Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Garcinol and Cell Culture Treatments
2.2. Cell Viability Assay
2.3. Western Blot Analysis
2.4. Semiquantitative Reverse Transcription Real-Time PCR
2.5. Determination of Cytokine and Mediator Secretion by ELISA Assay
2.6. Determination of NO and PGE2 Secretion
2.7. Statistical Analysis
3. Results
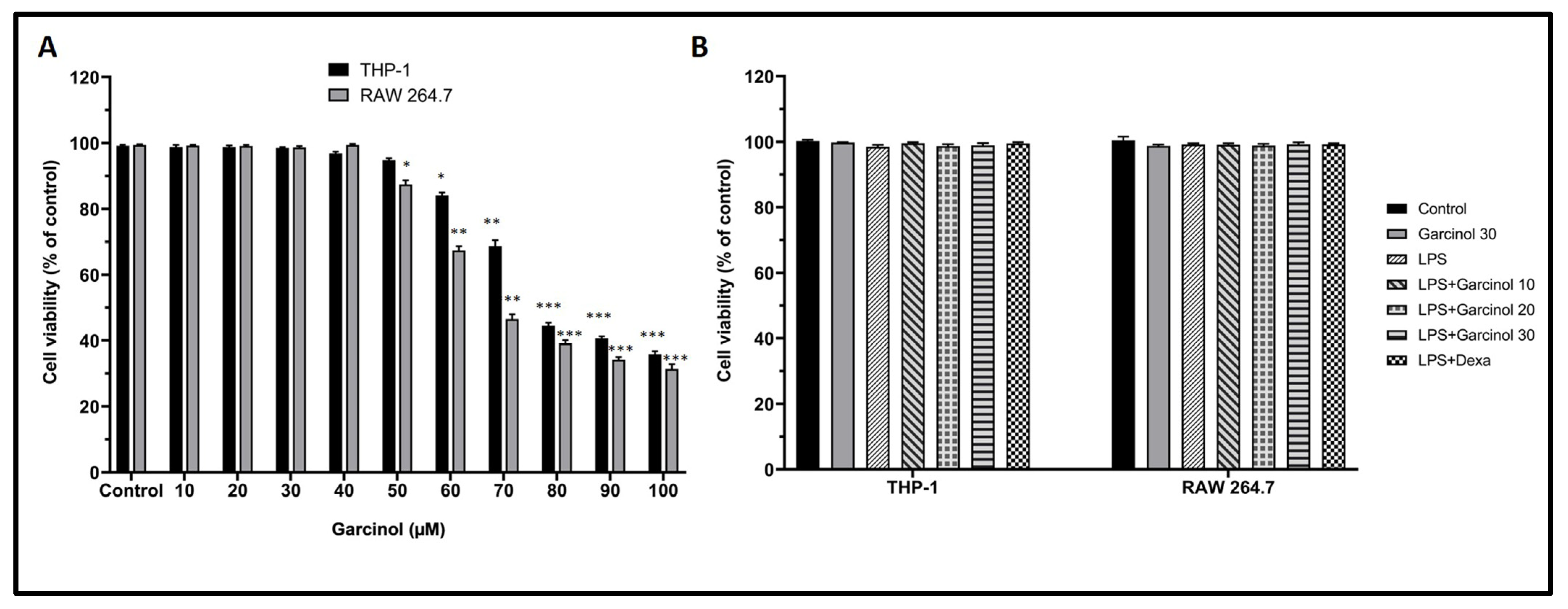
3.1. Effect of Garcinol on THP-1 and RAW 264.7 Cell Viability
3.2. Effect of Garcinol on the Expression of Inflammatory-Related Gene
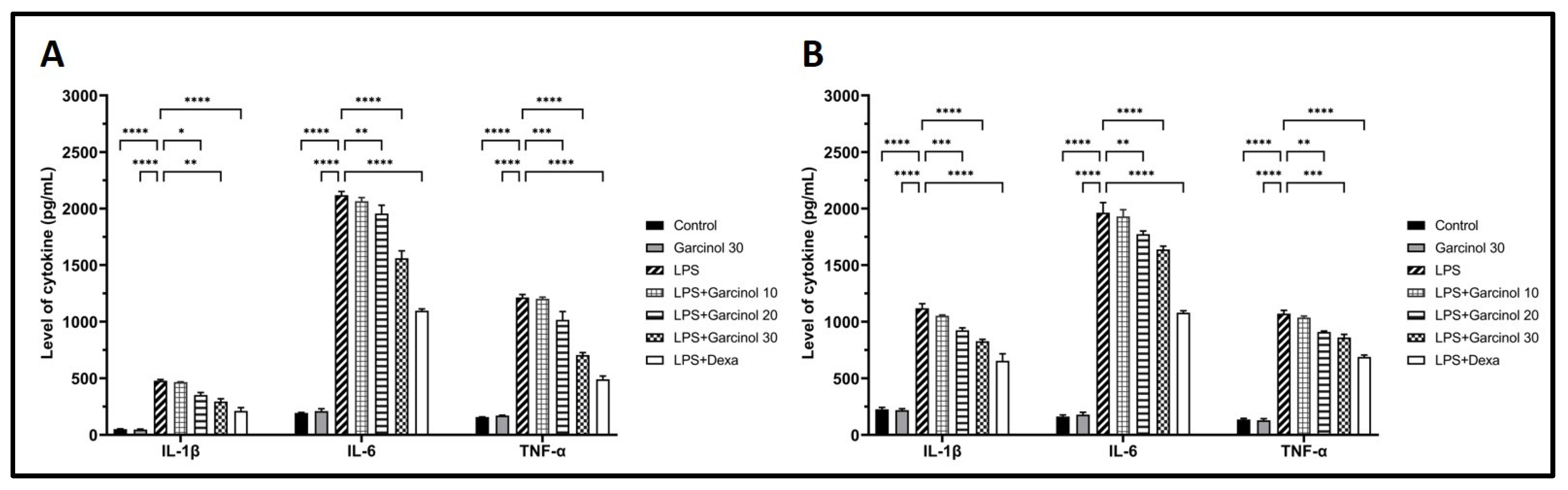
3.3. Effect of Garcinol on the Expression of Inflammatory-Related Cytokine and Mediators
3.4. Effect of Garcinol on the Secretion of Inflammatory Cytokines
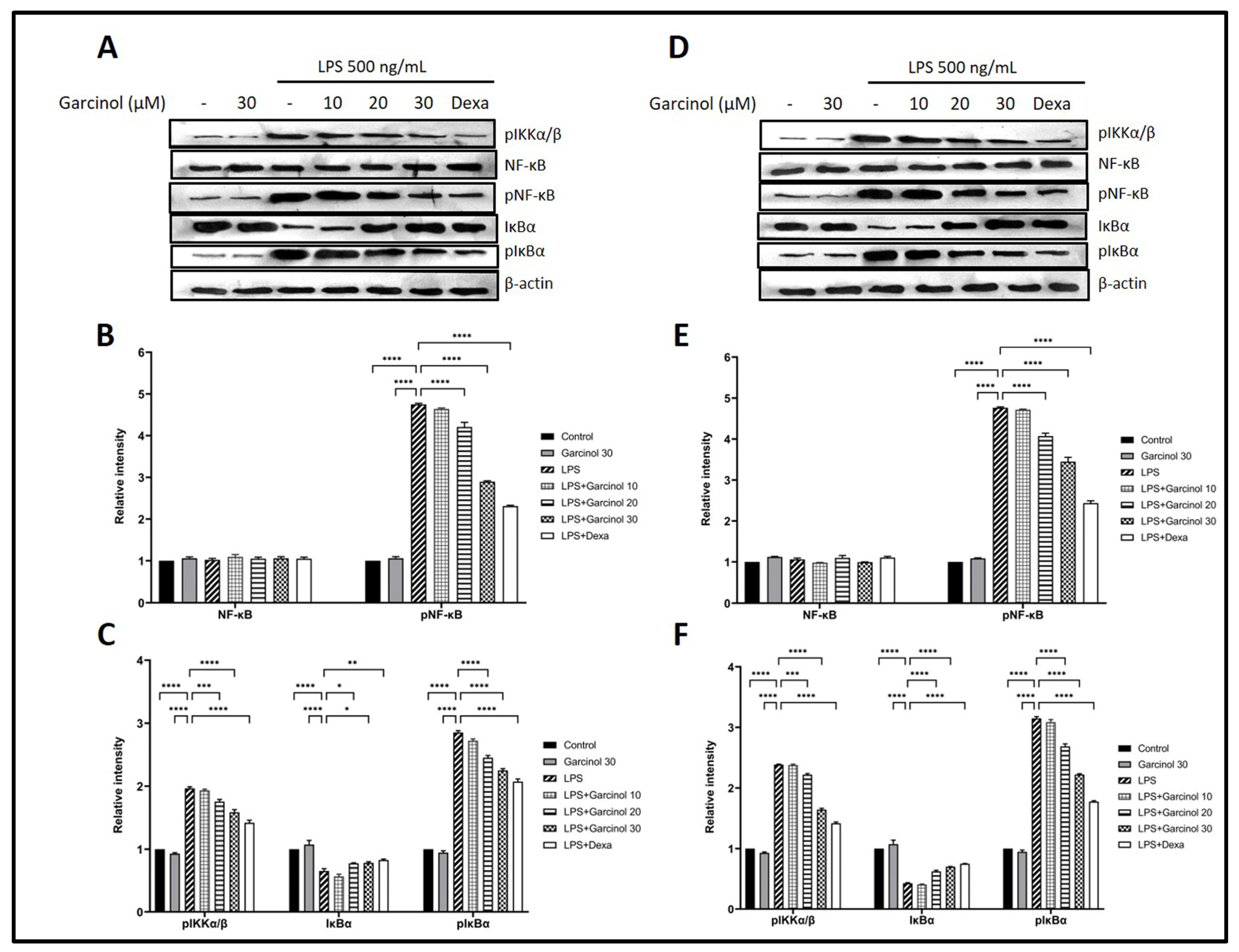
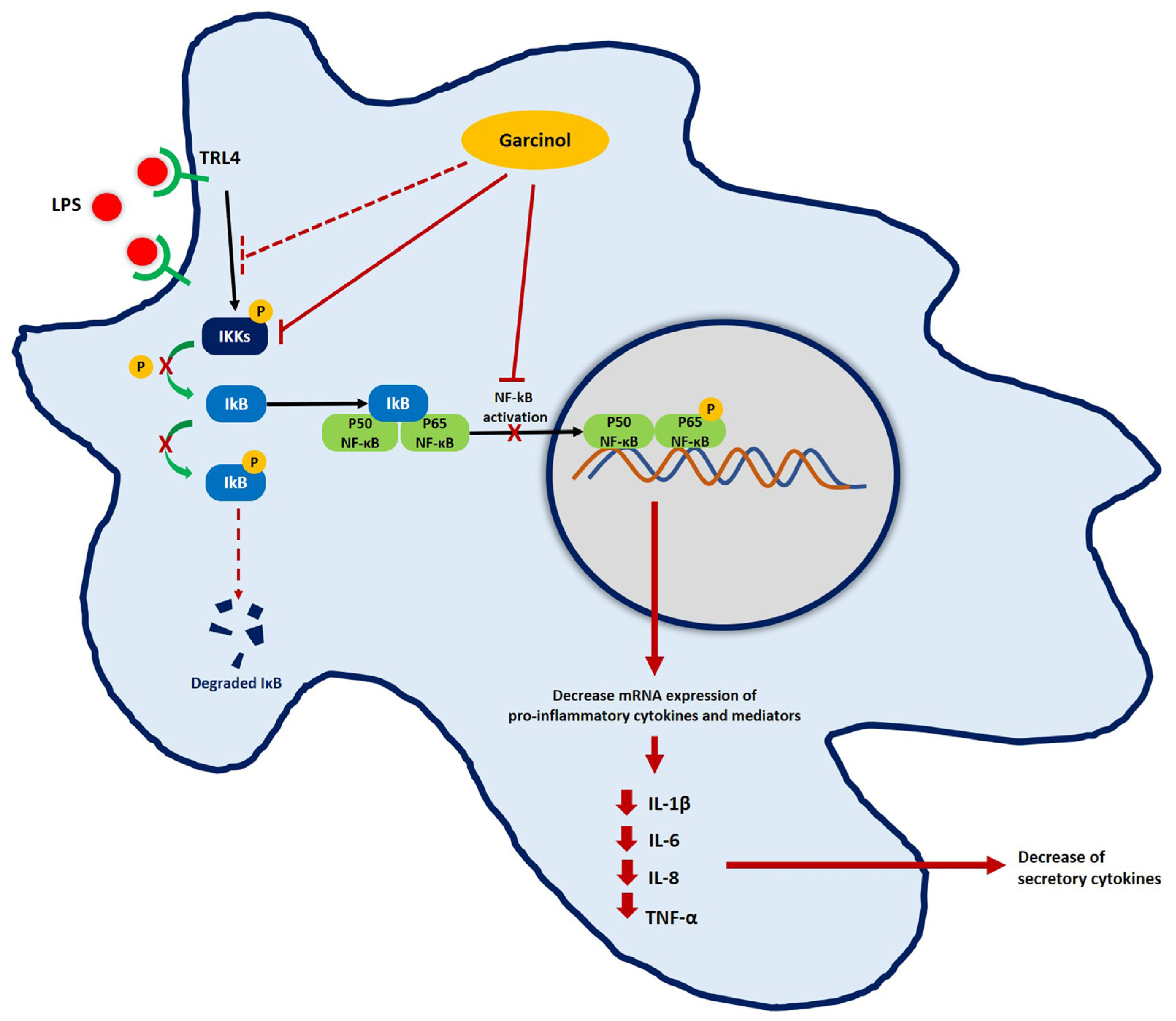
3.5. Effect of Garcinol on the Regulation of Nuclear Factor Kappa B (NF-κB) Signaling Pathway
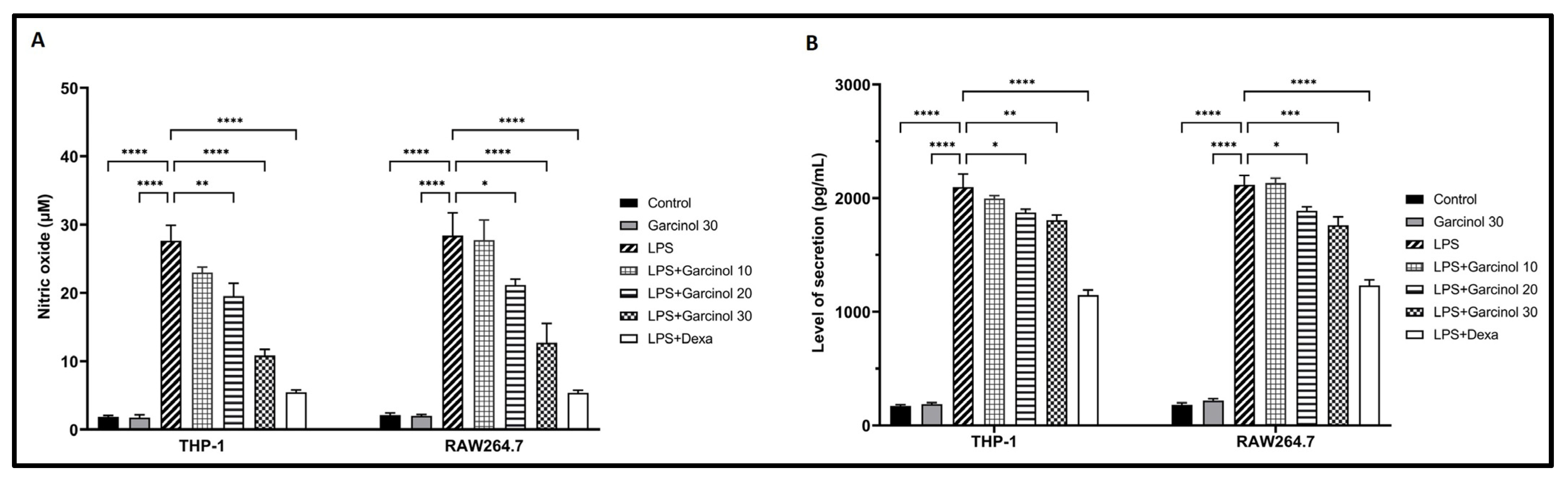
3.6. Effect of Garcinol on NO and PGE2 Production in LPS-Activated Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 1992, 13, 11–16. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C.A. Decoding the Patterns of Self and Nonself by the Innate Immune System. Science 2002, 296, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Reimer, T.; Brcic, M.; Schweizer, M.; Jungi, T.W. poly(I:C) and LPS induce distinct IRF3 and NF-κB signaling during type-I IFN and TNF responses in human macrophages. J. Leukoc. Biol. 2008, 83, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-H.; Jang, E.; Kang, S.-Y.; Shin, J.-S.; Han, H.-S.; Kim, T.-W.; Lee, D.H.; Lee, J.-H.; Jang, D.S.; Lee, K.-T. Anti-inflammatory potential of Patrineolignan B isolated from Patrinia scabra in LPS-stimulated macrophages via inhibition of NF-κB, AP-1, and JAK/STAT pathways. Int. Immunopharmacol. 2020, 86, 106726. