1. Introduction
Parkinson’s disease (PD) is the second most frequent neurodegenerative disorder that predominantly influences dopamine (DA) production areas of substantia nigra (SA) [
1]. Most cases of PD occur sporadically, but approximately 10–15% of patients have family histories of PD [
1]. The main features of PD are four motor symptoms, including resting tremors, bradykinesia, stiffness, and postural instability [
1]. While current therapies are intended to relieve symptoms and slow the progression of PD, the harmful motor complications of current PD drugs are still possible [
2]. Several ongoing studies are aimed at finding effective treatments with fewer side effects.
Numerous factors participate in the pathologic process of degeneration of DA neurons; most of this is oxidative stress, resulting from a disruption of the equilibrium between reactive oxygen species (ROS) generation and the antioxidant defense of the cell [
3]. The human antioxidant defense system is mainly composed primarily of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) [
4]. Reduced activity of several antioxidant enzymes was observed in SA of the Parkinsonian brain [
3]. Mitochondria are known to produce approximately 90% ROS within cells [
5]. Accumulation of ROS may activate several stress-sensitive serine kinase cascades, including c-Jun-N-terminal kinase (JNK), which is one of the mitogen-activated protein kinase (MAPK) [
6]. The mitochondrial transmembrane potential (ΔΨM) is subsequently destroyed upon activation of JNK, causing cytochrome c release to activate the downstream caspases, which eventually leads to apoptosis involving the progression of PD [
7]. It may be reasonably inferred to lessen the complex factors behind neurodegeneration, such as mitochondrial ROS/JNK/caspase signaling dysfunction, as discussed above, could be a therapeutic strategy for PD [
8].
Hispidin, 6-(3,4-dihydroxystyryl)-4-hydroxy-2-pyrone, is one of the phenolic substances widely distributed in edible and medicinal mushrooms of the Phellinus and Inonotus genera [
9].
Inonotus and
Phellinus are two genera in the Hymenochaetaceae that have been commonly used to treat a variety of disorders, such as cancers, heart, and hepatic disorders, diabetes, etc., in traditional medicine [
10]. Hispidin is a promising bioactive compound due to its antioxidant potency and possesses multiple biological effects, including anti-inflammatory and antitumor actions [
11,
12,
13]. Hispidin has anti-diabetic properties because of its powerful inhibitory activity of protein glycation [
14]. In fact, hispidin has significant neuroprotective properties. Hispidin has been documented to inhibit the generation of hydroxyl radicals against peroxynitrite-induced DNA lesions in primary rat astrocytes, the most abundant cells in the central nervous system [
15]. Hispidin inhibits ß-site amyloid precursor protein cleaving enzyme 1 to reduce the accumulation of ß-amyloid peptide [
16]. In addition, hispidin has been shown to inhibit nitric oxide generation caused by lipopolysaccharide in BV-2 cells that depend on MAPK signaling linked to ROS. [
17]. Although the above results provide additional guidance on the possibility that hispidin may serve as a targeted treatment to improve neuroinflammation or neural degeneration caused by oxidative stress, the protection of hispidin from PD is not fully apparent, and possible mechanisms need to be strongly clarified.
Cellular models generally develop pathology faster, more cost-effectively, and do not require ethical approval in comparison with animal models [
18]. Cellular models are therefore ideal for large-scale drug detection that may help focus on potential drug targets for later validation in animal models [
18]. A variety of neurotoxicity and genetic experimental models have been developed for PD studies [
18]. Neurotoxin-based models led to the rapid degeneration of nigrostriatal dopaminergic neurons, which mimic sporadic PD [
19]. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxin is known to be transformed into an active metabolite 1-methyl-4-phenylpyridinium (MPP
+) to damage the catecholaminergic neurons of the brain, resulting in PD-like motor symptoms [
20]. As a result, MPTP is currently the most useful and practical model for PD studies [
20]. MES23.5 cells have a number of characteristics similar to those of SN-derived primary neurons [
21]. Therefore, MPP
+ was used to induce a PD-like model in MES23.5 cells to provide an obvious direct correlation of hispidin on degenerated dopamine neurons with the mechanism of action.
2. Materials and Methods
2.1. Cell Culture and Treatment
MS23.5 cells acquired from the American Type Culture Collection (Manassas, VA, USA) were grown in DMEM/F12 (Sigma-Aldrich, St. Louis, MO, USA) with Sato’s components with 10% (v/v) fetal bovine serum, including 100 U/mL penicillin and 100 mg/mL streptomycin. Cells were grown at 37 °C in a humid environment with 5% CO2 and 95% air. In the experiments, the cells were seeded into plates of 6 wells at a density of 1 × 105 cells per well, and cultures were separated into 0.05% (w/v) trypsin in a saline phosphate buffer (PBS) at a pH of 7.4 at the confluence.
For the establishment of the PD model, MES23.5 cells were treated with MPP
+ (Sigma-Aldrich, St. Louis, MO, USA; Cat. # M0896) at a concentration of 0.5–2.5 mmol/L at 37 °C over a 24-h period. In the pretreatment studies, cells were incubated for 1 h with either hispidin at the indicated concentrations (5, 10, 20, and 40 µmol/L) or 1 µmol/L JNK-IN-8 (Sigma Chemical Co., St. Louis, MO, USA; Cat. # SML1246) and 25 µmol/L SR3576 (Sigma Chemical Co., St. Louis, MO, USA; Cat. # S8201), after exposure to 2 mmol/L of MPP
+ for 24 h. Untreated MES23.5 cells cultured during 24 h at 37 °C in normal conditions were used as controls. The stock solution of the experimental compounds at 1 mmol/L was prepared by dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA; Cat. # D8418) as a solvent, which was diluted in a culture medium at concentrations adequate for the following study. The final DMSO concentration was less than 0.1% (
v/
v), resulting in no toxicity to most cells [
22].
