Abstract
With the increase in human mean age, the prevalence of neurodegenerative diseases (NDs) also rises. This negatively affects mental and physiological health. In recent years, evidence has revealed that anthocyanins could regulate the functioning of the central nervous system (CNS) through the microbiome-gut-brain axis, which provides a new perspective for treating NDs. In this review, the protective effects and mechanisms of anthocyanins against NDs are summarized, especially the interaction between anthocyanins and the intestinal microbiota, and the microbial-intestinal-brain axis system is comprehensively discussed. Moreover, anthocyanins achieve the therapeutic purpose of NDs by regulating intestinal microflora and certain metabolites (protocateic acid, vanillic acid, etc.). In particular, the inhibitory effect of tryptophan metabolism on some neurotransmitters and the induction of blood-brain barrier permeability by butyrate production has a preventive effect on NDs. Overall, it is suggested that microbial-intestinal-brain axis may be a novel mechanism for the protective effect of anthocyanins against NDs.
1. Introduction
Recent investigations revealed an average global rise in life expectancy accompanied by the prevalence of neurodegenerative disease (NDs). The occurrence of NDs is mainly in middle and older age, due to which the number of people with diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic lateral sclerosis (ALS) is expected to rise soon. Despite the increasing prevalence of these diseases, current treatment approaches are mostly relatively conservative, and the treatment of NDs remains challenging. In this regard, polyphenols as multipotent agents have gained much attention. Particularly, anthocyanins have gained significant attention because they relieve many of the basic symptoms of neurodegenerative processes, including direct action on the central nervous system (CNS) and indirect action on the CNS through the gut microbiota. The claim that anthocyanins regulate neurological disorders via the microbiome-gut-brain axis has also been widely concerned.
Anthocyanins, a class of water-soluble flavonoids, which are natural color pigments found in various colorful plants, such as berry fruits and vegetables, have received extensive attention for their neuroprotective effects in the CNS [1]. Similarly, they have antioxidant, anti-aging [2], anti-inflammatory, antibacterial, vision protection, and other physiological functions, and could also reduce the level of oxidative stress, neuro-inflammation in the body [3], and improve the vitality of microglia. The color of anthocyanins usually changes with pH, and as the pH changes, its color will change from red to different colors such as purple, orange, colorless, and blue. As a result, they are widely used in the food, pharmaceutical, and cosmetic industries [4]. There are more than 20 known anthocyanins in nature, among them cyanidin, delphinidin, malvidin, peonidin, petunidin, and pelargonidin; these six are the most common anthocyanins. The stability of anthocyanins depends on the presence of the B-ring and hydroxyl or methoxy groups in their structure, with anthocyanin-3-glucoside (C3G) being the most widely distributed anthocyanin in nature (Figure 1) [5]. Anthocyanins are considered useful functional food ingredients, conferring various healthy benefits and emerging as promising ingredients for the food industry.
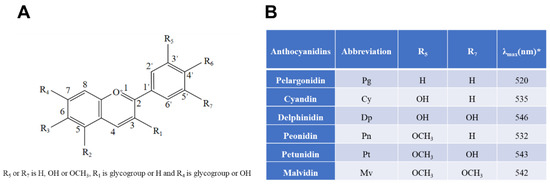
Figure 1.
Chemical structures of six common anthocyanidins (flavylium form) and their sources based on current information. (A) Basic structure; (B), six common anthocyanidins. * indicates that λmax means the wavelength at which a substance has its strongest photon absorption.
It is assessed that the number of human gut microbiota is made up of about 10 to 100 trillion microorganisms, 11 times that of human body cells, i.e., the body is made up of 10% of our cells and the other 90% is comprised of microbial cells [6]. The gut microbiota is generally classified into harmful, neutral, and beneficial bacteria. Studies have shown that the Bacteroides spp. and Firmicutes spp. are the most commonly known gut microbes, accounting for approximately 75% of all gut microbiota [7]. The gut microbiota is a complex micro-ecosystem in the human body. It is mutually restricted and interdependent with its host, thus maintaining the homeostasis of the human microecology. If affected by the changes of the host and external environment, microbial homeostasis will be altered, forming destructive physiological combinations and generating pathological combinations, resulting in intestinal microbiota disorders, and even inducing the production of many metabolic diseases, immune diseases, infectious diseases, and some neurological diseases [8,9]. The growing evidence reveals the importance of the gut microbiome in maintaining immune function and metabolic homeostasis in the body [9].
It has long been exhibited that the gastrointestinal tract can be regulated by brain via movement, blood flow, secretion, and absorption, while the gut can also affect the brain’s function and behavior. This two-way communication between the brain and the gut is called the gut-brain axis [10,11]. In addition to coordinating and integrating gastrointestinal functions, it also connects the brain and peripheral functions [12]. Many reactions occur in these processes, such as the activation of immune responses, the protection of intestinal permeability, and the transmission of gastrointestinal-endocrine signals [13]. The gut microbiota is currently recognized as a key regulator of a smooth two-way dialogue between the gut and the brain (gut-brain axis). A few preclinical examinations propose that the microbiome and its genome (microbiome) may assume a critical part in neurodevelopment and NDs. Furthermore, human gut microbiota composition alterations have also been linked to various neuropsychiatric conditions, including depression, autism spectrum disorder (ASD), and PD. Microbiome-gut-brain axis could explain how the gut microbiota mediates the interaction between the gastrointestinal tract and the brain and how the gut microbiota participates in signaling between the nervous, endocrine, and immune systems and the brain [14]. As a result, the microbiome-gut-brain axis has become a potential diagnostic and therapeutic target for many neurological disorders.
There is growing evidence that anthocyanins are therapeutically effective in neurological disorders via the gut microbiota, where the microbiome-gut-brain axis plays a key role. This review focuses on the protective effects of anthocyanins on neurological diseases, their interaction with the gut microbiota, and the possible mechanisms by which anthocyanins and/or their metabolites regulate neurological diseases through the microbial-gut-brain axis.
2. Protective Effects of Anthocyanins on Neurodegenerative Diseases
Progressive death of neurons or loss of myelin sheaths can trigger NDs that manifest as brain damage or physical dysfunction, resulting in central and internal nerve deterioration and muscle damage [15]. NDs can be classified into two types: acute and chronic. Acute NDs may cause permanent neurological damage in the short term [16]. Chronic NDs are the main diseases that hinder the quality of life of older adults, such as AD, PD, and ALS.
Oxidative stress and neuroinflammation are two key factors that aggravate nerve injury. In addition, abnormal protein aggregation, excitotoxicity, and neuronal apoptosis also trigger the occurrence and development of NDs. Furthermore, gut microbiota action also influences the CNS through the gut-brain axis (Figure 2). Advanced evidence suggests that anthocyanins could potentially be used in the clinical treatment of NDs. Anthocyanins have been shown to subside NDs by reducing oxidative stress and the viability of microglia. In addition, it can also prevent lipid peroxidation and inflammation caused by neurotoxins, thereby exerting its effects on the nervous system.
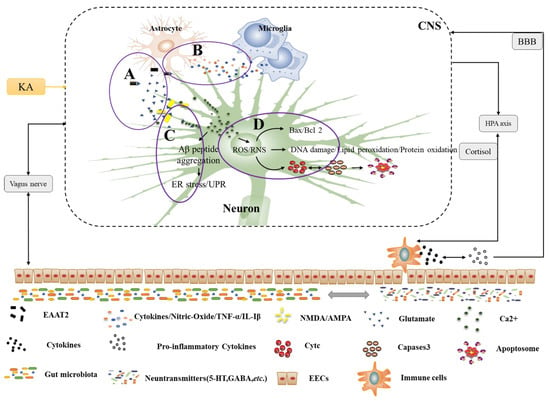
Figure 2.
Pathogenesis of NDs under the microbe-gut-brain axis system. A. Excitotoxins. Glutamate is released from neurons and binds to receptors (NMDA, AMPA), along with glutamate uptake by excitatory amino acid transporter 2 (EAAT2) in astrocytes and the action of external factors (e.g., KA) all lead to increased glutamate excitotoxicity, thus promoting Ca2+ flow, endoplasmic reticulum stress, and pro-apoptotic signaling cascades. B. Neuroinflammation. Interactions between glial cells predispose to neuroinflammation, especially astrocytes and microglia. They may produce several cytokines and oxidants (e.g., TNF-α and IL-Iβ by astrocytes, nitric oxide, and pro-inflammatory cytokines by microglia) that bind to cell surface death receptors and activate pro-apoptotic signaling cascades. C. Endoplasmic reticulum stress. There are many causes of endoplasmic reticulum stress, mainly abnormal folding of Aβ peptides and activation of the unfolded protein response (UPR). This induces abnormal protein aggregation and increases intracellular calcium efflux, which triggers a pro-apoptotic signaling cascade. D. Oxidative stress and mitochondrial dysfunction. Oxidative stress causes excessive levels of reactive oxygen and reactive nitrogen in the mitochondria, exacerbating abnormal mitochondrial function. This ultimately leads to the release of apoptosis-inducing factors and the pro-apoptotic signaling protein cyt c, which then activates the caspase cascade or other apoptotic proteins to form apoptotic vesicles, ultimately leading to neuronal apoptosis. HPA: hypothalamic-pituitary-adrenal axis; intestinal endocrine cells; EECs: BBB: blood-brain barrier; CNS: central nervous system; ROS/RNS: reactive oxygen species reactive nitrogen species.