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Wang, Y.; Zhai, J.; Chen, G.; Hu, K. Gambogic acid induces heme oxygenase-1 through Nrf2 signaling pathway and inhibits NF-κB and MAPK activation to reduce inflammation in LPS-activated RAW264.7 cells. Biomed. Pharmacother. 2019, 109, 555–562. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Yu, L.; Zhang, S.-D.; Ping, K.; Ni, H.-Y.; Qin, X.-Y.; Zhao, C.-J.; Wang, W.; Efferth, T.; Fu, Y.-J. Cryptochlorogenic acid attenuates LPS-induced inflammatory response and oxidative stress via upregulation of the Nrf2/HO-1 signaling pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2020, 83, 106436. [Google Scholar] [CrossRef]
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef]
- Choudhury, B.; Kandimalla, R.; Elancheran, R.; Bharali, R.; Kotoky, J. Garcinia morella fruit, a promising source of antioxidant and anti-inflammatory agents induces breast cancer cell death via triggering apoptotic pathway. Biomed. Pharmacother. 2018, 103, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Espirito Santo, B.L.S.d.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.d.O.; Bogo, D.; Freitas, K.d.C.; Guimarães, R.d.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.d.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, U.; Babu, K.; Kumar, R.; Ashis, G.R.; Mohan, S.; Va, P. Diversity of Indian Garcinia—A medicinally important spice crop in India. Acta Hortic. 2013, 979, 467–476. [Google Scholar] [CrossRef]
- Parthasarathy, U.; Nandakishore, O.P. Morphological Characterisation of Some Important Indian Garcinia Species. Dataset Pap. Sci. 2014, 2014, 823705. [Google Scholar] [CrossRef]
- Rama Rao, A.V.; Venkatswamy, G.; Pendse, D. Camboginol and Cambogin. Tetrahedron Lett. 1980, 21, 1975–1978. [Google Scholar] [CrossRef]
- Hong, J.; Sang, S.; Park, H.J.; Kwon, S.J.; Suh, N.; Huang, M.T.; Ho, C.T.; Yang, C.S. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis 2006, 27, 278–286. [Google Scholar] [CrossRef]