2.2. Cell Viability Assay
Cell viability was determined based on the Cell Counting Kit-8 (CCK-8) test (Cat. 96992) following the manufacturer’s protocol (Sigma Chemical Co., St. Louis, MO, USA). The cells were inoculated in 96-well plates with a density of 2 × 104 cells/mL. After treatment, 10 μL of the CCK-8 reagent was added to each well, and the absorbance value of each well at 450 nm was measured with a multifunction microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA) after incubation for 2 h at 37 °C. Cells treated with the vehicle were considered controls, and cell viability was defined to be 100%. The viability of the treatment group was expressed in percent of the control group.
2.3. Determination of DA
The DA levels were detected through high-performance liquid chromatography (HPLC) using a fluorescence detector (Waters 2475, Milford, MA, USA). Briefly, cells were taken after treatment and then were sonicated in 0.2 mol/L perchloric acid with isoproterenol, and homogenates obtained were centrifuged at 20,000× g for 15 min under 4 °C. The supernatant was then collected and filtered through a 0.22 μm filter, and 25 μL of the sample was injected into the column. An Atlantis T3 column (150 mm × 4.6 mm, 5 μm, Waters) was employed in the separation system (Waters 2695). The mobile phase has a flow rate of 0.6 mL/min, composed of acetonitrile and water (90:10% v/v) and 75 mmol/L pH 3.0 phosphate buffer containing octane sulfonic acid 1.8 mmol/L, EDTA 30 µmol/L, and triethylamine 0.015% (v/v). The standard solution was prepared fresh by dilution of the mobile phase stock solution. Serial concentrations of the standard solution determined the linearity ranges of DA before detection. Results were reported in ng/106 cells.
2.4. Cellular ROS Assay
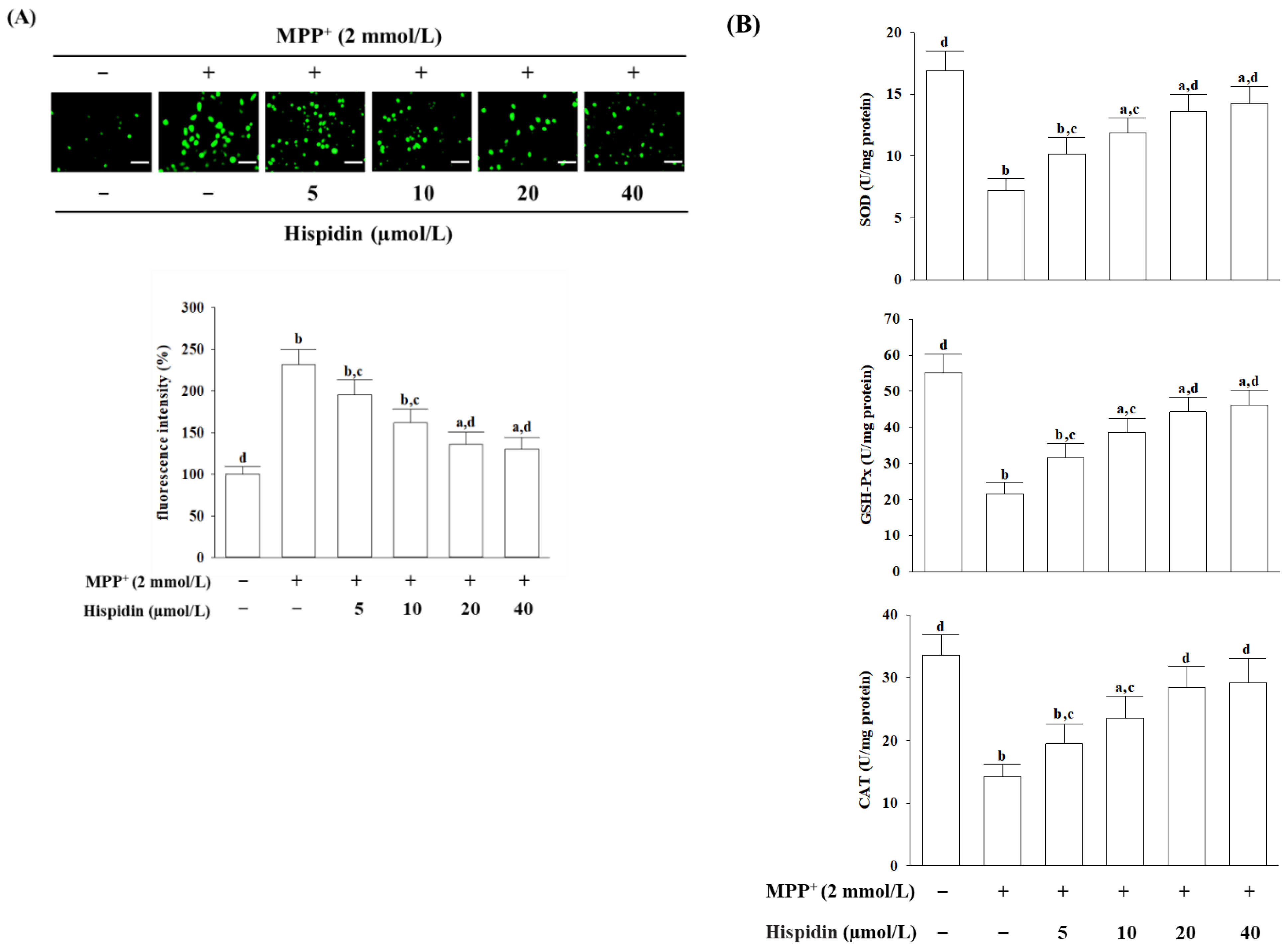
The ROS level of the cell was measured with dichloro-dihydro-fluorescein diacetate (DCFH-DA), as instructed by the manufacturer (Sigma-Aldrich, St. Louis, MO, USA) [
23]. In summary, DCFH-DA was diluted with DMEM at a final concentration of 10 μmol/L and then added to the cells for 20 min incubating at 37 °C. The cells were washed 3 times with PBS after treatment with DCFH-DA. A phase contrast fluorescence microscope (Nikon, model 80i) was used to capture the images. The DCF fluorescence was read from a multifunctional microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA) with 488 nm excitation and 525 nm emission wavelengths. The increase in ROS levels was expressed as a percentage of control.
