Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study
Abstract
1. Introduction
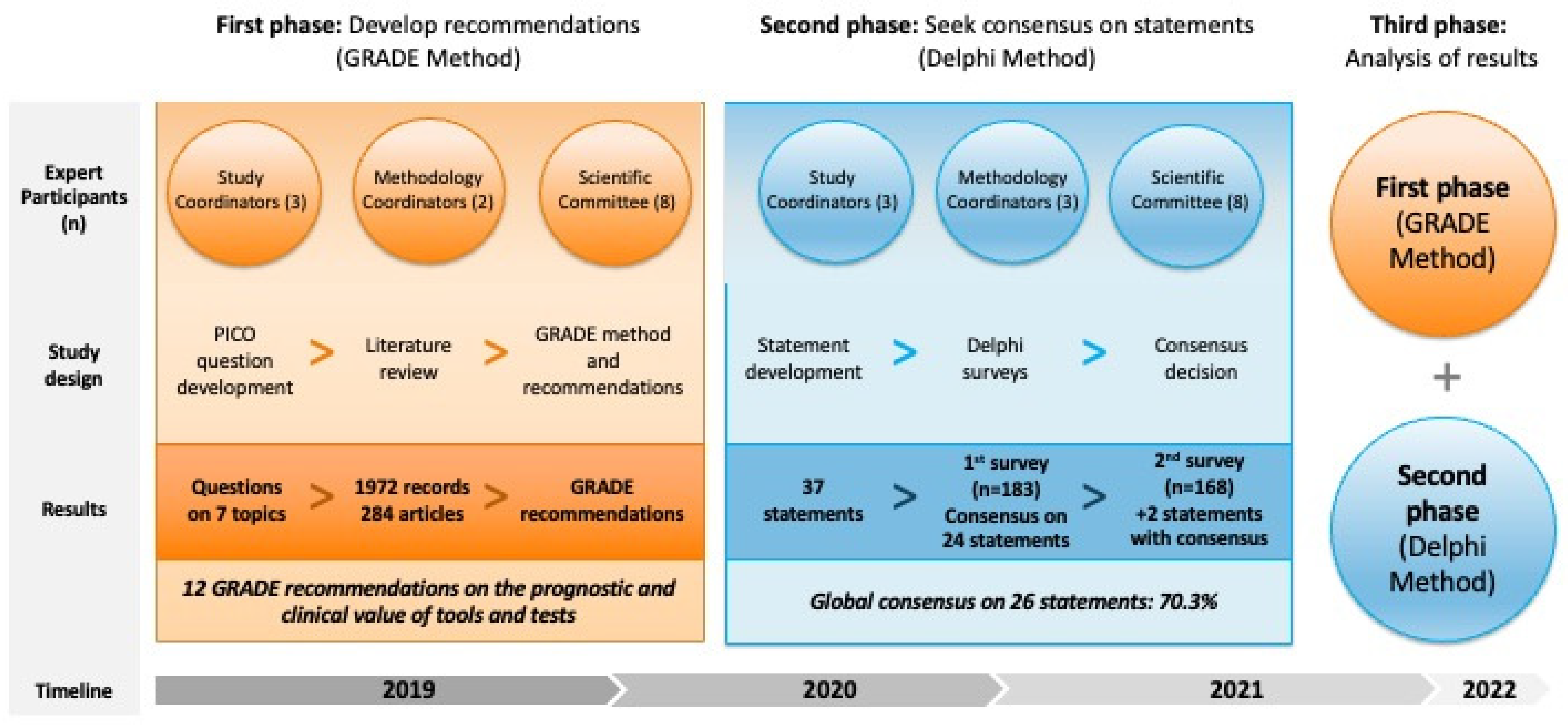
2. Materials and Methods
2.1. Study Design
2.2. Literature Search
2.3. GRADE Method
2.4. Delphi Method
2.5. Statistical Analysis
3. Results
3.1. Literature Review and GRADE Recommendations
3.2. Delphi Method
3.2.1. First Survey
3.2.2. Second Survey
3.3. Alignment of Delphi Consensus with GRADE Recommendations
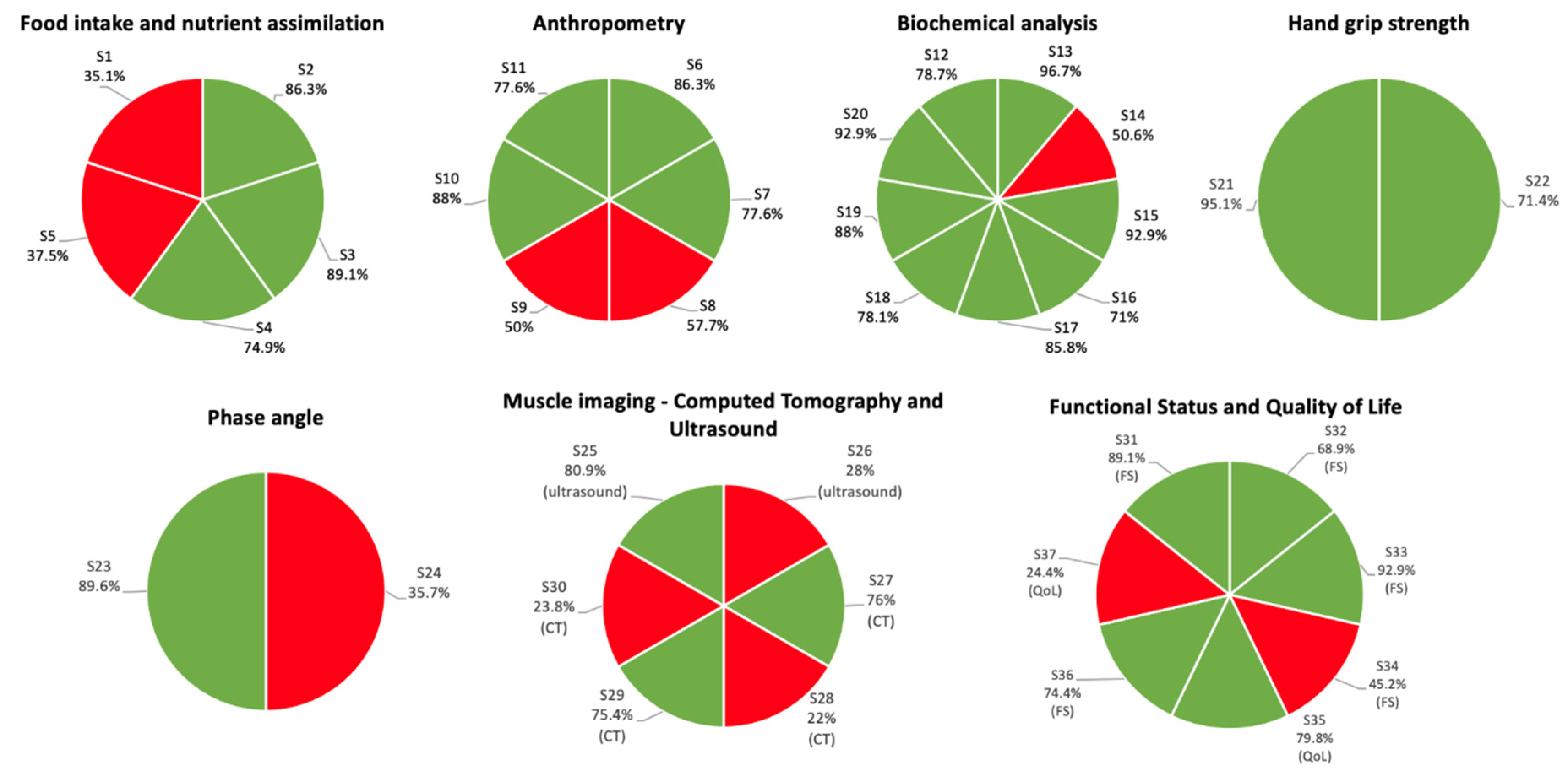
3.4. Subgroup Analysis of Statements with No Consensus
4. Discussion
4.1. Insights from the Scientific Committee on the Delphi Results
4.2. Implications for Clinical Practice
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Schindler, K.; Pernicka, E.; Laviano, A.; Howard, P.; Schütz, T.; Bauer, P.; Grecu, I.; Jonkers, C.; Kondrup, J.; Ljungqvist, O.; et al. How nutritional risk is assessed and managed in European hospitals: A survey of 21,007 patients findings from the 2007–2008 cross-sectional nutritionDay survey. Clin. Nutr. 2010, 29, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kim, J.H.; Ryu, S.-W.; Moon, J.Y.; Park, J.H.; Park, J.K.; Baik, H.-W.; Seo, J.-M.; Son, M.-W.; Song, G.A.; et al. Prevalence of Malnutrition in Hospitalized Patients: A Multicenter Cross-sectional Study. J. Korean Med Sci. 2018, 33, e10. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, I.M.; Gomes-Neto, A.W.; de Jong, M.F.; Jager-Wittenaar, H.; Navis, G.J. High prevalence of malnutrition both on hospital admission and predischarge. Nutrition 2020, 77, 110814. [Google Scholar] [CrossRef]
- Griffin, A.; O’Neill, A.; O’Connor, M.; Ryan, D.; Tierney, A.; Galvin, R. The prevalence of malnutrition and impact on patient outcomes among older adults presenting at an Irish emergency department: A secondary analysis of the OPTI-MEND trial. BMC Geriatr. 2020, 20, 455. [Google Scholar] [CrossRef]
- Murillo, A.Z.; Petrina-Jáuregui, M.E.; Ripa-Ciáurriz, C.; Sánchez, R.S.; Villazón-González, F.; González-Díaz, F.Á.; Fernández-López, C.; Calles-Romero, L.; Martín-Palmero, Á.; Riestra-Fernández, M.; et al. SeDREno study—prevalence of hospital malnutrition according to GLIM criteria, ten years after the PREDyCES study. Nutr. Hosp. 2021, 38, 1016–1025. [Google Scholar] [CrossRef]
- Ballesteros-Pomar, M.D.; Gajete-Martín, L.M.; Pintor-De-La-Maza, B.; González-Arnáiz, E.; González-Roza, L.; García-Pérez, M.P.; González-Alonso, V.; García-González, M.A.; de Prado-Espinosa, R.; Cuevas, M.J.; et al. Disease-Related Malnutrition and Sarcopenia Predict Worse Outcome in Medical Inpatients: A Cohort Study. Nutrients 2021, 13, 2937. [Google Scholar] [CrossRef]
- Burgos, R.; Sarto, B.; Elío, I.; Planas, M.; Forga, M.; Cantón, A.; Trallero, R.; Muñoz, M.J.; Pérez, D.; Bonada, A.; et al. Prevalence of malnutrition and its etiological factors in hospitals. Nutr. Hosp. 2012, 27, 469–476. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM Criteria Using Hand Grip Strength Adequately Predict Six-Month Mortality in Cancer Inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef]
- Correia, M.I.T.D.; Waitzberg, D.L.; Isabel, T.D.; Correia, M. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef]