- Yoshida, K.; Tanaka, T.; Hirose, Y.; Yamaguchi, F.; Kohno, H.; Toida, M.; Hara, A.; Sugie, S.; Shibata, T.; Mori, H. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer Lett. 2005, 221, 29–39. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Saito, M.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000, 48, 2320–2325. [Google Scholar] [CrossRef]
- Chatterjee, A.; Yasmin, T.; Bagchi, D.; Stohs, S.J. The bactericidal effects of Lactobacillus acidophilus, garcinol and Protykin® compared to clarithromycin, on Helicobacter pylori. Mol. Cell. Biochem. 2003, 243, 29–35. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Zhang, X.; Chen, C.-L.; Liu, Q.-Z.; Xu, J.-W.; Qian, Q.-Q.; Li, W.-Y.; Qian, Y.-N. Protective effects of Garcinol against neuropathic pain—Evidence from in vivo and in vitro studies. Neurosci. Lett. 2017, 647, 85–90. [Google Scholar] [CrossRef]
- Wong, W.W.W.; Chong, T.C.; Tananak, J.; Ramba, H.; Kalitu, N. Fruits of Sabah; Department of Agriculture: Sabah, Malaysia, 2007; Volume 1, pp. 71–73. [Google Scholar]
- Yapwattanaphun, C.; Subhadrabandhu, S.; Sugiura, A.; Yonemori, K.; Utsunomiya, N. Utilization of some Garcinia species in Thailand. Acta Hortic. 2002, 575, 563–570. [Google Scholar] [CrossRef]
- Deachathai, S.; Mahabusarakam, W.; Phongpaichit, S.; Taylor, W.C. Phenolic compounds from the fruit of Garcinia dulcis. Phytochemistry 2005, 66, 2368–2375. [Google Scholar] [CrossRef]
- Deachathai, S.; Phongpaichit, S.; Mahabusarakam, W. Phenolic compounds from the seeds of Garcinia dulcis. Nat. Prod. Res. 2008, 22, 1327–1332. [Google Scholar] [CrossRef]
- Mahabusarakam, W.; Mecawun, P.; Phongpaichit, S. Xanthones from the green branch of Garcinia dulcis. Nat. Prod. Res. 2016, 30, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Deachathai, S.; Mahabusarakam, W.; Phongpaichit, S.; Taylor, W.C.; Zhang, Y.J.; Yang, C.R. Phenolic compounds from the flowers of Garcinia dulcis. Phytochemistry 2006, 67, 464–469. [Google Scholar] [CrossRef]
