Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study
Abstract
1. Introduction
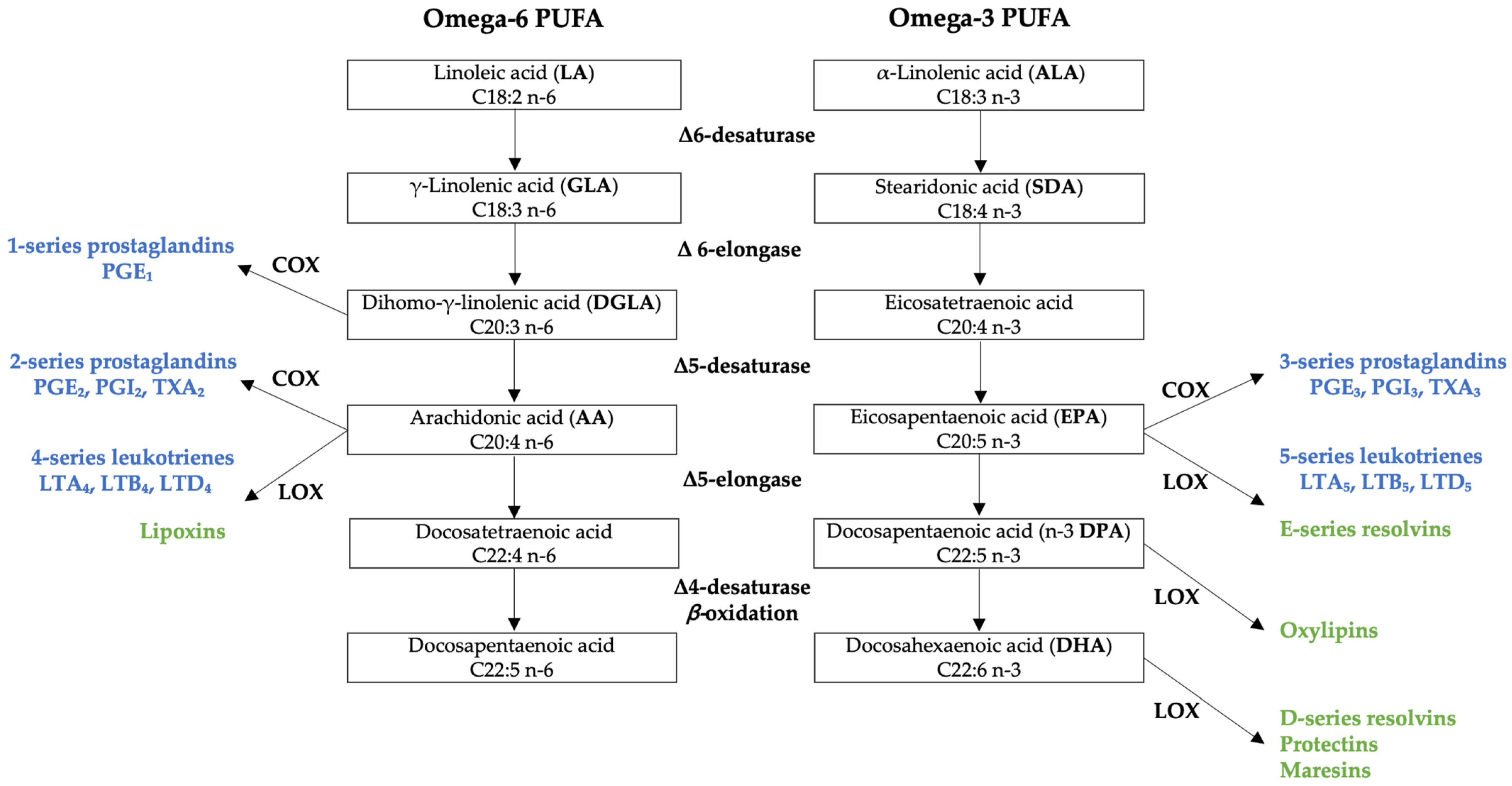
1.1. Metabolism of Omega-6 and Omega-3 Polyunsaturated Fatty Acids
1.2. Biological Functions and Adequate Intakes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids
2. Materials and Methods
2.1. Study Design and Sample
2.2. Dietary Survey and Data Collection
2.3. Anthropometric Measurements
2.4. Statistical Analysis
3. Results
3.1. Omega-3 and Omega-6 PUFA Dietary Intakes
3.2. Omega-3 and Omega-6 PUFA Dietary Sources
3.3. Combined Dietary Intake Adequacy of Omega-3 PUFA, Folic Acid (FA), Vitamin B12 and Choline
4. Discussion
4.1. Omega-3 and Omega-6 PUFA Dietary Intakes
4.2. Omega-3 and Omega-6 PUFA Dietary Sources
4.3. Combined Dietary Intake Adequacy of Omega-3 PUFA, Folic Acid (FA), Vitamin B12 and Choline
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef]
- Sheppard, K.W.; Cheatham, C.L. Omega-6/omega-3 fatty acid intake of children and older adults in the US: Dietary intake in comparison to current dietary recommendations and the Healthy Eating Index. Lipids Health Dis. 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Jauregibeitia, I.; Portune, K.; Rica, I.; Tueros, I.; Velasco, O.; Grau, G.; Trebolazabala, N.; Castaño, L.; Larocca, A.V.; Ferreri, C.; et al. Fatty Acid Profile of Mature Red Blood Cell Membranes and Dietary Intake as a New Approach to Characterize Children with Overweight and Obesity. Nutrients 2020, 12, 3446. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- De Groot, R.H.; Hornstra, G.; van Houwelingen, A.C.; Roumen, F. Effect of alpha-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am. J. Clin. Nutr. 2004, 79, 251–260. [Google Scholar] [CrossRef]
- Van Houwelingen, A.C.; Sørensen, J.D.; Hornstra, G.; Simonis, M.M.; Boris, J.; Olsen, S.F.; Secher, N.J. Essential fatty acid status in neonates after fish-oil supplementation during late pregnancy. Br. J. Nutr. 1995, 74, 723–731. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Gibson, R.A. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur. J. Clin. Nutr. 1996, 50, 352–357. [Google Scholar]
- Gramlich, L.; Ireton-Jones, C.; Miles, J.M.; Morrison, M.; Pontes-Arruda, A. Essential Fatty Acid Requirements and Intravenous Lipid Emulsions. JPEN J. Parenter. Enter. Nutr. 2019, 43, 697–707. [Google Scholar] [CrossRef]
- FAO Joint. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation, 10–14 November 2008, Geneva; FAO: Rome, Italy, 2010. [Google Scholar]
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Gura, K.M.; Puder, M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 165–170. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551. [Google Scholar] [CrossRef]
- Anderson, R.E. Lipids of ocular tissues: IV. A comparison of the phospholipids from the retina of six mammalian species. Exp. Eye Res. 1970, 10, 339–344. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, cd003402. [Google Scholar] [CrossRef]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef]
- Tahaei, H.; Gignac, F.; Pinar, A.; Fernandez-Barrés, S.; Romaguera, D.; Vioque, J.; Santa-Marina, L.; Subiza-Pérez, M.; Llop, S.; Soler-Blasco, R.; et al. Omega-3 Fatty Acid Intake during Pregnancy and Child Neuropsychological Development: A Multi-Centre Population-Based Birth Cohort Study in Spain. Nutrients 2022, 14, 518. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Kris-Etherton, P.M.; Harris, K.A. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr. Atheroscler. Rep. 2008, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.S.; Gammack, J.K.; Van Niekerk, J.; Dangour, A.D. Omega 3 fatty acid for the prevention of dementia. Cochrane Database Syst. Rev. 2006, 1, cd005379. [Google Scholar] [CrossRef]
- Issa, A.M.; Mojica, W.A.; Morton, S.C.; Traina, S.; Newberry, S.J.; Hilton, L.G.; Garland, R.H.; Maclean, C.H. The efficacy of omega–3 fatty acids on cognitive function in aging and dementia: A systematic review. Dement. Geriatr. Cogn. Disord. 2006, 21, 88–96. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, X.W.; Hou, W.S.; Li, M.; Tang, Z.Y. Omega-3 fatty acids and risk of cognitive decline in the elderly: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2016, 28, 165–166. [Google Scholar] [CrossRef]
