Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist
Abstract
1. Introduction
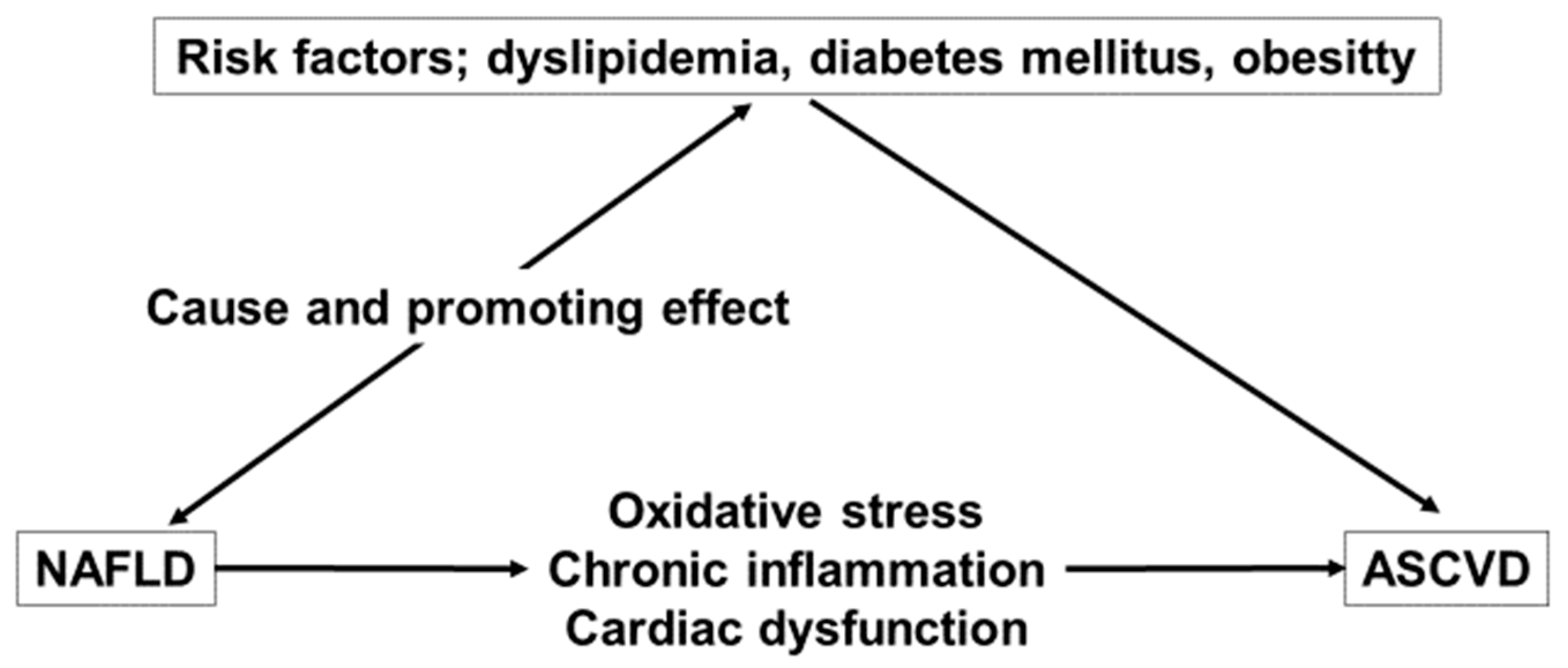
2. The Interlink Connections of ASCVD and NAFLD
2.1. Dyslipidemia
2.2. Oxidative Stress and Chronic Inflammation
2.3. Coagulation Disorders and Endothelial Dysfunction
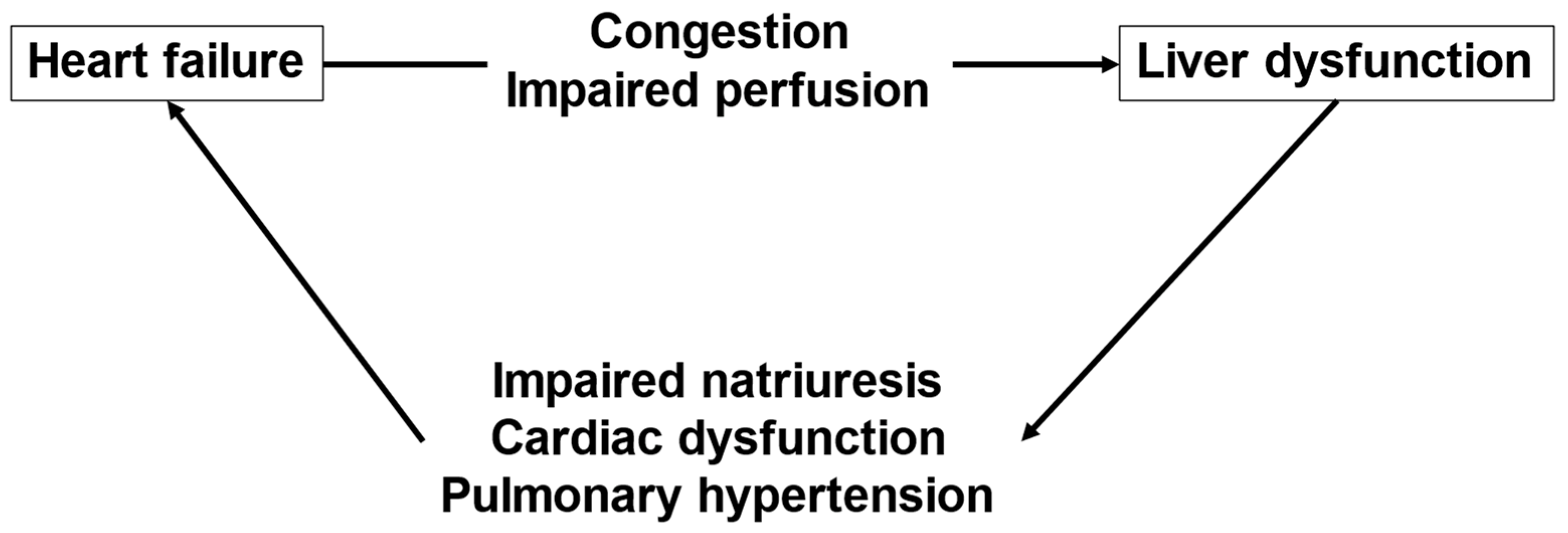
3. The Interactions between HF and Liver Dysfunction
4. Medical Therapy for CVD and Liver Damage
5. Clinical Applications
5.1. NAFLD Management and the Risk of CVD
5.2. Assessment of Liver Fibrosis as a Prognostic Marker for HF
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCVD | atherosclerotic cardiovascular disease |
| CCTA | coronary computed tomography angiography |
| CVD | cardiovascular disease |
| FALD | Fontan-associated liver disease |
| FIB-4 index | fibrosis-4 index |
| HCC | hepatocellular carcinoma |
| HDL | high-density lipoprotein |
| HF | heart failure |
| HRP | high-risk plaque |
| LDL | low-density lipoprotein |
| LV | left ventricular |
| NAFLD | non-alcoholic fatty liver disease |
| NFS | non-alcoholic fatty liver disease fibrosis score |
| PoPH | porto-pulmonary hypertension |
| RV | right ventricular |
| TG | triglyceride |
References
- Nakahara, T.; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD); Hyogo, H.; Yoneda, M.; Sumida, Y.; Eguchi, Y.; Fujii, H.; Ono, M.; Kawaguchi, T.; Imajo, K.; et al. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J. Gastroenterol. 2013, 49, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The Metabolic Syndrome as a Predictor of Nonalcoholic Fatty Liver Disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Gona, P.; Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Wang, T.J.; Tu, J.V.; Levy, D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation 2009, 119, 3070–3077. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Nichols, G.A.; Gullion, C.M.; Koro, C.E.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004, 27, 1879–1884. [Google Scholar] [CrossRef]
- Imajo, K.; Hyogo, H.; Yoneda, M.; Honda, Y.; Kessoku, T.; Tomeno, W.; Ogawa, Y.; Taguri, M.; Mawatari, H.; Nozaki, Y.; et al. LDL-Migration Index (LDL-MI), an Indicator of Small Dense Low-Density Lipoprotein (sdLDL), Is Higher in Non-Alcoholic Steatohepatitis than in Non-Alcoholic Fatty Liver: A Multicenter Cross-Sectional Study. PLoS ONE 2014, 9, e115403. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.W.; Mullens, W. Abdominal Contributions to Cardiorenal Dysfunction in Congestive Heart Failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef]
- Di Pasqua, L.G.; Cagna, M.; Berardo, C.; Vairetti, M.; Ferrigno, A. Detailed Molecular Mechanisms Involved in Drug-Induced Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis: An Update. Biomedicines 2022, 10, 194. [Google Scholar] [CrossRef]
- Mancuso, L.; Scordato, F.; Pieri, M.; Valerio, E.; Mancuso, A. Management of portopulmonary hypertension: New perspectives. World J. Gastroenterol. 2013, 19, 8252–8257. [Google Scholar] [CrossRef]
- Colle, I.O.; Moreau, R.; Godinho, E.; Belghiti, J.; Ettori, F.; Cohen-Solal, A.; Mal, H.; Bernuau, J.; Marty, J.; Lebrec, D.; et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: A prospective study. Hepatology 2003, 37, 401–409. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2012, 57, 1357–1365. [Google Scholar] [CrossRef]