- Thongsepee, N.; Mahabusarakam, W.; Hiranyachattada, S. Diuretic and hypotensive effect of morelloflavone from garcinia dulcis in Two-Kidneys-One-Clip (2K1C) hypertensive rat. Sains Malays. 2017, 46, 1479–1490. [Google Scholar] [CrossRef]
- Thongsepee, N.; Srisawat, U.; Mahabussarakam, W.; Ekarattanawong, S.; Suttirak, N.; Hiranyachattada, S. Effects of oral administration of Garcinia dulcis flower extract on arterial blood pressure and renal excretory functions in rats. ScienceAsia 2020, 46, 671–678. [Google Scholar] [CrossRef]
- Khamthong, N.; Hutadilok-Towatana, N. Phytoconstituents and Biological Activities of Garcinia dulcis (Clusiaceae): A Review. Nat. Prod. Commun. 2017, 12, 453–460. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.S.; Niranjana, S.R.; Girish, K.S. An overview on genus garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- Thongsepee, N.; Mahabusarakam, W.; Asa, W.T.; Hiranyachattada, S. Vasorelaxant mechanisms of camboginol from Garcinia dulcis in normotensive and 2-kidneys-1-clip hypertensive rat. Songklanakarin J. Sci. Technol. 2018, 40, 1248–1258. [Google Scholar] [CrossRef]
- Lund, M.E.; To, J.; O’Brien, B.A.; Donnelly, S. The choice of phorbol 12-myristate 13-acetate differentiation protocol influences the response of THP-1 macrophages to a pro-inflammatory stimulus. J. Immunol. Methods 2016, 430, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chantree, P.; Na-Bangchang, K.; Martviset, P. Anticancer Activity of Fucoidan via Apoptosis and Cell Cycle Arrest on Cholangiocarcinoma Cell. Asian Pac. J. Cancer Prev. 2021, 22, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Martviset, P.; Panrit, L.; Chantree, P.; Muhamad, P.; Na-Bangchang, K. Suppression of Cholangiocarcinoma Cell Growth and Proliferation by Atractylodes lancea (Thunb) DC. through ERK-Signaling Cascade. Asian Pac. J. Cancer Prev. 2021, 22, 3633–3640. [Google Scholar] [CrossRef]