- Bartrina, J.A.; Majem, L.S. Objetivos nutricionales para la población española: Consenso de la Sociedad Española de Nutrición Comunitaria 2011. Span. J. Community Nutr. 2011, 17, 178–199. [Google Scholar]
- Ortega, R.M.; González, L.G.; Villalobos, T.K.; Perea, J.M.; Aparicio, A.; López, A.M. Food sources and adequacy of intake of omega 3 and omega-6 fatty acids in a representative sample of Spanish adults. Nutr. Hosp. 2013, 28, 2236–2245. [Google Scholar]
- Aparicio, E.; Martín-Grau, C.; Bedmar, C.; Serrat, N.; Basora, J.; Arija, V. Maternal Factors Associated with Levels of Fatty Acids, Specifically n-3 PUFA during Pregnancy: ECLIPSES Study. Nutrients 2021, 13, 317. [Google Scholar] [CrossRef]
- Redruello-Requejo, M.; Carretero-Krug, A.; Rodríguez-Alonso, P.; Samaniego-Vaesken, M.L.; Partearroyo, T.; Varela-Moreiras, G. Dietary Intake Adequacy and Food Sources of Nutrients Involved in the Methionine-Methylation Cycle in Women of Childbearing Age from the ANIBES Spanish Population. Nutrients 2021, 13, 2958. [Google Scholar] [CrossRef]
- Strain, J.J.; Dowey, L.; Ward, M.; Pentieva, K.; McNulty, H. B-vitamins, homocysteine metabolism and CVD. Proc. Nutr. Soc. 2004, 63, 597–603. [Google Scholar] [CrossRef]
- Caudill, M.A.; Obeid, R.; Derbyshire, E.J.; Bernhard, W.; Lapid, K.; Walker, S.J.; Zeisel, S.H. Building better babies: Should choline supplementation be recommended for pregnant and lactating mothers? Literature overview and expert panel consensus. Gynecol. Obstet 2020, 2, 149–161. [Google Scholar]
- Cook, S. Choline: A Critical Prenatal Nutrient. Res. Guide 2017, 8. [Google Scholar]
- Ruiz, E.; Ávila, J.M.; Castillo, A.; Valero, T.; del Pozo, S.; Rodríguez, P.; Bartrina, J.A.; Gil, Á.; González-Gross, M.; Ortega, R.M. The ANIBES Study on Energy Balance in Spain: Design, Protocol and Methodology. Nutrients 2015, 7, 970–998. [Google Scholar] [CrossRef]
- Ruiz, E.; Ávila, J.M.; Valero, T.; del Pozo, S.; Rodríguez, P.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L. Energy Intake, Profile, and Dietary Sources in the Spanish Population: Findings of the ANIBES Study. Nutrients 2015, 7, 4739–4762. [Google Scholar] [CrossRef]
- Moreiras, O.; Carbajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos/Guía de Prácticas; Ediciones Pirámide: Madrid, Spain, 2019. [Google Scholar]
- USDA. Composition of Foods: Raw, Processed, Prepared. USDA National Nutrient Database for Standard Reference, Legacy; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2018. [Google Scholar]
- USDA. USDA Database for the Choline Content of Common Foods Release Two January 2008; U.S. Department of Agriculture, Agricultural Research Service: Beltsville, MD, USA, 2008. [Google Scholar]
- EFSA (European Food Safety Authority). Dietary Reference Values for nutrients. Summary Report. EFSA Support. Publ. 2017, 2017, e15121. [Google Scholar]
- Fernández, C.; Lorenzo, H.; Vrotsou, K.; Aresti, U.; Rica, I.; Sánchez, E. Estudio de Crecimiento de Bilbao: Curvas y Tablas de Crecimiento (Estudio Transversal); Fundación Faustino Orbegozo Eizaguirre: Bilbao, Spain, 2011. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Astorg, P.; Arnault, N.; Czernichow, S.; Noisette, N.; Galan, P.; Hercberg, S. Dietary intakes and food sources of n-6 and n-3 PUFA in french adult men and women. Lipids 2004, 39, 527–535. [Google Scholar] [CrossRef]
- Sioen, I.A.; Pynaert, I.; Matthys, C.; De Backer, G.; Van Camp, J.; De Henauw, S. Dietary intakes and food sources of fatty acids for Belgian women, focused on n-6 and n-3 polyunsaturated fatty acids. Lipids 2006, 41, 415–422. [Google Scholar] [CrossRef]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef]
- Partearroyo, T.; Samaniego-Vaesken, M.L.; Ruiz, E.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Current food consumption amongst the Spanish ANIBES study population. Nutrients 2019, 11, 2663. [Google Scholar] [CrossRef]
