAO) with Foods. Nutrients 2021, 13, 1426. [Google Scholar] [CrossRef] [PubMed]
- Krüger, R.; Merz, B.; Rist, M.J.; Ferrario, P.G.; Bub, A.; Kulling, S.E.; Watzl, B. Associations of current diet with plasma and urine TMAO in the KarMeN study: Direct and indirect contributions. Mol. Nutr. Food Res. 2017, 61, 1700363. [Google Scholar] [CrossRef]
- Ridker, P.M. Fish Consumption, Fish Oils, and Cardiovascular Events: Still Waiting for Definitive Evidence; Oxford University Press: Oxford, UK, 2016; Volume 104, pp. 951–952. [Google Scholar]
- Vázquez, C.; Botella-Carretero, J.I.; Corella, D.; Fiol, M.; Lage, M.; Lurbe, E.; Richart, C.; Fernández-Real, J.M.; Fuentes, F.; Ordóñez, A.; et al. White fish reduces cardiovascular risk factors in patients with metabolic syndrome: The WISH-CARE study, a multicenter randomized clinical trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.; Mortensen, E.L.; Henriksen, T.B.; Olsen, S.F. Fish consumption measured during pregnancy and risk of cardiovascular diseases later in life: An observational prospective study. PLoS ONE 2011, 6, e27330. [Google Scholar] [CrossRef]
- Marckmann, P.; Grønbaek, M. Fish consumption and coronary heart disease mortality. A systematic review of prospective cohort studies. Eur. J. Clin. Nutr. 1999, 53, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Pounis, G.D.; Panagiotakos, D.B.; Chrysohoou, C.; Aggelopoulos, P.; Tsiamis, E.; Pitsavos, C.; Stefanadis, C. Long-term fish consumption is associated with lower risk of 30-day cardiovascular disease events in survivors from an acute coronary syndrome. Int. J. Cardiol. 2009, 136, 344–346. [Google Scholar] [CrossRef]
- Gawrys-Kopczynska, M.; Konop, M.; Maksymiuk, K.; Kraszewska, K.; Derzsi, L.; Sozanski, K.; Holyst, R.; Pilz, M.; Samborowska, E.; Dobrowolski, L.; et al. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. eLife 2020, 9, e57028. [Google Scholar] [CrossRef]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429. [Google Scholar] [CrossRef]