- Nasr, P.; Ignatova, S.; Kechagias, S.; Ekstedt, M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatol. Commun. 2017, 2, 199–210. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Katsarou, A.; Moustakas, I.I.; Pyrina, I.; Lembessis, P.; Koutsilieris, M.; Chatzigeorgiou, A. Metabolic inflammation as an instigator of fibrosis during non-alcoholic fatty liver disease. World J. Gastroenterol. 2020, 26, 1993–2011. [Google Scholar] [CrossRef]
- Ogresta, D.; Mrzljak, A.; Berkovic, M.C.; Bilic-Curcic, I.; Stojsavljevic-Shapeski, S.; Virovic-Jukic, L. Coagulation and Endothelial Dysfunction Associated with NAFLD: Current Status and Therapeutic Implications. J. Clin. Transl. Hepatol. 2022, 10, 339–355. [Google Scholar] [CrossRef]
- Zou, Y.; Lan, J.; Zhong, Y.; Yang, S.; Zhang, H.; Xie, G. Association of remnant cholesterol with nonalcoholic fatty liver disease: A general population-based study. Lipids Health Dis. 2021, 20, 139. [Google Scholar] [CrossRef]
- Peng, K.; Mo, Z.; Tian, G. Serum Lipid Abnormalities and Nonalcoholic Fatty Liver Disease in Adult Males. Am. J. Med. Sci. 2017, 353, 236–241. [Google Scholar] [CrossRef]
- Nishihara, T.; Miyoshi, T.; Ichikawa, K.; Osawa, K.; Nakashima, M.; Miki, T.; Ito, H. Association of Oxidized Low-Density Lipoprotein in Nonalcoholic Fatty Liver Disease with High-Risk Plaque on Coronary Computed Tomography Angiography: A Matched Case–Control Study. J. Clin. Med. 2022, 11, 2838. [Google Scholar] [CrossRef]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef]
- Campanella, A.; Iacovazzi, P.A.; Misciagna, G.; Bonfiglio, C.; Mirizzi, A.; Franco, I.; Bianco, A.; Sorino, P.; Caruso, M.G.; Cisternino, A.M.; et al. The Effect of Three Mediterranean Diets on Remnant Cholesterol and Non-Alcoholic Fatty Liver Disease: A Secondary Analysis. Nutrients 2020, 12, 1674. [Google Scholar] [CrossRef]
- Cobbina, E.; Akhlaghi, F. Non-alcoholic fatty liver disease (NAFLD)-pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free. Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Cayón, A.; Fernández-Gil, P.; Hernández-Guerra, M.; Mayorga, M.; Domínguez-Díez, A.; Fernández-Escalante, J.C.; Pons-Romero, F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Yang, L.; van Rooijen, N.; Brenner, D.A.; Ohnishi, H.; Seki, E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology 2012, 57, 577–589. [Google Scholar] [CrossRef]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Kumar, V.; Prabhu, S.D.; Bansal, S.S. CD4+ T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 992653. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, H.; Xu, T.; Hao, J.; Chen, X.; Sun, S.; Yang, J.; Sun, J.; Lin, H.; Guo, H. Identification and verification of immune-related biomarkers and immune infiltration in diabetic heart failure. Front. Cardiovasc. Med. 2022, 9, 931066. [Google Scholar] [CrossRef]
- Kumar, V.; Rosenzweig, R.; Asalla, S.; Nehra, S.; Prabhu, S.D.; Bansal, S.S. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4+ T Lymphocytes During Ischemic Heart Failure. JACC: Basic Transl. Sci. 2022, 7, 1038–1049. [Google Scholar] [CrossRef]
- Lu, Y.; Xia, N.; Cheng, X. Regulatory T Cells in Chronic Heart Failure. Front. Immunol. 2021, 12, 732794. [Google Scholar] [CrossRef]
- Rurik, J.G.; Aghajanian, H.; Epstein, J.A. Immune Cells and Immunotherapy for Cardiac Injury and Repair. Circ. Res. 2021, 128, 1766–1779. [Google Scholar] [CrossRef]
- Northup, P.G.; Caldwell, S.H. Coagulation in Liver Disease: A Guide for the Clinician. Clin. Gastroenterol. Hepatol. 2013, 11, 1064–1074. [Google Scholar] [CrossRef]