2.1. Neuroprotective Effects of Anthocyanins on Neurotoxicities Induced by Oxidative Stress and Neuroinflammatory Response
Oxidative stress is a major contributor to neuronal cell death; its clinical manifestation is mainly found in learning and memory dysfunction. Excitatory amino acid and neurotransmitter metabolism and various causes (such as Aggregation of amyloid-β (Aβ) aggregation, calcium imbalance, etc.) can damage mitochondria, thus producing a large number of reactive oxygen species, causing mitochondrial oxidative stress [17]. Activation of glial cells, especially microglia, leads to local inflammation and the death of many neurons. Inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin (IL-Iβ) released from microglia first activate astrocytes; cytokines released from astrocytes activate microglia, thus inducing neural cell apoptosis and producing neuronal inflammation [18]. Excessive amounts of many neurotransmitters will produce excitotoxicity in the nervous system, such as glutamate, 2-amino-3-hydroxy-5-methyl-4-isoxazoliproic acid receptors (AMPA), and kainic acid (KA) receptors, and Ca2+ and K+ will interact with some neurotransmitters (such as glutamate), thereby inducing mitochondrial dysfunction [19,20].
Apoptosis is a direct cause of neurological disorders, and Aβ, lipopolysaccharide (LPS), and glutamate as toxins can cause neuronal cascade responses, including endoplasmic reticulum stress-mediated apoptosis and mitochondria-dependent apoptotic pathways. On the other hand, Aβ peptides induce the accumulation of unfolded proteins, leading to abnormal protein aggregation and increased intracellular calcium fluxes. A combination of glutamate and lipopolysaccharide then induces neuronal apoptosis. On the other hand, mitochondria release apoptosis-inducing factors and cytochrome c (cyt c) and cysteine-specific proteases (caspases) or other apoptosis-related proteins are activated to form apoptotic vesicles, which ultimately disrupt neuronal apoptosis [21].
Anthocyanins can modulate neurodegeneration by acting directly or indirectly on the nervous system. Anthocyanins extracted from bilberries and blackcurrants have been shown in vitro to reduce menadione-induced ROS production in human SHSY5Y neuroblastoma cells over-expressing APP751, thereby protecting SK-N-SH cells from Aβ-induced cellular toxicities [22]. Anthocyanins can restrain the production of pro-inflammatory mediators in microglia and avoid inflammatory cell damage caused by Aβ, thereby exerting neuroprotective effects. The related research mechanism shows that anthocyanins inhibit nuclear factor-κB (NF-κB) activity, c-Jun N-terminal kinase (JNK) activation, and microglial proliferation to suppress neuroinflammatory responses, suggesting that the NF-κB and JNK could be potential targets of anthocyanins [23]. Ullah, et al. [24] found that anthocyanins could reduce Ca2+ dysregulation, ROS accumulation, and AMP-dependent protein kinases in KA-treated mouse hippocampal cell line (HT22) and primary prenatal rat neurons (AMPK) activation and increase in the percentage of apoptotic cells. Song et al. [25] found that C3G not only inhibits the spontaneous aggregation of Aβ25-35 at the molecular level, but also effectively inhibits the binding of Aβ25-35 to the cell surface. In vitro experiments suggested that C3G suppressed reactive oxygen species formation, cellular apoptosis, cleaved caspase-3 expression, and Bcl-2-related X protein (Bax)/upregulated B-lymphocystoma-2 (Bcl-2) ratio, and increased the expression of cyt c release from mitochondria [26].
2.2. The Penetration of Anthocyanins through the Blood-Brain Barrier
The three membranes between blood, brain cells, and cerebrospinal fluid are collectively referred to as the blood-brain barrier (BBB), which maintains the stability of brain tissue, protects neural tissue from toxins and pathogens, and limits the penetration of drug compounds used to treat NDS [27]. The polarity of anthocyanins allows molecules to penetrate the BBB. Anthocyanins are transported to the CNS and their accumulation occurs mainly in brain endothelial cells, brain parenchyma, and many tissues, including the striatum, hippocampus, cerebellum, and cortex. In rodent models, anthocyanin accumulation levels of up to 0.21 nmol/g have been found in brain tissue [28,29], which suggests that anthocyanins can accumulate in the brain through the BBB. The above study shows that anthocyanins and their metabolites can transmit to the BBB to protect the brain. However, some recent studies have also found that anthocyanins and their metabolites have difficulty entering the brain through the BBB by giving oral blackcurrant or strawberry AC extracts to male Wistar rats; anthocyanins are not directly related to the prevention of β-amyloid-induced neurotoxicity [30].
There is bidirectional communication between the gut and the brain. The CNS regulates the intestinal epithelium mainly through the vagus nerve, the immune system, and chemical signals, while the intestinal microbiota and its metabolites act as chemical signals that feed back to the CNS through the BBB or the vagus nerve. Microbial metabolites can disrupt the integrity of the intestinal epithelial barrier, which can be transferred to the circulatory system and cause immune cells to produce pro-inflammatory cytokines, thereby inducing the development of NDs. Additionally, some metabolites and cytokines cross the BBB and act on the CNS.
3. Interactions between Anthocyanins and Gut Microbiota
Anthocyanins’ health benefits have been widely reported, particularly in preventing oxidative stress-related diseases such as cardiovascular disease, noncommunicable diseases, and inflammatory diseases. There are currently some reports that anthocyanins can affect the gut microbiota (by absorbing metabolism directly in tissues or organs [31]), thereby modulating immune or neural pathways to affect the CNS. Neuronal activity and cognitive function in the brain may be directly or indirectly influenced by neurotransmitter synthesis pathways in the gut [32]. The gut microbiota primarily produces short-chain fatty acids (SCFAs) and modulates the metabolism of bile acids, as well as some neurotransmitters such as gamma aminobutyric acid (GABA), serotonin (5-HT), glutamate, and dopamine. Although unable to cross the BBB, they can be converted into functional neurotransmitters, including dopamine and norepinephrine [33,34]. These findings suggest that the gut microbiota can alter brain function and cognition, primarily by regulating neurotransmitter synthesis and blood levels to influence neurotransmitter delivery to the brain.
In addition, recent evidence suggests that the health benefits of anthocyanins may also be related to the regulation of the gut microbiota. Anthocyanins are only partially absorbed (up to 35%) in the stomach and small intestine, but they interact with the gut microbiota to produce a large number of metabolites and catabolite products [35]. In the intestinal epithelium, liver, and kidney, anthocyanins absorbed by the body are metabolized to glucuronide, sulfate, and methylation by separate metabolizing enzymes [36]. In turn, the intestinal microbiota is involved in anthocyanin metabolism, vitamin synthesis, and carbohydrate catabolism, accompanied by some enzymes, such as α-L-rhamnosidase, β-D-glucosidase, and β-D-glucuronidase. Phenolic compounds produced by anthocyanin catabolism under the action of intestinal microbiota can also inhibit oxidative stress and inflammatory reaction. There is evidence that these metabolites can activate Nrf2 and reduce intestinal inflammation by affecting MAKP mediated by tak1 and the NF-κB pathway mediated by SphK/S1P [37]. Studies have shown that anthocyanins from Lycium barbarum can reduce intestinal inflammation, induce SCFAs production, and inhibit endotoxin production by inhibiting LPS/NF-κB/TLR4 pathway.
3.1. Metabolism of Anthocyanins by Gut Microbiota
The body absorbs a small number of anthocyanins ingested through the diet, and the vast majority of the ingested compounds reach the colon (pH 7.4–8), where they interact with the microbiota for biotransformation and metabolism before being absorbed across the intestinal mucosa [31]. In the first phase of anthocyanin bacterial hydrolysis, anthocyanin aglycon is formed, mainly due to the cleavage of its sugar group. In the second phase, anthocyanins are degraded to simple phenolic acids in the small intestine, involving the activity of two bacterial enzymes, specifically α-L-rhamnosidase and β-D-glucosidase [38]. The benefits of anthocyanins are manifested in three main ways: absorption from the upper gastrointestinal tract, direct metabolism by endogenous enzymes, and absorption by the colon of metabolites grown by the action of third world anthocyanins and microbiota.
In assessing bioactivity in vivo, the bioavailability of anthocyanins in the gut microbiota is crucial. The bioactivity of anthocyanins depends on their bioavailability, i.e., the amount of anthocyanin that reaches the systemic circulation, which usually refers to the absorption and metabolism of anthocyanins in the body. Because anthocyanin bioavailability is the foundation of their health-promoting effect, it is critical to investigate anthocyanin absorption, metabolism, biotransformation, and elimination. Anthocyanins have a low bioavailability, with only 5–10% of total polyphenol intake absorbed in the small intestine [39]. It is widely believed that the action of intestinal microbiota can increase not only the bioavailability of dietary phenolics, but their antioxidant activity can be increased about fourfold [40]. Anthocyanins benefit from degradation by colonic microbiota, which contribute to anthocyanin bioavailability and may be responsible for antioxidant activity [38]; inhibit angiotensin-converting enzymes from improving blood pressure; improve glucose metabolism, plasma lipid profile, and inflammation [41].
Anthocyanins have been widely reported to have low bioavailability. However, due to the unstable nature of anthocyanins, their absorption, distribution, metabolism, elimination, complex metabolic pathways, and subsequent large amounts of metabolites have not been fully defined. Major degradation products of delphinidin-3-O-glucoside, peonidin-3-O-glucoside, cyanidin-3-O-glucoside, pelargonidin-3-O-glucoside, and malvidin-3-O-glucoside have been identified as gallic acid, vanillic acid, protocatechuic acid, 4-hydroxybenzoic acid, and syringic acid, respectively [42]. Other metabolites produced by anthocyanin degradation include catechol, pyrogallol, resorcinol, tyrosol, 3-(3’-hydroxyphenyl) propionic acid, dihydrocaffeic acid, and 3-(4’-hydroxyphenyl) lactic acid [43]. Through monocarboxylic acid transport by epithelial cells, these acidic metabolites may be absorbed [31]. Three types of anthocyanins in mulberry trees (Cy-3-O-glucoside, Cy-3-O-ruevoside, Dp-3-O-ruetoside) are metabolized by intestinal microbes to produce aldehyde and phenolic acid metabolites (such as protocateic acid and vanillic acid), and the formation of aldehyde and phenolic acid metabolites is catalyzed by associated enzymes or spontaneous cleavage, resulting in a series of anthocyanin monomer transformation steps [44]. Studies have proved that the main absorption point of anthocyanins is jejunum ileum, and it is suggested that anthocyanins are carriers that transport phenols to the intestine, but this still requires further experimental proof [45].
