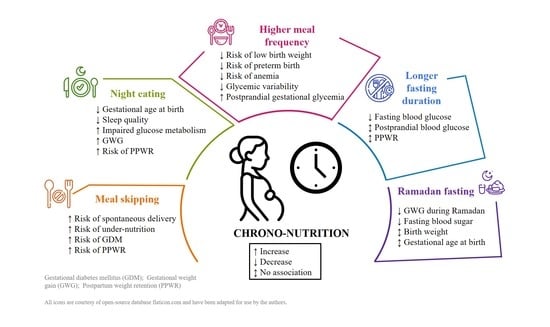
Chrononutrition during Pregnancy and Its Association with Maternal and Offspring Outcomes: A Systematic Review and Meta-Analysis of Ramadan and Non-Ramadan Studies
Highlights
- Meal skipping and night eating may adversely affect maternal and birth outcomes, leading to higher gestational weight gain, postpartum weight retention, impaired glucose metabolism, and higher risks of spontaneous preterm delivery, insomnia, and poor maternal nutritional status.
- Maternal fasting during Ramadan is associated with lower gestational weight gain and fasting blood sugar, but its impact on birth outcomes remains unclear.
- Meal skipping and night eating may negatively impact both maternal and birth outcomes. However, to establish reliable dietary recommendations for pregnant women, larger and well-conducted prospective cohort and intervention studies are needed.
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
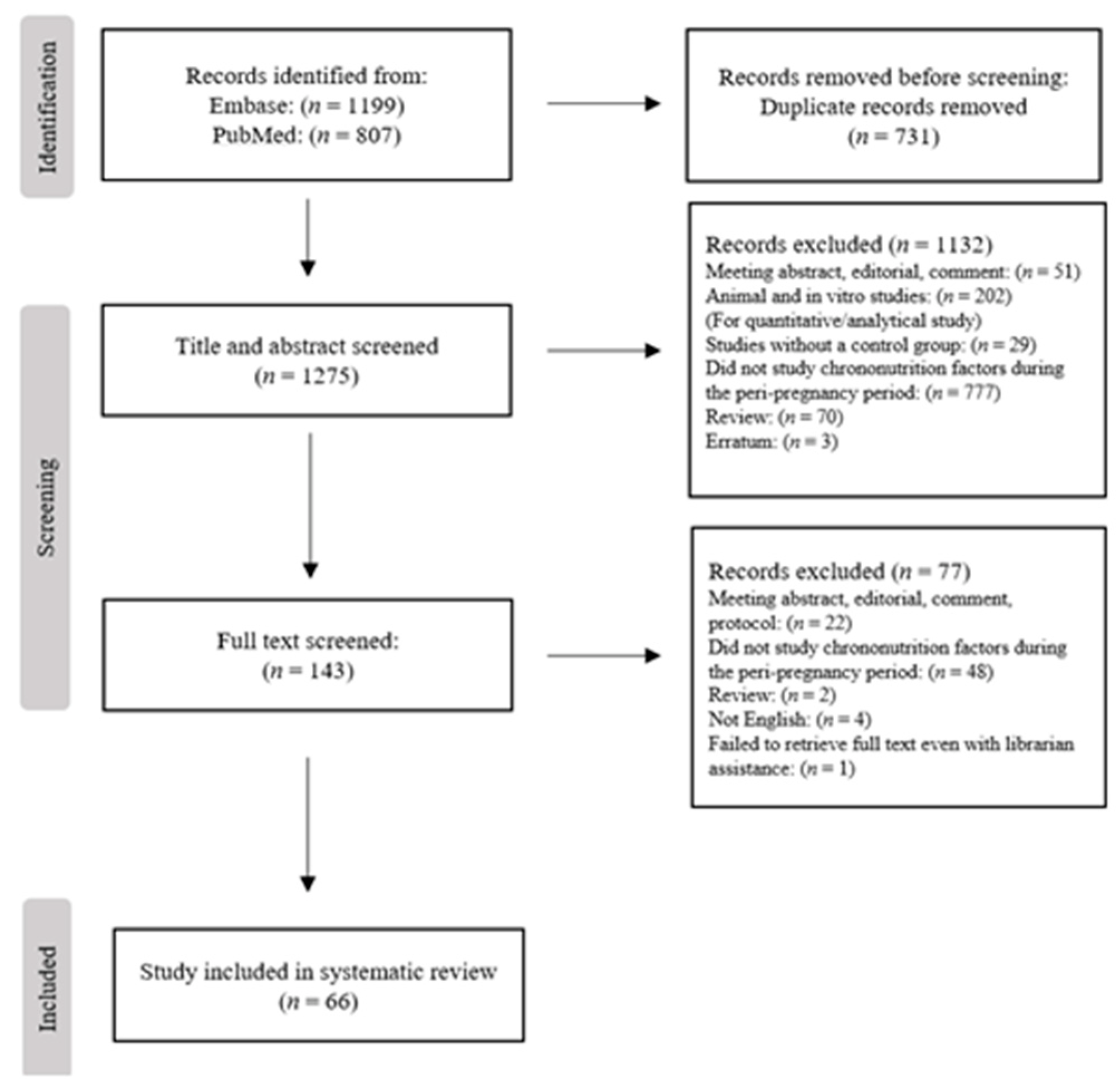
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Data Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Maternal Chrononutrition Factors and Maternal and Child Outcomes: Non-Ramadan Studies
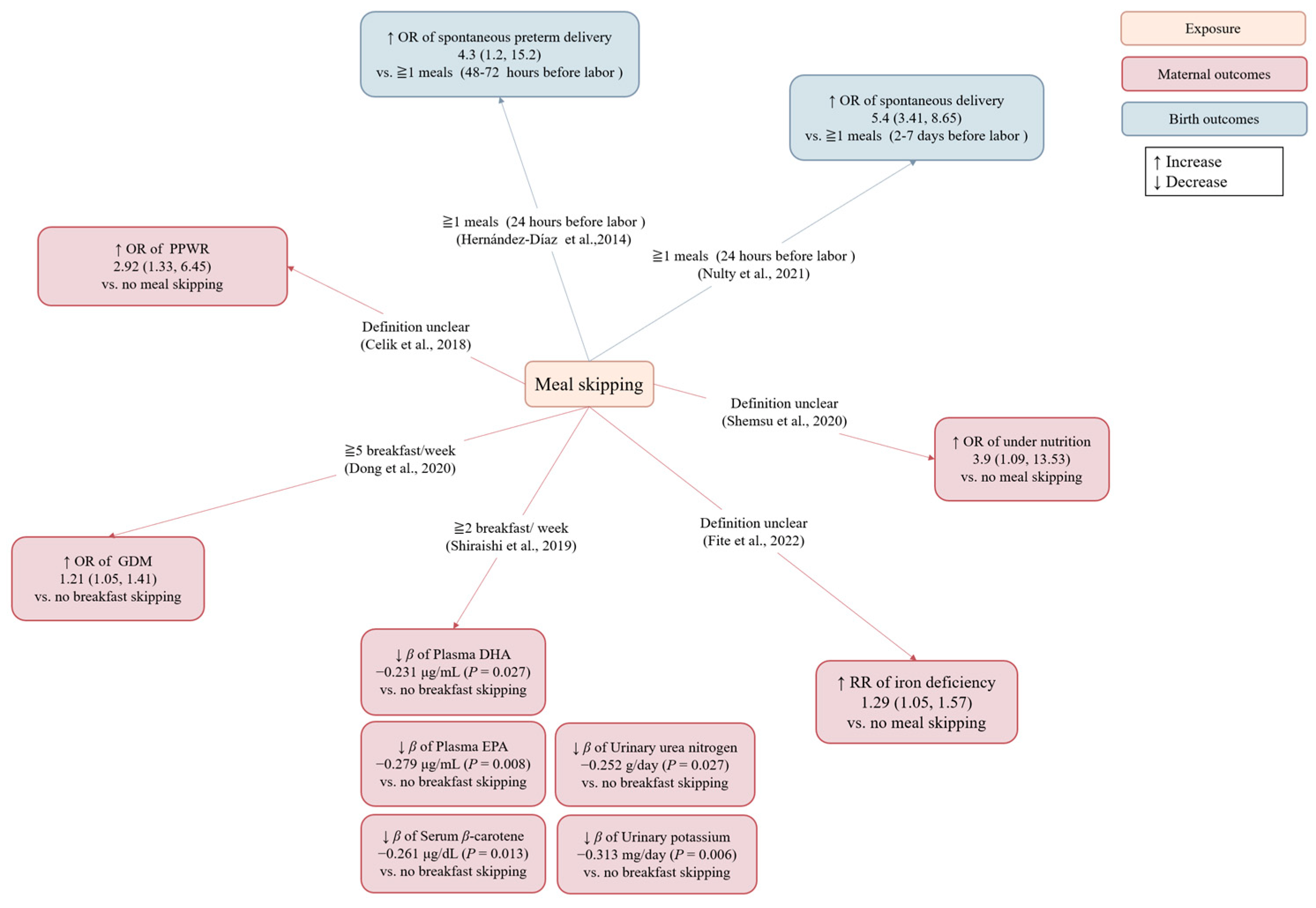
3.3.1. Meal Skipping
| Author (Country, Year of Publication) | Study Design | Population | Number of Participants | Age, y (mean) | BMI, kg/m2 | Period of Exposure Assessment | Comparison of Exposure | Outcomes | Covariates | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Nulty (United States, 2021) [20] | Case-crossover study | Pregnant women with spontaneous labor | 607 | 29.1 | 24.4 (Pre-pregnancy) | One week before delivery | Meal skipping ≥1 times/day within 24 h of labor vs. the week before labor | Spontaneous delivery | Na | Meal skipping later in pregnancy was associated with an increased likelihood of imminent spontaneous labor. |
| Hernández-Día (United States, 2014) [21] | Case-crossover study | Pregnant women with premature labor or preterm premature rupture of membranes | 100 | 31.5 | 25.6 (Pre-pregnancy) | 0–72 h before preterm delivery | Meal skipping ≥1 times/day within 24 h of labor vs. during the 48–72 h before labor | Spontaneous preterm labor and preterm premature rupture of membranes | Na | Meal skipping was associated with an increased risk for spontaneous preterm labor and preterm premature rupture of membranes within the subsequent 24 h. |
| Shemsu (Ethiopia, 2020) [22] | Cross-sectional study | Pregnant women | 378 | 28.9 | na | (Definition unclear) | Meal skipping vs. no meal skipping | Under-nutrition (MUAC < 21.0 cm) | Wealth data | Meal skipping was associated with an increased odds of under-nutrition. |
| Fite (Ethiopia, 2022) [23] | Cross-sectional study | Pregnant women | 446 | 24.8 | na | 1–40 weeks of gestation | Meal skipping vs. no meal skipping | Iron deficiency (serum ferritin <15 μg/L) | Inflammation | Meal skipping was associated with an increased risk of iron deficiency. |
| Shiraishi (Japan, 2019) [24] | Cross-sectional study | Healthy pregnant women | 97 | 35.1 | 20.4 (Pre-pregnancy) | 15–18 weeks of gestation | Breakfast skipping ≥2 times/week vs. no breakfast skipping | Circulating and urinary levels of nutrients | Educational levels, supplement use, LDL-C (only for serum vitamin E levels) | Breakfast skipping ≥2 times/week was associated with lower plasma EPA, plasma DHA, serum β-carotene, urinary urea nitrogen, and urinary potassium compared to no breakfast skipping |
| Dong (Japan, 2020) [25] | Cohort study | Healthy pregnant women | 84,669 | 30.7 | 21.1 (Pre-pregnancy) | 26–28 weeks of gestation | Breakfast skipping 1–2, 3–4, or 5–7 times/week vs. no breakfast skipping | GDM | Age, smoking status, drinking status, education level, occupation, household income, history of depression, history of having infants with macrosomia, history of polycystic ovarian syndrome, marital status, parity, physical activity, total daily energy intakes, Western dietary pattern scores, BMI | Breakfast skipping 5–7 times/week before and during early pregnancy was associated with an increased odds of developing GDM compared to no breakfast skipping. |
| Celik (Turkey, 2018) [26] | Cross-sectional study | The mothers were in the 12–18 month postpartum, and gave birth between the 37th and 42nd weeks. | 239 | 30.8 | 22.6 (Pre-pregnancy) | (Definition unclear) | Meal skipping vs. no meal skipping | PPWR (at 12–18 months) | na | Meal skipping was associated with higher PPWR. |
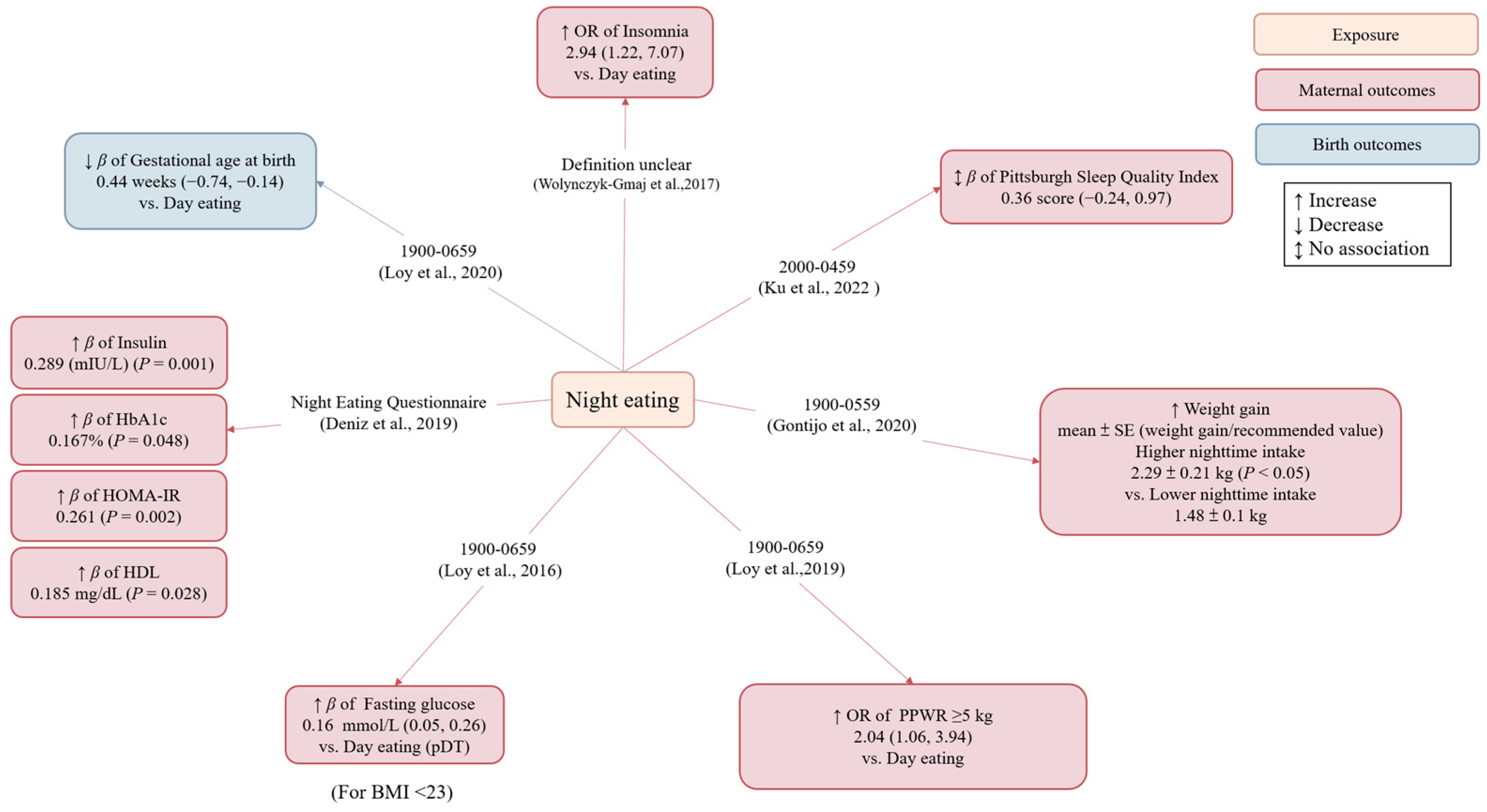
3.3.2. Night Eating
| Authors, Publication Date | Study Design | Population | Number of Participants | Participants’ Age, y | BMI, kg/m2 | Period of Exposure Assessment | Dietary Assessment Methods | Exposure | Outcomes | Covariates | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Loy (Singapore, 2020) [27] | Cohort study | Healthy pregnant women | 673 | 30.9 | <23: 53.2% ≥23: 46.8% | 26–28 weeks of gestation | 24 h dietary recall | Night eating (defined as consuming >50% of total daily energy intake during 1900–0659 h) vs. day eating | (1) Gestational age at birth (2) Preterm birth | Age, ethnicity, education, monthly household income, employment status, night shift, physical activity, early pregnancy BMI, anxiety score, eating episodes, total daily energy intake, infant sex, bedtime, GDM | Night eating was associated with shorter gestation length, but its association with preterm birth did not reach statistical significance. |