- Chantree, P.; Surarak, T.; Sangpairoj, K.; Aguilar, P.; Hitakomate, E. Antitumor Effects of Fucoidan Via Apoptotic and Autophagic Induction on HSC-3 Oral Squamous CellCarcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 2013, 3, 71–85. [Google Scholar]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Padhye, S.; Ahmad, A.; Oswal, N.; Sarkar, F.H. Emerging role of Garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J. Hematol. Oncol. 2009, 2, 38. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Allison, D.B.; Vasselli, J.R.; Pietrobelli, A.; Greenfield, D.; Nunez, C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: A randomized controlled trial. JAMA 1998, 280, 1596–1600. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Sakariah, K.K. Determination of (−) hydroxycitric acid in commercial samples of Garcinia cambogia extract by liquid chromatography with ultraviolet detection. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 915–923. [Google Scholar] [CrossRef]
- Liu, C.; Ho, P.C.L.; Wong, F.C.; Sethi, G.; Wang, L.Z.; Goh, B.C. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef]
- Tang, W.; Pan, M.H.; Sang, S.; Li, S.; Ho, C.T. Garcinol from garcinia indica: Chemistry and health beneficial effects. ACS Symp. Ser. 2013, 1129, 133–145. [Google Scholar] [CrossRef]
- Won, S.J.; Liu, C.T.; Tsao, L.T.; Weng, J.R.; Ko, H.H.; Wang, J.P.; Lin, C.N. Synthetic chalcones as potential anti-inflammatory and cancer chemopreventive agents. Eur. J. Med. Chem. 2005, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Lewis, Y.S.; Ravindranath, B. On the structures of garcinol, isogarcinol and camboginol. Tetrahedron Lett. 1981, 22, 793–796. [Google Scholar] [CrossRef]