3.2. Modulation of Gut Microbiota by Anthocyanins
There may be a relationship between the intake of anthocyanin-rich foods and the composition of the gut microbiome, including increasing the overall microbial population and the growth of specific microbial substances (Table 1). Different anthocyanins acted on different animals or were treated differently and fermented in vivo or in vitro, with similar effects on gut microbiota, even if the microbiota were of different origins. This can be easily detected by fecal microbiota transplantation or simulated in vitro fermentation. However, these studies mainly focus on the ability of anthocyanins to change intestinal flora and improve inflammation and rarely focus on the role of specific anthocyanins and microbiome in health.

Table 1.
Gut microbiome alterations following consumption of anthocyanins rich foods.
Anthocynins and their metabolites are known for both promotion and inhibition of intestinal microbiota, and can hinder the proliferation of pathogenic bacteria, both gram-positive (Bacillus subtilis, Staphylococcus aureus, and Listeria monocytogenes) and gram-negative (Escherichia coli, Salmonella enterica, and Citrobacter freundi [31]. Anthocyanins can stimulate the growth of beneficial bacteria, such as Bifidobaterium spp., Lactobacillus, Enterococcus spp. in vitro and in humans [62], which are recognized as one of the most important bacterial groups associated with human health, anti-obesity effects, and cholesterol regulation [63]. In vitro microbial cultivations revealed that anthocyanin monomers derived from peonidin and purple sweet potato anthocyanins could promote the growth of Bifidobacterium bifidum, Bifidobacterium adolescentis, Bifidobacterium infantis, and Lactobacillus acidophilus while inhibiting the growth of Staphylococcus aureus and Salmonella typhimurium [55]. Black fungus (Aronia melanocarpa (Michx.) Elliot) anthocyanins significantly increased the relative abundance of beneficial bacteria, especially Bifidobacteria, and decreased the quantity of harmful bacteria, thus improving the intestinal microecology [64]. A study found that in a mouse model of depression treatment, black rice anthocyanins achieved antidepressant effects by regulating the balance of the intestinal microbiota. The species richness and diversity of the intestinal microbiota were altered, with increased levels of Firmicutes and decreased levels of Bacteroidetes and Proteobacteria [65]. In the dextran sodium sulfate (DSS) murine model of colitis, the intake of anthocyanin-rich purple potatoes inhibits intestinal permeability, elevated mRNA expression of inflammatory interleukins IL-6 and IL-17, and elevated protein levels, as well as the relative abundance of specific pathogenic bacteria such as Enterobacteriaceae and the relative abundance of Akkermansia muciniphila (A. muciniphila) to improve some of the symptoms of inflammatory bowel disease [54].
Anthocyanins can also promote short-chain fatty acids to change in a better direction when they act on the intestinal tract. Recently, Sun, Zhang, Zhu, Lou, and He [52] reported a potential prebiotic effect of anthocyanins on gut microbiota, promoting double-ambiguous effects, including improved probiotics (Bifidobacterium and Lactobacillus) and SCFAs producers (Roseburia, Faecalibaculum, and Parabacteroides), which improved the colon environment and reorganized microbial structure. In vitro simulation of the digestion and fermentation of Lycium barbarum polysaccharide by human gut microbiota revealed that anthocyanins from L. ruthenicum interacted with gut microbiota to produce SCFAs. In addition, another study also showed that anthocyanins from L. ruthenicum promote the production of SCFAs (as evidenced by the promotion of acetate, propionate, and butyrate formation), demonstrating the dynamic regulation of the intestinal microbiota by anthocyanins [47]. Chen et al. showed that anthocyanins can stimulate the growth of beneficial bacteria (Bifidobacterium spp., Lactobacillus spp., and Enterococcus spp.) and inhibit the growth of Clostridium histolyticum perfringens (a potentially harmful bacteria) [66]. Therefore, it is generally accepted that dietary anthocyanins can alter the colonization of the gut microbiota, i.e., it has a prebiotic effect similar to regulating the combined position of the gut microbiota [67]. In addition, membrane and intracellular interactions of compounds also influence anthocyanin activity, such as decreasing the release of LPS molecules from the outer membrane of Gram-negative bacteria [68].
3.3. Physiological Activity Related to Modulation of Microbiota
It is well-known that in the human body, the gut microbiota is an important producer of vitamins and essential coenzymes for metabolic reactions. Biotin, cobalamin, folic acid, niacin, pantothenic acid, pyridoxine, riboflavin, and thiamine can all be synthesized by gut microbiota, as can vitamin K2.
In addition, vitamins appear to modulate gastrointestinal health, either by modulating the composition and metabolic activity of the gut microbiome or by affecting the gut barrier and immune system [69]. Similarly, beneficial bacteria can produce SCFAs (e.g., acetate, propionate, butyrate, etc.) and other metabolites (e.g., pyruvate, succinate, lactate, soluble oligosaccharides, gases, etc.), thus maintaining glucose homeostasis and intestinal integrity [70].
Li et al. [71] found that anthocyanin-containing potatoes attenuated colonic epithelial damage caused by colitis and maintained intestinal permeability in mice treated with antibiotics in a mouse model. Anthocyanins are bioactive molecules in the colon that maintain the balance of the intestinal microbiome and, in addition, they have similar mechanisms in the prevention and treatment of cardiovascular disease, osteoporosis, cancer and NDs [72]. As mentioned above, the benefits of anthocyanins are mainly achieved through regulating the homeostasis of the intestinal microbiota and producing some metabolites (SCFAs, etc.). Bifidobacterium is the main genus of human gut microbiota, and its abundance is closely related to the total number of gut microbiota [73]. By observing the pregnant rats who ingested dietary anthocyanins, Lactobacillus and Bifidobacterium were recovered in their mothers, NF-κB signaling pathway was down-regulated, and genomic DNA and ZO-1 gene were significantly expressed in their offspring [74,75].
The ability of anthocyanins to alleviate atherosclerosis may be related to the gut microbiota. A study showed that the adhesion of monocytes to endothelial cells (the first step in the development of atherosclerosis (AS)) can be regulated by anthocyanins, intestinal flora, and their metabolites [76]. Wang et al. [77] showed that, mediated by the gut microbiota and its metabolites, anthocyanins promote reverse cholesterol transport under macrophages and alleviate atherosclerotic lesions. In an 8-week vitamin and high-fat diet-induced rat model, oral administration of purified anthocyanins from L. ruthenicum (LRPA) significantly increased HDL-C concentrations and the abundance of Bifidobacteria, Lactobacillus spp., while decreasing serum levels of TG, TNF-α, IL-6, and atherogenic index, suggesting that LRPA ameliorates atherosclerosis in rats by remodeling the gut microbial community and regulating signaling pathways of arterial inflammation (NF-κB) and hepatic lipid metabolism (SREBP-2) [78]. Thus, it emphasizes the importance of the interaction between the gut microbiota and anthocyanins. Hence, modulating microbiota by probiotics may be a new area for functional food efforts, which aims to reduce the prevalence of cardiovascular, digestive, and neurological disorders.
4. Protective Effect of Anthocyanins on Neurodegenerative Diseases under the Microbial-Gut-Brain Axis System
The maturation and development of the human early immune system are related to the gut microbiota, and dysregulation of the microbe-gut-brain axis can cause metabolic diseases and NDs. In addition, the gut microbiota is extensively involved in the synthesis and release of various hormones, active metabolites, neurotransmitters, and other active compounds, in part by regulating the availability of circulating tryptophan, 5-HT, kynurenine, and SCFAs, the permeability of BBB, and the activation of peripheral immune cells and glial cells, thus affecting brain function and host behavior. In addition, altered gut microbiota composition may be involved in the pathogenesis of various neurological diseases, including autism AD, PD, ASD, etc. The gut microbiota interacts with the CNS via multiple parallel and interacting pathways that involve chemical, neuronal, and immune signaling (Figure 3). According to one study, anthocyanins modify host tryptophan metabolism to produce metabolites that control CNS inflammation. As a result, anthocyanins may act as microbe-gut-brain axis mediators to regulate neuroinflammation via gut microbial modulation. In addition, studies have also showed that anthocyanins can regulate the synthesis of certain metabolic products (such as tryptophan, SCFAs, etc.) and certain toxic substances (such as LPS, etc.) by regulating the gut microbiota, thereby increasing the integrity of the BBB and reducing the level of neuroinflammation in nerve cells to achieve the effect of treating NDs.
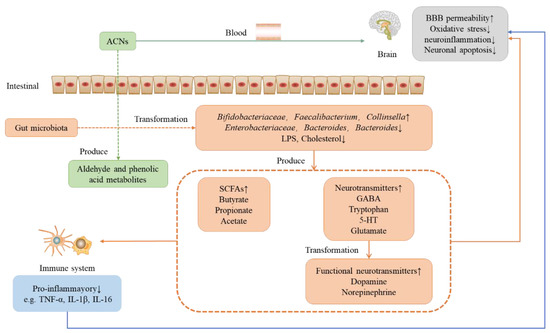
Figure 3.
Role of anthocyanins on the nervous system under the microbial-intestinal-brain axis system. ACNS: anthocyanins; LPS: lipopolysaccharide; GABA; gamma aminobutyric acid; 5-HT: serotonin; SCFAs: short-chain fatty acids.
