Cardioprotective Potential of Berries of Schisandra chinensis Turcz. (Baill.), Their Components and Food Products
Abstract
1. Introduction
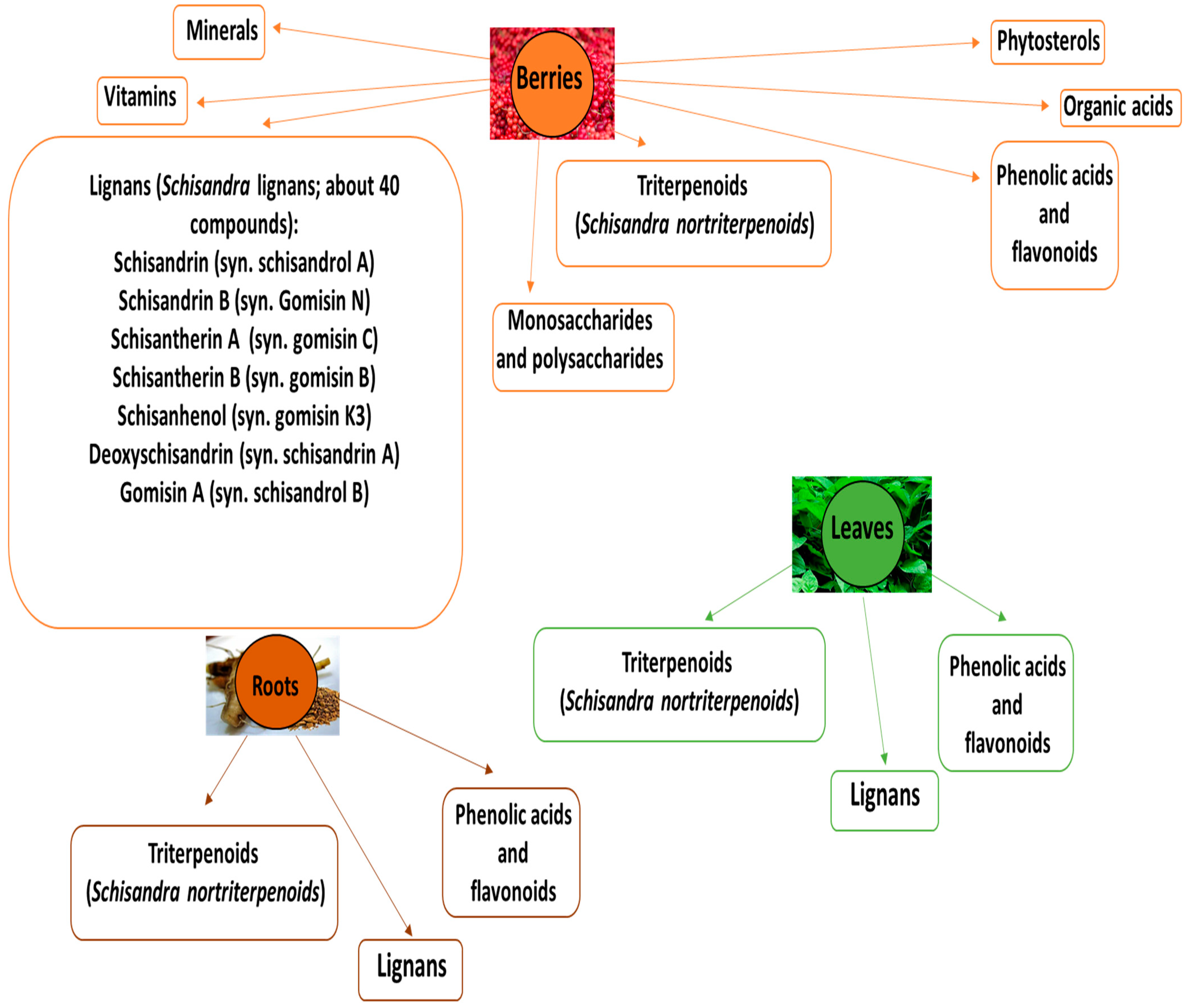
2. Phytochemical Characteristics of Various Parts of S. chinensis
3. Cardioprotective Properties of S. chinenis Berries, their Components, and Food Products
3.1. Cardioprotective Properties of S. chinensis Berry Preparations
3.2. Cardioprotective Properties of S. chinensis Components
3.2.1. Schisandrin (Schisandrol A)
3.2.2. Schisandrin A
3.2.3. Schisandrin B
3.2.4. Schisandrin C
3.2.5. Polysaccharides from S. chinensis
3.2.6. Other Bioactive Compounds of S. chinensis Berries
4. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (China magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Warzecha, A.; Klimek-Szczykutowicz, M.; Stanczyk, K.; Kubica, P.; Ekiert, H. Chinesa magnolia vine (Schisandra chinensis (Turcz.) Baill.)—From traditional Chinese medicine to modern phytotherapy. Herbalism 2018, 1, 101–119. [Google Scholar]
- Li, Z.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Ju, J.; Wang, Y.; Liu, Z.; Zhao, X.; Fang, Y. Schisandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am. J. Translat. Res. 2019, 11, 199–209. [Google Scholar]
- Zhang, L.; Chen, H.; Tian, J.; Chen, S. Antioxidant and anti-proliferative activities of five compounds from Schisandra chinensis fruit. Ind. Crop. Prod. 2013, 50, 690–693. [Google Scholar] [CrossRef]
- Ranouille, E.; Boutot, C.; Bony, E.; Bombarde, O.; Grosjean, S.; Lazewski, A.; Berthon, J.X.; Filaire, E. Schisandra chinensis protects the skin from global pollution by inflammatory and redox balance pathway modulations: An in vitro study. Cosmetics 2018, 5, 36. [Google Scholar] [CrossRef]
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; He, L.; Sun, J.; Wenbo, J.; Li, X.; Chen, J.; Wang, C. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Błasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of Schisandra chinensis (Turcz.) Baill. in human health and nutrition: A review of current knowledge and therapeutic perspectives. Nutrients 2019, 11, 333. [Google Scholar] [CrossRef]
- Nasser, M.I.; Zhu, S.; Chen, C.; Zhao, M.; Huang, H.; Zhu, P. A comprehensive review on schisandrin B and its biological properties. Oxid. Med. Cell. Long. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Liao, X.; Zhang, Y.; Wei, L.; Pang, Y. Schisandrin B regulates MC3T3-E1 subclone 14 cells proliferation and differentiation through BMP2-SMADs-RUNX2-SP7 signaling axis. Sci. Rap. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Fu, K.; Zhou, H.; Wang, C.; Gong, L.; Ma, C.; Zhang, Y.; Li, Y. A review: Pharmacology and pharmacokinetics of schisandrin A. Phytother. Res. 2022, 36, 2375–2393. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xiao, W.; Li, G. research progress on the pharmacological action of schisantherin. Evid. Based Comp. Alter. Med. 2022, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.N.; Cho, M.; So, I.; Jeon, J.H. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: A review of the molecular mechanisms. Fitoterapia 2014, 97, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Yuan, C. Schisandra chinensis: A comprehenosive review on its phytochemicals and biological activities. Arabian J. Chem. 2021, 14, 1–20. [Google Scholar]