2.5. Antioxidant Enzyme Activity Assay
The following commercial kits, based on an enzyme-linked immunosorbent assay (ELISA), were used to estimate antioxidant activity. The Activity Colorimetric Assay Kits of SOD (Cat. # K335), GSH-Px (Cat. # K762), and CAT (Cat. # K773) were purchased from Bio Vision, Inc. (San Francisco, CA, USA). The activities of SOD, GSH-Px, and CAT were calculated to measure the absorbance recorded at 450 nm, 340 nm, and 570 nm, respectively, by a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA). Enzymatic activities were normalized from the respective protein concentration of each group, expressed in units per milligram of protein. The protein level was quantified using a Bradford protein assay.
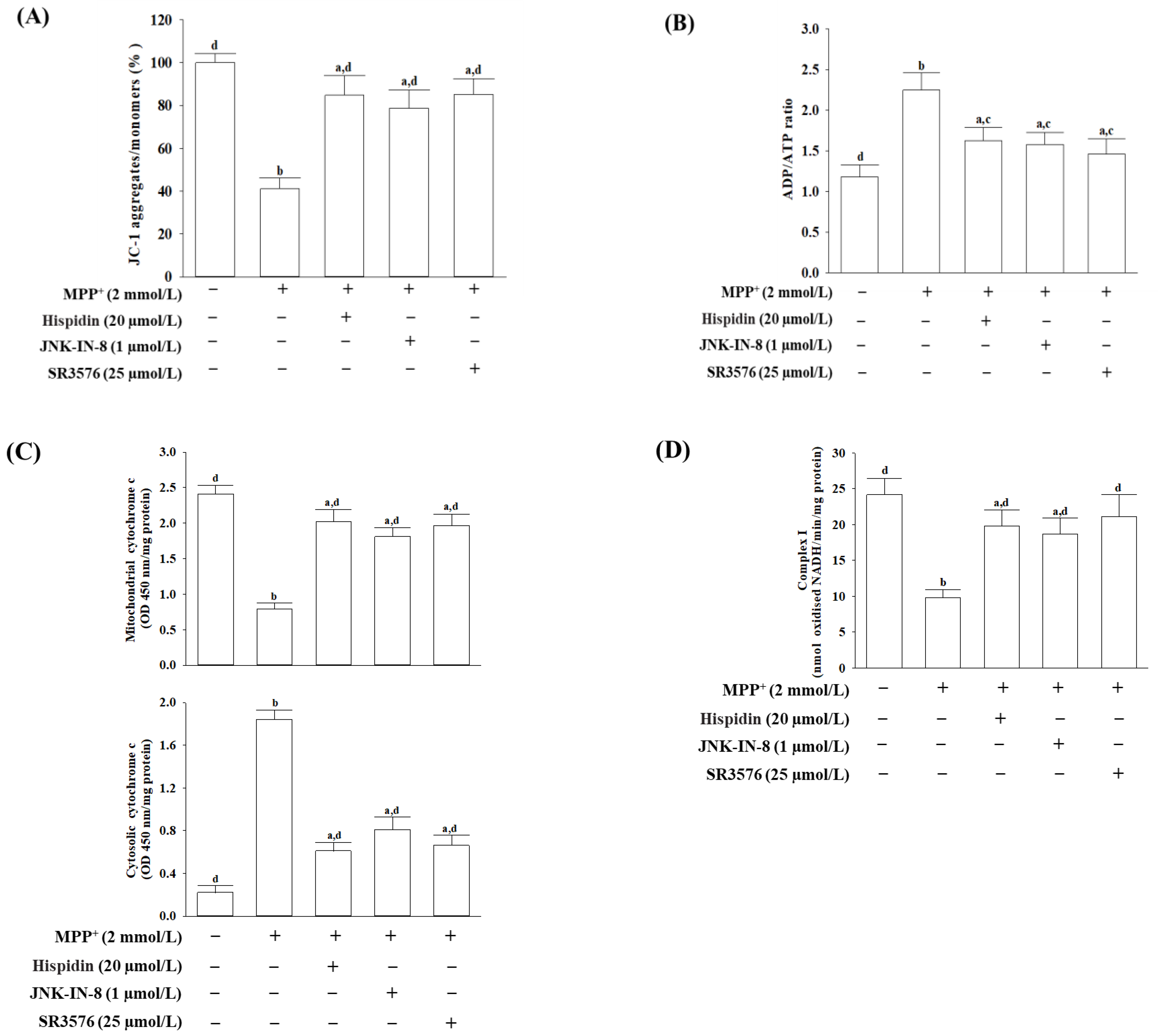
2.6. Mitochondrial Transmembrane Potential (ΔΨM) Assay
The ΔΨM was measured using an assay kit containing 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanin iodine (JC-1) dye (Abcam plc., Cambridge, MA, USA). Cells were seeded into a 96-well plate at a density of 1 × 10
4 cells/well, which was incubated with 20 µmol/L JC-1 at 37 °C for 30 min. The cells were then centrifuged at 2500 rpm for 5 min, and the pellets were resuspended in 0.5 mL PBS. The red aggregates emit at 590 nm, and the green-fluorescent monomer with a 530 nm emission was determined by a fluorescence spectrophotometer (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA). The ratio of J-aggregates to J-monomers was computed to determine the changes in the ΔΨM [
24]. The ratio of fluorescence intensity of J aggregates to the fluorescence intensity of monomers was used as an indicator to determine changes in ΔΨM.