| Wolynczyk-Gmaj (Poland, 2017) [28] | Cross-sectional study | Pregnant women | 202 | 30.6 | 26.9 (third trimester of pregnancy) | 28–41 weeks of gestation | Harvard Light Exposure Assessment questionnaire | Night eating (definition unclear) vs. day eating | Sleep quality (insomnia) | Tingling in the legs, nightmares, snoring, myoclonus, higher depression scores, higher hyperarousal | Night eating was associated with an increased odds of insomnia (defined as an Athens Insomnia Scale (AIS) score >8). |
| Ku (Singapore, 2022) [29] | Cross-sectional study | Healthy pregnant women | 299 | 31.09 | 22.89 (pre-pregnancy) | 18–24 weeks of gestation | Questionnaire | Night eating (defined as eating during 2000-0459 h) | Sleep quality | Education, employment status, working overtime, pre-pregnancy BMI, negative emotion | Night eating was associated with higher PSQI scores (reflecting poorer sleep). Further adjustment for negative emotions, lifestyle behaviors, maternal age, etc. attenuated the association between night eating and sleep quality |
| Gontijo (Brazil, 2020) [30] | Cohort study | Healthy pregnant women | 100 | 27.7 | 24.25 | The first/second/third trimester | 24 h dietary recall | Higher night eating vs. lower night eating [caloric consumption during 1900-0559 h was above (higher night eating) or below (lower night eating) the median of the population] | GWG | Age, pre-pregnancy BMI, education, chronotype, physical activity, frequency of nausea | Women in the higher night eating group gained more weight (weight gain/recommended value) in the third trimester compared to the lower night eating group |
| Loy (Singapore, 2019) [31] | Cohort study | Pregnant women | 687 | 31.3 | 23.6 (≤14 weeks gestation) | 26–28 weeks of gestation | 24 h dietary recall | Night eating (consuming >50% of total daily energy intake during 1900–0659 h) vs. day eating | PPWR (≥5 kg at 18 months) | Age, ethnicity, education, parity, night shift, total Edinburgh Postnatal Depression Scale score, total daily energy intake, BMI, bedtime, GDM, GWG, feeding in the first six months | Night eating was associated with higher odds of substantial PPWR |
| Loy (Singapore, 2016) [32] | Cohort study | Healthy pregnant women | 985 | 30.7 | <23: 54.2% ≥23: 45.8% | 26–28 weeks of gestation | 24 h dietary recall | pNT vs. pDT [consuming a greater percentage of calories from 0700 to 1859 (pDT) hours or from 1900 to 0659 h (pNT)] | Blood glucose | Age, education, ethnicity, physical activity, sleep duration, total daily energy intake | Night eating was associated with higher fasting glycemia in lean but not in overweight women. |
| Deniz (Turkey, 2019) [33] | Cross-sectional study | Healthy pregnant women | 148 | 28.9 | 30.66 | 28–38 weeks of gestation | Night Eating Questionnaire | Night eating | (1) Blood glucose (2) Blood lipid (3) Birth weight | na | Night eating was associated with higher HbA1c, insulin resistance, insulin, and HDL. |
| Messika (Israel, 2022) [34] | Randomized controlled trial | Pregnant women with GDM | 103 | 33.6 | 27.7 (pre-pregnancy) | Intervention and follow up from gestational week 25–29 until delivery | 24 h dietary recall | Intervention group: chrononutrition and sleep hygiene program vs. control group: usual GDM care | (1) Glycemic control (2) Birth weight (3) Gestational age at birth | Age, pre-pregnancy BMI, number of children, history of GDM, LGA birth | A significant reduction in suboptimal glycemic control in the intervention compared to control group. The intervention had no effect on birth weight or gestational age at birth. |
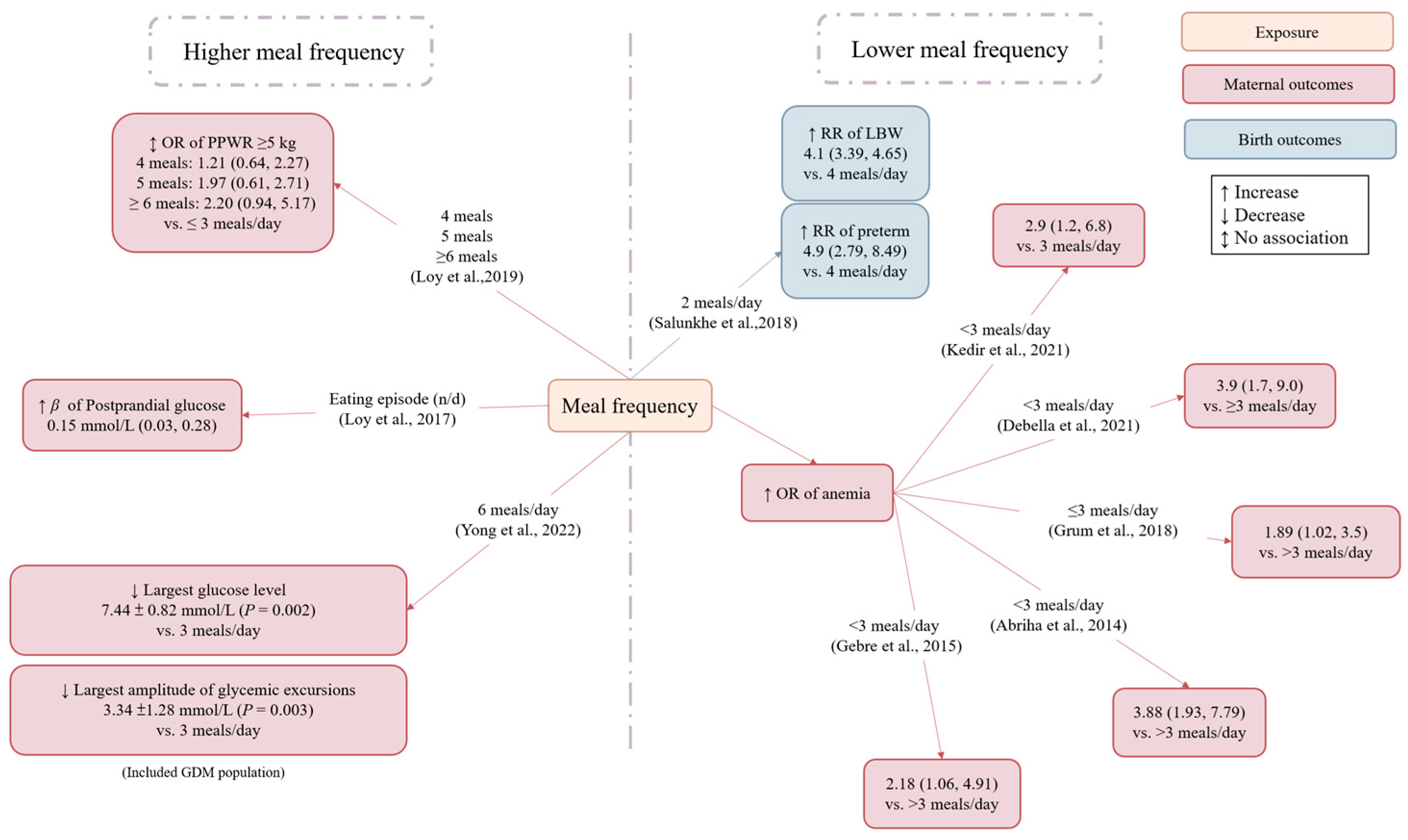
3.3.3. Meal Frequency
| Author (Country, Year of Publication) | Study Design | Population | Number of Participants | Participants’ Age, y | BMI, kg/m2 | Period of Exposure Assessment | Comparison of Exposure | Outcomes | Covariates | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Salunkhe (India, 2018) [36] | Cohort study | Healthy pregnant women | 380 | na | na | 14–26 weeks of gestation (the second trimester) | 2 or 3 meals/day vs. ≥4 meals/day | (1) Birth weight (2) Gestational age at birth (3) Low birth weight (4) Preterm birth | na | Increasing meal frequency from two to four meals was associated with higher birth weight, longer gestational age at birth, and a reduced risk of low birth weight and preterm birth delivery. |
| Englund (Ögge (Sweden, 2017) [37] | Cohort study | Healthy pregnant women | 65,487 | <35y: 83.2% ≥35y: 16.8% | <18.5: 3.3% 18.5–24.9: 66% 25–29.9: 20.2% ≥30: 8% | na | The second, third, and fourth quartiles of meal patterns [snack meal, main meal (breakfast, lunch, and dinner), evening meal] vs. the first quartile of each meal pattern | Preterm birth | Age, pre-pregnancy BMI, height, parity, total daily energy intake, education, marital status, smoking, income, history of preterm birth, other meal frequency patterns | Regular consumption of main meals (breakfast, lunch, and dinner) was associated with a lower preterm birth risk. |