- Zhou, Y.; Men, L.; Sun, Y.; Wei, M.; Fan, X. Pharmacodynamic effects and molecular mechanisms of ligans from Schisandra chinensis Turcz. (Baill.), a current review. Eur. J. Pharamcol. 2021, 5, 1–14. [Google Scholar]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacol. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Zhu, Z.; Zhao, L.; Fei, Y.; Jing, J.; Chai, Y. Determination of six lignans in Schisandra chinensis (Turcz.) Baill. fruits and related Chinese multiherb remedies by HPLC. Food Chem. 2009, 115, 735–739. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Li, W.; Wang, C.; Li, H.; Ju, W.; Chen, J.; Sun, J. Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenathera from different locations. Chem. Biodivers. 2018, 15, 1–7. [Google Scholar] [CrossRef]
- Liu, H.; Lai, H.; Jia, X.; Liu, J.; Zhang, Z.; Qi, Y.; Zhang, J.; Song, J.; Wu, C.; Zhang, B.; et al. Comprehensive chemical analysis of Schisandra chinensis by HPLC-DAD-MS combined with chemometrics. Phytomedicine 2013, 20, 1135–1143. [Google Scholar] [CrossRef]
- Yim, T.K.; Ko, K.M. Methylenedioxy group and cyclooctadiene ring as structural determinants of schisandrin in protecting against myocardial ischemia-reperfusion injury in rats. Biochem. Pharmacol. 1999, 57, 77–81. [Google Scholar] [CrossRef]
- Choi, Y.W.; Takamatsu, S.; Khan, S.I.; Srinivas, P.V.; Zhao, J.; Khan, I.A. Schisandrene, a dibenzocycloactadiene lignan from Schisandra chinensis: Structure-antioxidant activity relationship of dibenzocycloactadiene lignans. J. Nat. Prod. 2006, 69, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Crisan, G.; Vlase, L.; Crisan, O.; Vodnar, D.C.; Raita, O.; Gheldin, A.M.; Toiu, A.; Oprean, R.; Tilea, Y. Comparative studies on polyphenolic composition, antioxidant and anti-microbial activities of Schisandra chinensis leaves nd fruits. Molecules 2014, 19, 15162–15179. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Han, B.; Zhang, B.; Chu, Z.; Zhang, X.; Lu, Z.; Han, J. Schisandra chinensis polysaccharides prevent cardiac hypertrophy by dissociating thioredoxin-interacting protein/thioredoxin-1 complex and inhibiting oxidative stress. Biomed. Pharmacother. 2021, 139, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, S. Comprehensive analysis of the phospholipids nd phytosterols in Schisnadra chinensis oil by UPLC-Q/TOF-MS. Chem. Phys. Lipids 2019, 221, 15–23. [Google Scholar] [CrossRef]
- Alexander, J.S.; Wang, Y. Therapeutic potential of Schisandra chinensis extracts for treatment of hypertension. Hypertens. Res. 2012, 35, 892–893. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.Y.; Kim, M.K.; Koh, P.O.; Cho, K.W.; Kim, C.H.; Lee, K.S.; Chung, B.Y.; Kim, G.S.; Cho, J.H. Anti-obesity effect of Schisandra chinensis in 3T3-L1 cells and high fat diet-induced obese rats. Food Chem. 2012, 134, 227–234. [Google Scholar] [CrossRef]