2.7. Measurement of ADP and ATP Levels
The commercially available assay kit (Cat. # ELDT-100) from BioAssay Systems (Hayward, CA, USA) was used to determine the ability of luciferase to produce light in the presence of its luciferal substrate [
25]. After treatment, cells were lysed with 10% tricholoroacetic acid, then neutralized with 1 mol/L of KOH, and diluted with 100 mmol/L HEPES buffer (pH 7.4). The first step in the test was the luciferase-catalyzed response of cellular ATP and D-luciferin to produce a luminescent signal. Later, ADP was converted into ATP by enzyme reaction, while the newly formed ATP responded with D-luciferin. The second light intensity accounted for the total amount of ADP and ATP. The ratio of ADP/ATP was normalized based on the total protein content of the samples.
2.8. Measurement of Cytochrome C Release
The cells were homogenized following treatment. The lysate spun two times at 800× g for 20 min. The mitochondrial granule was produced from the supernatant after centrifugation at 10,000× g over a period of 15 min. The remaining supernatant was centrifuged at 16,000× g within 25 min for a cytosolic fraction. The cytochrome C ELISA kit was used to measure the cytochrome C level in mitochondria and cytosolic fraction as indicated by the manufacturer. Cytochrome c is immune-captured inside the wells, determined by the addition of a specific cytochrome c antibody conjugated to horseradish peroxidase. This peroxidase changes the substrate from colorless to blue, which was measured at 450 nm. The protein content was measured with Bio-Rad protein analysis.
2.9. Analyses of Mitochondrial Complex I Activity