- Virović-Jukić, L.; Stojsavljević-Shapeski, S.; Forgač, J.; Kukla, M.; Mikolašević, I. Non-alcoholic fatty liver disease-A procoagulant condition? Croat. Med. J. 2021, 62, 25–33. [Google Scholar] [CrossRef]
- Wanless, I.R.; Wong, F.; Blendis, L.M.; Greig, P.; Heathcote, E.J.; Levy, G. Hepatic and portal vein thrombosis in cirrhosis: Possible role in development of parenchymal extinction and portal hypertension. Hepatology 1995, 21, 1238–12347. [Google Scholar]
- Potze, W.; Siddiqui, M.S.; Boyett, S.L.; Adelmeijer, J.; Daita, K.; Sanyal, A.J.; Lisman, T. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 980–987. [Google Scholar] [CrossRef]
- Kotronen, A.; Joutsi-Korhonen, L.; Sevastianova, K.; Bergholm, R.; Hakkarainen, A.; Pietiläinen, K.; Lundbom, N.; Rissanen, A.; Lassila, R.; Yki-Järvinen, H. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int. 2010, 31, 176–183. [Google Scholar] [CrossRef]
- Lallukka, S.; Luukkonen, P.K.; Zhou, Y.; Isokuortti, E.; Leivonen, M.; Juuti, A.; Hakkarainen, A.; Orho-Melander, M.; Lundbom, N.; Olkkonen, V.M.; et al. Obesity/insulin resistance rather than liver fat increases coagulation factor activities and expression in humans. Thromb. Haemost. 2017, 117, 286–294. [Google Scholar] [CrossRef]
- Verrijken, A.; Francque, S.; Mertens, I.; Prawitt, J.; Caron, S.; Hubens, G.; Van Marck, E.; Staels, B.; Michielsen, P.; Van Gaal, L. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2013, 59, 121–129. [Google Scholar] [CrossRef]
- Tripodi, A.; Fracanzani, A.L.; Primignani, M.; Chantarangkul, V.; Clerici, M.; Mannucci, P.M.; Peyvandi, F.; Bertelli, C.; Valenti, L.; Fargion, S. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2014, 61, 148–154. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Scala, L.; Zenari, L.; Lippi, G.; Franchini, M.; Arcaro, G. Plasma PAI-1 Levels Are Increased in Patients With Nonalcoholic Steatohepatitis. Diabetes Care 2007, 30, e31–e32. [Google Scholar] [CrossRef]
- Fujimoto, M.; Shimizu, N.; Kunii, K.; Martyn, J.J.; Ueki, K.; Kaneki, M. A Role for iNOS in Fasting Hyperglycemia and Impaired Insulin Signaling in the Liver of Obese Diabetic Mice. Diabetes 2005, 54, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.D.; Seggara, G.; Vo, P.A.; Macallister, R.J.; Hobbs, A.J.; Ahluwalia, A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003, 17, 773–775. [Google Scholar] [CrossRef] [PubMed]
- Villanova, N.; Moscatiello, S.; Ramilli, S.; Bugianesi, E.; Magalotti, D.; Vanni, E.; Zoli, M.; Marchesini, G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Sherlock, S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br. Heart J. 1951, 13, 273–293. [Google Scholar] [CrossRef]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Samsky, M.D.; Patel, C.B.; DeWald, T.A.; Smith, A.D.; Felker, G.M.; Rogers, J.G.; Hernandez, A.F. Cardiohepatic interactions in heart failure: An overview and clinical implications. J. Am. Coll. Cardiol. 2013, 61, 2397–2405. [Google Scholar] [CrossRef]
- Henrion, J. Hypoxic Hepatitis. Liver Int. 2012, 32, 1039–1052. [Google Scholar] [CrossRef]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.-F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.-F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef]
- Ahloulay, M.; Dechaux, M.; Hässler, C.; Bouby, N.; Bankir, L. Cyclic AMP is a hepatorenal link influencing natriuresis and contributing to glucagon-induced hyperfiltration in rats. J. Clin. Investig. 1996, 98, 2251–2258. [Google Scholar] [CrossRef]
