Effect of Oral Intake of Carrot Juice on Cyclooxygenases and Cytokines in Healthy Human Blood Stimulated by Lipopolysaccharide
Abstract
1. Introduction
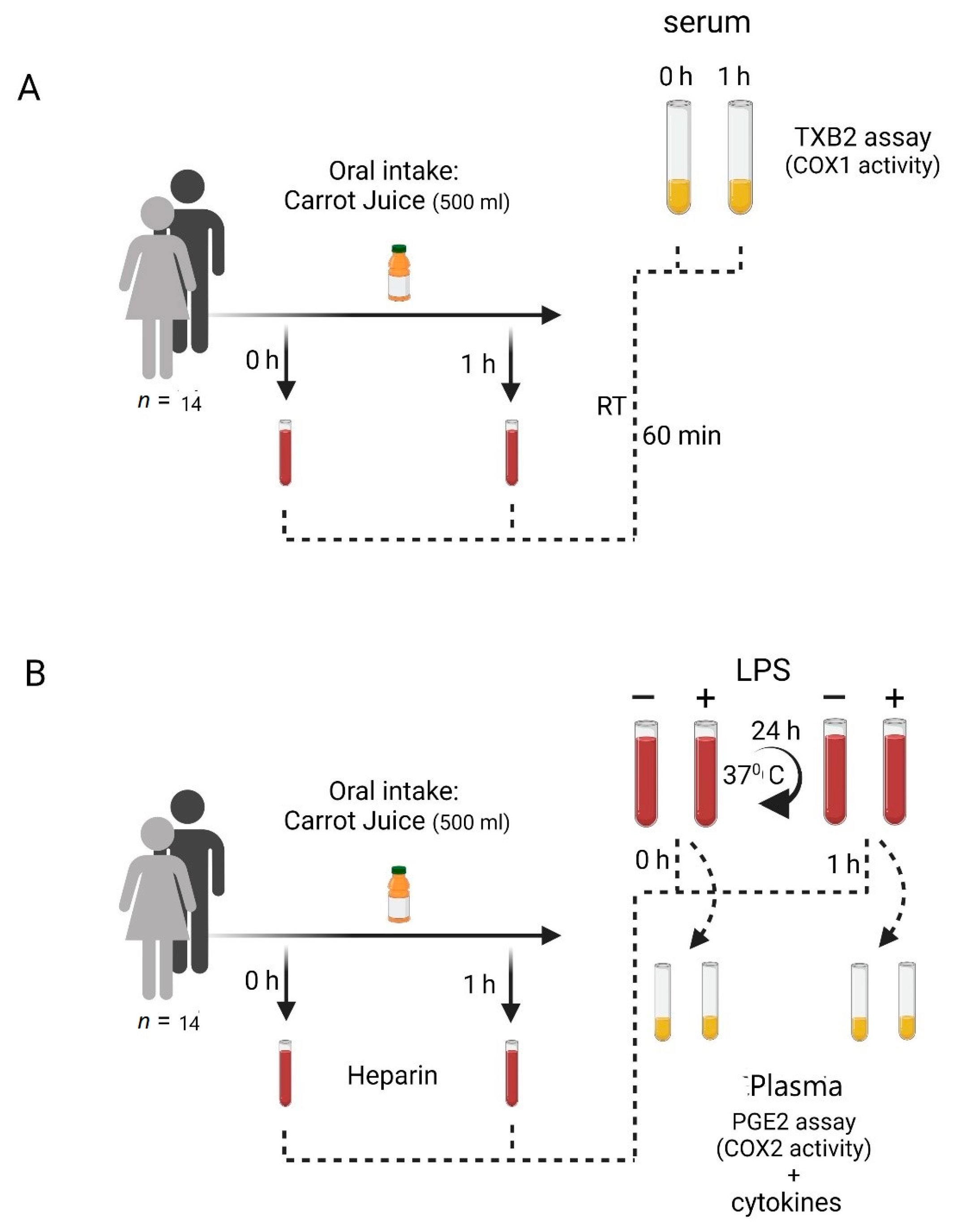
2. Materials and Methods
2.1. Study Subjects
2.2. Carrot Juice
2.3. Blood Sampling
2.4. Blood Handling
2.4.1. COX-1 Activity in Human Blood
2.4.2. COX-2 Induction in Human Blood
2.4.3. Cytokine Induction in Human Blood
2.5. Test Methods-ELISA and Electrochemiluminescence Assay
2.6. Statistical Analysis
3. Results
3.1. Levels of TXB2 in Coagulated Blood before and after Intake of Carrot Juice
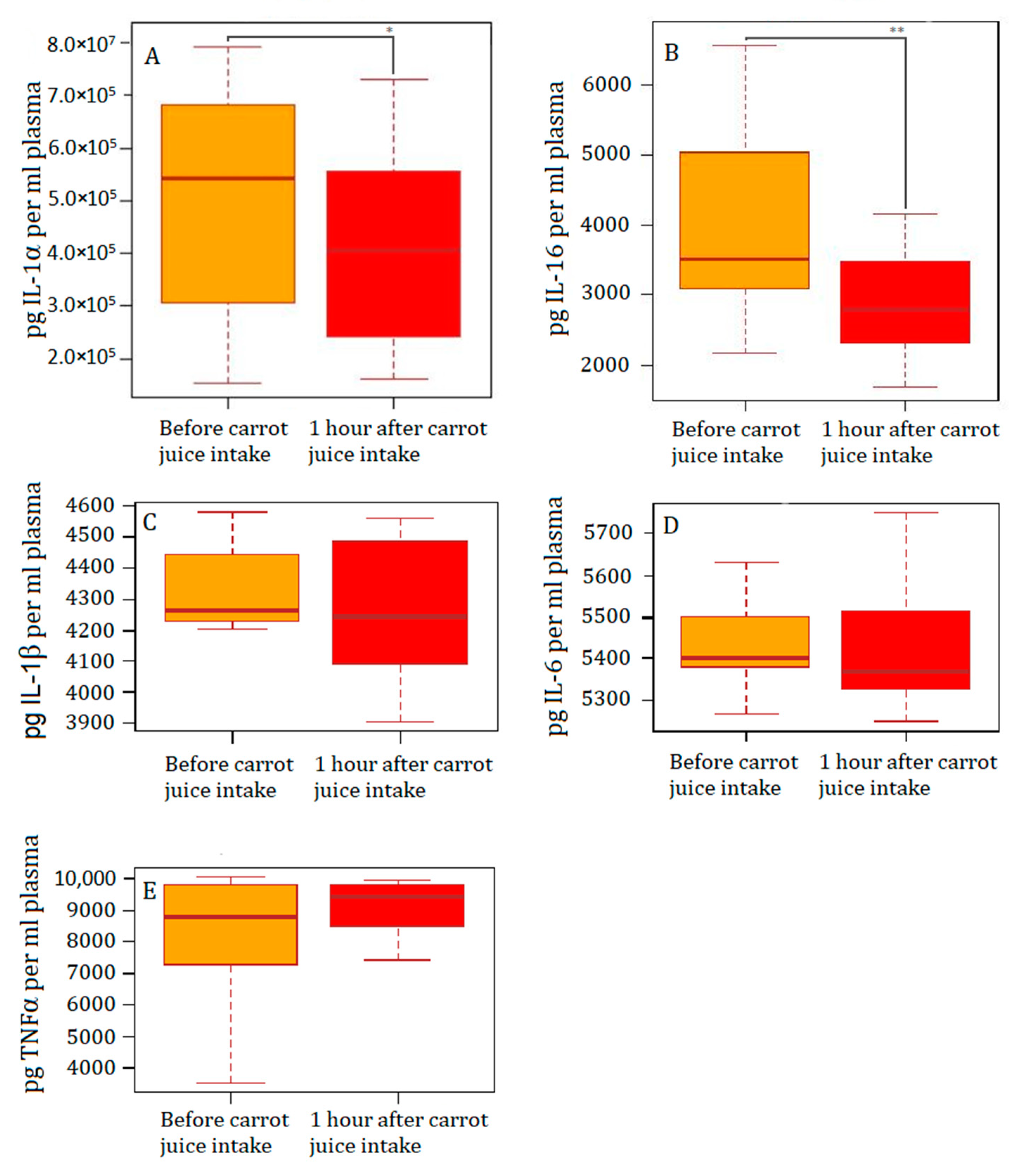
3.2. Levels of PGE2 and Inflammatory Markers in Plasma before and after Intake of Carrot Juice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C(1)(7)-Polyacetylenes in Carrots (Daucus carota L.): Current Knowledge and Future Perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- Kjellenberg, L.; Johansson, E.; Gustavsson, K.E.; Granstedt, A.; Olsson, M.E. Correlations between Polyacetylene Concentrations in Carrot (Daucus carota L.) and Various Soil Parameters. Foods 2016, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, U.; Kobaek-Larsen, M.; Kjoller, K.D.; Antonsen, S.; Baatrup, G.; Trelle, M.B. Quantification of the anti-neoplastic polyacetylene falcarinol from carrots in human serum by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1210, 123440. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef]
- Kang, H.; Bang, T.S.; Lee, J.W.; Lew, J.H.; Eom, S.H.; Lee, K.; Choi, H.Y. Protective effect of the methanol extract from Cryptotaenia japonica Hassk. against lipopolysaccharide-induced inflammation in vitro and in vivo. BMC Complement. Altern. Med. 2012, 12, 199. [Google Scholar] [CrossRef]