The commercial ELISA kit (Abcam plc., Cambridge, MA, USA; Cat. AB109721) was used to detect the activity of mitochondrial complex 1. The protein concentration was adjusted to 1 mg/mL in an incubation solution following cell lysis. The 200 μL diluted sample and the control were charged into the wells with a microplate pre-coated with complex I capture antibodies for 3 h at room temperature. Complex I activity was determined by measuring the oxidation of NADH to NAD+ and the simultaneous reduction of a dye, resulting in enhanced absorption at 450 nm. The activity of complex I was expressed as nmol oxidized NADH/min/mg protein. Protein contents were measured with a Bio-Rad protein assay.
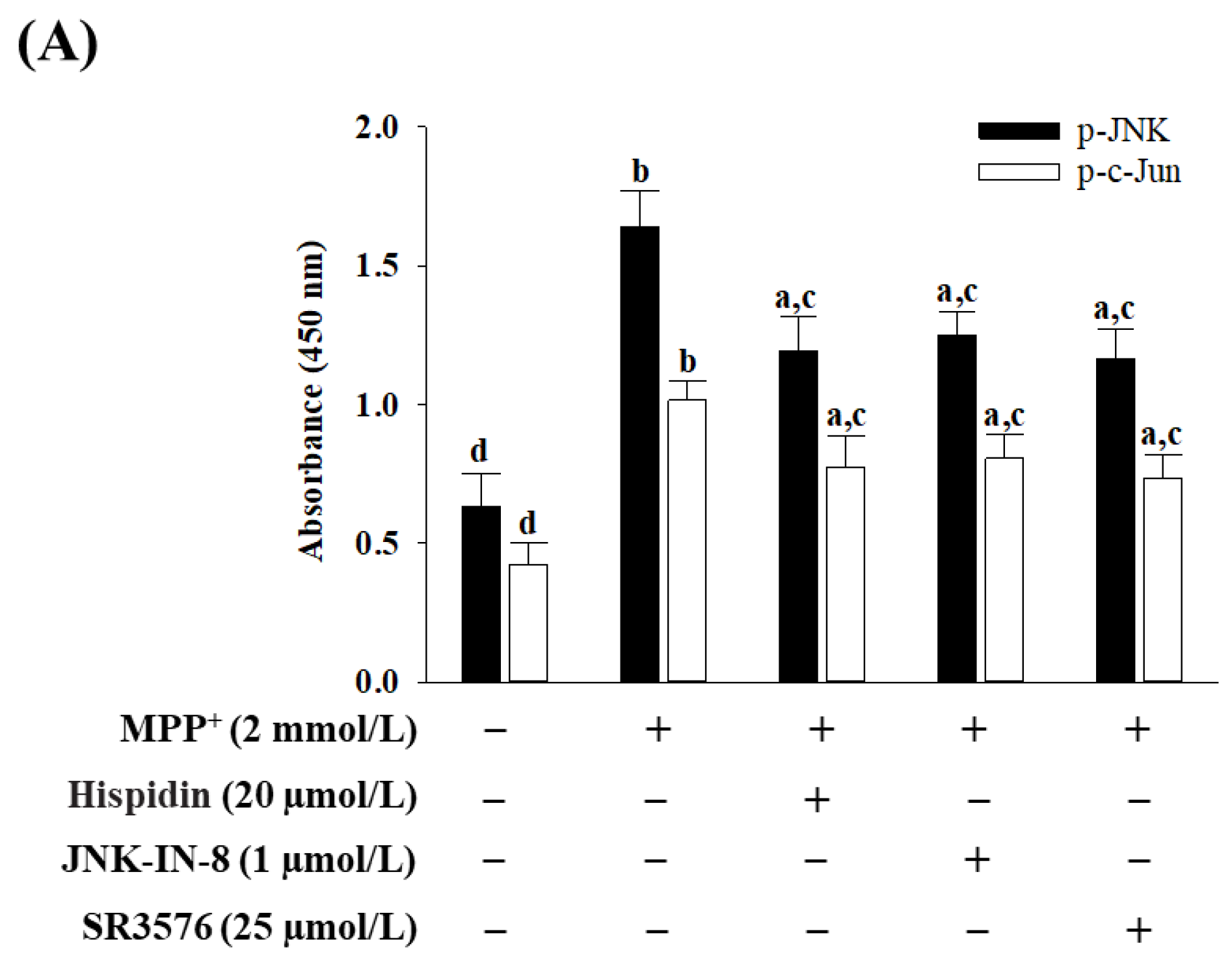
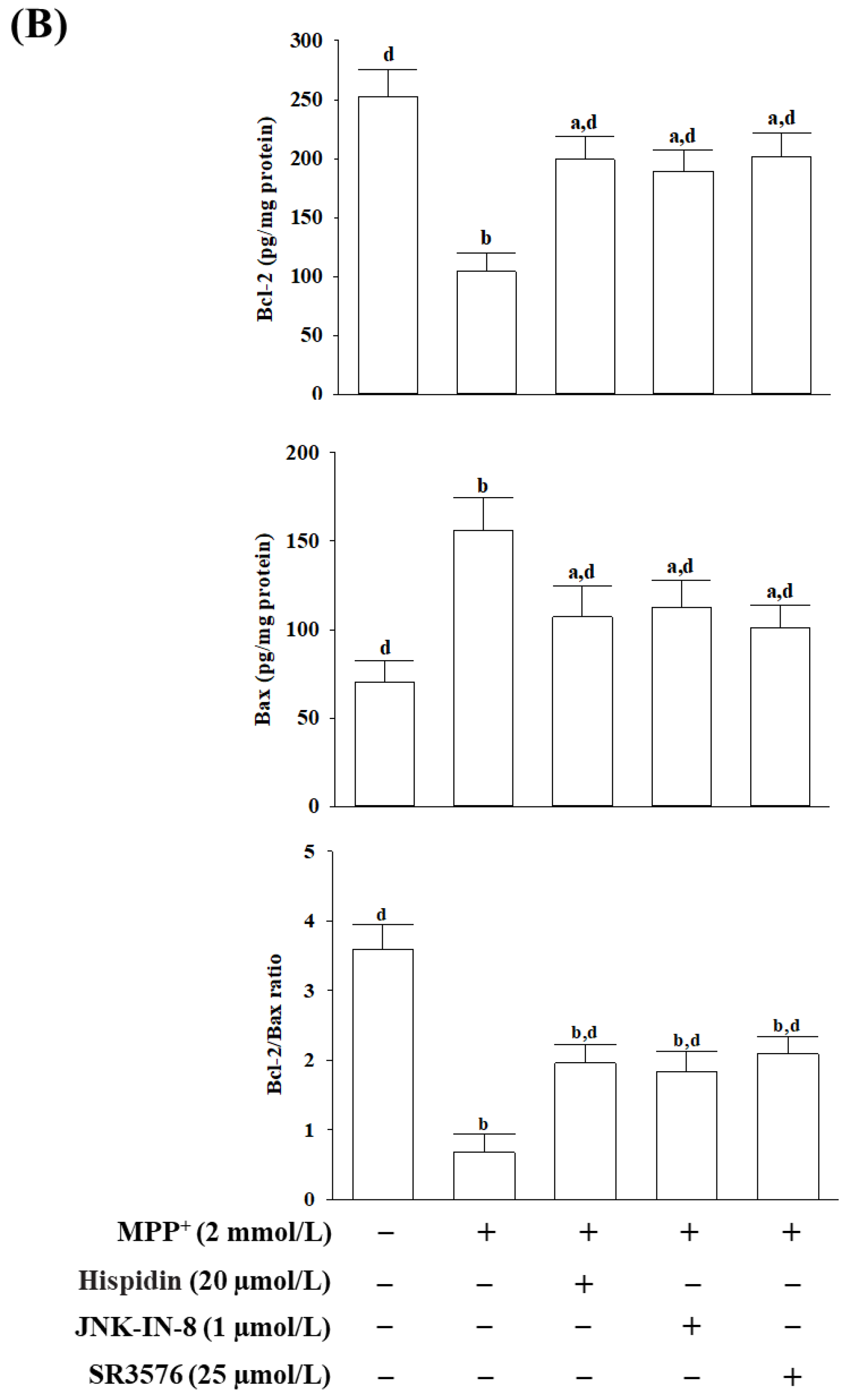
2.10. Analysis of the Levels of Activated JNK and c-Jun, and the Expressions of Bax and Bcl-2
The phosphorylation status of JNK and c-Jun was determined using the Phospho-JNK1/2/3 (Thr183 + Tyr185) Colorimetric Cell-Based ELISA Kit (Cat. # CBCAB00421) and c-Jun (Phospho-Ser63) Colorimetric Cell-Based ELISA kit (Cat. # CBCAB00581), respectively. The protein amounts of Bcl-2 (Cat. # CBCAB00158) and Bax (Cat. # CBCAB00157) were also measured with commercial ELISA kits. The listed ELISA kits were purchased from Assay Genie (Windsor Place, Dublin, Ireland) and performed as per the manufacturer’s protocols. The commercially available kit is based on primary target-specific antibodies and was detected by a secondary antibody conjugated with horseradish peroxidase. The optical density (OD) was determined on the target absorbance values at a wavelength of 450 nm with the aid of a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA).