4.1. The Microbiota-Gut-Brain Axis
4.1.1. Neuronal Pathways
Neural pathways are the connections formed by a mix of electric and chemical transmissions within the nervous system access, the direct pathways linking the gut and brain, primarily including the vagus nerve and enteric nervous system (ENS). The vagus nerve (80% of afferent fibers and 20% of efferent fibers) is the main component of the parasympathetic nervous system and the most direct pathway between the intestine and the brain, producing reactions such as adaptive/maladaptiveness, where the refractory responses are mainly manifested in gastrointestinal pathology and NDs [79]. Enteroendocrine cells interact directly with vagus nerve by releasing serotonin (activating 5-HT receptors in afferent fibers of vagus nerve) or gastrointestinal hormones. Significantly, these cells express TLRs, mediating microbial induction. For example, enteroendocrine cells can use LR4 to detect LPS and TLR2 to detect peptidoglycan to identify signals from gram-negative bacteria [80]. Similarly, a matched cohort study from Sweden shows that vagotomy may have a protective effect on PD [81]. However, another study has shown that there is no dependence of gut and brain on vagus nerve in antibacterial therapy, which indicates that there may be an independent neural mechanism in vagus nerve communication [82].
The ENS controls the behavior of the gastrointestinal tract (GI), and is an essential part of autonomic nervous systems, such as intestinal transit and secretion. ENS transmits these signals from the intestinal tract to the brain through the interaction of neurotransmitters (adrenaline, norepinephrine, and acetylcholine), anthocyanins, motor neurons, and the CNS. In addition, the ENS and the CNS are interconnected by the sympathetic and vagus nerve. Gut microflora can regulate the levels of intestinal peptides (such as glucagon-like peptide-1 (GLP-1) and 5-HT), which are secreted by intestinal endocrine cells (EECs) to influence the afferent pathways of vagus nerve, thus regulating intestinal metabolism through the microflora-gut-brain axis [83]. It may also affect the neuroanatomical structure of the ENS, and there is evidence that the immature ENS of germ-free (GF) mice can be normalized by colonization of the gut microbiota [84]. Following electrical stimulation, metabolic compounds of Bifidobacterium longum NCC3001 cause a decrease in action potential spikes in myenteric neurons [82]. The gut microbiota not only influences the intestinal neurons but also promotes the settlement and homeostasis of intestinal glial cells in the intestinal mucosa [77].
4.1.2. Neural Immune Pathways
The immune system interacts with the gut microbiota and the CNS. Enterocytes and GALT immune cells such as T cells and dendritic cells (DCs) are important components of the microbe-gut-brain axis, from which some of the microbial metabolites and neurotransmitters released can cross the blood-brain barrier and affect neurons and glial cells in the brain [85]. Microglia can cause neuroinflammation upon external stimulation and are innate immune effector cells of the CNS, while tissue macrophages of the CNS are responsible for maintaining neural networks and repairing injuries [86]. Furthermore, a recent study of GF mice showed that the immune system of CNS can be regulated by gut microbiota by regulating the activation and steady state of microglia [87]. Microbial tryptophan metabolites can influence CNS inflammation by activating astrocyte aryl hydrocarbon receptors [88].
By adjusting intestinal and peripheral immune cells, the gut microbiota can also communicate with the brain, and in some cases, such as brain injury, directly penetrate the BBB, or connect indirectly through afferent fibers and enteric nerve [89]. The abnormality of the gut microbiome leads to the secretion of amyloid and LPS, increases the intestinal permeability, makes other cytokines such as LPS and amyloid enter the intestinal wall, and stimulates TLR4 and other TLRs to produce inflammatory cytokines, thereby increasing the permeability of the BBB, entering the brain tissue, causing neuroinflammation, nerve damage, resulting in neuronal death, and directly affecting the function of the nervous system [90]. Another study reported the discovery of bacterial LPS in postmortem brain lysates from AD patients’ hippocampus and superior temporal neocortex [91].
The gut microbiota can also communicate with the brain by modulating intestinal and peripheral immune cells. The vagus nerve and circumventricular organs are affected by the systemic circulation of immune factors, cytokines, and chemokines. A study has shown that Bifidobacterium infantis could correct IL-10/IL-12 from the peripheral blood mononuclear cells of patients with irritable bowel syndrome [92]. Elevated levels of IL-6 in the brain can stimulate the differentiation of astrocytes, hippocampal neurons, and Schwann cells, and regulate autism-like behaviors through synapse formation, dendritic spine development, and defects in the balance of neuronal circuit [93]. Non-inflammatory cytokines also mediate the regulation of brain function by gut microbes. For example, plasma levels of granulocyte colony-stimulating factor were decreased in neonatal mice after exposure to antibiotics, thus reducing the stimulation of the BBB and playing a protective role in NDs [94,95].
4.1.3. Chemical Signaling Pathways
Gut microbiota can produce a spectrum of neurotransmitters, neuroactive compounds, and metabolites, which then regulate the homeostasis of the host CNS directly or indirectly through various chemical signals, such as SCFAs, 5-HT, bile acids, and GABA. Lactobacillus and Bifidobacterium species, for example, can produce GABA, as Escherichia, Bacillus, and Saccharomyces spp. can produce norepinephrine; Escherichia, Bacillus, Lactococcus, Lactobacillus, and Serratia can produce dopamine. Studies have shown that there is direct communication between gut microbiota and the hypothalamic-pituitary-adrenal axis (HPA axis, one of the main regulatory factors of human physiological stress response), which affects the CNS. SCFAs could interact with G protein-coupled receptors or histone deacetylase, and act on the brain through humoral, immune, and other pathways, thus affecting inflammation and hormone regulation and interact with the vagus nerve to have an effect on the nervous system [96]. SCFAs are demonstrated to possess immunomodulatory effects, which interact with the CNS to directly activate sympathetic neurons and indirectly affect behavior and neural signals through the BBB [97]. In addition, glial cells in the developing brain can take up SCFAs as a source of energy for cellular metabolism [98].
Similarly, the gut microbiota may play a role in neuropsychiatric disorders by regulating tryptophan levels. Under the action of the gut microbiota, tryptophan produces 5-HT, a neurotransmitter with diverse effects, which can act via both pro-inflammatory and anti-inflammatory mechanisms to affect the intestinal mucosal immunity [99]. Peripheral 5-HT could not cross the BBB, which participates in a variety of human physiological functions by stimulating specific 5-HT receptors. It is an important gastrointestinal signaling molecule that transmits signals from the intestine to intrinsic or extrinsic neurons, thereby influencing intestinal peristalsis, secretory reflexes, and absorption of nutrients. However, we still have no way of knowing the mechanism by which gut-derived 5-hydroxytryptamine affects the CNS [99]. GABA is a major inhibitory neurotransmitter, and GABA secretion by gut microbes may be influenced by intestinal pH, with anaphylactic Bacteroides showing the strongest potential for GABA production in the pH range of the human intestine [100]. Microbial production of GABA in the testis may be involved in the gut-brain connection via the intestinal enteroendocrine pathway and the neuroimmune pathway [101].
The HPA axis is a complex two-way communication network among hypothalamus, pituitary gland and adrenal gland, and regulates various bodily functions. It acts through a series of endocrine steps that lead to the production of cortisol (which inhibits the production of pro-inflammatory factors), which, together with the endocrine and its metabolites (such as adrenaline and norepinephrine), act on the body to maintain endocrine balance [102]. Gut microbiota directly act on the HPA axis to regulate stress and human endocrine, and maintain body homeostasis. HPA axis can also act on the intestinal tract, change the composition of gut microbiota, increase intestinal permeability, promote the infiltration of microbial metabolites into the blood, and produce inflammation [103]. GF mice showed significantly higher HPA axis activity under restrictive stress compared to specific pathogen-free (SPF) mice [104].
4.2. The Role of the Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders
Past studies have revealed that the microbial-gut-brain axis is a key pathway for the involvement of the gut microbiota in NDs (Table 2).

Table 2.
Characteristics of prior studies investigating the relation between microbiome and neuropsychiatric disorders.
4.2.1. Alzheimer Disease
AD is a form of dementia with memory loss and failure to develop normal daily life and behavior. It is the most common form of dementia in the elderly [116]. AD is a NDs caused by the aggregation of Aβ peptides leading to the loss of neurons. Similar to the CNS, the gut microbiota is a source of amyloid. There is growing evidence that probiotics and their metabolites can work through the immune pathway in order to improve neurological disorders. Recently, a study showed that AD’s patients with a combination of probiotics containing Bacillus B. longum and Lactobacillus spp. had improved cognitive function and metabolic status [117]. Taxa in the fecal microbiome of elderly patients with AD that cause the abundance of pro-inflammatory conditions is higher, which is associated with the production of Aβ. In a mouse model of AD, probiotics increased antioxidant and neuroprotective effects via the SIRT1 pathway, while attenuating cognitive impairment, brain damage, Aβ aggregation, and neuronal protein breakdown levels [106]. The combined effect of probiotics and selenium kawaii improved cognitive function (i.e., higher Mini-Mental State Examination scores) and slowed inflammation and oxidative stress levels, compared to AD’s patients in the selenium-only or placebo groups [105]. Overall, probiotics have potential efficacy to improve cognitive function in both AD patients and healthy populations.
In fact, loss of function of the BBB may also lead to the onset of AD, for example, the gut microbiota produce endotoxin to destroy BBB integrity, thus infiltrating inflammatory factors and microbial-derived metabolites (phenylalanine or isoleucine) to produce the pro-inflammatory effect [118]. It is well-known that age is the greatest risk factor for AD. With age, the permeability of BBB and the integrity of the gastrointestinal epithelium decrease, while gut microbiota homeostasis shifts in an unfavorable direction [119].