- Sauer, A.C.; Goates, S.; Malone, A.; Mogensen, K.M.; Gewirtz, G.; Sulz, I.; Moick, S.; Laviano, A.; Hiesmayr, M. Prevalence of Malnutrition Risk and the Impact of Nutrition Risk on Hospital Outcomes: Results From nutritionDay in the U.S. J. Parenter. Enter. Nutr. 2019, 43, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Hiesmayr, M.; Schindler, K.; Pernicka, E.; Schuh, C.; Schoeniger-Hekele, A.; Bauer, P.; Laviano, A.; Lovell, A.; Mouhieddine, M.; Schuetz, T.; et al. Decreased food intake is a risk factor for mortality in hospitalised patients: The NutritionDay survey 2006. Clin. Nutr. 2009, 28, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Makhlouf, A.-M.; Kossovsky, M.P.; Iavindrasana, J.; Chikhi, M.; Meyer, R.; Pittet, D.; Zingg, W.; Pichard, C. Healthcare-Associated Infections Are Associated with Insufficient Dietary Intake: An Observational Cross-Sectional Study. PLoS ONE 2015, 10, e0123695. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Abbasoglu, O.; Ioannou, E.; Meija, L.; Ottens-Oussoren, K.; Pichard, C.; Rothenberg, E.; Rubin, D.; Siljamäki-Ojansuu, U.; Vaillant, M.-F.; et al. ESPEN guideline on hospital nutrition. Clin. Nutr. 2021, 40, 5684–5709. [Google Scholar] [CrossRef]
- Fry, C.M.; Ramet, S.; Hubbard, G.P.; Stratton, R.J. GP patient databases show that malnutrition is under-reported and under-treated in patients with chronic disease. Clin. Nutr. ESPEN 2017, 22, 120–121. [Google Scholar] [CrossRef]
- Anghel, S.; Kerr, K.W.; Valladares, A.F.; Kilgore, K.M.; Sulo, S. Identifying patients with malnutrition and improving use of nutrition interventions: A quality study in four US hospitals. Nutrition 2021, 91–92, 111360. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef]
- García, C.G.; Almeida, J.M.G.; Aguilar, I.M.V.; Castañeda, V.B.; Guerrero, D.B. Morphofunctional assessment of patient nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition–A consensus report from the global clinical nutrition community. Clin Nutr 2019, 1, 1–9. [Google Scholar] [CrossRef]
- Almeida, J.M.G.; García, C.G.; Castañeda, V.B.; Guerrero, D.B. Nuevo enfoque de la nutrición. Valoración del estado nutricional del paciente: Función y composición corporal. Nutr. Hosp. 2018, 35, 1–14. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.M.G.; Bellido, D.; Botella, F. Valoración Morfofuncional de la Desnutrición Relacionada con la Enfermedad; Editorial Médica Panamericana: Madrid, Spain, 2022. [Google Scholar]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013; The GRADE Working Group, 2013. [Google Scholar]
- Niederberger, M.; Spranger, J. Delphi Technique in Health Sciences: A Map. Front. Public Health 2020, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Pendás, L.C.T.; Ortega, M.M.; Ortega, R.M.M.; Abreu, A.P.; Cánovas, A.M. El coeficiente de correlacion de los rangos de spearman caracterizacion. Rev. Habanera Cienc. Med. 2009, 8. [Google Scholar]