2.11. Measurement for Activities of Caspases and Poly (ADP-Ribose) Polymerase (PARP)
The activities of caspase-9 and caspase-3 were determined using the caspase colorimetric test kits based on spectrophotometric detection of the chromophore p-nitroanilide (p-NA) as a result of the cleavage of the labeled substrate acetyl-Leu-Glu-His-Asp-pNA and Asp-Glu-Val-Asp-pNA, respectively. The assay kits for caspases-9 (Cat. # ab65608) and caspase-3 (Cat. # ab39401) were purchased from Abcam plc. (Cambridge, MA, USA). Free pNA was identified with a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA) at 405 nm.
The PARP/Apoptosis colorimetric assay kit (R&D Systems, Minneapolis, MN, USA; Cat. # 4684-096-K) was used for the determination of PARP activity based on a semi-quantitative measurement of the amount of poly (ADP-ribose) deposited on the immobilized histone proteins. The absorbance values at 450 nm were obtained from a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA). All values were compared to the controls treated by the vehicle.
2.12. Study of Apoptotic DNA Fragmentation
DNA fragments associated with cytoplasmic histones resulting from induced cell death were quantitatively detected through the cell death detection ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany; Cat. #11774425001). Cytoplasmic cell extracts were treated with a primary mouse anti-histone monoclonal antibody coated with a microtiter plate and then a second mouse anti-DNA monoclonal antibody coupled with peroxidase. The quantity of peroxidase retained in the immune complex was determined by photometric analysis by incubation with 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate] as a substrate for 10 min at 20 °C. The color shift was measured at 405 nm with a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA).
2.13. Statistical Analysis
Data are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using Systat SigmaPlot version 14.0. (Systat Software Inc., San Jose, CA, USA). Three wells were tested in each experiment, and each experiment was performed a minimum of five times. Where no special description is available, significant differences from the vehicle controls were evaluated using one-way ANOVA analysis followed by Dunnett’s test as a post-hoc test. The differences were statistically significant at p < 0.05.
4. Discussion
The precise etiology of the degeneration and death of dopaminergic neurons in PD remains uncertain; the exacerbation of oxidative stress is considered a major contributor to the damage of dopaminergic neurons in the pathogenesis of PD [
1]. When the toxic metabolite of MPTP, MPP
+, continues to accumulate in the synaptosomal vesicles of dopaminergic neurons, it causes mitochondrial dysfunction, oxidative stress, and programmed cell death that simulates parkinsonian syndrome in cellular and animal models [
20]. Cell viability was first assessed to determine if hispidin saves cells from MPP
+-induced neurotoxicity was associated with combating oxidative stress. Our results demonstrated that hispidin acts as a cytoprotective on MPP
+-exposed MES23.5 cells as a result of reduced ROS production and improved cell viability. This is consistent with previous evidence that hispidin was protective for cells exposed to hydrogen-peroxide-induced oxidative stress [
26].
Even though ROS can cause oxidative damage to biomolecules at tightly regulated levels, they are needed to sustain redox cell homeostasis and are involved in adaptive signaling to overcome various stresses in a way that supports health [
27]. If the antioxidant system is unable to maintain ROS under control, high levels of ROS may cause oxidative stress leading to the activation of malignant signaling or cell death [
27]. Although antioxidant defense, including SOD, CAT, and GSH-Px, can scavenge ROS and reduce the oxidation of cellular molecules, thus against the deleterious effects of various oxidative stress, these antioxidants appear insufficient if higher levels of ROS activate cell death processes [
4]. MPP
+-induced ROS accumulation in MES23.5 cells was accompanied by a clear decline in SOD, CAT, and GSH-Px activity, providing that MPP
+-inducing oxidative stress is associated with a disruption of the initial antioxidant defense systems [
20]. The present study indicated that pretreatment with hispidin abolished the MPP
+-induced inhibition of SOD, CAT, and GSH-Px activities in MES23.5 cells. These results suggest that the protective effect of hispidin against MPP
+-induced neurotoxicity may be mediated by the enhancement of the first-line antioxidant defense of scavenging different radicals, thereby attenuating oxidative damage. Because DA is easily oxidized, the oxidative destruction in dopaminergic neurons leading to lower cellular DA content is central to PD development; antioxidants can, of course, protect neurons against oxidative damage to destroy dopaminergic neurons in PD [
28]. In this study, DA levels decreased after MPP
+ stimulation, but preconditioning hispidin significantly increased DA levels in MPP
+-stimulated MES23.5 cells. This could be due to the antioxidant effect of hispidin, which protects DA against oxidation as well as maintains cellular function.