- Hendy, G.N.; Tomlinson, S.; O’Riordan, J.L.H. Impaired responsiveness to the effect of glucagon on plasma adenosine 3′:5′-cyclic monophosphate in normal man. Eur. J. Clin. Investig. 1977, 7, 155–160. [Google Scholar] [CrossRef]
- Bernardi, M.; Maggioli, C.; Dibra, V.; Zaccherini, G. QT interval prolongation in liver cirrhosis: Innocent bystander or serious threat? Expert Rev. Gastroenterol. Hepatol. 2012, 6, 57–66. [Google Scholar] [CrossRef]
- Sessa, A.; Allaire, M.; Lebray, P.; Medmoun, M.; Tiritilli, A.; Iaria, P.; Cadranel, J.-F. From congestive hepatopathy to hepatocellular carcinoma, how can we improve patient management? JHEP Rep. 2021, 3, 100249. [Google Scholar] [CrossRef]
- Elder, R.W.; McCabe, N.M.; Hebson, C.; Veledar, E.; Romero, R.; Ford, R.M.; Mahle, W.T.; Kogon, B.E.; Sahu, A.; Jokhadar, M.; et al. Features of portal hypertension are associated with major adverse events in Fontan patients: The VAST study. Int. J. Cardiol. 2013, 168, 3764–3769. [Google Scholar] [CrossRef]
- Miike, H.; Ohuchi, H.; Hayama, Y.; Isawa, T.; Sakaguchi, H.; Kurosaki, K.; Nakai, M. Systemic Artery Vasoconstrictor Therapy in Fontan Patients with High Cardiac Output-Heart Failure: A Single-Center Experience. Pediatr. Cardiol. 2021, 42, 700–706. [Google Scholar] [CrossRef]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.-P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2020, 42, 563–645. [Google Scholar] [CrossRef]
- Ohuchi, H. Where Is the “Optimal” Fontan Hemodynamics? Korean Circ. J. 2017, 47, 842–857. [Google Scholar] [CrossRef]
- Ohuchi, H.; Ono, S.; Tanabe, Y.; Fujimoto, K.; Yagi, H.; Sakaguchi, H.; Miyazaki, A.; Yamada, O. Long-Term Serial Aerobic Exercise Capacity and Hemodynamic Properties in Clinically and Hemodynamically Good, “Excellent”, Fontan Survivors. Circ. J. 2012, 76, 195–203. [Google Scholar] [CrossRef]
- Herve, P.; Lebrec, D.; Brenot, F.; Simonneau, G.; Humbert, M.; Sitbon, O.; Duroux, P. Pulmonary vascular disorders in portal hypertension. Eur. Respir. J. 1998, 11, 1153–1166. [Google Scholar] [CrossRef]
- Krowka, M.J.; Swanson, K.L.; Frantz, R.P.; McGoon, M.D.; Wiesner, R.H. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology 2006, 44, 1502–1510. [Google Scholar] [CrossRef]
- Hadengue, A.; Benhayoun, M.K.; Lebrec, D.; Benhamou, J.-P. Pulmonary hypertension complicating portal hypertension: Prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991, 100, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Van Erven, L.; Schalij, M.J. Amiodarone: An effective antiarrhythmic drug with unusual side effects. Heart 2010, 96, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Pessayre, D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol. Ther. 1995, 67, 101–154. [Google Scholar] [CrossRef]
- Lewis, J.H.; Ranard, R.C.; Caruso, A.; Jackson, L.K.; Mullick, F.; Ishak, K.G.; Seeff, L.B.; Zimmerman, H.J. Amiodarone hepatotoxicity: Prevalence and clinicopathologic correlations among 104 patients. Hepatology 1989, 9, 679–685. [Google Scholar] [CrossRef]
- Deschamps, D.; DeBeco, V.; Fisch, C.; Fromenty, B.; Guillouzo, A.; Pessayre, D. Inhibition by perhexiline of oxidative phosphorylation and the beta-oxidation of fatty acids: Possible role in pseudoalcoholic liver lesions. Hepatology 1994, 19, 948–961. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.-A.; Borgne-Sanchez, A.; Fromenty, B. Drug-induced toxicity on mitochondria and lipid metabolism: Mechanistic diversity and deleterious consequences for the liver. J. Hepatol. 2011, 54, 773–794. [Google Scholar] [CrossRef]