- Altman, D.G. Practical Statistics for Medical Research; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Irving, G.; Neves, A.L.; Dambha-Miller, H.; Oishi, A.; Tagashira, H.; Verho, A.; Holden, J. International variations in primary care physician consultation time: A systematic review of 67 countries. BMJ Open 2017, 7, e017902. [Google Scholar] [CrossRef]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lenchik, L.; Heacock, L.; Weaver, A.A.; Boutin, R.D.; Cook, T.S.; Itri, J.; Filippi, C.G.; Gullapalli, R.P.; Lee, J.; Zagurovskaya, M.; et al. Automated Segmentation of Tissues Using CT and MRI: A Systematic Review. Acad. Radiol. 2019, 26, 1695–1706. [Google Scholar] [CrossRef]
- Parker, S.G.; Bechinger-English, D.; Jagger, C.; Spiers, N.; Lindesay, J. Factors affecting completion of the SF-36 in older people. Age Ageing 2006, 35, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Melville, M.R.; Lari, M.A.; Brown, N.; Young, T.; Gray, D. Quality of life assessment using the short form 12 questionnaire is as reliable and sensitive as the short form 36 in distinguishing symptom severity in myocardial infarction survivors. Heart 2003, 89, 1445–1446. [Google Scholar] [CrossRef]
- Hays, R.D.; Kallich, J.D.; Mapes, D.L.; Coons, S.J.; Amin, N.; Carter, W.B.; Kamberg, C. Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3: A Manual for Use and Scoring; RAND Corporation: Santa Monica, CA, USA, 1997. [Google Scholar]
- Fermont, J.M.; Mohan, D.; Fisk, M.; Bolton, C.E.; Macnee, W.; Cockcroft, J.R.; McEniery, C.; Fuld, J.; Cheriyan, J.; Tal-Singer, R.; et al. Short physical performance battery as a practical tool to assess mortality risk in chronic obstructive pulmonary disease. Age Ageing 2021, 50, 795–801. [Google Scholar] [CrossRef]
- Harris, K.M.; Krantz, D.S.; Kop, W.J.; Marshall, J.; Robinson, S.W.; Marshall, J.M.; Gottlieb, S.S. A New Clinically Applicable Measure of Functional Status in Patients with Heart Failure: The 60-Foot Walk Test. JACC Heart Fail. 2017, 5, 411–420. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Zocher, D.; Bosy-Westphal, A.; Szramek, A.; Scheufele, R.; Smoliner, C.; Pirlich, M. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am. J. Clin. Nutr. 2010, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef]
- Llames, L.; Baldomero, V.; Iglesias, M.L.; Rodota, L.P. Values of the phase angle by bioelectrical impedance; nutritional status and prognostic value. Nutr. Hosp. 2013, 28, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, Y.J.; Yang, J.-H.; Kim, C.-M.; Choi, W.-S. The Association between Phase Angle of Bioelectrical Impedance Analysis and Survival Time in Advanced Cancer Patients: Preliminary Study. Korean J. Fam. Med. 2014, 35, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Soundar, E.P.; Genton, L.; Pichard, C. Can phase angle determined by bioelectrical impedance analysis assess nutritional risk? A comparison between healthy and hospitalized subjects. Clin. Nutr. 2012, 31, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Hadda, V.; Kumar, R.; Khilnani, G.C.; Kalaivani, M.; Madan, K.; Tiwari, P.; Mittal, S.; Mohan, A.; Bhalla, A.S.; Guleria, R. Trends of loss of peripheral muscle thickness on ultrasonography and its relationship with outcomes among patients with sepsis. J. Intensive Care 2018, 6, 81. [Google Scholar] [CrossRef]