- Vyncke, K.E.; Libuda, L.; De Vriendt, T.; Moreno, L.A.; Van Winckel, M.; Manios, Y.; Gottrand, F.; Molnar, D.; Vanaelst, B.; Sjöström, M. Dietary fatty acid intake, its food sources and determinants in European adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Br. J. Nutr. 2012, 108, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Bisselou, K.S.; Appiah, A.K.; Kris-Etherton, P.; Skulas-Ray, A.C. Omega-3 fatty acid intake by age, gender, and pregnancy status in the United States: National health and nutrition examination survey 2003–2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary intakes of EPA and DHA omega-3 fatty acids among US childbearing-age and pregnant women: An analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Harika, R.K.; Eilander, A.; Alssema, M.; Osendarp, S.J.; Zock, P.L. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: A systematic review of data from 40 countries. Ann. Nutr. Metab. 2013, 63, 229–238. [Google Scholar] [CrossRef]
- Del Pozo, S.; García, V.; Cuadrado, C.; Ruiz, E.; Valero, T.; Ávila, J.M.; Varela, G. Valoración Nutricional de la Dieta Española de Acuerdo al Panel de Consumo Alimentario; Fundación Española de la Nutrición (FEN): Madrid, Spain, 2012; pp. 1–142. [Google Scholar]
- Geelen, A.; Schouten, J.M.; Kamphuis, C.; Stam, B.E.; Burema, J.; Renkema, J.M.; Bakker, E.J.; van’t Veer, P.; Kampman, E. Fish consumption, n-3 fatty acids, and colorectal cancer: A meta-analysis of prospective cohort studies. Am. J. Epidemiol. 2007, 166, 1116–1125. [Google Scholar] [CrossRef]
- Gerber, M. Background review paper on total fat, fatty acid intake and cancers. Ann. Nutr. Metab. 2009, 55, 140–161. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- Cottin, S.; Sanders, T.; Hall, W. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Ortega, R.; Rodríguez-Rodríguez, E.; López-Sobaler, A. Effects of omega 3 fatty acids supplementation in behavior and non-neurodegenerative neuropsychiatric disorders. Br. J. Nutr. 2012, 107, S261–S270. [Google Scholar] [CrossRef]
- Sioen, I.; van Lieshout, L.; Eilander, A.; Fleith, M.; Lohner, S.; Szommer, A.; Petisca, C.; Eussen, S.; Forsyth, S.; Calder, P.C. Systematic review on n-3 and n-6 polyunsaturated fatty acid intake in European countries in light of the current recommendations-Focus on specific population groups. Ann. Nutr. Metab. 2017, 70, 39–50. [Google Scholar] [CrossRef]
- Umesawa, M.; Yamagishi, K.; Iso, H. Intake of fish and long-chain n-3 polyunsaturated fatty acids and risk of diseases in a Japanese population: A narrative review. Eur. J. Clin. Nutr. 2021, 75, 902–920. [Google Scholar] [CrossRef]
- Goyens, P.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of α-linolenic acid in humans is influenced by the absolute amounts of α-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef]
- Vani, A.; Laxmi, R.; Sesikeran, B. Effects of dietary α-linolenic acid from blended oils on biochemical indices of coronary heart disease in Indians. Lipids 2002, 37, 1077–1086. [Google Scholar]
- Ubeda, N.; Achón, M.; Varela-Moreiras, G. Omega 3 fatty acids in the elderly. Br. J. Nutr. 2012, 107, S137–S151. [Google Scholar] [CrossRef]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar]
| n | Omega-3 Fatty Acids (ω3) (g/Day) | Alfa-Linolenic Acid (ALA) (C18: 3n-3) (g/Day) | Eicosapentaenoic Acid (EPA) (C20: 5n-3) (g/Day) | Docosahexaenoic Acid (DHA) (C22: 6n-3) (g/Day) | EPA + DHA (g/Day) | |||
|---|---|---|---|---|---|---|---|---|
| Total Population (9–75 years) | Total | 2009 | 0.81 | 0.61 | 0.03 | 0.06 | 0.09 | |
| (0.56–1.19) | (0.45–0.85) | (0.01–0.12) | (0.01–0.20) | (0.02–0.34) | ||||
| Men | 1013 | 0.86 *** | 0.65 *** | 0.03 | 0.06 | 0.10 | ||
| (0.60–1.26) | (0.50–0.89) | (0.01–0.12) | (0.01–0.22) | (0.02–0.36) | ||||
| Women | 996 | 0.75 | 0.57 | 0.03 | 0.06 | 0.09 | ||
| (0.52–1.11) | (0.42–0.79) | (0.01–0.11) | (0.01–0.19) | (0.03–0.33) | ||||