- Chen, X.; Cao, J.; Sun, Y.; Dai, Y.; Zhu, J.; Zhang, X.; Zhao, X.; Wang, L.; Zhao, T.; Li, Y.; et al. Ethanol extract of Schisandrae chinensis fructus ameliorates the extent of experimentally induced atherosclerosis in rats by increasing antioxidant capacity and improving endothelial dysfunction. Pharmaceut. Biol. 2018, 56, 612–619. [Google Scholar] [CrossRef]
- Demir, Y. The behaviour of some antihypertension drugs on human serum paraoxonase-1: An important protector enzyme against atherosclerosis. J. Pharm. Pharmacol. 2019, 71, 1576–1583. [Google Scholar] [CrossRef]
- Demir, Y. Napthroquinones, benzoquinonses, and antraquinones: Molecular docking, ADME and inhibition studies on human serum paraoxonase-1 associated with cardiovascular disease. Drug Develop. Res. 2020, 81, 628–636. [Google Scholar] [CrossRef]
- Hu, D.; Yang, Z.; Yao, X.; Wang, H.; Han, N.; Liu, Z.; Wang, Y.; Yang, J.; Yin, J. Dibenzocyclooctadiene lignans from Schisandra chinensis and their inhibitory activity on NO production in lipopolysaccharide-activated microglia cells. Phytochemistry 2014, 104, 72–78. [Google Scholar] [CrossRef]
- Lin, Q.; Qin, X.; Shi, M.; Qin, Z.; Meng, Y.; Qin, Z.; Guo, S. Schisandrin B inhibits LPS-induced inflammatory response in human umbilical vein endothelial cells by activating Nrf2. Internat. Immunophar. 2017, 49, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jia, J.; Liu, L.; Yang, F.; Fan, Y.; Zhang, S.; Yan, D.; Bu, R.; Li, G.; Gao, Y.; et al. Schisandrin B displays a protective role against primary pulmonary hypertension by targeting transforming growth factor β1. J. Am. Soc. Hypertens. 2017, 11, 148–157. [Google Scholar] [CrossRef]
- Demir, Y.; Koksal, Z. The inhibition effects of same sulfonamides on human serum paraoxonase-1 (hPON1). Pharmacutical reports 2019, 71, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.; Balci, N.; Gurbuz, M. Differential effects of selective serotonin reuptake inhibitors on paraoxonase-1 enzyme activity: An in vitro study. Comp. Biochem. Physiol. Part C Toxicol. Pharamacol. 2019, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Luo, J.; Ge, J.; Liu, Y.; Xu, P.; Zhang, R.; Zhu, G.; Yang, M.; Ai, Z.; Song, Y. Raw and wine processed Schisandra chinensis regulate NREM-sleep and alleviate cardiovascular dysfunction assoiciated with insomnia by modulating HPA axis. Planta Med. 2021, 15, 1–12. [Google Scholar]
- Zhang, X.; Zhao, Y.; Bai, D.; Yuan, X.; Cong, S. Schizandrin protects H9c2 cells against lipopolysaccharide-induced injury by downregulating Smad3. J. Biochem. Mol. Toxicol. 2019, 33, 1–9. [Google Scholar] [CrossRef]
- Lai, Q.; Yuan, G.Y.; Wang, H.; Liu, Z.I.; Kou, J.P.; Yu, B.Y.; Li, F. Exploring the protective effects of schizandrol A in acute myocardial ischemia mice by comprehensive metabolomics profiling integrated with molecular mechanism studies. Acta Pharmacol. Sinica 2020, 41, 1058–1072. [Google Scholar] [CrossRef]