- Erez, N.; Hubel, E.; Avraham, R.; Cohen, R.; Fishman, S.; Bantel, H.; Manns, M.; Tirosh, B.; Zvibel, I.; Shibolet, O. Hepatic Amiodarone Lipotoxicity Is Ameliorated by Genetic and Pharmacological Inhibition of Endoplasmatic Reticulum Stress. Toxicol. Sci. 2017, 159, 402–412. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Nassif, M.E.; Qintar, M.; Windsor, S.L.; Jermyn, R.; Shavelle, D.M.; Tang, F.; Lamba, S.; Bhatt, K.; Brush, J.; Civitello, A.; et al. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation 2021, 143, 1673–1686. [Google Scholar] [CrossRef]
- Nakashima, M.; Miyoshi, T.; Ejiri, K.; Kihara, H.; Hata, Y.; Nagano, T.; Takaishi, A.; Toda, H.; Nanba, S.; Nakamura, Y.; et al. Effects of luseogliflozin on estimated plasma volume in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 9, 712–720. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sjöström, C.D.; Sugg, J.; Parikh, S. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes. Metab. 2014, 16, 159–169. [Google Scholar] [CrossRef]
- Yki-Järvinen, H.; Ryysy, L.; Kauppila, M.; Kujansuu, E.; Lahti, J.; Marjanen, T.; Niskanen, L.; Rajala, S.; Salo, S.; Seppälä, P.; et al. Effect of obesity on the response to insulin therapy in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1997, 82, 4037–4043. [Google Scholar] [CrossRef]
- Katsuyama, H.; Hamasaki, H.; Adachi, H.; Moriyama, S.; Kawaguchi, A.; Sako, A.; Mishima, S.; Yanai, H. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Metabolic Parameters in Patients With Type 2 Diabetes: A Chart-Based Analysis. J. Clin. Med. Res. 2016, 8, 237–243. [Google Scholar] [CrossRef]
- Komiya, C.; Tsuchiya, K.; Shiba, K.; Miyachi, Y.; Furuke, S.; Shimazu, N.; Yamaguchi, S.; Kanno, K.; Ogawa, Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016, 11, e0151511. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Mikami, S.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; Okubo, T.; Hayama, K.; et al. Effect of sodium-glucose cotransporter 2 inhibitor in patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus: A propensity score-matched analysis of real-world data. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211000243. [Google Scholar] [CrossRef]
- Shinozaki, S.; Tahara, T.; Lefor, A.K.; Ogura, M. Long-term empagliflozin therapy improves levels of hepatic fibrosis marker in patients with non-alcoholic fatty liver disease complicated by type 2 diabetes mellitus. J. Med. Investig. 2020, 67, 280–284. [Google Scholar] [CrossRef]
- Taheri, H.; Malek, M.; Ismail-Beigi, F.; Zamani, F.; Sohrabi, M.; Babaei, M.R.; Khamseh, M.E. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2020, 37, 4697–4708. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Petracca, G.; Csermely, A.; Beatrice, G.; Targher, G. Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Metabolites 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, Y.; Chen, J.; Hu, Y.; Xu, Y. Verapamil Ameliorates Hepatic Metaflammation by Inhibiting Thioredoxin-Interacting Protein/NLRP3 Pathways. Front. Endocrinol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Shimamura, M.; Miyake, T.; Shimosato, T.; Minobe, N.; Moritani, T.; Osako, M.K.; Nakagami, F.; Koriyama, H.; Kyutoku, M.; et al. Nifedipine prevents hepatic fibrosis in a non-alcoholic steatohepatitis model induced by an L-methionine-and choline-deficient diet. Mol. Med. Rep. 2011, 5, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M.; Stål, P.; Kechagias, S.; Hultcrantz, R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef]