| Age | 9–12 years | Total | 213 | 0.77 | 0.61 | 0.03 a,b | 0.05 a,b | 0.09 a,b,c |
| (0.57–1.02) | (0.48–0.79) | (0.01–0.10) | (0.01–0.17) | (0.02–0.26) | ||||
| Men | 126 | 0.81 | 0.62 | 0.03 | 0.05 | 0.10 | ||
| (0.61–1.02) | (0.49–0.82) | (0.01–0.09) | (0.01–0.19) | (0.02–0.29) | ||||
| Women | 87 | 0.72 | 0.57 | 0.04 | 0.05 | 0.09 | ||
| (0.55–1.02) | (0.46–0.78) | (0.01–0.10) | (0.01–0.13) | (0.02–0.22) | ||||
| 13–17 years | Total | 211 | 0.80 | 0.63 | 0.02 b | 0.04 a | 0.06 b | |
| (0.55–1.10) | (0.50–0.90) | (0.01–0.07) | (0.01–0.12) | (0.01–0.21) | ||||
| Men | 137 | 0.85 ** | 0.67 *** | 0.02 | 0.04 | 0.07 | ||
| (0.59–1.14) | (0.54–0.98) | (0.01–0.08) | (0.01–0.13) | (0.01–0.21) | ||||
| Women | 74 | 0.68 | 0.54 | 0.02 | 0.03 | 0.05 | ||
| (0.49–1.04) | (0.45–0.75) | (0.01–0.07) | (0.00–0.09) | (0.02–0.19) | ||||
| 18–64 years | Total | 1655 | 0.82 | 0.62 | 0.03 b | 0.06 b | 0.09 a,c | |
| (0.56–1.20) | (0.46–0.86) | (0.01–0.12) | (0.01–0.20) | (0.02–0.34) | ||||
| Men | 798 | 0.87 *** | 0.66 *** | 0.03 | 0.06 | 0.09 | ||
| (0.60–1.27) | (0.50–0.90) | (0.01–0.12) | (0.01–0.21) | (0.02–0.36) | ||||
| Women | 857 | 0.76 | 0.58 | 0.03 | 0.05 | 0.09 | ||
| (0.53–1.12) | (0.42–0.79) | (0.01–0.11) | (0.01–0.20) | (0.02–0.33) | ||||
| 65–75 years | Total | 206 | 0.81 | 0.58 | 0.05 a | 0.09 c | 0.15 d | |
| (0.55–1.25) | (0.41–0.82) | (0.01–0.15) | (0.03–0.25) | (0.05–0.39) | ||||
| Men | 99 | 0.95 ** | 0.61 * | 0.06 ** | 0.13 * | 0.18 | ||
| (0.62–1.55) | (0.45–0.84) | (0.01–0.19) | (0.03–0.30) | (0.05–0.48) | ||||
| Women | 107 | 0.79 | 0.50 | 0.04 | 0.08 | 0.13 | ||
| (0.51–1.08) | (0.38–0.79) | (0.01–0.09) | (0.03–0.17) | (0.04–0.26) | ||||
| n | Omega-3 Fatty Acids (ω3) (g/Day) | Alfa-Linolenic Acid (ALA) (C18: 3n-3) (g/Day) | Eicosapentaenoic Acid (EPA) (C20: 5n-3) (g/Day) | Docosahexaenoic Acid (DHA) (C22: 6n-3) (g/Day) | EPA + DHA (g/Day) | |
|---|---|---|---|---|---|---|
| Total women of childbearing age | 552 | 0.74 | 0.58 | 0.02 | 0.05 | 0.07 |
| (0.51–1.04) | (0.42–0.77) | (0.01–0.10) | (0.01–0.18) | (0.02–0.28) | ||
| Younger women (18–30 years) | 209 | 0.69 * | 0.56 | 0.01 *** | 0.03 ** | 0.05 ** |
| (0.49–0.95) | (0.42–0.77) | (0.00–0.07) | (0.01–0.14) | (0.02–0.22) | ||
| Older women (31–45 years) | 343 | 0.76 | 0.59 | 0.03 | 0.06 | 0.09 |
| (0.53–1.09) | (0.41–0.79) | (0.01–0.12) | (0.01–0.20) | (0.03–0.33) |
| n | Omega-6 Fatty Acids (ω6) (g/Day) | Linoleic Acid (la) (c18: 2n-6) (g/Day) | Arachidonic Acid (aa) (c20: 4n-6) (g/Day) | |||
|---|---|---|---|---|---|---|
| Total Population (9–75 years) | Total | 2009 | 10.07 | 10.00 | 0.08 | |
| (7.00–14.01) | (6.93–13.90) | (0.05–0.13) | ||||
| Men | 1013 | 10.78 *** | 10.69 *** | 0.09 *** | ||
| (7.65–14.86) | (7.53–14.75) | (0.06–0.14) | ||||
| Women | 996 | 9.16 | 9.07 | 0.07 | ||
| (6.41–12.78) | (6.34–12.73) | (0.04–0.11) | ||||
| Age | 9–12 years | Total | 213 | 11.58 a | 11.51 a | 0.09 a |
| (8.63–14.52) | (8.52–14.42) | (0.06–0.13) | ||||
| Men | 126 | 11.64 | 11.53 | 0.09 | ||
| (8.92–14.49) | (8.82–14.36) | (0.06–0.14) | ||||
| Women | 87 | 11.44 | 11.27 | 0.09 | ||
| (8.12–14.84) | (8.02–14.76) | (0.06–0.13) | ||||
| 13–17 years | Total | 211 | 11.33 b | 11.16 a | 0.09 a | |
| (8.07–16.13) | (7.95–16.00) | (0.06–0.13) | ||||
| Men | 137 | 12.75 * | 12.60 * | 0.10 ** | ||
| (9.21–16.21) | (9.06–16.04) | (0.07–0.14) | ||||
| Women | 74 | 10.23 | 10.21 | 0.07 | ||
| (7.32–15.77) | (7.21–15.70) | (0.05–0.12) | ||||
| 18–64 years | Total | 1655 | 10.18 c | 10.10 b | 0.08 b | |
| (7.16–14.12) | (7.06–14.04) | (0.05–0.13) | ||||
| Men | 798 | 10.88 | 10.75 *** | 0.09 *** | ||
| (7.72–15.02) *** | (7.67–14.88) | (0.06–0.14) | ||||
| Women | 857 | 9.35 | 9.25 | 0.07 | ||
| (6.62–12.94) | (6.53–12.83) | (0.04–0.11) | ||||
| 65–75 years | Total | 206 | 7.91 d | 7.79 c | 0.07 b | |
| (5.22–11.25) | (5.13–11.19) | (0.04–0.11) | ||||
| Men | 99 | 8.24 | 8.15 | 0.09 *** | ||
| (5.87–11.98) | (5.68–11.92) | (0.06–0.14) | ||||
| Women | 107 | 7.73 | 7.66 | 0.06 | ||
| (4.69–11.13) | (4.60–10.95) | (0.04–0.09) | ||||