- Shiao, Y.J.; Lin, Y.L.; Sun, Y.H.; Chi, C.W.; Chen, C.F.; Wang, C.N. Falcarindiol impairs the expression of inducible nitric oxide synthase by abrogating the activation of IKK and JAK in rat primary astrocytes. Br. J. Pharmacol. 2005, 144, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.T.; Barnes, D.M. Polyacetylene diversity and bioactivity in orange market and locally grown colored carrots (Daucus carota L.). J. Agric. Food Chem. 2009, 57, 11134–11139. [Google Scholar] [CrossRef]
- Young, J.F.; Christensen, L.P.; Theil, P.K.; Oksbjerg, N. The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose Response 2008, 6, 239–251. [Google Scholar] [CrossRef]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on caco-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Zidar, N.; Odar, K.; Glavac, D.; Jerse, M.; Zupanc, T.; Stajer, D. Cyclooxygenase in normal human tissues—Is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2009, 13, 3753–3763. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, H.M.; Te Morsche, R.H.; van Heumen, B.W.; Nagengast, F.M.; Peters, W.H. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014, 14, 9005. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Baatrup, G.; KhataeiNotabi, M.; El-Houri, R.B.; Pipó-Ollé, E.; Christensen Arnspang, E.; Christensen, L.P. Dietary Polyacetylenic Oxylipins Falcarinol and Falcarindiol Prevent Inflammation and Colorectal Neoplastic Transformation: A Mechanistic and Dose-Response Study in A Rat Model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef]
- Stefanson, A.; Bakovic, M. Dietary polyacetylene falcarinol upregulated intestinal heme oxygenase-1 and modified plasma cytokine profile in late phase lipopolysaccharide-induced acute inflammation in CB57BL/6 mice. Nutr. Res. 2020, 80, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Stefanson, A.L.; Bakovic, M. Falcarinol Is a Potent Inducer of Heme Oxygenase-1 and Was More Effective than Sulforaphane in Attenuating Intestinal Inflammation at Diet-Achievable Doses. Oxidative Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1alpha and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Patrignani, P.; Panara, M.R.; Greco, A.; Fusco, O.; Natoli, C.; Iacobelli, S.; Cipollone, F.; Ganci, A.; Créminon, C.; Maclouf, J. Characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. Adv. Prostaglandin Thromboxane Leukot. Res. 1995, 23, 129–131. [Google Scholar]