| Ainscough (Ireland, 2020) [38] | Cohort study | Pregnant women with overweight and obesity | 143 | 32.1 | 29.1 | 16–28 weeks of gestation | Meals pattern [main meal pattern (3 main meals + 0–3 snacks), large meal pattern (≤2 main meals + <2 snacks), and snack pattern (3 main meals + >3 snacks or ≤2 main meals + ≥2 snacks)] | (1) Blood glucose (2) GWG (3) Gestational age at birth (4) Macrosomia | na | Women with a large meal pattern at 16 weeks gestation had lower GWG than those with the main meal or snack pattern. Women with a large meal pattern at 28 weeks gestation were more likely to give birth to a macrosomic infant than those with the main meal or snack pattern. Meal patterns were not associated with blood glucose or gestational age at birth. |
| Loy (Singapore, 2017) [39] | Cross-sectional study | Healthy pregnant women | 1061 | 30.7 | 23.6 (≤14 weeks gestation) | 26–28 weeks of gestation | Eating episodes (n/day) | Blood glucose | Age, ethnicity, education, employment status, night shift status, parity, BMI, physical activity, sleep duration, bedtime, total daily energy intake, % energy during nighttime, % energy from protein, % energy from fat | Each additional daily eating episode was associated with higher 2 h glucose but not with fasting glucose. |
| Yong (China, 2022) [40] | Randomized crossover study | Pregnant women with GDM | 10 | 30.1 | 30.1 | 26.7 weeks (Average) | (1) Three meals a day (2) Six meals a day | Blood glucose | na | Increasing the number of meals decreased the standard deviation and mean amplitude of glycemic excursions, the largest amplitude of glycemic excursions, coefficient variation, and peak glucose level but not the mean glucose level or the lowest glucose level |
| Loy (Singapore, 2019) [31] | Cohort study | Pregnant women | 687 | 31.3 | 23.6 (≤14 weeks gestation) | 26–28 weeks of gestation | ≥6 or 5 or 4 meals/day vs. ≤3 meals/day | PPWR (≥5 kg at 18 months) | Age, ethnicity, education, parity, night shift, total Edinburgh Postnatal Depression Scale score, total daily energy intake, BMI, bedtime, GDM, GWG, feeding in the first six months | Eating episodes were not associated with 18-month PPWR. |
| Kedir (Ethiopia, 2021) [41] | Cross-sectional study | Pregnant women | 284 | 33.1% 18–22 y 53.5% 23–34 y 13.4% ≥ 35 y | na | 1–40 weeks of gestation (the first to third trimester) | <3 meals/day vs. 3 meals/day | Anemia | A history of heavy menstrual bleeding, the lack of animal-origin food at least once a week, short birth interval, education | Eating <3 meals a day increased anemia risk. |
| Debella (Ethiopia, 2021) [42] | Cross-sectional study | Healthy pregnant women | 405 | 26.6 | na | (Definition unclear) | <3 meals/day vs. ≥3 meals/day | Anemia | Place of residence, marital status, ANC visit, birth interval, history of contraceptive use, IFA supplementation, blood loss in the current pregnancy, drinking alcohol, eating leafy vegetables, drinking milk with tea after meals | Eating <3 meals a day increased anemia risk. |
| Grum (Ethiopia, 2018) [43] | Cross-sectional study | Pregnant women | 638 | 27 | na | (Definition unclear) | ≤3 meals/day vs. >3 meals/day | Anemia | Education, birth interval, malaria attack in last one year, excessive menstrual bleeding, pregnancy-related complication | Eating ≤ 3 meals a day increased anemia risk. |
| Abriha (Ethiopia, 2014) [44] | Cross-sectional study | Healthy pregnant women | 619 | 27.4 | na | (Definition unclear) | 3 or <3 meals/day vs. >3 meals/day | Anemia | Age category, family monthly income, marital status, occupational status | Eating <3 meals a day increased anemia risk. |
| Gebre (Ethiopia, 2015) [45] | Cross-sectional study | Healthy pregnant women | 714 | 25.8 | ≤20: 21.8% 20–24.9: 20.9% ≥25: 57.3% | 26.7 (Average) | 3 or < 3 meals/day vs. >3 meals/day | Anemia | Marital status, residence, education, family monthly income, number of visits, age of the women at first marriage, BMI, iron supplementation, nutrition education | Eating <3 meals a day increased anemia risk. |
3.3.4. Non-Ramadan (Night) Fasting Duration
3.4. Ramadan Fasting
3.4.1. Maternal Outcomes: Quantitative Analyses
Weight Gain
Fasting Blood Glucose
3.4.2. Birth Outcomes: Quantitative Analyses
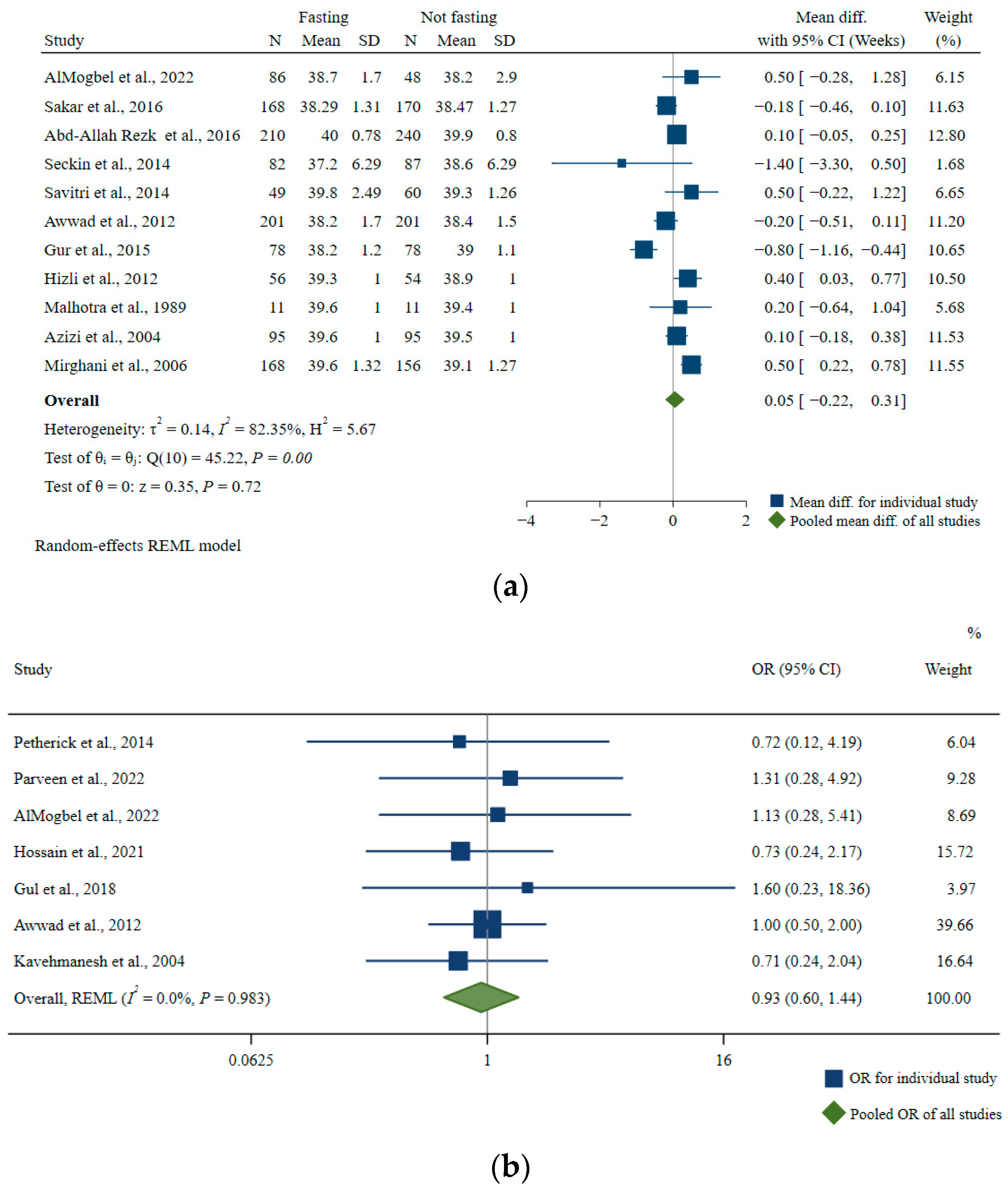
Gestational Age at Birth
| Characteristics | Number of Studies | Pooled Mean Difference, Week | 95% CI, Week | I2 | p-For Heterogeneity |
|---|---|---|---|---|---|
| All studies | 11 | 0.05 | −0.22, 0.31 | 82.35 | <0.01 |
| Number of participants a | |||||
| ≤219 | 7 | 0.04 | −0.39, 0.47 | 77.74 | <0.01 |
| >219 | 4 | 0.06 | −0.25, 0.37 | 84.14 | <0.01 |
| Age (years old) a | |||||
| ≤27.2 | 8 | −0.03 | −0.35, 0.29 | 82.54 | <0.01 |
| >27.2 | 3 | 0.23 | −0.26, 0.72 | 80.39 | <0.01 |
| Duration of fasting per day (hours) a | |||||
| ≤15.5 | 7 | 0.21 | 0.00, 0.42 | 63.89 | 0.02 |
| >15.5 | 4 | −0.40 | −0.90, 0.11 | 70.40 | 0.02 |
| Region | |||||
| Middle East/Northeast Africa | 4 | 0.13 | −0.13, 0.39 | 77.74 | 0.01 |
| Oceania/Europe | 7 | −0.02 | −0.45, 0.42 | 78.60 | <0.01 |
| BMI (kg/m2) | |||||
| <25 | 2 | −0.49 | −1.08, 0.09 | 83.46 | 0.01 |
| ≥25 | 3 | 0.17 | −0.28, 0.63 | 70.29 | 0.03 |
| MMAT (risk of bias) | |||||
| Low | 7 | −0.08 | −0.48, 0.33 | 75.90 | <0.01 |
| Medium/High | 4 | 0.19 | −0.10, 0.49 | 81.29 | <0.01 |
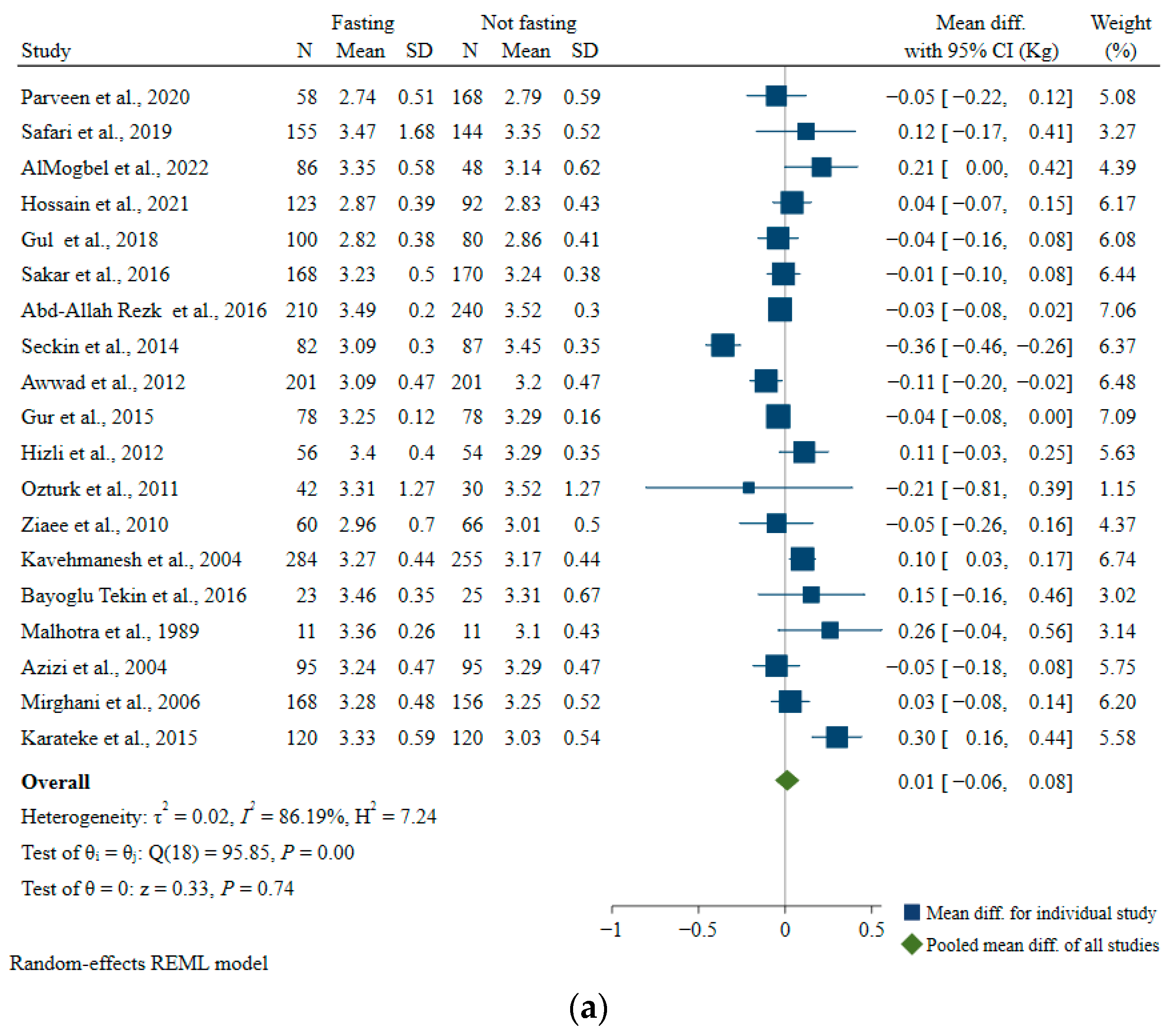