- Lin, X.; Tian, D.; Fu, Y.; Li, Y.; Huang, L.; Gu, W.; Song, J.; Li, Y.; Ben-David, Y.; Wen, M.; et al. Synthesis of novel guttiferone E and xanthochymol derivatives with cytotoxicities by inducing cell apoptosis and arresting the cell cycle phase. Eur. J. Med. Chem. 2019, 162, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Lu, F.; Xie, J.; Wu, M.; Cai, B.; Liu, Y.; Zhang, H.; Tan, H.; Pan, Y.; Xu, H. Cambogin exerts anti-proliferative and pro-apoptotic effects on breast adenocarcinoma through the induction of NADPH oxidase 1 and the alteration of mitochondrial morphology and dynamics. Oncotarget 2016, 7, 50596–50611. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Kim, N.M.; Jiang, Y.W.; Zhang, H.; Zheng, D.; Zhu, F.X.; Liang, R.; Li, B.; Xu, H.X. Cambogin suppresses dextran sulphate sodium-induced colitis by enhancing Treg cell stability and function. Br. J. Pharmacol. 2018, 175, 1085–1099. [Google Scholar] [CrossRef]
- Yamaguchi, F.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000, 48, 180–185. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Cao, J.; Han, C.-M.; Li, S.-W.; Zhang, C.; Du, Y.-D.; Zhou, Q.-Q.; Zhang, X.-Y.; Chen, X. The C8 side chain is one of the key functional group of Garcinol for its anti-cancer effects. Bioorganic Chem. 2017, 71, 74–80. [Google Scholar] [CrossRef]
- Lee, J.; Cacalano, G.; Camerato, T.; Toy, K.; Moore, M.W.; Wood, W.I. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 1995, 155, 2158. [Google Scholar] [CrossRef]
- Wang, L.; Gu, J.; Zong, M.; Zhang, Q.; Li, H.; Li, D.; Mou, X.; Liu, P.; Liu, Y.; Qiu, F.; et al. Anti-inflammatory action of physalin A by blocking the activation of NF-κB signaling pathway. J. Ethnopharmacol. 2021, 267, 113490. [Google Scholar] [CrossRef]