- Martín, C.A.G.; Zelaya, R.D.C.U.; Zepeda, E.M.; Méndez, O.A.L. ROUNDS Studies: Relation of OUtcomes with Nutrition Despite Severity—Round One: Ultrasound Muscle Measurements in Critically Ill Adult Patients. J. Nutr. Metab. 2018, 2018, 7142325. [Google Scholar] [CrossRef]
- Mueller, N.; Murthy, S.; Tainter, C.; Lee, J.; Riddell, K.; Fintelmann, F.J.; Grabitz, S.D.; Timm, F.P.; Levi, B.; Kurth, T.; et al. Can Sarcopenia Quantified by Ultrasound of the Rectus Femoris Muscle Predict Adverse Outcome of Surgical Intensive Care Unit Patients as well as Frailty? A Prospective, Observational Cohort Study. Ann. Surg. 2016, 264, 1116–1124. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.-S.; Ren, D.; Li, T.; Yao, H.-C.; Ma, S.-J. Low serum prealbumin levels on admission can independently predict in-hospital adverse cardiac events in patients with acute coronary syndrome. Medicine 2018, 97, e11740. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Tang, X.; Hao, G.; Xie, Y.; Ma, S.; Luo, J.; Guo, D.; Ding, F. Serum prealbumin and its changes over time are associated with mortality in acute kidney injury. Sci. Rep. 2017, 7, 41493. [Google Scholar] [CrossRef]
- Chermesh, I.; Papier, I.; Karban, A.; Kluger, Y.; Eliakim, R. Identifying patients at risk for malnutrition is a MUST: A multidisciplinary approach. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2011, 6, e41–e44. [Google Scholar] [CrossRef]
- Ruiz, A.J.; Buitrago, G.; Rodríguez, N.; Gómez, G.; Sulo, S.; Gómez, C.; Partridge, J.; Misas, J.; Dennis, R.; Alba, M.J.; et al. Clinical and economic outcomes associated with malnutrition in hospitalized patients. Clin. Nutr. 2019, 38, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kaegi-Braun, N.; Tribolet, P.; Baumgartner, A.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Hoess, C.; et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 731–740. [Google Scholar] [CrossRef]
- Skipper, A.; Coltman, A.; Tomesko, J.; Charney, P.; Porcari, J.; Piemonte, T.A.; Handu, D.; Cheng, F.W. Adult Malnutrition (Undernutrition) Screening: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 669–708. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P.; Jensen, G.L.; the ASPEN Malnutrition Committee. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
| No. | Topic | Strength of Recommendation | Quality of Evidence | Recommendation |
|---|---|---|---|---|
| R1 | Food intake and nutrient assimilation | Strong | Moderate | Oral food intake questionnaires, especially those included in MNA and SGA, should be used in routine nutritional assessment of malnourished patients or patients at risk of malnutrition. |
| R2 | Anthropometry (skinfolds and circumference) | Strong | Moderate | Anthropometry—including skinfold and circumference measurements—should be conducted during nutritional assessment to predict the prognosis of patients who are malnourished or who have diseases that increase the risk of disease-related malnutrition. |