Birth Weight
| Characteristics | Number of Studies | Mean Difference, kg | 95% CI, kg | I2 | p-For Heterogeneity |
|---|---|---|---|---|---|
| All studies | 19 | 0.01 | −0.06, 0.08 | 86.19 | <0.01 |
| Number of participants a | |||||
| ≤225 | 11 | −0.01 | −0.11, 0.10 | 85.07 | <0.01 |
| >225 | 8 | 0.03 | −0.05, 0.12 | 83.13 | <0.01 |
| Age (years old) a | |||||
| ≤27.3 | 10 | 0.00 | −0.11, 0.11 | 92.50 | <0.01 |
| >27.3 | 9 | 0.02 | −0.05, 0.10 | 55.18 | 0.03 |
| Duration of fasting per day (hours) a | |||||
| ≤15.4 | 11 | 0.01 | −0.04, 0.06 | 56.38 | 0.01 |
| >15.4 | 8 | 0.03 | −0.13, 0.18 | 91.84 | <0.01 |
| Region | |||||
| Middle East/Northeast Africa | 7 | −0.01 | −0.07, 0.06 | 62.38 | 0.02 |
| South Asia | 3 | −0.01 | −0.08, 0.06 | 0.00 | 0.53 |
| Oceania/Europe | 9 | 0.05 | −0.10, 0.20 | 91.49 | <0.01 |
| BMI (kg/m2) | |||||
| <25 | 6 | 0.03 | −0.09, 0.16 | 85.87 | <0.01 |
| ≥25 | 7 | 0.05 | −0.02, 0.11 | 40.94 | 0.11 |
| MMAT (risk of bias) | |||||
| Low | 12 | 0.02 | −0.09, 0.13 | 90.48 | <0.01 |
| Medium/High | 7 | −0.01 | −0.05, 0.02 | 0.02 | 0.52 |
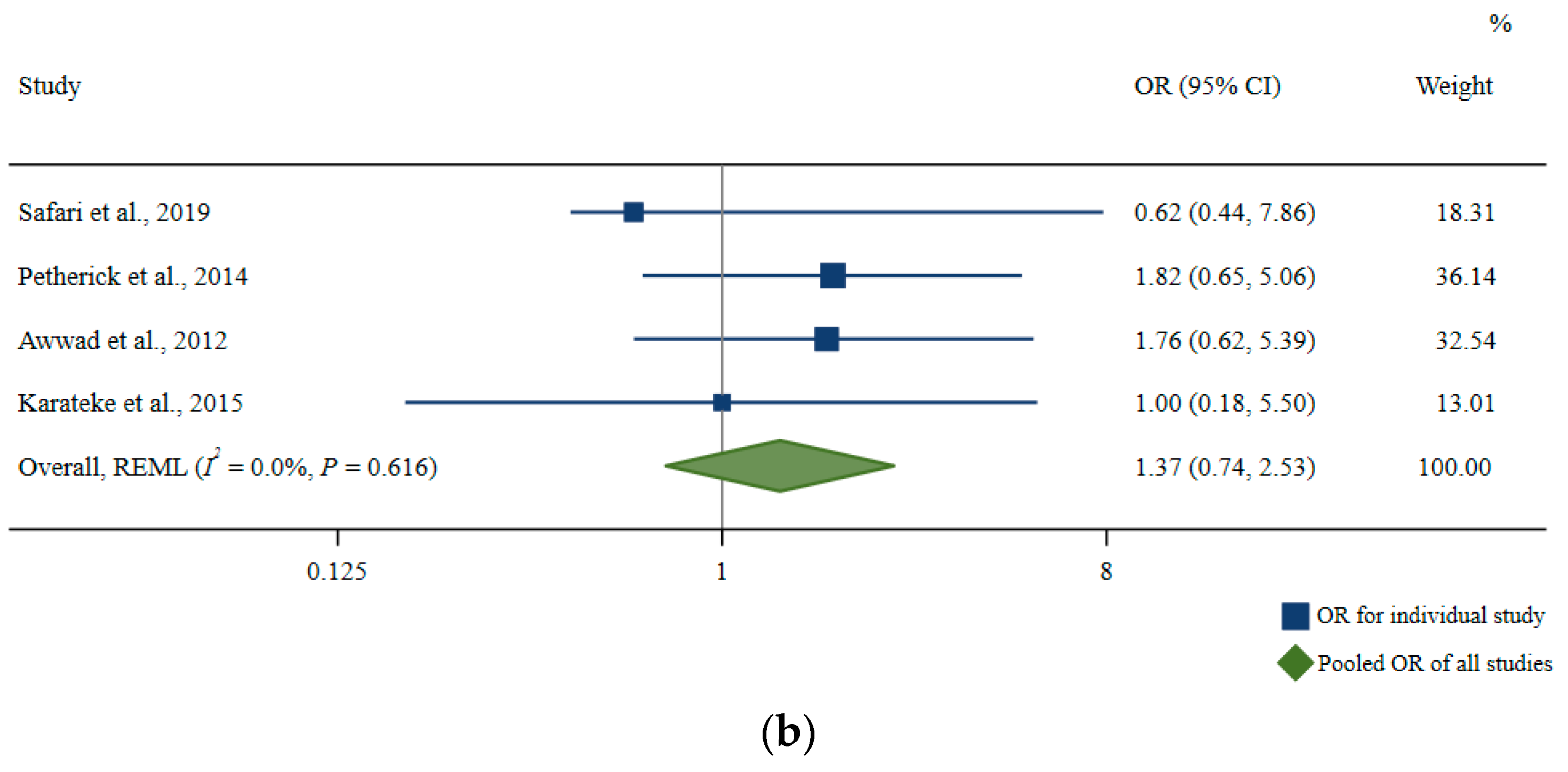
3.4.3. Other Outcomes for Ramadan Studies: Qualitative Analyses
Maternal Health Outcomes
Fetal Outcomes
Birth Outcomes
Childhood Outcomes
4. Discussion
4.1. Principal Findings
4.2. Meal Skipping
4.3. Night Eating
4.4. Meal Frequency
4.5. Ramadan Fasting
4.6. Strengths, Limitations, and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gluckman, P.; Hanson, M.; Seng, C.Y.; Chong, Y.S.; Bardsley, A. Nutrition and Lifestyle for Pregnancy and Breastfeeding; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Clarke, G.S.; Gatford, K.L.; Young, R.L.; Grattan, D.R.; Ladyman, S.R.; Page, A.J. Maternal adaptations to food intake across pregnancy: Central and peripheral mechanisms. Obesity 2021, 29, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, E.; Guo, J.; Pan, L.; Li, B.; Wang, P.; Liu, J.; Wang, Y.; Liu, G.; Baccarelli, A.A. Maternal Prepregnancy Body Mass Index and Gestational Weight Gain on Pregnancy Outcomes. PLoS ONE 2013, 8, e82310. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Tilling, K.; Macdonald-Wallis, C.; Sattar, N.; Brion, M.-J.; Benfield, L.; Ness, A.; Deanfield, J.; Hingorani, A.; Nelson, S.M. Association of Maternal Weight Gain in Pregnancy with Offspring Obesity and Metabolic and Vascular Traits in Childhood. Circulation 2010, 121, 2557–2564. [Google Scholar] [CrossRef]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-nutrition: From molecular and neuronal mechanisms to human epidemiology and timed feeding patterns. J. Neurochem. 2021, 157, 53–72. [Google Scholar] [CrossRef]
- Chaudhari, A.; Gupta, R.; Makwana, K.; Kondratov, R. Circadian clocks, diets and aging. Nutr. Healthy Aging 2017, 4, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.; Johnston, J.; Morgan, P.; Johnstone, A. The Big Breakfast Study: Chrono-nutrition influence on energy expenditure and bodyweight. Nutr. Bull. 2018, 43, 174–183. [Google Scholar] [CrossRef]
- Garbarino, S.; Garbarino, E.; Lanteri, P. Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions. Nutrients 2022, 14, 3113. [Google Scholar] [CrossRef]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. Int. Rev. J. 2019, 10, 30–42. [Google Scholar] [CrossRef]
- Zheng, X.; Sehgal, A. AKT and TOR Signaling Set the Pace of the Circadian Pacemaker. Curr. Biol. 2010, 20, 1203–1208. [Google Scholar] [CrossRef]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef]
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, clock genes, and chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–212. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, D.; Faris, M.E.; Hassanein, M.; Shakir, A.Z.; Yusuf, A.M.; Almeneessier, A.S.; BaHammam, A.S. Impact of Ramadan Diurnal Intermittent Fasting on Hypoglycemic Events in Patients with Type 2 Diabetes: A Systematic Review of Randomized Controlled Trials and Observational Studies. Front. Endocrinol. 2021, 12, 624423. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F. Research in Islamic Fasting and Health. Ann. Saudi Med. 2002, 22, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Almond, D.; Mazumder, B. Health Capital and the Prenatal Environment: The Effect of Ramadan Observance During Pregnancy. Am. Econ. J. Appl. Econ. 2011, 3, 56–85. [Google Scholar] [CrossRef]
- Van Bilsen, L.A.; Savitri, A.I.; Amelia, D.; Baharuddin, M.; Grobbee, D.E.; Uiterwaal, C.S. Predictors of Ramadan fasting during pregnancy. J. Epidemiol. Glob. Health 2016, 6, 267–275. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Group, C.S.M. Analysing Data and Undertaking Meta-Analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar]
- Kiziltan, G.; Karabudak, E.; Tuncay, G.; Avsar, F.; Tuncay, P.; Mungan, O.; Meral, P. Dietary intake and nutritional status of Turkish pregnant women during Ramadan. Saudi Med. J. 2005, 26, 1782–1787. [Google Scholar]
- Nulty, A.K.; Bovbjerg, M.L.; Herring, A.H.; Siega-Riz, A.M.; Thorp, J.M., Jr.; Evenson, K.R. Meal patterning and the onset of spontaneous labor. Birth 2022, 49, 123–131. [Google Scholar] [CrossRef]
- Hernández-Díaz, S.; Boeke, C.E.; Romans, A.T.; Young, B.; Margulis, A.V.; McElrath, T.F.; Ecker, J.L.; Bateman, B.T. Triggers of spontaneous preterm delivery—Why today? Paediatr. Perinat. Epidemiol. 2014, 28, 79–87. [Google Scholar] [CrossRef]