- Melegh, B.; Hermann, R.; Bock, I. Generation of hydroxytrimethyllysine from trimethyllysine limits the carnitine biosynthesis in premature infants. Acta Paediatr. 1996, 85, 345–350. [Google Scholar] [CrossRef]
- Fischer, M.; Hirche, F.; Kluge, H.; Eder, K. A moderate excess of dietary lysine lowers plasma and tissue carnitine concentrations in pigs. Br. J. Nutr. 2009, 101, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Where does N(ε)-trimethyllysine for the carnitine biosynthesis in mammals come from? PLoS ONE 2014, 9, e84589. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J.; Lehman, L.J.; Olson, L. epsilon-N-trimethyllysine availability regulates the rate of carnitine biosynthesis in the growing rat. J. Nutr. 1986, 116, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, N.R.; Gidlund, M.A.; Goto, H.; Dias, C.T.; Okawabata, F.S.; Abdalla, D.S. Casein and soy protein isolate in experimental atherosclerosis: Influence on hyperlipidemia and lipoprotein oxidation. Ann. Nutr. Metab. 2001, 45, 38–46. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.; Van Horn, L.; Harris, W.; Kris-Etherton, P.; Winston, M. American Heart Association Nutrition Committee: Soy protein, isoflavones, and cardiovascular health: An American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation 2006, 113, 1034–1044. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef]
- Cross, T.-W.L.; Kasahara, K.; Rey, F.E. Sexual dimorphism of cardiometabolic dysfunction: Gut microbiome in the play? Mol. Metab. 2018, 15, 70–81. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a German adult population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef]
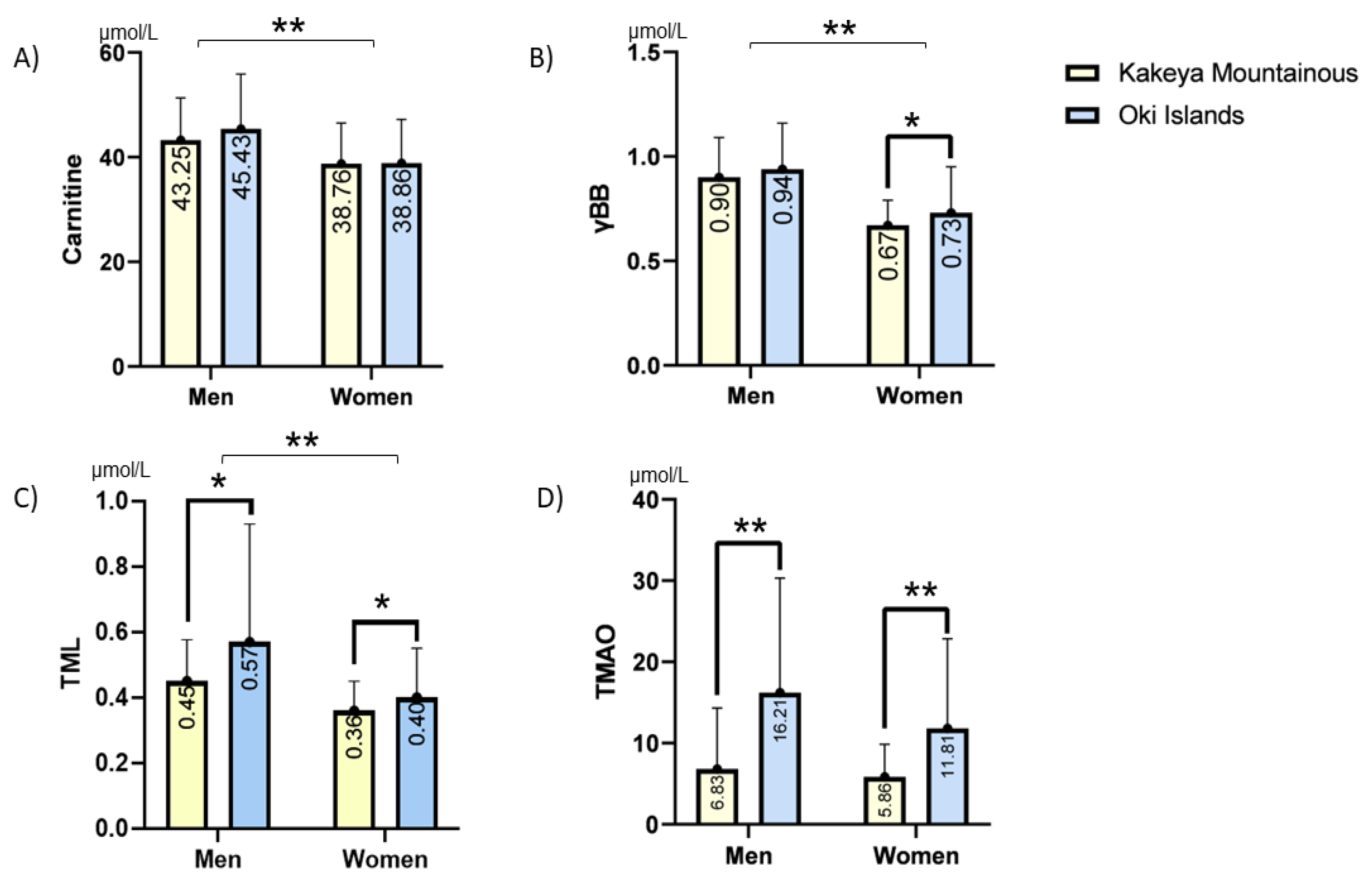
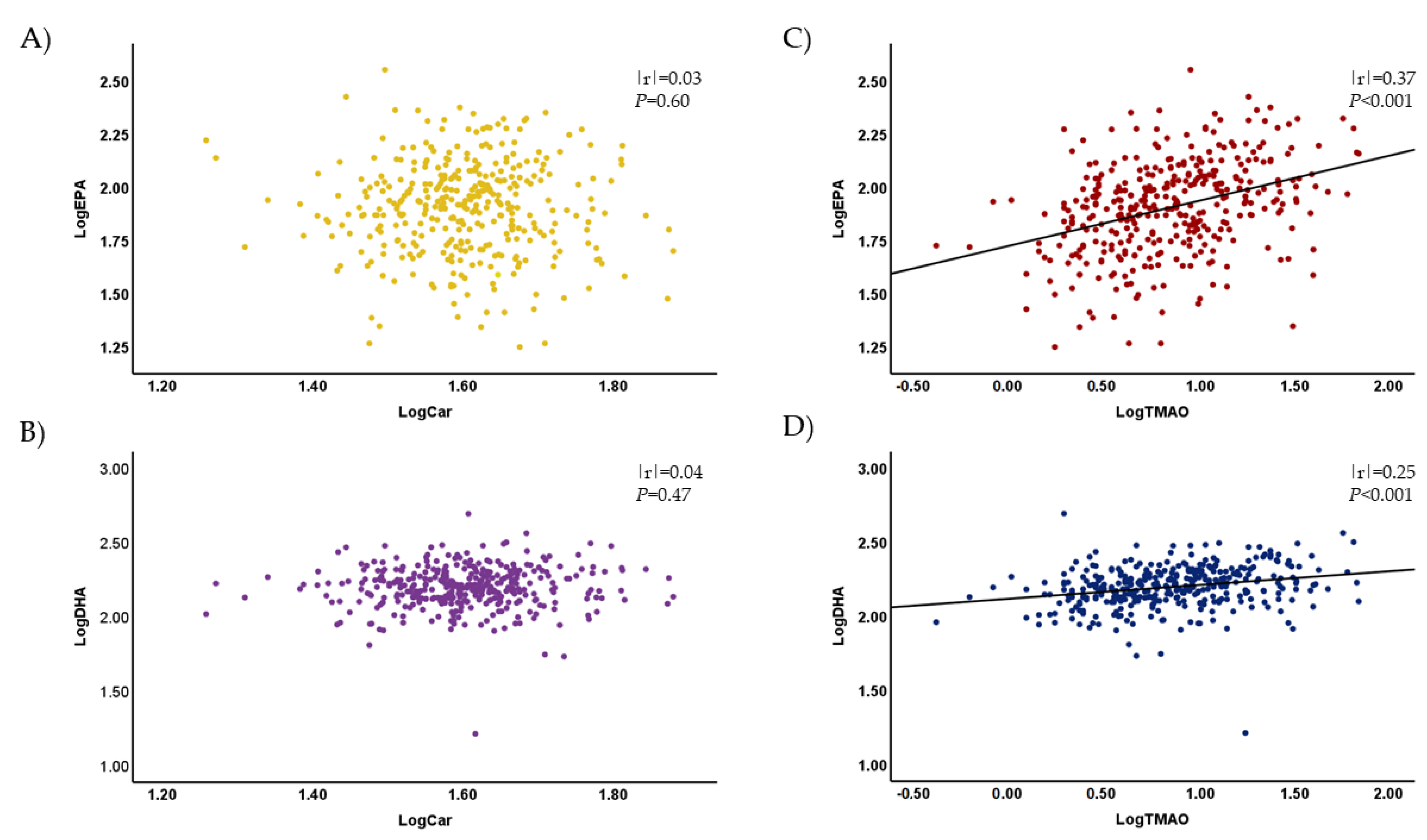
| Total (n: 364) | Men (n:142) | Women (n:222) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Men (n: 142) | Women (n: 222) | p-Value | Kakeya (n: 72) | Oki (n: 70) | p-Value | Kakeya (n: 85) | Oki (n: 137) | p-Value |