- Saadeh, S.; Cammell, G.; Carey, W.D.; Younossi, Z.; Barnes, D.; Easley, K. The role of liver biopsy in chronic hepatitis C. Hepatology 2001, 33, 196–200. [Google Scholar] [CrossRef]
- Bravo, A.A.; Sheth, S.G.; Chopra, S. Liver biopsy. N. Engl. J. Med. 2001, 344, 495–500. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Nash Clinical Research Network. Comparison of Noninvasive Markers of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Barbosa, J.V.; Milligan, S.; Frick, A.; Broestl, J.; Younossi, Z.; Afdhal, N.; Lai, M. Fibrosis-4 Index Can Independently Predict Major Adverse Cardiovascular Events in Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2022, 117, 453–461. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Kim, K.J.; Yoo, M.E.; Kim, G.; Yoon, H.-J.; Jo, K.; Youn, J.-C.; Yun, M.; Park, J.Y.; Shim, C.Y.; et al. Association of non-alcoholic steatohepatitis with subclinical myocardial dysfunction in non-cirrhotic patients. J. Hepatol. 2018, 68, 764–772. [Google Scholar] [CrossRef]
- Song, D.S.; Chang, U.I.; Kang, S.-G.; Song, S.-W.; Yang, J.M. Noninvasive Serum Fibrosis Markers are Associated with Coronary Artery Calcification in Patients with Nonalcoholic Fatty Liver Disease. Gut Liver 2019, 13, 658–668. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Ezponda, A.; Núñez-Córdoba, J.M.; Herrero, J.I.; Bastarrika, G.; Frühbeck, G.; Escalada, J. Transient elastography and serum markers of liver fibrosis associate with epicardial adipose tissue and coronary artery calcium in NAFLD. Sci. Rep. 2022, 12, 6564. [Google Scholar] [CrossRef]
- Kim, D.; Choi, S.-Y.; Park, E.H.; Lee, W.; Kang, J.H.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Jeong, S.H.; Lee, D.H.; et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012, 56, 605–613. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.S.; Cho, Y.K.; Kim, E.H.; Lee, M.J.; Bae, I.Y.; Jung, C.H.; Park, J.-Y.; Kim, H.-K.; Lee, W.J. Association between noninvasive assessment of liver fibrosis and coronary artery calcification progression in patients with nonalcoholic fatty liver disease. Sci. Rep. 2020, 10, 18323. [Google Scholar] [CrossRef]
- Puchner, S.B.; Lu, M.T.; Mayrhofer, T.; Liu, T.; Pursnani, A.; Ghoshhajra, B.; Truong, Q.A.; Wiviott, S.D.; Fleg, J.L.; Hoffmann, U.; et al. High-Risk Coronary Plaque at Coronary CT Angiography Is Associated with Nonalcoholic Fatty Liver Disease, Independent of Coronary Plaque and Stenosis Burden: Results from the ROMICAT II Trial. Radiology 2015, 274, 693–701. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Toda, H.; Ejiri, K.; Yoshida, M.; Nakamura, K.; Morita, H.; Ito, H. Incremental prognostic value of non-alcoholic fatty liver disease over coronary computed tomography angiography findings in patients with suspected coronary artery disease. Eur. J. Prev. Cardiol. 2021, 28, 2059–2066. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Nakamura, K.; Ito, H. Prognostic Value of Coronary Computed Tomographic Angiography in Patients With Nonalcoholic Fatty Liver Disease. JACC: Cardiovasc. Imaging 2020, 13, 1628–1630. [Google Scholar] [CrossRef]