- Shemsu, S.; Argaw, A.; Zinab, B. Dietary Practice and Nutritional Status Among Pregnant Women Attending Antenatal Care at Mettu Karl Referral Hospital, Southwest Ethiopia. Open Public Health J. 2020, 13, 538–546. [Google Scholar] [CrossRef]
- Fite, M.B.; Bikila, D.; Habtu, W.; Tura, A.K.; Yadeta, T.A.; Oljira, L.; Roba, K.T. Beyond hemoglobin: Uncovering iron deficiency and iron deficiency anemia using serum ferritin concentration among pregnant women in eastern Ethiopia: A community-based study. BMC Nutr. 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Haruna, M.; Matsuzaki, M. Effects of skipping breakfast on dietary intake and circulating and urinary nutrients during pregnancy. Asia Pac. J. Clin. Nutr. 2019, 28, 99–105. [Google Scholar]
- Dong, J.-Y.; Ikehara, S.; Kimura, T.; Cui, M.; Kawanishi, Y.; Kimura, T.; Ueda, K.; Iso, H.; Environment, J.; Group, C.s.S. Skipping breakfast before and during early pregnancy and incidence of gestational diabetes mellitus: The Japan Environment and Children’s Study. Am. J. Clin. Nutr. 2020, 111, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Atan, S.U. Investigation of factors affecting postpartum maternal weight retention: A cross-sectional study. J. Pak. Med. Assoc. 2018, 68, 1578–1583. [Google Scholar] [PubMed]
- Loy, S.L.; Cheung, Y.B.; Cai, S.; Colega, M.T.; Godfrey, K.M.; Chong, Y.-S.; Shek, L.P.-C.; Tan, K.H.; Chong, M.F.-F.; Yap, F. Maternal night-time eating and sleep duration in relation to length of gestation and preterm birth. Clin. Nutr. 2020, 39, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Wołyńczyk-Gmaj, D.; Różańska-Walędziak, A.; Ziemka, S.; Ufnal, M.; Brzezicka, A.; Gmaj, B.; Januszko, P.; Fudalej, S.; Czajkowski, K.; Wojnar, M. Insomnia in Pregnancy Is Associated With Depressive Symptoms and Eating at Night. J. Clin. Sleep Med. 2017, 13, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.W.; Loo, R.S.X.; Tiong, M.M.Y.; Eng, S.Y.C.; Cheung, Y.B.; Ong, L.S.; Tan, K.H.; Chong, M.F.-F.; Chan, J.K.Y.; Yap, F.; et al. Nocturnal Lifestyle Behaviours and Risk of Poor Sleep during Pregnancy. Nutrients 2022, 14, 2348. [Google Scholar] [CrossRef]
- Gontijo, C.A.; Balieiro, L.C.T.; Teixeira, G.P.; Fahmy, W.M.; Crispim, C.A.; Maia, Y.C.D.P. Higher energy intake at night effects daily energy distribution and contributes to excessive weight gain during pregnancy. Nutrition 2020, 74, 110756. [Google Scholar] [CrossRef]
- Loy, S.L.; Cheung, Y.B.; Colega, M.T.; Chia, A.; Han, C.Y.; Godfrey, K.M.; Chong, Y.-S.; Shek, L.P.-C.; Tan, K.H.; Lek, N. Associations of Circadian Eating Pattern and Diet Quality with Substantial Postpartum Weight Retention. Nutrients 2019, 11, 2686. [Google Scholar] [CrossRef]
- Loy, S.L.; Cheng, T.S.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Gluckman, P.D.; Kwek, K.; Saw, S.M.; Chong, Y.-S.; Padmapriya, N.; et al. Predominantly night-time feeding and maternal glycaemic levels during pregnancy. Br. J. Nutr. 2016, 115, 1563–1570. [Google Scholar] [CrossRef]
- Deniz, Ç.D.; Özler, S.; Sayın, F.K.; Eryılmaz, M.A. Associations between night eating syndrome and metabolic parameters in pregnant women. Turk. J. Obstet. Gynecol. 2019, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Messika, A.; Toledano, Y.; Hadar, E.; Shmuel, E.; Tauman, R.; Shamir, R.; Froy, O. Relationship among chrononutrition, sleep, and glycemic control in women with gestational diabetes mellitus: A randomized controlled trial. Am. J. Obstet. Gynecol. MFM 2022, 4, 100660. [Google Scholar] [CrossRef] [PubMed]
- Milano, W.; De Rosa, M.; Milano, L.; Capasso, A. Night eating syndrome: An overview. J. Pharm. Pharmacol. 2012, 64, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.H.; Pratinidhi, A.; Salunkhe, J.A.; Kakade, S.; Mohite, V.R.; Hiremath, P. Frequency and Nutrient Content of Meals of the Mothers and the Birth Weight and Gestational Age of the Baby. J. Krishna Inst. Med. Sci. (JKIMSU) 2018, 7, 33–41. [Google Scholar]
- Englund-Ögge, L.; Birgisdottir, B.E.; Sengpiel, V.; Brantsæter, A.L.; Haugen, M.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Meal frequency patterns and glycemic properties of maternal diet in relation to preterm delivery: Results from a large prospective cohort study. PLoS ONE 2017, 12, e0172896. [Google Scholar] [CrossRef]
- Ainscough, K.M.; Kennelly, M.A.; Lindsay, K.L.; O’Brien, E.C.; O’Sullivan, E.J.; Mehegan, J.; Gibney, E.R.; McAuliffe, F.M. An observational analysis of meal patterns in overweight and obese pregnancy: Exploring meal pattern behaviours and the association with maternal and fetal health measures. Ir. J. Med. Sci. 2020, 189, 585–594. [Google Scholar] [CrossRef]
- Loy, S.L.; Chan, J.K.Y.; Wee, P.H.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Kwek, K.; Saw, S.M.; Chong, Y.-S.; Natarajan, P. Maternal Circadian Eating Time and Frequency Are Associated with Blood Glucose Concentrations during Pregnancy. J. Nutr. 2017, 147, 70–77. [Google Scholar] [CrossRef]
- Yong, G.; Jing, Q.; Yao, Q.; Yang, K.; Ye, X. Changing Meal Sequence Affects Glucose Excursions in Gestational Diabetes Mellitus. J. Diabetes Res. 2022, 2022, 7083106. [Google Scholar] [CrossRef]