MPP
+ exerts its neurotoxicity mainly by inhibiting the activity of mitochondrial respiratory chain complexes I [
29]. In the current study, we observed that hispidin could restore mitochondrial respiratory chain complexes I activity, suggesting that the restoration of mitochondrial respiratory chain complexes I activity might contribute to the neuroprotective effects of hispidin. In order to verify this hypothesis, further investigations on hispidin blocking the concentration of MPP
+ into mitochondria or competitively combined to hydrophobic binding site on NADH dehydrogenase need to be further clarified.
Once MPP
+ damages mitochondrial respiratory enzymes, mitochondrial respiration becomes dysfunctional, and the potential of the mitochondrial membrane decreases, leading to a reduction in ATP production and increasing mitochondrial permeability [
29]. It is well known that the Bcl-2 family of proteins plays an important role in intracellular apoptotic signal transduction by regulating the permeability of the mitochondrial membrane [
30]. Among members of the Bcl-2 family, Bax can especially regulate the permeability of the outer mitochondrial membrane, leading to increased cytochrome c release from mitochondria to trigger apoptosis cascades [
30]. Bcl-2 acts as an inhibitor of mitochondrial permeability by changing the conformation within the mitochondrial membrane to bind the Bax and block the oligomerization of the Bax [
31]. The Bcl-2/Bax ratio is regarded as a better predictor of apoptosis than either Bcl-2 or Bax alone [
32]. Cytochrome c in the cytosol participates in caspase activation via binding to Apaf-1, and caspase 9 forms the apoptosome that activates downstream effector caspases 3 to conduct the process of apoptosis [
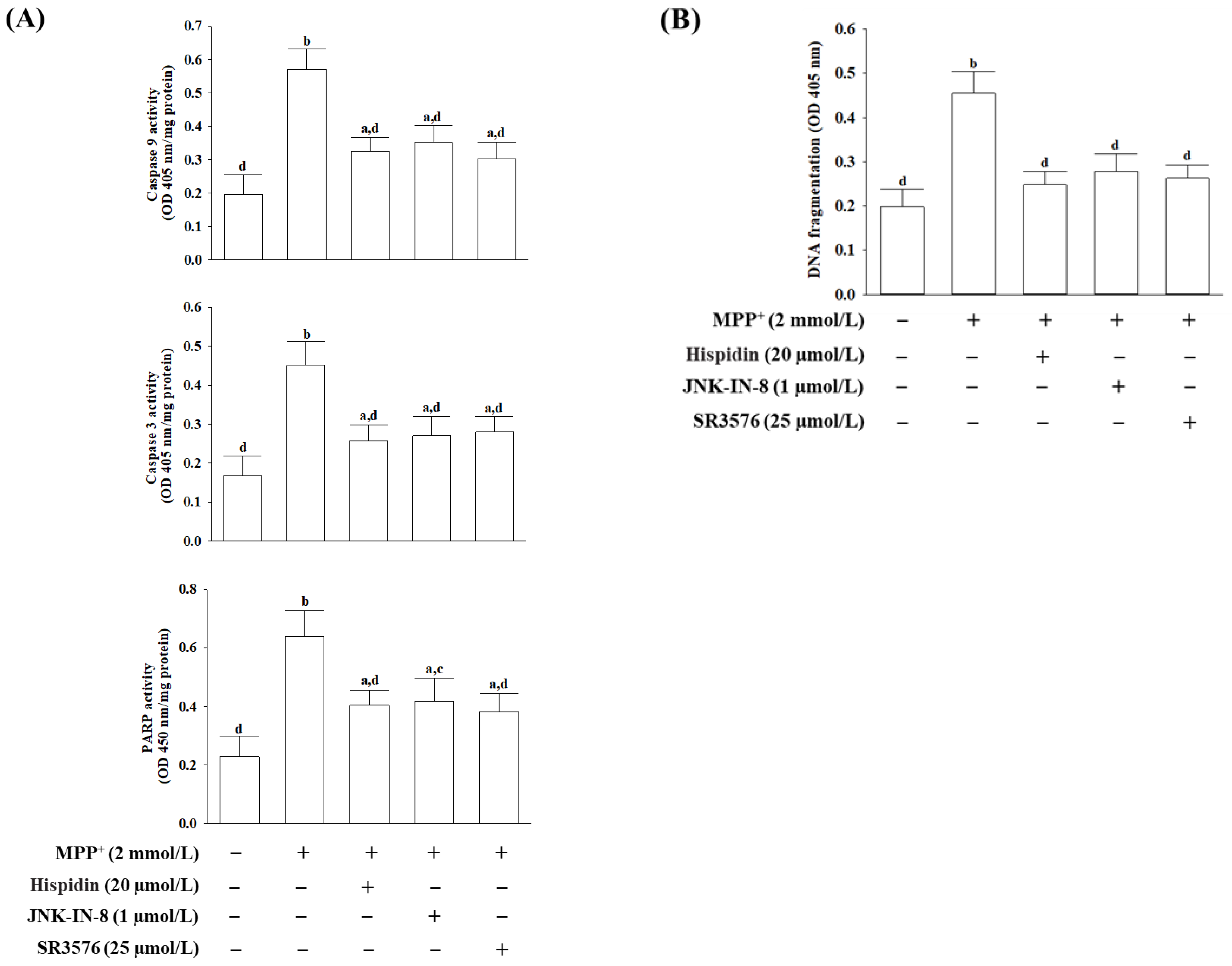
33]. Activated caspase-3 can also cleave PARP-1, which could suppress DNA repair and facilitate caspase-mediated DNA fragmentation; this phenomenon has been proven in several neurological diseases [
34]. Mitochondrial therapies targeted at restoring mitochondrial function and promoting the survival of neuronal cells are tendencies of drug development in the prevention or treatment of neurodegeneration [
35]. The mitochondrial-mediated apoptotic phenomenon has been shown in MES23.5 cells exposed to MPP
+, as shown by mitochondrial membrane potential loss, ATP depletion, cytochrome c release, cleavage, and activation of initiator caspase-9, effector caspase-3, and PARP proteolysis. Down-regulation of the Bcl-2/Bax ratio, coupled with significant DNA fragmentation, was also evidence of apoptosis in MPP
+-exposed MES23.5 cells. All of the above events associated with MPP
+-induced mitochondria-dependent caspase cascades were attenuated under cells receiving hispidin pretreatment. Hispidin could therefore be seen as being able to target mitochondrial defects from oxidative damage to prevent neuronal death and enhance existing cellular function leading to reducing the risk of neurodegeneration.