- Sharma, D.L.; Lakhani, H.V.; Klug, R.L.; Snoad, B.; El-Hamdani, R.; Shapiro, J.I.; Sodhi, K. Investigating Molecular Connections of Non-alcoholic Fatty Liver Disease with Associated Pathological Conditions in West Virginia for Biomarker Analysis. J. Clin. Cell. Immunol. 2017, 8, 523. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyoshi, T.; Nakashima, M.; Nishihara, T.; Osawa, K.; Miki, T.; Toda, H.; Yoshida, M.; Ito, H. Prognostic value of pericoronary adipose tissue attenuation in patients with non-alcoholic fatty liver disease with suspected coronary artery disease. Heart Vessels 2022, 37, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Morimitsu, Y.; Akagi, N.; Nakashima, M.; Ito, H. Association between higher pericoronary adipose tissue attenuation measured by coronary computed tomography angiography and nonalcoholic fatty liver disease: A matched case-control study. Medicine 2021, 100, e27043. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Ng, C.S.; Wu, T.T.; Ayers, G.D.; Curley, S.A.; Abdalla, E.K.; Vauthey, J.N.; Charnsangavej, C. Comparison of CT Methods for Determining the Fat Content of the Liver. Am. J. Roentgenol. 2007, 188, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Zeb, I.; Li, D.; Nasir, K.; Katz, R.; Larijani, V.N.; Budoff, M.J. Computed tomography scans in the evaluation of fatty liver disease in a population based study: The multi-ethnic study of atherosclerosis. Acad. Radiol. 2012, 19, 811–818. [Google Scholar] [CrossRef]
- Van Deursen, V.M.; Damman, K.; Hillege, H.L.; van Beek, A.P.; Van Veldhuisen, D.J.; Voors, A.A. Abnormal Liver Function in Relation to Hemodynamic Profile in Heart Failure Patients. J. Card. Fail. 2010, 16, 84–90. [Google Scholar] [CrossRef]
- Ferraioli, G.; Tinelli, C.; Dal Bello, B.; Zicchetti, M.; Filice, G.; Filice, C.; on behalf of the Liver Fibrosis Study Group. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology 2012, 56, 2125–2133. [Google Scholar] [CrossRef]
- Nakayama, R.; Takaya, Y.; Nakamura, K.; Toh, N.; Ito, H. Efficacy of shear wave elastography for assessment of liver function in patients with heart failure. ESC Heart Fail. 2021, 8, 1751–1758. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sakata, Y.; Ohtani, T.; Mizote, I.; Takeda, Y.; Asano, Y.; Masuda, M.; Minamiguchi, H.; Kanzaki, M.; Ichibori, Y.; et al. Usefulness of Transient Elastography for Noninvasive and Reliable Estimation of Right-Sided Filling Pressure in Heart Failure. Am. J. Cardiol. 2013, 113, 552–558. [Google Scholar] [CrossRef]
- Saito, Y.; Kato, M.; Nagashima, K.; Monno, K.; Aizawa, Y.; Okumura, Y.; Matsumoto, N.; Moriyama, M.; Hirayama, A. Prognostic Relevance of Liver Stiffness Assessed by Transient Elastography in Patients With Acute Decompensated Heart Failure. Circ. J. 2018, 82, 1822–1829. [Google Scholar] [CrossRef]
- Nakayama, R.; Takaya, Y.; Nakamura, K.; Takemoto, R.; Toh, N.; Ito, H. Assessment of congestion and clinical outcomes in patients with chronic heart failure using shear wave elasticity. ESC Heart Fail. 2022, 9, 1279–1286. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Tomiyama, H.; Shiina, K.; Matsumoto, C.; Kimura, K.; Fujii, M.; Takata, Y.; Yamashina, A.; Chikamori, T. Liver stiffness and arterial stiffness/abnormal central hemodynamics in the early stage of heart failure. IJC Heart Vasc. 2018, 20, 32–37. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshihisa, A.; Kanno, Y.; Watanabe, S.; Yokokawa, T.; Abe, S.; Misaka, T.; Sato, T.; Suzuki, S.; Oikawa, M.; et al. Liver stiffness assessed by Fibrosis-4 index predicts mortality in patients with heart failure. Open Heart 2017, 4, e000598. [Google Scholar] [CrossRef]
- Nakashima, M.; Sakuragi, S.; Miyoshi, T.; Takayama, S.; Kawaguchi, T.; Kodera, N.; Akai, H.; Koide, Y.; Otsuka, H.; Wada, T.; et al. Fibrosis-4 index reflects right ventricular function and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 2240–2247. [Google Scholar] [CrossRef]