- Kedir, R.D.; Halil, H.M.; Reta, A.E.; Helill, S.E.; Abdo, R.A. Prevalence and Factors Associated with Anaemia Among Pregnant Women in Hossana Town, Southern Ethiopia: A Cross-Sectional Study. J. Nepal Paediatr. Soc. 2021, 41, 218–225. [Google Scholar] [CrossRef]
- Debella, A.; Dheresa, M.; Geda, B.; Tiruye, G.; Fage, S.G. A Third of Pregnant Women are Affected by Anemia in Eastern Ethiopia: A Facility-Based Study. J. Blood Med. 2021, 12, 299–306. [Google Scholar] [CrossRef]
- Grum, T.; Brhane, E.; Hintsa, S.; Kahsay, G. Magnitude and factors associated with anemia among pregnant women attending antenatal care in public health centers in central zone of Tigray region, northern Ethiopia: A cross sectional study. BMC Pregnancy Childbirth 2018, 18, 433. [Google Scholar] [CrossRef] [PubMed]
- Abriha, A.; Yesuf, M.E.; Wassie, M.M. Prevalence and associated factors of anemia among pregnant women of Mekelle town: A cross sectional study. BMC Res. Notes 2014, 7, 888. [Google Scholar] [CrossRef] [PubMed]
- Gebre, A.; Mulugeta, A. Prevalence of Anemia and Associated Factors among Pregnant Women in North Western Zone of Tigray, Northern Ethiopia: A Cross-Sectional Study. J. Nutr. Metab. 2015, 2015, 165430. [Google Scholar] [CrossRef] [PubMed]
- Savitri, A.I.; Amelia, D.; Painter, R.C.; Baharuddin, M.; Roseboom, T.J.; Grobbee, D.E.; Uiterwaal, C.S.P.M. Ramadan during pregnancy and birth weight of newborns. J. Nutr. Sci. 2018, 7, e5. [Google Scholar] [CrossRef] [PubMed]
- Safari, K.; Piro, T.J.; Ahmad, H.M. Perspectives and pregnancy outcomes of maternal Ramadan fasting in the second trimester of pregnancy. BMC Pregnancy Childbirth 2019, 19, 128. [Google Scholar]
- AlMogbel, T.A.; Ross, G.; Wu, T.; Molyneaux, L.; Constantino, M.I.; McGill, M.; Harding, A.J.; Pech, C.; Alrasheed, A.A.; Wong, J. Ramadan and gestational diabetes: Maternal and neonatal outcomes. Acta Diabetol. 2021, 59, 21–30. [Google Scholar] [CrossRef]
- Azizi; Sadeghipour; Siahkolah; Ghaleh, R. Intellectual Development of Children Born of Mothers who Fasted in Ramadan during Pregnancy. Int. J. Vitam. Nutr. Res. 2004, 74, 374–380. [Google Scholar] [CrossRef]
- Awwad, J.; Usta, I.; Succar, J.; Musallam, K.; Ghazeeri, G.; Nassar, A. The effect of maternal fasting during Ramadan on preterm delivery: A prospective cohort study. BJOG: Int. J. Obstet. Gynaecol. 2012, 119, 1379–1386. [Google Scholar] [CrossRef]
- Sakar, M.N.; Gultekin, H.; Demir, B.; Bakir, V.L.; Balsak, D.; Vuruskan, E.; Acar, H.; Yucel, O.; Yayla, M. Ramadan fasting and pregnancy: Implications for fetal development in summer season. J. Périnat. Med. 2015, 43, 319–323. [Google Scholar] [CrossRef]
- Moradi, M. The effect of Ramadan fasting on fetal growth and Doppler indices of pregnancy. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2011, 16, 165. [Google Scholar]
- Engin-Ustun, Y.; Caglayan, E.K.; Kara, M.; Gocmen, A.Y.; Polat, M.F.; Aktulay, A. The effect of Ramadan fasting on sirtuin and visfatin levels. Interv. Med. Appl. Sci. 2016, 8, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Baynouna Al Ketbi, L.M.; Niglekerke, N.J.; Zein Al Deen, S.M.; Mirghani, H. Diet restriction in Ramadan and the effect of fasting on glucose levels in pregnancy. BMC Res. Notes 2014, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Gur, E.; Turan, G.; Ince, O.; Karadeniz, M.; Tatar, S.; Kasap, E.; Sahin, N.; Guclu, S. Effect of Ramadan fasting on metabolic markers, dietary intake and abdominal fat distribution in pregnancy. Hippokratia 2016, 19, 298–303. [Google Scholar]
- Malhotra, A.; Scott, P.; Scott, J.; Gee, H.; Wharton, B. Metabolic changes in Asian Muslim pregnant mothers observing the Ramadan fast in Britain. Br. J. Nutr. 1989, 61, 663–672. [Google Scholar] [CrossRef]
- Sakar, M.; Balsak, D.; Verit, F.; Zebitay, A.; Buyuk, A.; Akay, E.; Turfan, M.; Demir, S.; Yayla, M. The effect of Ramadan fasting and maternal hypoalbuminaemia on neonatal anthropometric parameters and placental weight. J. Obstet. Gynaecol. 2016, 36, 483–486. [Google Scholar] [CrossRef]
- Abd-Allah Rezk, M.; Sayyed, T.; Abo-Elnasr, M.; Shawky, M.; Badr, H. Impact of maternal fasting on fetal well-being parameters and fetal–neonatal outcome: A case–control study. J. Matern. Neonatal Med. 2016, 29, 2834–2838. [Google Scholar] [CrossRef]
- Seckin, K.D.; Yeral, M.I.; Karslı, M.F.; Gultekin, I.B. Effect of maternal fasting for religious beliefs on fetal sonographic findings and neonatal outcomes. Int. J. Gynecol. Obstet. 2014, 126, 123–125. [Google Scholar] [CrossRef]
- Savitri, A.I.; Yadegari, N.; Bakker, J.; van Ewijk, R.J.; Grobbee, D.E.; Painter, R.C.; Uiterwaal, C.S.; Roseboom, T.J. Ramadan fasting and newborn’s birth weight in pregnant Muslim women in The Netherlands. Br. J. Nutr. 2014, 112, 1503–1509. [Google Scholar] [CrossRef]
- Hızlı, D.; Yılmaz, S.S.; Onaran, Y.; Kafalı, H.; Danışman, N.; Mollamahmutoğlu, L. Impact of maternal fasting during Ramadan on fetal Doppler parameters, maternal lipid levels and neonatal outcomes. J. Matern. Neonatal Med. 2012, 25, 975–977. [Google Scholar] [CrossRef]
- Mirghani, H.M.; Hamud, O.A. The Effect of Maternal Diet Restriction on Pregnancy Outcome. Am. J. Perinatol. 2005, 23, 021–024. [Google Scholar] [CrossRef]