| Age (y) | 73.0 ± 8.5 | 72.5 ± 7.2 | 0.512 | 73.1 ± 8.4 | 73.0 ± 8.7 | 0.961 | 72.7 ± 6.3 | 72.3 ± 7.8 | 0.665 |
| Height (cm) | 162.6 ± 6.7 | 149.6 ± 5.7 | <0.001 | 163.8 ± 6.5 | 161.5 ± 6.8 | <0.05 | 150.1 ± 5.7 | 149.2 ± 5.8 | 0.312 |
| Weight (kg) | 61.5 ± 10.3 | 50.8 ± 8.7 | <0.001 | 61.1 ± 10.1 | 62.0 ± 10.5 | 0.566 | 48.2 ± 8.0 | 52.4 ± 8.7 | <0.001 |
| BMI (kg/m2) | 23.2 ± 3.2 | 22.7 ± 3.5 | 0.158 | 22.7 ± 3.2 | 23.7 ± 3.1 | 0.064 | 21.4 ± 3.3 | 23.5 ± 3.5 | <0.001 |
| DL (%) | 15.5 | 35.1 | <0.001 | 15.3 | 15.7 | 0.218 | 28.2 | 39.4 | <0.05 |
| DM (%) | 10.6 | 7.2 | 0.264 | 8.3 | 12.9 | <0.05 | 7.1 | 7.3 | 0.653 |
| HT (%) | 52.8 | 40.1 | <0.05 | 47.2 | 58.6 | <0.05 | 36.5 | 42.3 | 0.120 |
| CVD (%) | 9.64 | 5.23 | <0.001 | 9.55 | 9.71 | 0.288 | 3.18 | 6.8 | 0.264 |
| Habitual drinking (%) | 44.4 | 6.3 | <0.001 | 47.2 | 41.4 | 0.491 | 9.4 | 4.4 | 0.135 |
| Current smoking (%) | 8.5 | 1.4 | <0.001 | 9.7 | 7.1 | 0.584 | 1.2 | 1.5 | 0.860 |
| EPA (μg/mL) | 95.9 ± 56.7 | 87.82 ± 38.6 | 0.108 | 81.2 ± 45.2 | 111.1 ± 63.31 | 0.002 | 73.3 ± 32,0 | 96.9 ± 39.6 | <0.001 |
| DHA (μg/mL) | 164.21 ± 63.4 | 166.42 ± 43.82 | 0.695 | 142.9 ± 41.1 | 186.2 ± 74.2 | <0.001 | 143.8 ± 32.8 | 180.4 ± 44.0 | <0.001 |
| Max-IMT (mm) | 2.08 (1.92–2.24) | 1.74 (1.64–1.84) | <0.001 | 1.92 (1.72–2.11) | 1.98 (1.74–2.22) | 0.706 | 1.75 (1.59–1.92) | 1.73 (1.61–1.85) | 0.861 |
| PS (mm) | 5.42 (4.63–6.21) | 3.26 (2.90–3.61) | <0.001 | 4.81 (3.79–5.83) | 4.81 (3.67–5.96) | 0.996 | 3.18 (2.67–3.70) | 3.31 (2.83–3.78) | 0.483 |
| Variables | Spearman’s ρ | Model 1 | Model 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρ | p-Value | β | SE | Standard β | t | p-Value | VIF | β | SE | Standard β | t | p-Value | VIF | |
| Carnitine | 0.122 | 0.020 | 0.009 | 0.005 | 0.096 | 1.725 | 0.085 | 1.152 | 0.006 | 0.005 | 0.072 | 1.312 | 0.190 | 1.274 |
| γBB | 0.162 | 0.002 | 0.337 | 0.221 | 0.091 | 1.527 | 0.128 | 1.327 | −0.120 | 0.232 | −0.033 | −0.516 | 0.606 | 1.664 |