- Asfaha, S.; Dubeykovskiy, A.N.; Tomita, H.; Yang, X.; Stokes, S.; Shibata, W.; Friedman, R.A.; Ariyama, H.; Dubeykovskaya, Z.A.; Muthupalani, S.; et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology 2013, 144, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yu, Y.; Shi, Y.; Sun, W.; Xie, M.; Ge, N.; Mao, R.; Chang, A.; Xu, G.; Schneider, M.D.; et al. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J. Biol. Chem. 2010, 285, 5347–5360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Wang, C.; He, C.; Ma, Q.; Li, J.; Wang, W.; Xu, Y.T.; Wang, T. Qingwenzhike Prescription Alleviates Acute Lung Injury Induced by LPS via Inhibiting TLR4/NF-kB Pathway and NLRP3 Inflammasome Activation. Front Pharm. 2021, 12, 790072. [Google Scholar] [CrossRef]
- Lee, E.H.; Shin, M.H.; Gi, M.; Park, J.; Song, D.; Hyun, Y.M.; Ryu, J.H.; Seong, J.K.; Jeon, Y.; Han, G.; et al. Inhibition of Pendrin by a small molecule reduces Lipopolysaccharide-induced acute Lung Injury. Theranostics 2020, 10, 9913–9922. [Google Scholar] [CrossRef]
- Li, X.; Shan, C.; Wu, Z.; Yu, H.; Yang, A.; Tan, B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm. Res. 2020, 69, 365–373. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, W.; Wang, S.; Li, B.; Li, G.; Shi, Q. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway. Int. Immunopharmacol. 2020, 88, 106974. [Google Scholar] [CrossRef]
- Simu, S.Y.; Alam, M.B.; Kim, S.Y. The Activation of Nrf2/HO-1 by 8-Epi-7-deoxyloganic Acid Attenuates Inflammatory Symptoms through the Suppression of the MAPK/NF-κB Signaling Cascade in In Vitro and In Vivo Models. Antioxidants 2022, 11, 1765. [Google Scholar] [CrossRef]
- Masullo, M.; Menegazzi, M.; Di Micco, S.; Beffy, P.; Bifulco, G.; Dal Bosco, M.; Novelli, M.; Pizza, C.; Masiello, P.; Piacente, S. Direct interaction of garcinol and related polyisoprenylated benzophenones of Garcinia cambogia fruits with the transcription factor STAT-1 as a likely mechanism of their inhibitory effect on cytokine signaling pathways. J. Nat. Prod. 2014, 77, 543–549. [Google Scholar] [CrossRef]