| n | Omega-6 Fatty Acids (ω6) (g/Day) | Linoleic Acid (LA) (C18: 2n-6) (g/Day) | Arachidonic Acid (AA) (C20: 4n-6) (g/Day) | |
|---|---|---|---|---|
| Total women of childbearing age | 552 | 9.88 | 9.74 | 0.07 |
| (6.85–13.65) | (6.80–13.53) | (0.04–0.11) | ||
| Younger women (18–30 years) | 209 | 9.93 | 9.76 | 0.07 |
| (7.26–13.42) | (7.20–13.42) | (0.04–0.11) | ||
| Older women (31–45 years) | 343 | 9.84 | 9.70 | 0.08 |
| (6.73–13.79) | (6.70–13.68) | (0.05–0.12) |
| n | Omega-6/Omega-3 | EPA/DHA | |||
|---|---|---|---|---|---|
| Total Population (9–75 years) | Total | 2009 | 12.13 | 0.57 | |
| (8.69–16.32) | (0.32–0.94) | ||||
| Men | 1013 | 12.40 * | 0.58 | ||
| (8.89–16.67) | (0.32–0.95) | ||||
| Women | 996 | 11.83 | 0.57 | ||
| (8.57–15.89) | (0.31–0.93) | ||||
| Age | 9–12 years | Total | 213 | 14.30 a | 0.61 |
| (10.94–18.86) | (0.35–1.08) | ||||
| Men | 126 | 13.89 | 0.61 | ||
| (11.26–18.84) | (0.31–1.10) | ||||
| Women | 87 | 14.99 | 0.61 | ||
| (10.76–18.90) | (0.38–1.06) | ||||
| 13–17 years | Total | 211 | 14.24 a | 0.62 | |
| (10.76–19.11) | (0.41–1.13) | ||||
| Men | 137 | 14.06 | 0.62 | ||
| (10.64–18.52) | (0.44–1.06) | ||||
| Women | 74 | 14.74 | 0.52 | ||
| (10.89–21.50) | (0.32–1.49) | ||||
| 18–64 years | Total | 1655 | 12.22 b | 0.56 | |
| (8.87–16.37) | (0.30–0.96) | ||||
| Men | 798 | 12.59 * | 0.56 | ||
| (8.92–16.90) | (0.28–0.96) | ||||
| Women | 857 | 11.94 | 0.56 | ||
| (8.79–15.83) | (0.30–0.95) | ||||
| 65–75 years | Total | 206 | 9.36 c | 0.59 | |
| (6.50–12.63) | (0.34–0.88) | ||||
| Men | 99 | 9.18 | 0.60 | ||
| (5.94–12.22) | (0.38–0.84) | ||||
| Women | 107 | 9.58 | 0.54 | ||
| (7.31–13.12) | (0.23–0.89) | ||||
| n | Omega-6/Omega-3 | EPA/DHA | |
|---|---|---|---|
| Total women of childbearing age | 552 | 12.78 | 0.56 |
| (9.61–17.52) | (0.29–1.04) | ||
| Younger women (18–30 years) | 209 | 13.86 *** | 0.50 |
| (10.60–18.13) | (0.23–0.97) | ||
| Older women (31–45 years) | 343 | 11.94 | 0.59 |
| (8.67–16.08) | (0.34–1.08) |
| Total Population (9–75 Years) | |||||
|---|---|---|---|---|---|
| Omega-3 Fatty Acids (ω3) (g/Day) | Omega-6 Fatty Acids (ω6) (g/Day) | Omega-6/Omega-3 | EPA/DHA | ||
| BMI | Underweight n = 42 | 0.74 | 10.40 a | 13.88 a | 0.62 |
| (0.56–0.93) | (8.05–14.97) | (11.70–17.26) | (0.47–1.46) | ||
| Normal n = 854 | 0.84 | 10.68 a,b | 12.20 a,b | 0.58 | |
| (0.58–1.19) | (7.39–14.58) | (8.94–16.66) | (0.32–0.94) | ||
| Overweight n = 713 | 0.80 | 9.86 a,b | 12.12 a,b | 0.59 | |
| (0.56–1.21 | (6.85–13.88) | (8.56–16.09) | (0.32–1.00) | ||
| Obesity n = 400 | 0.77 | 9.15 b | 11.85 b | 0.51 | |
| (0.53–1.21) | (6.67–12.86) | (8.42–15.83) | (0.29–0.83) | ||
| Total Population (9–75 Years) | ||||
|---|---|---|---|---|
| Omega-3 Fatty Acids (ω3) (g/Day) | Omega-6 Fatty Acids (ω6) (g/Day) | Omega-6/Omega-3 | EPA/DHA | |
| Barcelona (Metropolitan Area) n = 129 | 0.82 | 9.64 | 10.96 | 0.59 |
| (0.56–1.45) | (7.47–12.96) | (7.87–15.79) | (0.42–0.89) | |
| Canary Islands n = 93 | 0.71 | 8.77 | 11.80 | 0.46 |
| (0.53–1.15) | (6.77–11.60) | (8.36–15.85) | (0.23–1.55) | |
| Central n = 191 | 0.81 | 10.05 | 12.44 | 0.59 |
| (0.59–1.16) | (6.73–13.99) | (8.76–17.55) | (0.37–0.84) | |
| Levante (East) n = 335 | 0.87 | 10.71 | 11.78 | 0.55 |
| (0.55–1.33) | (6.87–14.79) | (8.52–15.88) | (0.32–0.74) | |
| Madrid (Metropolitan Area) n = 264 | 0.77 | 9.51 | 11.86 | 0.62 |
| (0.54–1.13) | (6.59–13.30) | (8.59–15.74) | (0.38–0.88) | |
| Northeast n = 240 | 0.74 | 10.57 | 12.98 | 0.59 |
| (0.56–1.10) | (7.65–13.93) | (9.95–17.51) | (0.33–0.87) | |
| Northwest n = 152 | 0.81 | 9.66 | 11.62 | 0.58 |
| (0.55–1.32) | (6.54–14.29) | (8.16–15.88) | (0.27–1.44) | |
| North Central n = 162 | 0.90 | 11.00 | 12.08 | 0.59 |
| (0.60–1.24) | (7.29–15.18) | (8.67–15.88) | (0.31–1.27) | |
| South n = 443 | 0.82 | 9.93 | 12.73 | 0.50 |
| (0.56–1.14) | (7.14–14.22) | (8.94–16.54) | (0.24–1.01) | |
| Nutritional Goals 1 | Non-Compliance with Nutritional Goals | Total | Men | Women | 9–12 Years | 13–17 Years | 18–64 Years | 65–75 Years | |
|---|---|---|---|---|---|---|---|---|---|