| R3 | Biochemical analysis | Strong | Moderate | Serum albumin should be evaluated prior to a major surgery to predict complications and mortality. |
| R4 | Biochemical analysis | Strong | Moderate | Serum albumin should be evaluated in patients with acute diseases and in the elderly to predict complications and mortality. |
| R5 | Hand grip strength | Strong | Low–Moderate | Routine nutritional assessment of patients with acute or chronic diseases should include the hand-grip strength, given its prognostic value and ease of use (it is affordable and can be standardized). |
| R6 | Phase angle | Strong | Low–Moderate | The phase angle, measured by bioelectrical impedance analysis, can be used for predicting mortality in patients with disease-related malnutrition. |
| R7 | Phase angle | Strong | Low–Moderate | The phase angle, measured by bioelectrical impedance analysis, can be used for predicting complications in patients with disease-related malnutrition. |
| R8 | Muscle imaging | Moderate | Very Low–Low | Evaluation of the quantity and quality of muscle mass with ultrasound is suggested for predicting clinical prognosis when other body composition measurement methods are not available. |
| R9 | Muscle imaging | Weak | Low | Evaluation of quantity and quality of muscle mass (low attenuation/myosteatosis) with computed tomography is suggested for predicting clinical prognosis when this technique is routinely used. |
| R10 | Muscle imaging | Strong | Moderate | Evaluation of change in muscle mass with computed tomography is recommended for predicting clinical prognosis when this technique is routinely used. |
| R11 | Functional status and quality of life | Strong | Low–Moderate | Functional tests should be added to the routine nutritional assessment to predict mortality and complications in malnourished patients with acute or chronic diseases. |
| R12 | Functional status and quality of life | Moderate | Very Low–Low | Quality of life test may be added to the routine nutritional assessment for predicting mortality and complications in malnourished patients with acute or chronic diseases. |
| Characteristic | N (%) |
|---|---|
| Age (mean ± SD), years | 42.7 ± 10.2 |
| Female | 108 (64.3) |
| Professional experience (median [IQR]), years | 11 (5–22) |
| Type of healthcare professional | |
| Medical doctor | 165 (98.2) |
| Nurse | 1 (0.6) |
| Nutritionist | 2 (1.2) |
| Specialty | |
| Endocrinology and nutrition | 164 (97.6) |
| Internal medicine | 1 (0.6) |
| Not specified | 3 (1.8) |
| Position | |
| Head of department | 27 (16.1) |
| Consultant | 130 (77.4) |
| Resident | 5 (3) |
| Others | 6 (3.6) * |
| Type of hospital (financing) | |
| Public | 141 (83.9) |
| Private | 2 (1.2) |
| Mixed | 25 (14.9) |
| Type of hospital (level of healthcare) | |
| District | 18 (10.7) |
| General | 54 (32.1) |
| Tertiary | 96 (57.1) |
| Works in a nutrition unit | 149 (88.7) |
| Healthcare professional profiles working in the nutrition units | |
| Medical doctor | 149 (100) |
| Nurse | 106 (71.1) |
| Nutritionist | 94 (63.1) |
| No. | Statement | Median (Q1–Q3) | Min–Max | Median Range | Accept Statement; n (%) | Reject Statement; n (%) | Consensus/Decision |