- Li, X.; Fries, S.; Li, R.; Lawson, J.A.; Propert, K.J.; Diamond, S.L.; Blair, I.A.; FitzGerald, G.A.; Grosser, T. Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2014, 111, 16830–16835. [Google Scholar] [CrossRef]
- Jespersen, B.; Thiesson, H.C.; Henriksen, C.; Therland, K.; Falk, C.; Poulsen, T.; Fogh, B.; Madsen, K.; Walther, S.; Jensen, B.L. Differential effects of immunosuppressive drugs on COX-2 activity in vitro and in kidney transplant patients in vivo. Nephrol. Dial. Transplant. 2009, 24, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Panara, M.R.; Greco, A.; Fusco, O.; Natoli, C.; Iacobelli, S.; Cipollone, F.; Ganci, A.; Créminon, C.; Maclouf, J. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J. Pharmacol. Exp. Ther. 1994, 271, 1705–1712. [Google Scholar] [PubMed]
- Clausen, B.H.; Wirenfeldt, M.; Hogedal, S.S.; Frich, L.H.; Nielsen, H.H.; Schroder, H.D.; Ostergaard, K.; Finsen, B.; Kristensen, B.W.; Lambertsen, K.L. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol. Commun. 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Voronov, E.; Dinarello, C.A.; Apte, R.N.; Cohen, I. Alarmins: Feel the Stress. J. Immunol. 2017, 198, 1395–1402. [Google Scholar] [CrossRef]
- Chiu, J.W.; Binte Hanafi, Z.; Chew, L.C.Y.; Mei, Y.; Liu, H. IL-1alpha Processing, Signaling and Its Role in Cancer Progression. Cells 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.; Tuzova, M.; Cruikshank, W.; Center, D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J. Cell. Physiol. 2014, 229, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-B.; Rao, L.; Wang, Y.-Y.; Liang, W.-B.; Li, C.; Xue, H.; Zhou, B.; Sun, H.; Li, Y.; Lv, M.-L.; et al. The association of interleukin-16 polymorphisms with IL-16 serum levels and risk of colorectal and gastric cancer. Carcinogenesis 2009, 30, 295–299. [Google Scholar] [CrossRef]