| TMAO | −0.018 | 0.726 | 0.004 | 0.005 | 0.048 | 0.793 | 0.428 | 1.351 | 0.008 | 0.005 | 0.107 | 1.759 | 0.080 | 1.543 |
| TML | 0.061 | 0.249 | −0.069 | 0.228 | −0.018 | −0.303 | 0.762 | 1.280 | −0.588 | 0.228 | −0.152 | −2.581 | 0.010 | 1.447 |
| Age | 0.314 | <0.001 | 0.029 | 0.006 | 0.279 | 5.138 | <0.001 | 1.234 | ||||||
| BMI | 0.008 | 0.880 | −0.007 | 0.013 | −0.030 | −0.550 | 0.582 | 1.223 | ||||||
| DL | −0.044 | 0.402 | −0.003 | 0.088 | −0.002 | −0.037 | 0.971 | 1.174 | ||||||
| DM | 0.033 | 0.536 | 0.030 | 0.123 | 0.013 | 0.246 | 0.806 | 1.096 | ||||||
| HT | 0.257 | <0.001 | 0.202 | 0.089 | 0.122 | 2.264 | 0.024 | 1.212 | ||||||
| Drinker | 0.107 | 0.041 | 0.041 | 0.111 | 0.021 | 0.369 | 0.712 | 1.330 | ||||||
| Smoker | 0.014 | 0.791 | 0.088 | 0.205 | 0.022 | 0.427 | 0.670 | 1.074 | ||||||
| Sex #1 | 0.214 | <0.001 | 0.364 | 0.109 | 0.221 | 3.328 | 0.001 | 1.839 | ||||||
| Area #2 | −0.014 | 0.785 | 0.001 | 0.088 | 0.001 | 0.012 | 0.991 | 1.236 | ||||||
| Variables | IMT | PS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | Standard β | t | p-Value | VIF | β | SE | Standard β | t | p-Value | VIF | |
| Carnitine | 0.017 | 0.006 | 0.204 | 2.941 | 0.004 | 1.207 | 0.066 | 0.022 | 0.213 | 3.015 | 0.003 | 1.207 |
| γBB | −0.395 | 0.281 | −0.108 | −1.405 | 0.161 | 1.472 | −0.851 | 1.049 | −0.063 | −0.811 | 0.418 | 1.472 |
| TMAO | 0.009 | 0.007 | 0.125 | 1.383 | 0.168 | 2.047 | 0.023 | 0.024 | 0.087 | 0.942 | 0.347 | 2.047 |
| TML | −0.826 | 0.454 | −0.153 | −1.819 | 0.070 | 1.789 | −3.172 | 1.694 | −0.161 | −1.873 | 0.063 | 1.789 |
| Age | 0.028 | 0.007 | 0.294 | 4.199 | <0.001 | 1.230 | 0.100 | 0.025 | 0.289 | 4.051 | <0.001 | 1.230 |
| BMI | −0.006 | 0.014 | −0.031 | −0.434 | 0.665 | 1.296 | 0.005 | 0.052 | 0.007 | 0.089 | 0.929 | 1.296 |
| DL | −0.093 | 0.092 | −0.068 | −1.013 | 0.312 | 1.116 | −0.221 | 0.341 | −0.044 | −0.646 | 0.519 | 1.116 |