|---|---|---|---|---|---|---|---|
| S1 | The assessment of food intake alone during anamnesis in patients with malnutrition or at risk of malnutrition has uncertain usefulness in predicting prognosis | 5 (3–7) | 1–9 | 4–6 | 59 (35.1%) | 66 (39.3%) | No consensus |
| S2 | Screening tools that include food intake assessment (MUST, NRS2002, SNAQ and MNA-SF) are the tools of choice for initial assessment of patients with malnutrition or at risk of malnutrition, given their clinical prognostic value | 8 (7–9) | 1–9 | 7–9 | 158 (86.3%) | 7 (3.8%) | Consensus/Accept statement |
| S3 | Nutritional assessment tools that include food intake evaluation (MNA and SGA) are the tools of choice for evaluating patients with malnutrition or at risk of malnutrition, given their clinical prognostic value | 8 (7–9) | 2–9 | 7–9 | 163 (89.1%) | 6 (3.3%) | Consensus/Accept statement |
| S4 | Malabsorption and maldigestion tests are useful for the diagnosis of diseases that deteriorate the nutritional status of patients and for adapting the nutritional treatment | 7 (6–8) | 1–9 | 7–9 | 137 (74.9%) | 13 (7.1%) | Consensus/Accept statement |
| S5 | Malabsorption and maldigestion tests in patients with malnutrition or at risk of malnutrition have uncertain usefulness in predicting prognosis | 5 (3–7) | 1–9 | 4–6 | 63 (37.5%) | 55 (32.7%) | No consensus |
| S6 | Height and weight measurements as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition are useful for predicting prognosis | 8 (7–9) | 1–9 | 7–9 | 158 (86.3%) | 3 (1.6%) | Consensus/Accept statement |
| S7 | Height and weight measurements are feasible in routine clinical practice | 8 (7–9) | 1–9 | 7–9 | 142 (77.6%) | 6 (3.3%) | Consensus/Accept statement |
| S8 | Skinfold measurement as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 7 (5–8) | 1–9 | 7–9 | 97 (57.7%) | 23 (13.7%) | No consensus |
| S9 | Skinfold measurement is feasible in routine clinical practice | 6.5 (4–8) | 1–9 | 7–9 | 84 (50%) | 32 (19%) | No consensus |
| S10 | Arm and calf circumference measurements as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition are useful for predicting prognosis | 8 (7–9) | 2–9 | 7–9 | 161 (88.0%) | 2 (1.1%) | Consensus/Accept statement |
| S11 | Arm and calf circumference measurements are feasible in routine clinical practice | 8 (7–8) | 1–9 | 7–9 | 142 (77.6%) | 11 (6.0%) | Consensus/Accept statement |
| S12 | Evaluation of preoperative serum albumin as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 8 (7–9) | 1–9 | 7–9 | 144 (78.7%) | 13 (7.1%) | Consensus/Accept statement |
| S13 | Evaluation of preoperative serum albumin is feasible in routine clinical practice | 9 (8–9) | 3–9 | 7–9 | 177 (96.7%) | 1 (0.5%) | Consensus/Accept statement |
| S14 | Evaluation of serum albumin when patients with malnutrition or at risk of malnutrition and an acute disease are hospitalized is useful for predicting prognosis | 7 (4–8) | 1–9 | 7–9 | 85 (50.6%) | 41 (24.4%) | No consensus |
| S15 | Evaluation of serum albumin when patients with malnutrition or at risk of malnutrition and an acute disease are hospitalized is feasible in routine clinical practice | 9 (8–9) | 1–9 | 7–9 | 170 (92.9%) | 3 (1.6%) | Consensus/Accept statement |