| Ex Vivo Normal Conditions | Ex Vivo Inflammation (LPS) | |||||
|---|---|---|---|---|---|---|
| Biomarker | No Carrots Median | Carrots Median | p-Value | No Carrots Median | Carrots Median | p-Value |
| IL-1α (pg/mL) | 1441 | 1496 | p = 0.6812 | 542,144 | 405,623 | p = 0.0419 |
| IL-1β (pg/mL) | 70 | 120 | p = 0.9032 | 4260 | 4268 | p = 0.9032 |
| IL-6 (pg/mL) | 358 | 947 | p = 04263 | 5431 | 5422 | p = 0.9515 |
| IL-16 (pg/mL) | 2042 | 2126 | p = 0.9032 | 3520 | 2794 | p = 0.0085 |
| TNFα(pg/mL) | 18 | 18 | p = 0.5830 | 8099 | 8479 | p = 0.1531 |
| PgE2 (pg/mL) | 1,607,925 | 1,668,736 | p = 0.1294 | 68,154,921 | 83,403,951 | p = 0.1040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deding, U.; Clausen, B.H.; Al-Najami, I.; Baatrup, G.; Jensen, B.L.; Kobaek-Larsen, M. Effect of Oral Intake of Carrot Juice on Cyclooxygenases and Cytokines in Healthy Human Blood Stimulated by Lipopolysaccharide. Nutrients 2023, 15, 632. https://doi.org/10.3390/nu15030632
Deding U, Clausen BH, Al-Najami I, Baatrup G, Jensen BL, Kobaek-Larsen M. Effect of Oral Intake of Carrot Juice on Cyclooxygenases and Cytokines in Healthy Human Blood Stimulated by Lipopolysaccharide. Nutrients. 2023; 15(3):632. https://doi.org/10.3390/nu15030632
Chicago/Turabian StyleDeding, Ulrik, Bettina Hjelm Clausen, Issam Al-Najami, Gunnar Baatrup, Boye Lagerbon Jensen, and Morten Kobaek-Larsen. 2023. "Effect of Oral Intake of Carrot Juice on Cyclooxygenases and Cytokines in Healthy Human Blood Stimulated by Lipopolysaccharide" Nutrients 15, no. 3: 632. https://doi.org/10.3390/nu15030632
APA StyleDeding, U., Clausen, B. H., Al-Najami, I., Baatrup, G., Jensen, B. L., & Kobaek-Larsen, M. (2023). Effect of Oral Intake of Carrot Juice on Cyclooxygenases and Cytokines in Healthy Human Blood Stimulated by Lipopolysaccharide. Nutrients, 15(3), 632. https://doi.org/10.3390/nu15030632