- Tsai, M.L.; Chiou, Y.S.; Chiou, L.Y.; Ho, C.T.; Pan, M.H. Garcinol suppresses inflammation-associated colon carcinogenesis in mice. Mol. Nutr. Food Res. 2014, 58, 1820–1829. [Google Scholar] [CrossRef]
- Liao, C.H.; Sang, S.; Liang, Y.C.; Ho, C.T.; Lin, J.K. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 in downregulating nuclear factor-kappa B pathway by garcinol. Mol. Carcinog. 2004, 41, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Shimada, R.; Kagami, S.; Yamaguchi, F.; Kataoka, S.; Ariga, T.; Murakami, A.; Koshimizu, K.; Ohigashi, H. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis 2000, 21, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Pang, C.; Zhao, K.; Jiang, J.; Zhang, T.; Peng, J.; Sun, P.; Qian, Y. Garcinol Suppresses IL-1β-Induced Chondrocyte Inflammation and Osteoarthritis via Inhibition of the NF-κB Signaling Pathway. Inflammation 2019, 42, 1754–1766. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantree, P.; Martviset, P.; Thongsepee, N.; Sangpairoj, K.; Sornchuer, P. Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway. Nutrients 2023, 15, 575. https://doi.org/10.3390/nu15030575
Chantree P, Martviset P, Thongsepee N, Sangpairoj K, Sornchuer P. Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway. Nutrients. 2023; 15(3):575. https://doi.org/10.3390/nu15030575
Chicago/Turabian StyleChantree, Pathanin, Pongsakorn Martviset, Nattaya Thongsepee, Kant Sangpairoj, and Phornphan Sornchuer. 2023. "Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway" Nutrients 15, no. 3: 575. https://doi.org/10.3390/nu15030575
APA StyleChantree, P., Martviset, P., Thongsepee, N., Sangpairoj, K., & Sornchuer, P. (2023). Anti-Inflammatory Effect of Garcinol Extracted from Garcinia dulcis via Modulating NF-κB Signaling Pathway. Nutrients, 15(3), 575. https://doi.org/10.3390/nu15030575