- Gong, S.; Liu, J.; Wan, S.; Yang, W.; Zhang, Y.; Yu, B.; Li, F.; Kou, J. Schisnadrol A attenuates myocardial ischemia/reperfusion-induced myocardial apoptosis through upregulation of 14-3-3θ. Oxid. Med. Cell. Long. 2021, 1, 1–15. [Google Scholar]
- Chang, R.; Li, Y.; Yang, X.; Yue, Y.; Dou, L.; Wang, Y.; Zhang, W.; Li, X. Protective role of deoxyschizandrin and schisantherin A against myocardial ischemia-reperfusion injury in rats. PLoS ONE 2013, 8, e61590. [Google Scholar] [CrossRef]
- Shengban, Y.; Jianchang, Q.; Gaojun, W.; Yuanyuan, Q.; Zhengxian, W.; Taiwei, C.; Jingying, W.; Weijian, H.; Gung, L. Schizandrin B attenuates angiotensin II induced endothelial to mesenchymal transition in vascular endothelium by suppressing NF-κB activation. Phytomedicine 2019, 62, 1–11. [Google Scholar]
- Chiu, P.Y.; Mak, D.H.F.; Poon, M.K.T.; Ko, K.M. Role of cytochrome P-450 in schisandrin B-induced antioxidant and heat shock responses in mouse liver. Life Sci. 2005, 77, 2887–2895. [Google Scholar] [CrossRef] [PubMed]
- Thandavarayan, R.A.; Giridharan, V.V.; Arumugam, S.; Suzuki, K.; Ko, K.M.; Krishnamurthy, P.; Watanabe, K.; Konishi, T. Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PLoS ONE 2015, 10, e0119214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xiang, Y.; Cai, C.; Zhou, A.; Zhu, N.; Zeng, C. Schisandrin B protects against myocardial ischemia/reperfusion injury via the PI3K/Akt pathway in rats. Mol. Med. Rep. 2017, 17, 556–561. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Z.; Meng, F. Schisandrin B ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Inflammation 2017, 40, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.Y.; Ko, K.M. Time-dependent enhancement in mitochondrial glutathione status and ATP generation capacity by schisandrin B treatment decreases the susceptibility of rat hearts to ischemia-reperfusion injury. BioFactors 2003, 19, 43–51. [Google Scholar] [CrossRef]
- Chen, N.; Ko, M. Schisandrin B –induced glutathione antioxidant response and cardioprotection are mediated by reactive oxygen species production in rat hearts. Biol. Pharm. Bull. 2010, 33, 825–889. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Wu, J.; Su, T.; Chao, X.J.; Yu, H.; Liu, B.; Fu, X.; Tse, A.K.W.; Chan, C.L.; Fong, W.F.; et al. Schisndrin B regulates lipid metabolism in subcutaneous adipocytes. Sci. Rap. 2017, 7, 1–16. [Google Scholar]
- Han, J.; Shi, X.; Du, Y.; Shi, F.; Zhang, B.; Zheng, Z.; Xu, J.; Jiang, L. Schisandrin C targets Keap1 and attenuates oxidative stress by activating Nrf2 pathway in Ang II-challenged vascular endothelium. Phytother. Res. 2019, 33, 779–790. [Google Scholar] [CrossRef]
- Yue, C.; Chen, J.; Hou, R.; Liu, J.; Li, X.; Gao, Z.; Liu, C.; Wang, D.; Lu, Y.; Li, H.; et al. Effects of selenylation modification on antioxidative activities of Schisandra chinensis polysaccharide. Pub. Lib. Sci. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Yue, C.; Chen, J.; Hou, R.; Tian, W.; Liu, K.; Wang, D.; Lu, Y.; Liu, J.; Wu, Y.; Hu, Y. The antioxidant action and mechanism of selenizing Schisandra chinenisis polysaccharide in chicken embryo hepatocyte. Inter. J. Biol. Macromol. 2017, 98, 506–514. [Google Scholar] [CrossRef]