- Petherick, E.S.; Tuffnell, D.; Wright, J. Experiences and outcomes of maternal Ramadan fasting during pregnancy: Results from a sub-cohort of the Born in Bradford birth cohort study. BMC Pregnancy Childbirth 2014, 14, 335. [Google Scholar] [CrossRef]
- Parveen, R.; Khakwani, M.; Latif, M.; Tareen, A.U. Maternal and Perinatal outcome after Ramadan Fasting. Pak. J. Med. Sci. 2020, 36, 894–898. [Google Scholar] [CrossRef]
- Hossain, N.; Samuel, M.; Mughal, S.; Shafique, K. Ramadan Fasting: Perception and maternal outcomes during Pregnancy. Pak. J. Med. Sci. 2021, 37, 1262. [Google Scholar] [CrossRef]
- Gul, Z.; Rajar, S.; Shaikh, Z.F.; Shafique, K.; Hossain, N. Perinatal outcome among fasting and non fasting mothers during the month of Ramadan. Pak. J. Med. Sci. 2018, 34, 989–993. [Google Scholar] [CrossRef]
- Kavehmanesh, Z.; Abolghasemi, H. Maternal Ramadan Fasting and Neonatal Health. J. Perinatol. 2004, 24, 748–750. [Google Scholar] [CrossRef][Green Version]
- Ozturk, E.; Balat, O.; Ugur, M.G.; Yazicioglu, C.; Pençe, S.; Erel, O.; Kul, S. Effect of Ramadan fasting on maternal oxidative stress during the second trimester: A preliminary study. J. Obstet. Gynaecol. Res. 2011, 37, 729–733. [Google Scholar] [CrossRef]
- Ziaee, V.; Kihanidoost, Z.; Younesian, M.; Akhavirad, M.-B.; Bateni, F.; Kazemianfar, Z.; Hantoushzadeh, S. The Effect of Ramadan Fasting on Outcome of Pregnancy. Iran. J. Pediatr. 2010, 20, 181–186. [Google Scholar]
- Karateke, A.; Kaplanoglu, M.; Avci, F.; Kurt, R.K.; Baloglu, A. The effect of Ramadan fasting on fetal development. Pak. J. Med. Sci. 2015, 31, 1295. [Google Scholar] [CrossRef]
- Bayoglu Tekin, Y.; Guvendag Guven, E.S.; Mete Ural, U.; Yazici, Z.A.; Kirbas, A.; Kir Sahin, F. Evaluation of the effects of fasting associated dehydration on maternal NGAL levels and fetal renal artery Doppler parameters. J. Matern. Neonatal Med. 2015, 29, 629–632. [Google Scholar] [CrossRef]
- Dikensoy, E.; Balat, O.; Cebesoy, B.; Ozkur, A.; Cicek, H.; Can, G. The effect of Ramadan fasting on maternal serum lipids, cortisol levels and fetal development. Arch. Gynecol. Obstet. 2009, 279, 119–123. [Google Scholar] [CrossRef]
- Khalaf, M.; Tammam, A.E.; Ibrahem, I.; Habib, D.M.; Abdellah, M.S.; Bahlol, M.; Khairy, M.; El Saman, A.M. Effect of Ramadan fasting on amniotic fluid index in last month of pregnancy. Middle East Fertil. Soc. J. 2015, 20, 54–56. [Google Scholar] [CrossRef]
- Kamyabi, Z.; Naderi, T. The effect of Ramadan fasting on amniotic fluid volume. Saudi Med. J. 2004, 25, 45–46. [Google Scholar]
- Makvandi, S.; Karimi, L.; Mahdavian, M.; Bastami, A. No Differences in Hematological Parameters of Fasting and Non-Fasting Pregnant Women Three Months after Ramadan. Int. J. Vitam. Nutr. Res. 2018, 88, 258–262. [Google Scholar] [CrossRef]
- Mirghani, H.; Weerasinghe, D.; Ezimokhai, M.; Smith, J. The effect of maternal fasting on the fetal biophysical profile. Int. J. Gynecol. Obstet. 2003, 81, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.M.; Salem, M.; Weerasinghe, S.D. Effect of maternal fasting on uterine arterial blood flow. J. Obstet. Gynaecol. Res. 2007, 33, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.M.; Weerasinghe, S.; Al-Awar, S.; Abdulla, L.; Ezimokhai, M. The Effect of Intermittent Maternal Fasting on Computerized Fetal Heart Tracing. J. Perinatol. 2005, 25, 90–92. [Google Scholar] [CrossRef]
- Zeballos, E.; Todd, J.E. The effects of skipping a meal on daily energy intake and diet quality. Public Health Nutr. 2020, 23, 3346–3355. [Google Scholar] [CrossRef]
- Petraglia, F.; Imperatore, A.; Challis, J.R. Neuroendocrine Mechanisms in Pregnancy and Parturition. Endocr. Rev. 2010, 31, 783–816. [Google Scholar] [CrossRef]
- Bartlett, D.J.; Biggs, S.N.; Armstrong, S.M. Circadian rhythm disorders among adolescents: Assessment and treatment options. Med. J. Aust. 2013, 199, S16–S20. [Google Scholar] [CrossRef][Green Version]
- Birketvedt, G.S.; Florholmen, J.; Sundsfjord, J.; Østerud, B.; Dinges, D.; Bilker, W.; Stunkard, A. Behavioral and Neuroendocrine Characteristics of the Night-Eating Syndrome. JAMA 1999, 282, 657–663. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, R.; Hallmann, A.; Sokołowska, E.; Kaletha, K.; Klimek, J. Melatonin enhances antioxidant action of α-tocopherol and ascorbate against NADPH- and iron-dependent lipid peroxidation in human placental mitochondria. J. Pineal Res. 2010, 49, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Menet, J.S.; Poirel, V.-J.; Streicher, D.; Gauer, F.; Vivien-Roels, B.; Klosen, P.; Pévet, P.; Masson-Pévet, M. Melatonin induces Cry1 expression in the pars tuberalis of the rat. Mol. Brain Res. 2003, 114, 101–106. [Google Scholar] [CrossRef]
- Gimeno, M.; Landa, A.; Sterin-Speziale, N.; Cardinali, D.; Gimeno, A. Melatonin blocks in vitro generation of prostaglandin by the uterus and hypothalamus. Eur. J. Pharmacol. 1980, 62, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef]
- Supply, W.U.J.W.; Programme, S.M. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Mekary, R.A.; Giovannucci, E.; Willett, W.C.; Van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012, 95, 1182–1189. [Google Scholar] [CrossRef]
- Mekary, R.A.; Giovannucci, E.; Cahill, L.; Willett, W.C.; van Dam, R.M.; Hu, F.B. Eating patterns and type 2 diabetes risk in older women: Breakfast consumption and eating frequency. Am. J. Clin. Nutr. 2013, 98, 436–443. [Google Scholar] [CrossRef]