4.2.2. Parkinson’s Disease
PD, known as tremor paralysis, is characterized by motor and non-motor symptoms. Gastrointestinal dysfunction is prevalent in PD patients, the initial symptom seen before motor symptoms. Some scholars believe gut microbiota plays a key function in the aggregation of beta-synaptic nuclear proteins and neuroinflammation [120]. During PD, the first and most common nerves affected by alphasynuclein pathology are the enteric and parasympathetic nerves, respectively. Recent research has found that, in the typical pathological changes in PD, the α-Syn in Lewy (Protein aggregate consisting of multiple proteins such as α-synuclein-positive) are expressed in enteric neurons and enteroendocrine. Mouse experiments show that-Syn can be transported across the BBB to the brain, transported from the intestinal mucosa to the CNS, and infusion of Syn FPP into rodent gut trace findings that the risk of PD can be reduced by cutting off the vagus nerve, which blocks its transmission pathways [81,120]. Compared with healthy individuals, changes in the gut microbiota of the PD patients are less Brlautia and Rosaceae, less faecobacterium, and increased Lactobacillaceae, Amancella, and Bifidobacterium [108]. Animal experiments show the movement of PD model mice undergoing fecal transplantation of healthy mice. With improved function, increased striatal neurotransmitters, and decreased levels of neuroinflammation [121]. Another study showed dysregulation of gut microbiota homeostasis and reduced serum LPS binding protein levels in PD’s patients [122]. In a placebo-controlled clinical trial, the investigators found an increase in enzymatic defenses and a decrease in levels of high-sensitivity C-reactive protein and oxidative damage in PD’s patients treated with probiotics (Lactobacillus acidophilus, B. bififidum, L. reuteri, and Lactobacillus fermentum) [107].
Therefore, regulating the gut microbiota and improving the function of the gut microbiota-gut-brain axis may be important for the prevention and treatment of Parkinson’s disease.
4.2.3. Autism Spectrum Disorder
ASD is characterized explicitly by limitations and repetition of personal interests or activities, deficits in language learning, and social skills [123]. However, the vast majority of ASD patients, especially children, are accompanied by gastrointestinal disease such as diarrhea and constipation. Several studies have demonstrated the role of GI microbiota in ASD symptomatology [124]. While genes generally cause ASD, environmental factors can also contribute to the development of similar conditions, such as anxiety [123]. More and more evidence has revealed that the gut microbiota of patients with ASD and metabolites (including neurotransmitters) differ from healthy controls [124]. A comparison of stools from 58 ASD patients and 39 normal children revealed that the ASD patients had higher levels of Lactobacillus and lower levels of SCFAs (such as acetate, propionate, and valerate) and Bifidobacteria [125]. Numerous studies have shown that probiotics positively affect ASD symptoms, improving neurobehavioral symptoms such as anxiety or inattention and/or gastrointestinal symptoms [103]. Fecal microbiota transplantation (FMT) resulted in an approximate 80% reduction in GI symptom scale scores in patients with ASD, and clinical evaluation showed that behavioral ASD symptoms improved and were maintained for at least 8 weeks [109]. The microbial-gut-brain axis of ASD has much to be discovered, especially fecal microbiota transplantation (FMT), and is promising in the treatment of gastrointestinal symptoms in ASD. A recent study showed that oral Lactobacillus rhamnosus (JB-1) promotes increased functional brain metabolites such as GABA, N-acetylaspartate, and glutamate, and one step proved that microorganisms can affect brain activity through metabolic routes [126]. Thus, it is observed that the gut microbiota can influence ASD behavior by producing neuroactive metabolites to regulate the excitation-inhibition balance in the brain.
4.2.4. Anxiety and Depression
Anxiety often accompanies mental illness and various physical illnesses, especially among stress-related illnesses. Depression is low mood and/or loss of interest or fun in daily activities for at least 2 weeks, and also includes symptoms such as depression, worthlessness and/or despair, decreased energy, decreased appetite, sleep disturbances, altered psychomotor function, and suicidality and/or action [127]. Major depressive disorder (MDD) is one of the leading causes of disability, with symptoms including decreased appetite and sleep, irritability, poor concentration, and depressed mood [128]. Chronic GIT can lead to responsiveness to stress and MDD in the body [129]. However, many antidepressants are prone to drug resistance, and more scholars are focusing on the intestinal microbiota.
Numerous studies have demonstrated that anxiety is closely related to intestinal microbiota dysbiosis. Behavioral and stress tests on microbiota were conducted on GF zebrafish larvae and found that motor and anxiety behaviors improved with probiotic treatment [130]. Naseribafrouei et al. found that disturbances in the intestinal-brain axis are associated with the pathophysiology of depression [131].
In a study by Jiang et al., by comparing the 16S rRNA sequences of fecal bacteria from 37 depressed patients, it was found that lower levels of anaphylactic bacteria translated to higher levels of Arthrobacter and Enterobacter [132]. In addition, in a placebo-controlled clinical trial, including 40 patients with MDD, their depression was significantly improved by probiotic treatment (Lactobacillus acidophilus, Bacillus casei, and Bifidobacterium bifidum) [111]. Oscillobacter spp. is known to produce valeric acid, a compound similar to GABA, which produces valeric acid and binds to the GABA (a) receptor. The decrease in corticosterone and IL-6 levels in the cecum was accompanied by an increase in SCFAs levels and an increase in plasma IL-10 levels, which may explain the anxiolytic and antidepressant properties of Oscillobacter spp. [130].
4.2.5. Schizophrenia
SCZ is a mental disorder which manifests as abnormal thinking, perceptual impairment, memory impairment, psychological abnormalities, and emotional expression disorders [133]. The pathogenesis of SCZ is still unclear, and some studies believe that SCZ has abnormalities in neurotransmitter systems such as dopamine, glutamate, and beta-aminobutyric acid (BABA) [134]. Peng et al. found lower glutamate, higher glutamine, and γ-aminobutyric acid in the hippocampus of mice that received SCZ microbiota fecal transplants and displayed schizophrenic-like behavior [112]. The literature also reported that SCZ’s patients exhibit complex gut microbiota changes compared to the healthy group, such as lower levels of Haemophilus spp., Stachybotrys spp., and Proteobacteria phylum, and higher levels of anaerobic bacteria spp. [135]. In addition, both anxiety and depressive symptoms in SCZ’s patients can be effectively improved by probiotic therapy. It was found that intestinal discomfort caused by C. albicans was more pronounced in men, and probiotic treatment significantly reduced C. albicans antibody in men, but not in women [112]. However, in a randomized, placebo-controlled trial of SCZ-assisted probiotics, the patient’s stomach bowel function improved, but the severity of psychiatric symptoms did not change [113]. Differences in data on altered gut microbiota in SCZ may be interfered with by other external factors, requiring further experimental studies and more clinical trials to improve the treatment of such diseases.
4.2.6. Bipolar Disorder
BD is a complex disorder involving mood disorders, neuropsychological deficits, and disorders of the immune system if severe enough, which can lead to mania. BD patients are at a high risk of suicide, and it is one of the leading causes of disability worldwide. The pathogenesis of BD is still not fully known, but genetics and environment are important influencing factors.
Changes in flavonoid levels could play a role in BD. Coello et al. [136] showed that the abundance of Flavonifractor became higher in patients with BD. A study by Painold et al. reported higher levels of Actinobacteria and Coriobacteria in BD’s patients compared to healthy controls [114]. The results of Lu et al. [115] revealed that BD patients had a lower intermediate level in Enterobacteriaceae, which suggests that the homeostasis of the gut microbiota and brain function are altered in BD patients. A recent study found that probiotic clinical interventions with Lactobacillus and Bifidobacterium lactis strains resulted in significantly lower readmission rates in patients with mania, with a particularly pronounced relief of the inflammatory response [136].
4.3. The Protective Effect of Anthocyanins on the Nervous System under the Microbial-Entero-Brain Axis System
Accumulating evidence suggests that for NDs, partly because of oxidative stress and neuroinflammation, etc., neuroinflammatory processes are modulated by peripheral inflammation, particularly from the gut microbiota. There is evidence that NDS may have originated in the gut. In addition, changes in gut microbiota homeostasis are also closely related to the anti-inflammatory properties of anthocyanins. It regulates the gut microbiome through the microbial-gut-brain axis and acts as a preventive role in NDs. Under the microbial-gut-brain axis system, the effect of anthocyanins on the nervous system is shown in Figure 3.
Anthocyanins may be slowing neurodegeneration through their regulation of the gut microbiota. An analysis revealed that consuming blackberry anthocyanin-rich extract (BE) decreased TCK-1 expression in the hippocampus of rats, while expanding Pseudofavonifractor and Sporobacter modulated gut microbiota composition. BE alters CNS inflammation through the use of tryptophan microbial metabolites. In a study, tryptophan (one of the identified metabolites) was decreased in the groups processed by BE, which suggests that BE, gut microbiota, and tryptophan metabolism are closely related [137]. Tryptophan is the precursor of serotonin and kynurenine, which, as an essential amino acid, can affect the CNS’s function through the microbiome-gut-brain axis [138]. The vast majority of tryptophan is metabolized via the kynurenine pathway, about 90% [139]. Tryptophan and kynulic acid (anti-inflammatory properties) can be converted into each other, which could regulate the glutamate level [140]. The gut microbiome modulates serum and urinary levels of kynurenine quinolinic acid, which may alter the state of the CNS [141]. In addition, tryptophan has been shown to regulate neurotransmitter synthesis and maintain the integrity of the intestinal barrier by promoting serotonin synthesis and indole production in the intestine [142]. The gut microbiome can regulate levels of serotonin, while at the same time regulating some gastrointestinal dysfunction due to intestinal axial imbalances and neuro-endocrine-immune stimulation [143]. In summary, anthocyanins not only influence host tryptophan metabolism, but they also produce metabolites that regulate CNS inflammation.