- Maeda, D.; Sakane, K.; Ito, T.; Kanzaki, Y.; Sohmiya, K.; Hoshiga, M. Fibrosis-4 index reflects right-sided filling pressure in patients with heart failure. Heart Vessels 2019, 35, 376–383. [Google Scholar] [CrossRef]
- Kawahira, M.; Tamaki, S.; Yamada, T.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Seo, M.; et al. Prognostic value of impaired hepato-renal function and liver fibrosis in patients admitted for acute heart failure. ESC Heart Fail. 2021, 8, 1274–1283. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.L.; Anand, I.S.; Sweitzer, N.K.; Fang, J.C.; O’Meara, E.; Shah, S.J.; Desai, A.S.; Lewis, E.F.; et al. Association of Natriuretic Peptides With Cardiovascular Prognosis in Heart Failure With Preserved Ejection Fraction: Secondary Analysis of the TOPCAT Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 1000–1005. [Google Scholar] [CrossRef]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef]
- Yoshihisa, A.; Sato, Y.; Yokokawa, T.; Sato, T.; Suzuki, S.; Oikawa, M.; Kobayashi, A.; Yamaki, T.; Kunii, H.; Nakazato, K.; et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2017, 5, 262–270. [Google Scholar] [CrossRef]
- Nakashima, M.; Tanakaya, M.; Miyoshi, T.; Saito, T.; Katayama, Y.; Sakuragi, S.; Ito, H. The Fibrosis-4 Index Predicts Cardiovascular Prognosis in Patients With Severe Isolated Tricuspid Regurgitation. Circ. J. 2022, 86, 1777–1784. [Google Scholar] [CrossRef]
- Nakayama, R.; Takaya, Y.; Nakamura, K.; Takemoto, R.; Toh, N.; Ito, H. Efficacy of shear wave elasticity for predicting clinical outcomes in patients with significant tricuspid regurgitation. Heart Vessels 2022, 37, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.; Kozhuharov, N.; Coats, A.J.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, N.; Matsubara, T.; Sato-Matsubara, M.; Fujii, H.; Enomoto, M.; Kawada, N. Anti-fibrotic treatments for chronic liver diseases: The present and the future. Clin. Mol. Hepatol. 2021, 27, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
| Indices | Equation |
|---|---|
| FIB-4 index | (Age(years) × AST(U/L))/(platelet (109) × ALT1/2 (U/L)) |
| NFS | −1.675 + 0.037 × age (years) + 0.094 × body mass index (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1; no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet count (109/L) − 0.66 × serum albumin (g/dL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, M.; Nakamura, K.; Nishihara, T.; Ichikawa, K.; Nakayama, R.; Takaya, Y.; Toh, N.; Akagi, S.; Miyoshi, T.; Akagi, T.; et al. Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist. Nutrients 2023, 15, 748. https://doi.org/10.3390/nu15030748
Nakashima M, Nakamura K, Nishihara T, Ichikawa K, Nakayama R, Takaya Y, Toh N, Akagi S, Miyoshi T, Akagi T, et al. Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist. Nutrients. 2023; 15(3):748. https://doi.org/10.3390/nu15030748
Chicago/Turabian StyleNakashima, Mitsutaka, Kazufumi Nakamura, Takahiro Nishihara, Keishi Ichikawa, Rie Nakayama, Yoichi Takaya, Norihisa Toh, Satoshi Akagi, Toru Miyoshi, Teiji Akagi, and et al. 2023. "Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist" Nutrients 15, no. 3: 748. https://doi.org/10.3390/nu15030748
APA StyleNakashima, M., Nakamura, K., Nishihara, T., Ichikawa, K., Nakayama, R., Takaya, Y., Toh, N., Akagi, S., Miyoshi, T., Akagi, T., & Ito, H. (2023). Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist. Nutrients, 15(3), 748. https://doi.org/10.3390/nu15030748