| n = 2009 | n = 1013 | n = 996 | n = 213 | n = 211 | n = 1655 | n = 206 | |||
| Omega-3 PUFA: | |||||||||
| Total Omega-3 PUFA (%) | 0.5–2% TE | <0.5% TE | 65.1 | 65.6 | 64.5 | 78.9 a | 78.2 a | 64.5 b | 54.9 c |
| >2% TE | 0.4 | 0.5 | 0.4 | 0.0 | 0.0 | 0.5 | 0.5 | ||
| Alfa-Linolenic acid (ALA) (%) | >0.5% TE | <0.5% TE | 87.0 | 87.8 | 86.2 | 95.3 a | 90.0 a | 87.0 b | 81.6 c |
| EPA + DHA (%) | 0.25–2 g/day | <0.25 g/day | 83.3 | 82.3 | 84.3 | 88.3 a,c | 90.5 b | 83.1 c | 83.0 c |
| >2 g/day | 1.0 | 1.3 | 0.8 | 0.0 | 0.5 | 1.1 | 1.0 | ||
| Omega-6 PUFA: | |||||||||
| Linoleic acid (LA) (%) | 2.5–9% TE | <2.5% TE | 4.5 | 4.0 | 5.0 | 2.3 a | 2.4 a | 4.0 a | 10.2 b |
| >9% TE | 5.9 | 5.8 | 5.9 | 5.2 | 6.2 | 6.0 | 5.3 |
| Nutritional Goals 1 | Non-Compliance with Nutritional Goals | Total Women of Childbearing Age (18–45 Years) | Younger Women (18–30 Years) | Older Women (31–45 Years) | |
|---|---|---|---|---|---|
| n = 552 | n = 209 | n = 343 | |||
| Omega-3 PUFA: | |||||
| Total Omega-3 PUFA (%) | 0.5–2% TE | <0.5% TE | 69.6 | 75.1 * | 66.2 |
| >2% TE | 0.2 | 0.0 | 0.3 | ||
| Alfa-Linolenic acid (ALA) (%) | >0.5% TE | <0.5% TE | 88.9 | 90.9 | 88.7 |
| EPA + DHA (%) | 0.25–2 g/day | <0.25 g/day | 83.5 | 90.0 | 84.5 |
| >2 g/day | 1.0 | 0.5 | 0.9 | ||
| Omega-6 PUFA: | |||||
| Linoleic acid (LA) (%) | 2.5–9% TE | <2.5% TE | 4.0 | 3.8 | 4.1 |
| >9% TE | 6.5 | 7.7 | 5.8 |
| Food Sources (g/Day) | Lipids | SFA | MUFA | PUFA |
|---|---|---|---|---|
| Oils and fats | 22.7 | 3.9 | 13.3 | 3.7 |
| (16.7–29.6) | (2.8–5.6) | (9.6–17.6) | (3.2–6.2) | |
| Meat and meat products | 16.5 | 5.8 | 7.1 | 2.1 |
| (9.8–25.4) | (3.3–9.0) | (4.1–10.9) | (1.3–3.3) | |
| Cereals and derivatives | 6.3 | 1.9 | 1.8 | 1.2 |
| (3.0–11.2) | (0.7–3.8) | (0.8–3.3) | (0.7–2.3) | |
| Fish and shellfish | 1.9 | 0.3 | 0.4 | 0.5 |
| (0.1–4.7) | (0.0–0.9) | (0.0–1.4) | (0.0–2.2) | |
| Eggs | 2.2 | 0.6 | 0.8 | 0.3 |
| (0.4–4.2) | (0.1–1.2) | (0.1–1.5) | (0.1–0.6) | |
| Milk and dairy products | 9.1 | 5.2 | 2.3 | 0.2 |
| (5.5–13.5) | (3.2–7.9) | (1.4–3.5) | (0.1–0.4) | |
| Vegetables | 0.3 | 0.0 | 0.0 | 0.1 |
| (0.1–0.5) | (0.0–0.1) | (0.0–0.0) | (0.1–0.2) | |
| Ready-to-eat meals | 0.7 | 0.1 | 0.2 | 0.1 |
| (0.0–5.7) | (0.0–1.9) | (0.0–2.2) | (0.0–0.9) | |
| Sauces and condiments | 0.8 | 0.1 | 0.2 | 0.0 |
| (0.0–3.7) | (0.0–0.5) | (0.0–2.1) | (0.3–0.8) | |
| Fruits | 0.1 | 0.0 | 0.0 | 0.0 |
| (0.0–0.7) | (0.0–0.1) | (0.0–0.2) | (0.0–0.2) | |
| Legumes | 0.0 | 0.0 | 0.0 | 0.0 |
| (0.0–0.4) | (0.0–0.0) | (0.0–0.0) | (0.0–0.2) | |
| Appetizers | 0.0 | 0.0 | 0.0 | 0.0 |
| (0.0–0.9) | (0.0–0.1) | (0.0–0.6) | (0.0–0.1) | |
| Sugars and sweets | 0.2 | 0.1 | 0.1 | 0.0 |
| (0.0–1.4) | (0.0–0.7) | (0.0–0.4) | (0.0–0.1) | |
| Supplements and meal replacement | 0.0 | 0.0 | 0.0 | 0.0 |
| (0.0–0.0) | (0.0–0.0) | (0.0–0.0) | (0.0–0.0) | |
| Non-alcoholic beverages | 0.0 | 0.0 | 0.0 | 0.0 |
| (0.0–0.1) | (0.0–0.0) | (0.0–0.0) | (0.0–0.0) | |
| Alcoholic beverages | 0.0 | 0.0 | 0.0 | 0.0 |
| (0.0–0.0) | (0.0–0.0) | (0.0–0.0) | (0.0–0.0) |
| Food Sources (g/Day) | Omega-3 Fatty Acids (ω3) | Alfa-linolenic Acid (ALA) (C18: 3n-3) | Eicosapentaenoic Acid (EPA) (C20: 5n-3) | Docosahexaenoic Acid (DHA) (C22: 6n-3) |
|---|---|---|---|---|
| Oils and fats | 0.09 | 0.09 | 0.00 | 0.00 |
| (0.06–0.14) | (0.06–0.14) | (0.00–0.00) | (0.00–0.00) | |
| Meat and meat products | 0.15 | 0.14 | 0.00 | 0.00 |
| (0.08–0.23) | (0.08–0.23) | (0.00–0.00) | (0.00–0.00) | |
| Cereals and derivatives | 0.07 | 0.03 | 0.00 | 0.00 |
| (0.07–0.17) | (0.07–0.17) | (0.00–0.00) | (0.00–0.00) | |
| Fish and shellfish | 0.15 | 0.03 | 0.03 | 0.05 |
| (0.01–0.41) | (0.00–0.12) | (0.00–0.10) | (0.00–0.20) | |
| Eggs | 0.03 | 0.03 | 0.00 | 0.00 |
| (0.00–0.06) | (0.00–0.06) | (0.00–0.00) | (0.00–0.00) | |
| Milk and dairy products | 0.04 | 0.04 | 0.00 | 0.00 |
| (0.02–0.07) | (0.02–0.06) | (0.00–0.00) | (0.00–0.00) | |
| Vegetables | 0.01 | 0.01 | 0.00 | 0.00 |
| (0.00–0.03) | (0.00–0.03) | (0.00–0.00) | (0.00–0.00) | |