| S16 | Evaluation of serum albumin as part of the nutritional assessment of elderly patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 7 (6–8) | 1–9 | 7–9 | 130 (71.0%) | 11 (6.0%) | Consensus/Accept statement |
| S17 | Evaluation of serum albumin in elderly patients with malnutrition or at risk of malnutrition is feasible in routine clinical practice | 8 (7–9) | 3–9 | 7–9 | 157 (85.8%) | 2 (1.1%) | Consensus/Accept statement |
| S18 | Evaluation of prealbumin is feasible in routine clinical practice | 8 (7–9) | 2–9 | 7–9 | 143 (78.1%) | 9 (4.9%) | Consensus/Accept statement |
| S19 | Evaluation of C-reactive protein together with albumin as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 9 (8–9) | 1–9 | 7–9 | 161 (88.0%) | 4 (2.2%) | Consensus/Accept statement |
| S20 | Evaluation of C-reactive protein is feasible in routine clinical practice | 9 (8–9) | 2–9 | 7–9 | 170 (92.9%) | 1 (0.5%) | Consensus/Accept statement |
| S21 | Use of hand grip strength as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 9 (8–9) | 5–9 | 7–9 | 174 (95.1%) | 0 (0%) | Consensus/Accept statement |
| S22 | Use of hand grip strength is feasible in routine clinical practice | 8 (6–9) | 2–9 | 7–9 | 120 (71.4%) | 12 (7.1%) | Consensus/Accept statement |
| S23 | The phase angle measured by bioelectrical impedance assessment in patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 8 (7–9) | 3–9 | 7–9 | 164 (89.6%) | 2 (1.1%) | Consensus/Accept statement |
| S24 | Measurement of the phase angle by bioelectrical impedance assessment is feasible in routine clinical practice | 6 (3–7) | 1–9 | 4–6 | 60 (35.7%) | 42 (25%) | No consensus |
| S25 | Ultrasound evaluation of the quantity and quality of muscle as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 8 (7–9) | 1–9 | 7–9 | 148 (80.9%) | 3 (1.6%) | Consensus/Accept statement |
| S26 | Ultrasound evaluation of the quantity and quality of muscle is feasible in routine clinical practice | 5 (3–7) | 1–9 | 4–6 | 47 (28%) | 53 (31.5%) | No consensus |
| S27 | Computed tomography evaluation of the quantity and quality of muscle as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 8 (7–9) | 1–9 | 7–9 | 139 (76.0%) | 14 (7.7%) | Consensus/Accept statement |
| S28 | Computed tomography evaluation of the quantity and quality of muscle, when clinically indicated for follow-up, is feasible in routine clinical practice | 5 (3–6) | 1–9 | 4–6 | 37 (22%) | 59 (35.1%) | No consensus |
| S29 | Computed tomography evaluation of changes in muscle mass (when this technique is available for diagnosis/follow-up of the disease) as part of the nutritional assessment of patients is useful for predicting prognosis | 8 (7–8) | 1–9 | 7–9 | 138 (75.4%) | 11 (6.0%) | Consensus/Accept statement |
| S30 | When computed tomography is required for follow-up of patients, measuring changes in muscle mass is feasible in routine clinical practice | 5 (3–6) | 1–9 | 4–6 | 40 (23.8%) | 59 (35.1%) | No consensus |