- Jang, M.A.; Lee, S.J.; Baek, S.E.; Park, S.Y.; Choi, Y.W.; Kim, C.D. α-Iso-cubebene inhibits PDGF-induced vascular smooth muscle cell proliferation by suppressing osteopontin expression. PLoS ONE 2017, 23, e0170699. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qin, J.; Huang, Z.; Yu, Z.; Wang, W.; Hu, H.; You, Y. A comprehensive review ofethnophrmacology, phytochemistry, pharmacology, and pharmacokinetics of Schisandra chinensis (Turcz.) Baill and Schisandra sphenantera Rend. At Wils. J. Ethnopharmacol. 2022, 284, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G. Pharmacology of Schisandrin chinensis Bail: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Valickova, J.; Zezulka, S.; Marslkova, E.; Kotlik, J.; Marsalek, B.; Opatrilova, R. Bioactive compounds from Scisandra chinensis—Risk for aquatic plants. Aquat. Toxicol. 2023, 254, 1–10. [Google Scholar] [CrossRef]

| Component of S. chinensis Berries | Biological Properties for Cardioprotective Action | References |
|---|---|---|
| Schisandrin (Schisandrol A, wuweizisu A) | Antioxidant properties (↓ROS and lipid peroxidation; in vitro model and in vivo (mouse) model) Anti-inflammatory properties (↓COX and NO°; in vitro model and in vivo (mouse) model) | [37,38,39] |
| Schisandrin A (deoxyschisandrin) | Antioxidant properties (↓lipid peroxidation and ↑CAT and SOD; in vitro model and in vivo model) Anti-inflammatory properties (in vitro model and in vivo model) | [4,15,39] |
| Schisandrin B (gomisin N, wuwezisu B, γ-schisandrin) | Anti-inflammatory properties (↓prostaglandins and NO°; in vivo model) | [43] |
| Schisandrin B (gomisin N, wuwezisu B, γ-schisandrin) | Anti-aggregation properties (in vitro model) Anti-inflammatory properties (in vitro model and in vivo model) Antioxidant properties (↓lipid peroxidation; in vitro model and in vivo model) | [31,40,41,42] |
| Schisandrin C | Antioxidant properties (↓lipid peroxidation; in vitro model and in vivo (mouse) model) Anti-inflammatory properties (↓COX and NO°; in vitro model and in vivo (mouse) model) | [48] |
| Polysaccharides | Antioxidant properties (in vitro model and in vivo model) | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B. Cardioprotective Potential of Berries of Schisandra chinensis Turcz. (Baill.), Their Components and Food Products. Nutrients 2023, 15, 592. https://doi.org/10.3390/nu15030592
Olas B. Cardioprotective Potential of Berries of Schisandra chinensis Turcz. (Baill.), Their Components and Food Products. Nutrients. 2023; 15(3):592. https://doi.org/10.3390/nu15030592
Chicago/Turabian StyleOlas, Beata. 2023. "Cardioprotective Potential of Berries of Schisandra chinensis Turcz. (Baill.), Their Components and Food Products" Nutrients 15, no. 3: 592. https://doi.org/10.3390/nu15030592
APA StyleOlas, B. (2023). Cardioprotective Potential of Berries of Schisandra chinensis Turcz. (Baill.), Their Components and Food Products. Nutrients, 15(3), 592. https://doi.org/10.3390/nu15030592