After absorption in the gastrointestinal tract, anthocyanins can also affect brain structures by crossing the BBB and localizing to different areas [30]. Anthocyanins enter the colon and are hydrolyzed by the microbiome; the health benefits of anthocyanins are mainly attributed to their interaction with the intestinal microbiota. Upon entering the colon, anthocyanins are hydrolyzed by intestinal microbes, producing colonic metabolites that, in turn, act on anthocyanins. Positive changes occur in the gut microbiome through the intake of anthocyanin-rich foods, such as increased growth of beneficial bacteria (Bifidobacterium spp.) and increased growth of specific microbial metabolites (SCFAs) [144]. In vitro experiments have shown that the gut microbiota can produce neurotransmitters under certain conditions, such as Lactobacillus and Bifidobacterium secreting GABA and Candida, Streptococcus, Escherichia coli, and Enterococcus secreting 5-HT [145]. It is possible that consuming foods rich in anthocyanins could reduce total plasma cholesterol, TNF-α, and the concentration of LPS, as well as lead to a greater production of fecal SCFAs and favorably modulate the gut microbiota [146]. SCFAs are one of the key factors in the “gut-brain axis” that regulate many host processes, particularly in the CNS, such as mitochondrial function, gene expression, cell-cell interaction, microglia activation, and neurotransmitter synthesis and release [147]. SCFAs produced under the action of the gut microbiota stimulate the intestinal nerves and acts as a protector of the CNS by metabolically regulating immune cells [148]. In addition, it can cross the BBB and interact with microglia to reduce the inflammatory response of microglia [149]. GF mice showed more severe BBB permeability than their SPF counterparts due to reduced expression of endothelial tight junction proteins in the BBB [150]. Anthocyanins-rich supplements (brand name Metabolaid®) have been shown to increase the abundance of the main genera of the gut microbiota, Bifidobacterium, Blautia, and Faecalibacterium, as well as other minor genera like Prevotella and Akkermansia, promote an increase in SCFAs, particularly butyric acid [151]. Subsequent studies revealed that one of the key factors in maintaining the integrity of the BBB is the production of butyrate by gut bacteria [145]. Although the mechanisms are unknown, anthocyanidins gut microbiota metabolites can cross the BBB and reach different brain regions. However, it is worth noting that anthocyanidins have a prebiotic effect that helps regulate gut microbiota and its metabolites, thereby inducing a protective nervous system effect.
Between the “gut-brain axis”, the gut microbiota plays a key role in bi-directional communication, i.e., forming the “microbe-gut-brain axis”. The disorders of the gut microbiota are more associated with a variety of NDs. Cerebral ischemia due to acute brain injury leads to dysregulation of the gut microbiota, which leads to altered species diversity of the gut microbiota and increased intestinal barrier permeability. In addition, dysfunction and neuroinflammation in ICH mice are ameliorated by reprocessing of the healthy microbiota treatment [152]. A recent study found that using anthocyanin-rich blueberries and FMT treatments could modulate the gut microbiota of obese mice to improve metabolic health [153]. Many factors contribute to the homeostasis of the gut microbiota, such as oxidative stress, intestinal pathogens, and chemical exposure. LPS secreted by dysbiosis of the gut microbiota that exaggerate AD pathology, and if microglia receptors (TLR2, TLR4, and CD14) bind to LPS on the bacterial surface, NF-κB transcription factors are activated. Activation of NF-κB produces pro-inflammatory cytokines, which trigger a neuroinflammatory response and produce various NDs [91,154]. In addition, it may disrupt the BBB and increase the level of LPS, which activates the production of cytokines by dendritic cells in the intestinal barrier, such as the production of Aβ peptides in the presence of Salmonella, E. coli, and Citrobacter, etc. These signals may be transmitted to parasympathetic neurons of the vagus and enter the nervous system through retrograde axon transport, thus contributing to peripheral and systemic inflammation [155,156]. In an animal model of LPS-induced neurotoxicity (24 mg/kg/day for 2 weeks: 1 week before LPS and 1 week of LPS), anthocyanin treatment increased the survival of p-Akt and p-GSK3β proteins [23]. In a rat model treated with LPS, the use of FMT can balance the gut microbiome and produce higher levels of short-chain fatty acids, thus preventing neuroinflammation, reduced gut motility, mental weakness, and impaired gut barrier function [157]. Anthocyanin treatment was found to reduce the relative abundance of the Prevotellaceae family, including the Prevotella 9 and Prevotella 2 genera, in an LPS-treated weanling model, and to enrich the Lactobacillus family while increasing the Firmicutes/Bacteroidetes ratio, thereby reducing the inflammatory response [158].
In the intestine, anthocyanins react with the gut microbiota to produce aldehyde and phenolic acid metabolites, and the colonization of the gut microbiota is altered (e.g., increased levels of Bifidobacteriaceae, Fasciola, and Corynebacteriaceae and decreased levels of Enterobacteriaceae, Bacteroidetes, and Bacteroidetes), along with the production of microbial metabolites such as SCFAs and neurotransmitters. In the presence of gut microbiota, some neurotransmitters can be converted into functional neurotransmitters that cross the BBB and act on the CNS; SCFAs can also function on the immune system and decrease the levels of pro-inflammatory factors in turn affect the CNS. Tryptophan promotes the synthesis of neurotransmitters in the intestine, especially 5-HT and glutamate, while acetate promotes the permeability of the BBB. In addition, anthocyanins can also act directly on the CNS to prevent and treat NDs.
5. Conclusions and Expectations
The increased incidence of NDs poses a threat and a heavy burden to human society. In this study, we reviewed the mechanism of action of anthocyanins on NDs and their interaction with the gut microbiome, and it is shown that anthocyanins can achieve a protective effect on the nervous system under the microbial-intestinal-brain axis system by regulating the intestinal microbiota and through the action of certain metabolites. Previous studies have shown the health benefits of anthocyanins and the gut microbiome, particularly in preventing and treating diseases affecting the elderly, including cardiovascular disease, certain cancers, NDs, and age-related disorders such as bone loss. It has been increasingly demonstrated that anthocyanins regulate the CNS through the gastrointestinal tract or through the immune, neurological, and endocrine systems. It is also worth exploring the mechanism by which anthocyanins pass through the BBB to regulate the microbial-intestinal-brain axis.
Although the microbiome-gut-brain axis has developed rapidly in recent years, it is still in its infancy; there are still some challenges for future development. The underlying mechanism of anthocyanins of different structures for NDs under the microbial-intestinal-brain axis system needs further study, and other structures of anthocyanins may have different targeting molecules and initial mechanisms. The molecular mechanisms of the intricate interaction of anthocyanins and different kinds of gut microbes on the beneficial or pathogenic effects of neurological diseases should be more deeply elaborated. Tackling these challenging questions will help validate new causes of microbial-gut-brain axis-mediated neurological diseases and help explore anthocyanin therapies and other potential treatments.
Author Contributions
Conceptualization, H.Z. and J.X.; methodology, M.Y.; software, X.L.; validation, M.H.; formal analysis, H.Z.; investigation, H.Z.; resources, H.Z.; data curation, H.Z.; writing—original draft preparation, H.Z.; writing—review and editing, M.H. and F.F.; visualization, J.X.; supervision, R.G.; project administration, R.G.; funding acquisition, R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (No. 32172202) and Key Technology Research and Development Program of Natural Science Foundation of Zhejiang Province (No. 2021C04032).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rashid, K.; Wachira, F.N.; Nyabuga, J.N.; Wanyonyi, B.; Murilla, G.; Isaac, A.O. Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutr. Neurosci. 2014, 17, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Du, J.; Du, L.; Luo, Q.; Xiong, J. Anti-fatigue activity of purified anthocyanins prepared from purple passion fruit (P. edulis Sim) epicarp in mice. J. Funct. Foods 2020, 65, 103725. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Fang, C. The gut microbiota: A treasure for human health. Biotechnol. Adv. 2016, 34, 1210–1224. [Google Scholar] [CrossRef]
- Eckburg, P.; Bik, E.; Bernstein, C.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.; Nelson, K.; Relman, D. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wu, J.; Jiang, X.; Tang, H. Modulation of Gut Microbiota in Pathological States. Engineering 2017, 3, 83–89. [Google Scholar] [CrossRef]
- Joseph, S.; Curtis, M.A. Microbial transitions from health to disease. Periodontology 2021, 86, 201–209. [Google Scholar] [CrossRef]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524. [Google Scholar] [CrossRef]
- Fröhlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jačan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Fröhlich, E.; et al. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Pothoulakis, C.; Mayer, E. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Cryan, J.; Mahony, S. The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimer Dis. 2014, 42, S125. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Brenner, M.C.; Noelle, P.; Michael, S.; Caleb, B.; Yingyot, A.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kamil, K.K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, X.; Qu, S. Glutamate, Glutamate Transporters, and Circadian Rhythm Sleep Disorders in Neurodegenerative Diseases. ACS Chem. Neurosci. 2018, 10, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Lin, T.Y.; Wang, S.J. 11-Keto-β-boswellic acid attenuates glutamate release and kainic acid-induced excitotoxicity in the rat hippocampus. Planta Med. 2020, 86, 434–441. [Google Scholar] [CrossRef]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, A.; Ramassamy, C. Innovative anthocyanin/anthocyanidin formulation protects SK-N-SH cells against the amyloid-β peptide-induced toxicity: Relevance to Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem. -Cent. Nerv. Syst. Agents) 2016, 16, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Chung, J.I.; Kim, M.O. Anthocyanins Improve Hippocampus-Dependent Memory Function and Prevent Neurodegeneration via JNK/Akt/GSK3β Signaling in LPS-Treated Adult Mice. Mol. Neurobiol. 2018, 56, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. Cns Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, L.; Chen, W.; Zhu, H.; Deng, W.; Han, Y.; Guo, J.; Qin, C. Cyanidin 3- O -β-glucopyranoside activates peroxisome proliferator-activated receptor-γ and alleviates cognitive impairment in the APP swe /PS1 ΔE9 mouse model. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 1786–1800. [Google Scholar] [CrossRef]
- Wei, J.; Wu, H.; Zhang, H.; Fang, L.; Chen, S.; Hou, B. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in db/db mice. Int. J. Mol. Med. 2018, 41, 1608–1618. [Google Scholar] [CrossRef]
- Gosselet, F.; Loiola, R.A.; Roig, A.; Rosell, A.; Culot, M. Central nervous system delivery of molecules across the blood-brain barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin Metabolism in Rats and Their Distribution to Digestive Area, Kidney, and Brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; Mcdonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.; Graf, B.A.; O’Leary, J.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Tana, C.; Nouvenne, A.; Prati, B.; Lauretani, F.; Meschi, T. Gut microbiota, cognitive frailty and dementia in older individuals: A systematic review. Clin. Interv. Aging 2018, 13, 1497–1511. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.; Peng, Y.; Huang, Z.; Zhu, W. Antibiotics—Induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 2018, 146, 219–324. [Google Scholar] [CrossRef]
- Jameson, K.G.; Olson, C.A.; Kazmi, S.A.; Hsiao, E.Y. Toward Understanding Microbiome-Neuronal Signaling. Mol. Cell 2020, 78, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, L.; Bai, W.; Chen, W.; Chen, F.; Shu, C. Unveiling the Metabolic Modulatory Effect of Anthocyanin and Gut Microbiota Involvement; Springer: Singapore, 2021. [Google Scholar]
- Felgines, C.; Talaveéra, S.v.; Gonthier, M.-P.; Texier, O.; Scalbert, A.; Lamaison, J.-L.; Reémeésy, C. Strawberry anthocyanins are recovered in urine as glucuro-and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling Anthocyanin Bioavailability for Human Health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Coin-Araguez, L.; Roca-Rodriguez, M.; Delgado-Lista, J.; Cardona, F.; Andres-Lacueva, C.; Tinahones, F.J. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am. J. Clin. Nutr. 2013, 97, 1053–1061. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Freitas, V.D.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- González-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic Catabolism of Ellagitannins, Ellagic Acid, and Raspberry Anthocyanins: In Vivo and In Vitro Studies. Drug Metab. Dispos. Biol. Fate Chem. 2011, 39, 1680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Liu, C.; Gao, W.; Li, Z.; Qin, G.; Qi, S.; Jiang, H.; Li, X.; Liu, M.; Yan, F.; et al. Anthocyanins Are Converted into Anthocyanidins and Phenolic Acids and Effectively Absorbed in the Jejunum and Ileum. J. Agric. Food Chem. 2021, 69, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhou, W.; Xu, W.; Peng, Y.; Yan, Y.; Lu, L.; Mi, J.; Zeng, X.; Cao, Y. The Main Anthocyanin Monomer from Lycium ruthenicum Murray Fruit Mediates Obesity via Modulating the Gut Microbiota and Improving the Intestinal Barrier. Foods 2021, 11, 98. [Google Scholar] [CrossRef]
- Yu, W.; Gao, J.; Hao, R.; Yang, J.; Wei, J. Effects of simulated digestion on black chokeberry (Aronia melanocarpa (Michx.) Elliot) anthocyanins and intestinal flora. J. Food Sci. Technol. 2021, 58, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, B.; Zhong, C.; Guo, J.; Zhang, L.; Mu, T.; Zhang, Q.; Bi, X. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 2018, 39, 471–481. [Google Scholar] [CrossRef]
- Podsędek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix Effects on the Stability and Antioxidant Activity of Red Cabbage Anthocyanins under Simulated Gastrointestinal Digestion. BioMed Res. Int. 2014, 2014, 365738. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, C.; Chen, D.; Zeng, X.; Cao, Y. Prebiotic effects in vitro of anthocyanins from the fruits of Lycium ruthenicum Murray on gut microbiota compositions of feces from healthy human and patients with inflammatory bowel disease. LWT 2021, 149, 111829. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.P.; Reddivari, L. Anthocyanin-containing purple potatoes ameliorate DSS-induced colitis in mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Pascual-Teresa, S.D. Metabolism of Anthocyanins by Human Gut Microflora and Their Influence on Gut Bacterial Growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, Y.Z.; Wu, Y.X.; Zhang, M.Y.; Li, F.T.; He, Y.; Wen, L.k.; Yue, H. Effect of stabilization malvids anthocyanins on the gut microbiota in mice with oxidative stress. J. Food Biochem. 2021, 45, 4892–4902. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE 2018, 13, e0195754. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota In Vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef]
- Flores, G.; del Castillo, M.L.R.; Costabile, A.; Klee, A.; Guergoletto, K.B.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Santos-Martín, M.; Fernández-Recamales, Á. High-Throughput Method for Wide-Coverage and Quantitative Phenolic Fingerprinting in Plant-Origin Foods and Urine Samples. J. Agric. Food Chem. 2022, 70, 7796–7804. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, M.; Yang, F.; Liu, J. Antioxidant activity of high purity blueberry anthocyanins and the effects on human intestinal microbiota. LWT 2020, 117, 108621. [Google Scholar] [CrossRef]
- Boto- Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortu?o, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef]
- Blanco, G.; Ruiz, L.; Ruasmadiedo, P.; Fdezriverola, F.; Margolles, A. Revisiting the Metabolic Capabilities of Bifidobacterium longum susbp. longum and Bifidobacterium longum subsp. infantis from a Glycoside Hydrolase Perspective. Microorganisms 2020, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Kang, I.; Lee, Y.; Habib, M.A.; Choi, B.J.; Kang, J.S.; Park, D.-S.; Kim, Y.-S. Bifidobacterium longum subsp. infantis YB0411 Inhibits Adipogenesis in 3T3-L1 Pre-adipocytes and Reduces High-Fat-Diet-Induced Obesity in Mice. J. Agric. Food Chem. 2021, 69, 6032–6042. [Google Scholar] [CrossRef] [PubMed]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 1934578X1100600136. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Zhai, Y.; Liu, K.; Chen, J.; Yuan, F. Effects of black rice anthocyanins on the behavior and intestinal microbiota of mice with chronic unpredictable mild stress. IOP Conf. Ser. Earth Environ. Sci. 2021, 792, 012005–012006. [Google Scholar] [CrossRef]
- Si, X.; Bi, J.; Chen, Q.; Cui, H.; Bao, Y.; Tian, J.; Shu, C.; Wang, Y.; Tan, H.; Zhang, W.; et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021, 69, 3658–3666. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, Y.; Tang, J.; Mi, J.; Lu, L.; Li, X.; Ran, L.; Zeng, X.; Cao, Y. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J. Funct. Foods 2018, 48, 533–541. [Google Scholar] [CrossRef]
- Hosomi, K.; Kunisawa, J. The Specific Roles of Vitamins in the Regulation of Immunosurveillance and Maintenance of Immunologic Homeostasis in the Gut. Immune Netw. 2017, 17, 13. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Mo, Q.; Liu, T.; Fu, A.; Ruan, S.; Zhong, H.; Tang, J.; Zhao, M.; Li, Y.; Zhu, S.; Cai, H.; et al. Novel Gut Microbiota Patterns Involved in the Attenuation of Dextran Sodium Sulfate-Induced Mouse Colitis Mediated by Glycerol Monolaurate via Inducing Anti-inflammatory Responses. mBio 2021, 12, e02148-21. [Google Scholar] [CrossRef]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, Microbiome and Health Benefits in Aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, Y.; Xu, Z.; Na, L.; Zhu, B. An examination of data from the American Gut Project reveals that the dominance of the genus Bifidobacterium is associated with the diversity and robustness of the gut microbiota. MicrobiologyOpen 2019, 8, e939. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.A.; Oyama, L.M.; Conrado, R.D.M.; Rosso, V.D.; Nascimento, C.D.; Pisani, L.P. Polyphenols-rich fruit in maternal diet modulates inflammatory markers and the gut microbiota and improves colonic expression of ZO-1 in offspring. Food Res. Int. 2015, 77, 186–193. [Google Scholar] [CrossRef]
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K.; Zhang, J.; Paturi, G.; Hedderley, D.; Bovy, A.; et al. Dietary Flavonoids from Modified Apple Reduce Inflammation Markers and Modulate Gut Microbiota in Mice. J. Nutr. 2013, 144, 146–154. [Google Scholar] [CrossRef]
- Krga, I.; Monfoulet, L.E.; Konic-Ristic, A.; Mercier, S.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites reduce the adhesion of monocyte to TNFα-activated endothelial cells at physiologically relevant concentrations. Arch. Biochem. Biophys. 2016, 599, 51–59. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967. [Google Scholar] [CrossRef]
- Yang, L.A.; Jlf, A.; Ke, Y.; Shj, B.; Ying, G.C. Ameliorative effect of purified anthocyanin from Lycium ruthenicum on atherosclerosis in rats through synergistic modulation of the gut microbiota and NF-κB/SREBP-2 pathways. J. Funct. Foods 2019, 59, 223–233. [Google Scholar] [CrossRef]
- Eisenstein, M. Microbiome: Bacterial broadband. Nature 2016, 533, S104–S106. [Google Scholar] [CrossRef]