| S31 | Functional status questionnaires (Barthel index, Katz index) as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition are useful for predicting prognosis | 8 (7–9) | 2–9 | 7–9 | 163 (89.1%) | 2 (1.1%) | Consensus/Accept statement |
| S32 | The use of functional status questionnaires (Barthel index, Katz index) is feasible in routine clinical practice | 7 (6–8) | 2–9 | 7–9 | 126 (68.9%) | 6 (3.3%) | Consensus/Accept statement |
| S33 | The use of one or several functional tests (6-min walk test, 10-m walk test, short physical performance battery, timed up and go test, one-leg standing time) as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition is useful for predicting prognosis | 8 (7–9) | 4–9 | 7–9 | 170 (92.9%) | 0 (0%) | Consensus/Accept statement |
| S34 | The use of one or several functional tests (6-min walk test, 10-m walk test, short physical performance battery, timed up and go test, one-leg standing time) is feasible in routine clinical practice | 6 (4–7) | 1–9 | 4–6 | 76 (45.2%) | 30 (17.9%) | No consensus |
| S35 | Quality of life questionnaires (ECOG performance status, Karnofsky scale, SF-36, KDQOL-SF, EQ-5D-5L) as part of the nutritional assessment of patients with malnutrition or at risk of malnutrition are useful for predicting prognosis | 8 (7–9) | 2–9 | 7–9 | 146 (79.8%) | 3 (1.6%) | Consensus/Accept statement |
| S36 | The use of short functional tests (ECOG performance status or Karnofsky scale) is feasible in routine clinical practice | 8(6–8) | 2–9 | 7–9 | 125 (74.4%) | 3 (1.8%) | Consensus/Accept statement |
| S37 | The use of long questionnaires of quality of life (SF-36, KDQOL-SF, EQ-5D-5L) is feasible in routine clinical practice | 5.5 (3–6) | 1–9 | 4–6 | 41 (24.4%) | 42 (25%) | No consensus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Almeida, J.M.; García-García, C.; Ballesteros-Pomar, M.D.; Olveira, G.; Lopez-Gomez, J.J.; Bellido, V.; Bretón Lesmes, I.; Burgos, R.; Sanz-Paris, A.; Matia-Martin, P.; et al. Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study. Nutrients 2023, 15, 612. https://doi.org/10.3390/nu15030612
García-Almeida JM, García-García C, Ballesteros-Pomar MD, Olveira G, Lopez-Gomez JJ, Bellido V, Bretón Lesmes I, Burgos R, Sanz-Paris A, Matia-Martin P, et al. Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study. Nutrients. 2023; 15(3):612. https://doi.org/10.3390/nu15030612
Chicago/Turabian StyleGarcía-Almeida, José Manuel, Cristina García-García, María D. Ballesteros-Pomar, Gabriel Olveira, Juan J. Lopez-Gomez, Virginia Bellido, Irene Bretón Lesmes, Rosa Burgos, Alejandro Sanz-Paris, Pilar Matia-Martin, and et al. 2023. "Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study" Nutrients 15, no. 3: 612. https://doi.org/10.3390/nu15030612
APA StyleGarcía-Almeida, J. M., García-García, C., Ballesteros-Pomar, M. D., Olveira, G., Lopez-Gomez, J. J., Bellido, V., Bretón Lesmes, I., Burgos, R., Sanz-Paris, A., Matia-Martin, P., Botella Romero, F., Ocon Breton, J., Zugasti Murillo, A., & Bellido, D. (2023). Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study. Nutrients, 15(3), 612. https://doi.org/10.3390/nu15030612