| DM | −0.080 | 0.150 | −0.034 | −0.529 | 0.598 | 1.044 | 0.182 | 0.561 | 0.021 | 0.324 | 0.747 | 1.044 |
| HT | 0.239 | 0.097 | 0.172 | 2.478 | 0.014 | 1.206 | 0.428 | 0.360 | 0.084 | 1.189 | 0.236 | 1.206 |
| Drinker | −0.012 | 0.185 | −0.004 | −0.065 | 0.948 | 1.091 | 0.130 | 0.691 | 0.013 | 0.188 | 0.851 | 1.091 |
| Smoker | 0.050 | 0.385 | 0.008 | 0.129 | 0.898 | 1.063 | 0.966 | 1.435 | 0.045 | 0.673 | 0.502 | 1.063 |
| Area #1 | 0.010 | 0.099 | 0.007 | 0.099 | 0.921 | 1.243 | 0.264 | 0.369 | 0.051 | 0.716 | 0.475 | 1.243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuiya, J.; Notsu, Y.; Kobayashi, H.; Shibly, A.Z.; Sheikh, A.M.; Okazaki, R.; Yamaguchi, K.; Nagai, A.; Nabika, T.; Abe, T.; et al. Neither Trimethylamine-N-Oxide nor Trimethyllysine Is Associated with Atherosclerosis: A Cross-Sectional Study in Older Japanese Adults. Nutrients 2023, 15, 759. https://doi.org/10.3390/nu15030759
Bhuiya J, Notsu Y, Kobayashi H, Shibly AZ, Sheikh AM, Okazaki R, Yamaguchi K, Nagai A, Nabika T, Abe T, et al. Neither Trimethylamine-N-Oxide nor Trimethyllysine Is Associated with Atherosclerosis: A Cross-Sectional Study in Older Japanese Adults. Nutrients. 2023; 15(3):759. https://doi.org/10.3390/nu15030759
Chicago/Turabian StyleBhuiya, Jubo, Yoshitomo Notsu, Hironori Kobayashi, Abu Zaffar Shibly, Abdullah Md. Sheikh, Ryota Okazaki, Kazuto Yamaguchi, Atsushi Nagai, Toru Nabika, Takafumi Abe, and et al. 2023. "Neither Trimethylamine-N-Oxide nor Trimethyllysine Is Associated with Atherosclerosis: A Cross-Sectional Study in Older Japanese Adults" Nutrients 15, no. 3: 759. https://doi.org/10.3390/nu15030759
APA StyleBhuiya, J., Notsu, Y., Kobayashi, H., Shibly, A. Z., Sheikh, A. M., Okazaki, R., Yamaguchi, K., Nagai, A., Nabika, T., Abe, T., Yamasaki, M., Isomura, M., & Yano, S. (2023). Neither Trimethylamine-N-Oxide nor Trimethyllysine Is Associated with Atherosclerosis: A Cross-Sectional Study in Older Japanese Adults. Nutrients, 15(3), 759. https://doi.org/10.3390/nu15030759