- Bogunovic, M.; Davé, S.; Tilstra, J.; Chang, D.; Harpaz, N.; Xiong, H.; Mayer, L.; Plevy, S. Enteroendocrine cells express functional Toll-like receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1770–G1783. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, F.; Pedersen, N.L.; Tillander, A.; Ludvigsson, J.F.; Ekbom, A. Vagotomy and Parkinson disease A Swedish register-based matched-cohort study. Neurology 2017, 88, 1996–2002. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Adeli, K. Role of Gut Microbiota in Neuroendocrine Regulation of Carbohydrate and Lipid Metabolism via the Microbiota-Gut-Brain-Liver Axis. Microorganisms 2020, 8, 527. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Grasset, E.; Mannerås Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E.; Bäckhed, F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA 2018, 115, 6458–6463. [Google Scholar] [CrossRef] [PubMed]
- Diamond, B.; Huerta, P.T.; Tracey, K.; Volpe, B.T. It takes guts to grow a brain: Increasing evidence of the important role of the intestinal microflora in neuro- and immune-modulatory functions during development and adulthood. BioEssays 2011, 33, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Angelis, A.L.H.D.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.; Bunse, L.; Takenaka, M.; Kenison, J.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143–159. [Google Scholar] [CrossRef]
- Logsdon, A.F.; Erickson, M.A.; Rhea, E.M.; Salameh, T.S.; Banks, W.A. Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. 2017, 243, 159–165. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- O’Mahony, L.; Mccarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.; Kiely, B.; Collins, J.; Shanahan, F. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Wei, H.; Chadman, K.K.; McCloskey, D.P.; Sheikh, A.M.; Malik, M.; Brown, W.T.; Li, X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 831–842. [Google Scholar] [CrossRef]
- Deshmukh, H.S.; Liu, Y.; Menkiti, O.R.; Mei, J.; Dai, N.; O’Leary, C.E.; Oliver, P.M.; Kolls, J.K.; Weiser, J.N.; Worthen, G.S. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat. Med. 2014, 20, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, W.; Sebastian, P.; Claudia, P.; Herbert, R.; Ulrich, B.; Armin, S. The Granulocyte-colony stimulating factor has a dual role in neuronal and vascular plasticity. Front. Cell Dev. Biol. 2015, 3, 48. [Google Scholar] [CrossRef]
- Yang, L.; Lin, H.; Lin, W.; Xu, X. Exercise Ameliorates Insulin Resistance of Type 2 Diabetes through Motivating Short-Chain Fatty Acid-Mediated Skeletal Muscle Cell Autophagy. Biology 2020, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- David, R.-C.; Patricia, R.-M.; Abelardo, M.; Miguel, G.; G, d.l.R.-G.C.; Nuria, S. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Rafiki, A.; Boulland, J.; Halestrap, A.; Ottersen, O.; Bergersen, L. Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neuroscience 2003, 122, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.; Mawe, G. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.; Terekhova, D.; Liu, J.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef]
- Młynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The Role of the Microbiome-Brain-Gut Axis in the Pathogenesis of Depressive Disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef]
- Shaaban, S.Y.; El Gendy, Y.G.; Mehanna, N.S.; El-Senousy, W.M.; El-Feki, H.S.A.; Saad, K.; El-Asheer, O.M. The role of probiotics in children with autism spectrum disorder: A prospective, open-label study. Nutr. Neurosci. 2017, 21, 676–681. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice: Commensal microbiota and stress response. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Berardi, S.; Scarpona, S.; Rossi, G.; Eleuteri, A. SLAB51 Probiotic Formulation Activates SIRT1 Pathway Promoting Antioxidant and Neuroprotective Effects in an AD Mouse Model. Mol. Neurobiol. 2018, 55, 7987–8000. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R.; Kouchaki, E.; Bahmani, F.; Borzabadi, S.; Oryan, S.; Mafi, A.; Asemi, Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Sheu, J.C.; Venkatachalam, A.; Runge, J.K.; Luna, R.A.; Calarge, C.A. Gut microbiome in adolescent depression. J. Affect. Disord. 2021, 292, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zeng, B.; Liu, M.; Chen, J.; Peng, X. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Christina, L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 2016, 62, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Annamaria, P.; Sabrina, M.; Karl, K.; Bettina, H.; Nina, D.; Susanne, B.; Armin, B.; Frederike, F.; Martina, P.; Robert, Q. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2018, 21, 40–49. [Google Scholar] [CrossRef]
- Lu, Q.; Lai, J.; Lu, H.; Ng, C.; Hu, S. Gut Microbiota in Bipolar Depression and Its Relationship to Brain Function: An Advanced Exploration. Front. Psychiatry 2019, 10, 784. [Google Scholar] [CrossRef]
- Alzheimer’s, A. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Elmira, A.; Zatollah, A.; Reza, D.K.; Fereshteh, B.; Ebrahim, K.; Reza, T.O.; Ali, H.G.; Mahmoud, S. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Cryan, J.; O’Riordan, K.; Cowan, C.; Sandhu, K.; Dinan, T. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Tran, L.; Greenwood-Van, M.B. Age-Associated Remodeling of the Intestinal Epithelial Barrier. J. Gerontol. 2013, 68, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhu, Y.; Zhou, Z.; Jia, X.; Xu, Y.; Yang, Q.; Cui, C.; Shen, Y. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, J. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 2016, 324, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liang, J.; Liang, M.; Dai, J.; Wang, J.; Luo, Z. Altered Gut Microbiota in Chinese Children With Autism Spectrum Disorders. Front. Cell Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Janik, R.; Thomason, L.; Stanisz, A.; Forsythe, P.; Stanisz, G. Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate and glutamate. NeuroImage 2015, 125, 988–995. [Google Scholar] [CrossRef]
- Kroner, Z. Vitamins and Minerals: Fact Versus Fiction; ABC-CLIO: Santa Barbara, CA, USA, 2018. [Google Scholar]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.L.; Vos, T.; Whiteford, H.A. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Baker, G.B.; Dursun, S.M. The Relationship Between the Gut Microbiome-Immune System-Brain Axis and Major Depressive Disorder. Front. Neurol. 2021, 12, 721126. [Google Scholar] [CrossRef]
- Molteni, R.; Macchi, F.; Zecchillo, C.; Dell”Agli, M.; Colombo, E.; Calabrese, F.; Guidotti, G.; Racagni, G.; Riva, M.A. Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2013, 23, 1645–1655. [Google Scholar] [CrossRef]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linlkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Borelli, C.M.; Solari, H. Schizophrenia. JAMA 2019, 322, 1322. [Google Scholar] [CrossRef] [PubMed]
- Genedi, M.; Janmaat, I.E.; Haarman, B.B.C.M.; Sommer, I.E.C. Dysregulation of the gut-brain axis in schizophrenia and bipolar disorder: Probiotic supplementation as a supportive treatment in psychiatric disorders. Curr. Opin. Psychiatry 2019, 32, 185–195. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Ma, X. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 2020, 11, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Coello, K.; Hansen, T.H.; Sørensen, N.; Munkholm, K.; Kessing, L.V.; Pedersen, O.; Vinberg, M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav. Immun. 2018, 75, 112–118. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Wu, H.Q.; Pereira, E.; Bruno, J.P.; Pellicciari, R.; Schwarcz, R. The Astrocyte-Derived α7 Nicotinic Receptor Antagonist Kynurenic Acid Controls Extracellular Glutamate Levels in the Prefrontal Cortex. J. Mol. Neurosci. 2010, 40, 204–210. [Google Scholar] [CrossRef]
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742. [Google Scholar] [CrossRef]
- Whitfield-Cargile, C.M.; Cohen, N.D.; Chapkin, R.S.; Weeks, B.R.; Davidson, L.A.; Goldsby, J.S.; Hunt, C.L.; Steinmeyer, S.H.; Menon, R.; Suchodolski, J.S. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes 2016, 7, 246–261. [Google Scholar] [CrossRef]
- Stasi, C.; Sadalla, S.; Milani, S. The Relationship Between the Serotonin Metabolism, Gut-Microbiota and the Gut-Brain Axis. Curr. Drug Metab. 2019, 20, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.W.; Wang, L.S. Black Raspberries and Their Anthocyanin and Fiber Fractions Alter the Composition and Diversity of Gut Microbiota in F-344 Rats. Nutr. Cancer 2017, 69, 943. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef]
- Yang, L.L.; Millischer, V.; Rodin, S.; Macfabe, D.F.; Villaescusa, J.C.; Lavebratt, C. Enteric shor—Chain fatty acids promote proliferation of human neural progenitor cells. J. Neurochem. 2020, 154, 635–646. [Google Scholar] [CrossRef]
- Park, J.; Qin, W.; Qi, W.; Yang, M.; Chang, H.K. Bidirectional regulatory potentials of short-chain fatty acids and their G-protein-coupled receptors in autoimmune neuroinflammation. Sci. Rep. 2019, 9, 8837. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Toth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Cueva, C.; Alba, C.; Rodriguez, J.M.; de Pascual-Teresa, S.; Jones, J.; Caturla, N.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut microbiome-modulating properties of a polyphenol-enriched dietary supplement comprised of hibiscus and lemon verbena extracts. Monitoring of phenolic metabolites. J. Funct. Foods 2022, 91, 105016. [Google Scholar] [CrossRef]
- Khan, M.S.; Ikram, M.; Park, J.S.; Park, T.J.; Kim, M.O. Gut microbiota, its role in induction of Alzheimer’s disease pathology, and possible therapeutic interventions: Special focus on anthocyanins. Cells 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Morissette, A.; Kropp, C.; Songpadith, J.P.; Moreira, R.J.; Marette, A. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. AJP Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Knorr, D.A.; Haptonstall, K.M. Alzheimer’s disease and symbiotic microbiota: An evolutionary medicine perspective. Ann. N. York Acad. Sci. 2019, 1449, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Marta, S.; Katarzyna, D.Y.; Satler, D.B.; Donata, K.; Ewa, B.; Jerzy, L. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2018, 56, 1841–1851. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2018, 23, 570. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Su, J.; Gao, X.; Yang, H.; Weng, R.; Ni, W.; Gu, Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 2022, 10, 62. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).