| Ready-to-eat meals | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.03) | (0.00–0.02) | (0.00–0.00) | (0.00–0.00) | |
| Sauces and condiments | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.02) | (0.00–0.02) | (0.00–0.00) | (0.00–0.00) | |
| Fruits | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.02) | (0.00–0.02) | (0.00–0.00) | (0.00–0.00) | |
| Legumes | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Appetizers | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Sugars and sweets | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Supplements and meal replacement | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Non-alcoholic beverages | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Alcoholic beverages | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | (0.00–0.00) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Meat and meat products | 0.16 a | 0.18 a | 0.15 b | 0.12 c |
| (0.11–0.24) | (0.12–0.26) | (0.08–0.23) | (0.06–0.19) | |
| Fish and shellfish | 0.11 a | 0.11 a | 0.15 b | 0.17 b |
| (0.00–0.31) | (0.00–0.33) | (0.01–0.41) | (0.03–0.42) | |
| Oils and fats | 0.09 a | 0.08 a | 0.09 a | 0.11 b |
| (0.06–0.13) | (0.05–0.12) | (0.06–0.14) | (0.07–0.16) | |
| Cereals and derivatives | 0.07 a | 0.09 a | 0.06 b | 0.05 b |
| (0.07–0.16) | (0.04–0.20) | (0.03–0.18) | (0.02–0.14) | |
| Milk and dairy products | 0.06 a | 0.05 a | 0.04 b | 0.03 c |
| (0.03–0.08) | (0.02–0.07) | (0.01–0.06) | (0.01–0.05) | |
| Eggs | 0.03 | 0.03 | 0.03 | 0.03 |
| (0.00–0.06) | (0.00–0.05) | (0.00–0.05) | (0.01–0.06) | |
| Vegetables | 0.01 a | 0.01 a | 0.01 a | 0.02 b |
| (0.00–0.02) | (0.00–0.02) | (0.00–0.03) | (0.00–0.04) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Meat and meat products | 0.15 a,b | 0.18 b | 0.14 a | 0.11 c |
| (0.11–0.23) | (0.12–0.25) | (0.08–0.23) | (0.06–0.19) | |
| Oils and fats | 0.09 a | 0.08 a | 0.09 a | 0.11 b |
| (0.06–0.13) | (0.05–0.13) | (0.06–0.14) | (0.07–0.15) | |
| Cereals and derivatives | 0.08 a,b | 0.10 b | 0.07 a | 0.05 c |
| (0.05–0.16) | (0.04– 0.20) | (0.03–0.18) | (0.02–0.13) | |
| Milk and dairy products | 0.05 a | 0.05 a | 0.04 b | 0.03 c |
| (0.03–0.08) | (0.02–0.07) | (0.02–0.06) | (0.01–0.05) | |
| Fish and shellfish | 0.01 | 0.01 | 0.03 | 0.03 |
| (0.00–0.09) | (0.00–0.10) | (0.00–0.12) | (0.00–0.10) | |
| Eggs | 0.03 | 0.03 | 0.03 | 0.03 |
| (0.00–0.06) | (0.00–0.05) | (0.00–0.06) | (0.01–0.06) | |
| Vegetables | 0.01 a | 0.01 a | 0.01 b | 0.02 b |
| (0.00–0.02) | (0.00–0.03) | (0.00–0.03) | (0.00–0.04) | |
| Ready-to-eat meals | 0.00 a,b | 0.01 a | 0.00 b | 0.00 b |
| (0.00–0.03) | (0.00–0.04) | (0.00–0.02) | (0.00–0.02) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Fish and shellfish | 0.011 a | 0.002 a | 0.023 b | 0.033 b |
| (0.000–0.070) | (0.000–0.080) | (0.000–0.106) | (0.000–0.112) | |
| Milk and dairy products | 0.002 a | 0.002 a,b | 0.001 b | 0.001 b |
| (0.001–0.003) | (0.001–0.003) | (0.001–0.002) | (0.000–0.002) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Fish and shellfish | 0.04 a | 0.03 a | 0.05 a,b | 0.07 b |
| (0.00–0.15) | (0.00–0.06) | (0.000–0.20) | (0.02–0.22) |
| Food Sources (g/Day) | Omega-6 Fatty Acids (ω6) | Linoleic Acid (LA) (C18: 2n-6) | Arachidonic Acid (AA) (C20: 4n-6) |
|---|---|---|---|
| Oils and fats | 3.32 | 3.32 | 0.00 |
| (1.87–5.83) | (1.87–5.82) | (0.00–0.00) | |
| Meat and meat products | 1.63 | 1.58 | 0.05 |
| (0.92–2.60) | (0.89–2.51) | (0.02–0.09) | |
| Cereals and derivatives | 0.99 | 0.99 | 0.00 |
| (0.62–2.03) | (0.61–2.01) | (0.00–0.00) | |
| Fish and shellfish | 0.07 | 0.03 | 0.00 |
| (0.00–1.43) | (0.00–1.41) | (0.00–0.02) | |
| Eggs | 0.27 | 0.27 | 0.00 |
| (0.05–0.54) | (0.05–0.54) | (0.00–0.00) | |
| Milk and dairy products | 0.18 | 0.17 | 0.00 |
| (0.10–0.28) | (0.10–0.26) | (0.00–0.00) | |
| Vegetables | 0.04 | 0.04 | 0.00 |
| (0.00–0.07) | (0.01–0.07) | (0.00–0.00) | |
| Ready-to-eat meals | 0.09 | 0.09 | 0.00 |
| (0.00–0.77) | (0.00–0.77) | (0.00–0.00) | |
| Sauces and condiments | 0.30 | 0.30 | 0.00 |
| (0.00–0.76) | (0.00–0.76) | (0.00–0.00) | |
| Fruits | 0.00 | 0.00 | 0.00 |