- Ahola, A.J.; Mutter, S.; Forsblom, C.; Harjutsalo, V.; Groop, P.-H. Meal timing, meal frequency, and breakfast skipping in adult individuals with type 1 diabetes–associations with glycaemic control. Sci. Rep. 2019, 9, 20063. [Google Scholar] [CrossRef]
- Joosoph, J.; Abu, J.; Yu, S. A survey of fasting during pregnancy. Singap. Med. J. 2004, 45, 583–586. [Google Scholar]
- Arab, M.; Nasrollahi, S. Interrelation of Ramadan fasting and birth weight. Med. J. Islam. Acad. Sci. 2001, 14, 91–95. [Google Scholar]
- Yang, X. The festival of fast-breaking Eid al-Fitr in the Great Mosque of Lhasa. Some observations. Études Mong. Et Sibériennes Cent. Et Tibétaines 2016, 47. [Google Scholar] [CrossRef]
- Fernando, H.A.; Zibellini, J.; Harris, R.A.; Seimon, R.V.; Sainsbury, A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Seiermann, A.U.; Al-Mufti, H.; Waid, J.L.; Wendt, A.S.; Sobhan, S.; Gabrysch, S. Women’s fasting habits and dietary diversity during Ramadan in rural Bangladesh. Matern. Child Nutr. 2021, 17, e13135. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Lane, J.M.; Qian, J.; Mignot, E.; Redline, S.; Scheer, F.A.; Saxena, R. Genetics of circadian rhythms and sleep in human health and disease. Nat. Rev. Genet. 2022, 24, 4–20. [Google Scholar] [CrossRef]
- Aqeel, M.M.; Guo, J.; Lin, L.; Gelfand, S.B.; Delp, E.J.; Bhadra, A.; Richards, E.A.; Hennessy, E.; Eicher-Miller, H.A. Temporal Dietary Patterns Are Associated with Obesity in US Adults. J. Nutr. 2020, 150, 3259–3268. [Google Scholar] [CrossRef] [PubMed]

| Maternal Outcomes | Fetal Outcomes | Birth Outcomes | Childhood Outcomes | |
|---|---|---|---|---|
| Higher/direct association | Hematological parameter/lipid profile TC [19,72] TG [19,72] Urea [19] LDL [19] Albumin [19] BUN [71] Visfatin1 [53] NGAL [71] Mode of delivery Risk of caesarean section [62] Risk of induction labor [62] Oxidative stress marker TAS [68] Pregnant women’s health Ketonuria [50,61] Risk of GDM [62] | Biophysical profile Amniotic fluid index [59,73,74] Doppler flow indices [71] Renal artery PI [71] Renal artery RI [71] Placenta Weight of placenta [57] | Neonatal health indicator Fifth minutes APGAR score [65] Hematological parameter Risk of neonatal hyperbilirubinemia * [48] | NA |
| No difference/association | Hematological parameter/lipid profile [65,75] Cortisol [72] TC [61] LDL [72] HDL [19,61,72] VLDL [61,72] LDL/HDL [72] RBC [75] Hb [75] Hct [75] MCV [75] MCH [75] MCHC [75] Plt [75] Risk of hypoglycemia [50] Mode of delivery * [47,48,58,59,61,64,70] Oxidative stress marker TOS [68] OSI [68] Pregnant women’s health Risk of GDM [64,65] Risk of pre-eclampsia [47,64] Risk of pregnancy-induced hypertension [48,62,65] | Biophysical profile [72,76] Amniotic fluid index [51,52,58,61,72,76] [70] (third trimester) Doppler flow indices [51,52,58,59,61,77] Umbilical artery S/D ratio [70,71,72] Renal artery S/D [71] Fetal growth Abdominal circumference [51,52,59] Fetal weight gain [51,52,59,70,72] Biparietal diameter [52,59,72] [70] (second trimester) Femur length [52,59,70,72] Heart tracing Heart rate [78] Placenta Placental location [77] Weight of placenta [64,66] | Neonatal health indicator First minutes APGAR score [62,65,70] Fifth minutes APGAR score [47,55,58,62,70] Risk of macrosomia * [48] Risk of admission to NICU [58,59,61,70] Birth anthropometry Height [47,57,64,65,66,67,69] Head circumference [47,57,64,65,66,69] Mid arm circumference [64,66] | Brain development IQ [49] Children growth Weight for age [49] Height for age [49] BMI for age [49] |
| Lower/inverseassociation | Hematological parameter/lipid profile TG [61] VLDL [61] Protein [19] Sirtuin1 [53] Mode of delivery Risk of caesarean delivery [50] Pregnant women’s health Risk of GDM [47] | Biophysical profile Amniotic fluid index [70] (second trimester) Breath movement [76] Fetal growth Biparietal diameter [51] Femur length [51] Head circumference [51] Heart tracing Large accelerations [78] | Hematological parameter Risk of neonatal hypoglycaemia * [48] | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-E.; Loy, S.L.; Chen, L.-W. Chrononutrition during Pregnancy and Its Association with Maternal and Offspring Outcomes: A Systematic Review and Meta-Analysis of Ramadan and Non-Ramadan Studies. Nutrients 2023, 15, 756. https://doi.org/10.3390/nu15030756
Chen Y-E, Loy SL, Chen L-W. Chrononutrition during Pregnancy and Its Association with Maternal and Offspring Outcomes: A Systematic Review and Meta-Analysis of Ramadan and Non-Ramadan Studies. Nutrients. 2023; 15(3):756. https://doi.org/10.3390/nu15030756
Chicago/Turabian StyleChen, Yu-En, See Ling Loy, and Ling-Wei Chen. 2023. "Chrononutrition during Pregnancy and Its Association with Maternal and Offspring Outcomes: A Systematic Review and Meta-Analysis of Ramadan and Non-Ramadan Studies" Nutrients 15, no. 3: 756. https://doi.org/10.3390/nu15030756
APA StyleChen, Y.-E., Loy, S. L., & Chen, L.-W. (2023). Chrononutrition during Pregnancy and Its Association with Maternal and Offspring Outcomes: A Systematic Review and Meta-Analysis of Ramadan and Non-Ramadan Studies. Nutrients, 15(3), 756. https://doi.org/10.3390/nu15030756