Neurotoxins and oxidative stress have been shown to phosphorylate JNK and soon afterward activate multiple apoptosis-related transcriptional factors such as c-Jun [
36]. JNK-mediated apoptosis also through effects stimulates the expression of pro-apoptotic genes and reduces the expression of pro-survival genes [
37]. In addition to regulating the transcription of genes associated with apoptosis, JNK also, through transcription-independent mechanisms, mediates apoptosis [
37]. Inhibition of abnormal c-JNK signaling overactivation can therefore prevent cell death, making JNK a promising target for extending pharmacological intervention [
37]. Comparisons with JNK1 and JNK2 are expressed throughout the body; JNK 3 is mainly expressed within the nervous system [
38]. Therefore, JNK3 inhibitors have been identified as a potential therapeutic target for neurodegenerative diseases, although they are not yet in clinical use [
38]. Hispidin has the same tendency as a pan-JNK inhibitor JNK-IN-8 or JNK3 inhibitor SR3576 to mitigate mitochondrial dysfunctions and subsequent activation of the intrinsic apoptotic pathway in MPP
+-exposed MES23.5 cells, while SR3576 seems to be more efficient. In fact, hispidin could not completely block the MPP
+-induced mitochondrial ROS and the intrinsic apoptotic pathway effectors. Hispidin contributes to the partial protection against neurons from MPP
+-induced impairments by improving the mitochondrial JNK pathway can be considerable. In addition, considering that the most prominent mitochondrial inducers of JNK signaling ROS, the neuroprotective effect of hispidin is that it is an antioxidant capable of removing ROS, which indirectly results in the inactivation of the JNK pathway, or being a primary blockage target at JNK requires further study to clarify.
ELISA showing excellent quantitative features with reproducibility is often used to quantify a specific protein that exists in a mixture of different proteins [
39]. The ELISA is therefore considered more valid for accurate quantification of quantitative change. Our study aimed to investigate the quantitative changes of endogenous proteins depending on the revealed activation degrees in MPP
+-mediated ROS-dependent apoptosis, including the JNK-Bax-caspase-3 pathway in MES23.5 cells by hispidin pretreatment. Using ELISA enables qualitative analysis to be considered an appropriate technique for the experiment depending on the objective of the study.
Although our study proved the neuroprotective role of hispidin in MPP
+-induced MES23.5 cells, an in vitro PD-like model, the feasibility of clinical application requires further evaluation. Generally, molecules can cross the blood–brain barrier (BBB) and could be used for brain disorders [
40]. Research on CNS disease-modifying treatments has given rise to a cemetery of ineffective drugs, discarded in part because of their inability to cross the BBB [
40]. Developing effective delivery strategies to address the issue of transporting drugs to BBB remains a challenge in the treatment of neurological diseases [
41]. In general, only lipophilic molecules of low molecular weight (less than 400 Da) and positive charge may cross the BBB [
42]. Based on structural analysis, phenolic compounds containing at least one hydroxy group that is directly bound to an aromatic ring, thus theoretically, be not easy to cross the BBB [
43]. Hispidin actually contains more than one hydroxyl group and is not small enough to cross the BBB [
44]. Modifying hispidin to improve lipid solubility through the addition of lipid groups or functional groups to the polar ends of molecules can be useful in improving across BBB [
41]. Additionally, hispidin can be positively charged with a cationic molecule for further penetration into BBB for in vivo use [
41].
Since PD is a chronic disease that requires long-term therapy, further assessment of the safety of hispidin becomes a critical issue. In a toxicity study, it demonstrated that mushroom mycelium enriched with 3 mg/g of hispidin has very low toxicity based on the results obtained in the Ames test, in vitro chromosome aberration test, acute oral toxicity test, and bone marrow micronucleus test [
45]. Hispidin could be regarded as a bioactive compound used safely for a therapeutic strategy on neural disorders and other diseases [
9].
The effect of pretreatment of hispidin against MPP+-induced cell lesions in MES23.5 cells was clarified in this study. Although this discovery provides new insights into the prophylactic impact of hispidin on neural degeneration, it is unclear whether hispidin can play a therapeutic role after MPP+-induced neuronal damage, giving a new direction to our future study.
In conclusion, our results conferred a neuroprotective role on hispidin against the MPP+-induced cell model. This neuroprotective impact of hispidin may be attributed to the restoration of the mitochondrial complex respiratory chain I activity to improve mitochondrial dysfunction in addition to its potent antioxidant capacity. The downregulation of oxidation-dependent JNK signaling thus leads to caspase cascade suppression, which is the mediated mechanism of hispidin against MPP+-triggered apoptosis in MES23.5 cells. Our findings suggest that hispidin may represent a new therapeutic strategy for preventative and/or complementary PD therapies.