| (0.00–0.01) | (0.00–0.02) | (0.00–0.00) | |
| Legumes | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Appetizers | 0.00 | 0.00 | 0.00 |
| (0.00–0.08) | (0.00–0.08) | (0.00–0.00) | |
| Sugars and sweets | 0.00 | 0.00 | 0.00 |
| (0.00–0.07) | (0.00–0.06) | (0.00–0.00) | |
| Supplements and meal replacement | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Non-alcoholic beverages | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) | |
| Alcoholic beverages | 0.00 | 0.00 | 0.00 |
| (0.00–0.00) | (0.00–0.00) | (0.00–0.00) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Oils and fats | 4.02 a | 3.45 a,b | 3.39 a,b | 2.92 b |
| (2.16–6.39) | (1.86–5.87) | (1.89–5.83) | (1.68–4.92) | |
| Meat and meat products | 1.77 a | 2.01 b | 1.63 a | 1.29 c |
| (1.22–2.64) | (1.44–2.87) | (0.92–2.65) | (0.71–2.16) | |
| Cereals and derivates | 1.23 a | 1.37 a | 0.99 b | 0.77 b |
| (0.83–2.50) | (0.76–2.99) | (0.62–2.02) | (0.51–1.69) | |
| Ready-to-eat meals | 0.20 a | 0.17 a | 0.08 b | 0.06 b |
| (0.00–1.03) | (0.00–0.26) | (0.00–0.73) | (0.00–0.39) | |
| Milk and dairy products | 0.24 a | 0.21 a | 0.18 b | 0.16 b |
| (0.16–0.36) | (0.12–0.33) | (0.10–0.28) | (0.08–0.24) | |
| Sauces and condiments | 0.37 a | 0.42 a | 0.37 a | 0.00 b |
| (0.00–0.77) | (0.00–0.76) | (0.00–0.66) | (0.00–0.49) | |
| Eggs | 0.30 a | 0.27 a,b | 0.28 b | 0.32 b |
| (0.06–0.56) | (0.04–0.51) | (0.04–0.54) | (0.10–0.56) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Oils and fats | 4.01 a | 3.45 a,b | 3.39 a,b | 2.91 b |
| (2.16–6.39) | (1.86–5.87) | (1.89–5.83) | (1.68–4.91) | |
| Meat and meat products | 1.74 a,b | 1.97 a | 1.59 b | 1.23 c |
| (1.18–2.56) | (1.38–2.78) | (0.89–2.56) | (0.69–2.06) | |
| Cereals and derivates | 1.23 a | 1.37 a | 0.99 b | 0.77 c |
| (0.83–2.50) | (0.76–2.99) | (0.62–2.02) | (0.51–1.69) | |
| Ready-to-eat meals | 0.20 a | 0.16 a | 0.08 b | 0.06 b |
| (0.00–1.03) | (0.00–1.26) | (0.00–0.73) | (0.00–0.39) | |
| Sauces and condiments | 0.37 a | 0.42 a | 0.37 a | 0.00 b |
| (0.00–0.77) | (0.00–0.76) | (0.00–0.76) | (0.00–0.49) | |
| Eggs | 0.30 | 0.27 | 0.28 | 0.32 |
| (0.06–0.58) | (0.04–0.54) | (0.04–0.54) | (0.10–0.56) | |
| Milk and dairy products | 0.23 a | 0.20 a | 0.17 b | 0.15 b |
| (0.15–0.34) | (0.12–0.31) | (0.10–0.26) | (0.08–0.23) |
| Food Sources (g/Day) | Children | Adolescents | Adults | Elderly |
|---|---|---|---|---|
| Meat and meat products | 0.053 a,b | 0.067 a | 0.050 b | 0.044 c |
| (0.034–0.082) | (0.034–0.093) | (0.024–0.087) | (0.016–0.077) | |
| Milk and dairy products | 0.006 a | 0.005 a,b | 0.004 b | 0.004 b |
| (0.003–0.009) | (0.003–0.009) | (0.002–0.007) | (0.002–0.007) | |
| Fish and shellfish | 0.001 | 0.001 | 0.001 | 0.002 |
| (0.000–0.019) | (0.000– 0.014) | (0.000–0.015) | (0.000–0.025) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redruello-Requejo, M.; Samaniego-Vaesken, M.d.L.; Puga, A.M.; Montero-Bravo, A.; Ruperto, M.; Rodríguez-Alonso, P.; Partearroyo, T.; Varela-Moreiras, G. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2023, 15, 562. https://doi.org/10.3390/nu15030562
Redruello-Requejo M, Samaniego-Vaesken MdL, Puga AM, Montero-Bravo A, Ruperto M, Rodríguez-Alonso P, Partearroyo T, Varela-Moreiras G. Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients. 2023; 15(3):562. https://doi.org/10.3390/nu15030562
Chicago/Turabian StyleRedruello-Requejo, Marina, María de Lourdes Samaniego-Vaesken, Ana M. Puga, Ana Montero-Bravo, Mar Ruperto, Paula Rodríguez-Alonso, Teresa Partearroyo, and Gregorio Varela-Moreiras. 2023. "Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study" Nutrients 15, no. 3: 562. https://doi.org/10.3390/nu15030562
APA StyleRedruello-Requejo, M., Samaniego-Vaesken, M. d. L., Puga, A. M., Montero-Bravo, A., Ruperto, M., Rodríguez-Alonso, P., Partearroyo, T., & Varela-Moreiras, G. (2023). Omega-3 and Omega-6 Polyunsaturated Fatty Acid Intakes, Determinants and Dietary Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients, 15(3), 562. https://doi.org/10.3390/nu15030562









