Sleep, Nutrition, and Injury Risk in Adolescent Athletes: A Narrative Review
Abstract
1. Introduction
2. Importance of Sleep Health
3. Sleep Adaptations during Adolescence
4. Growth, Maturation, and Energy Demands in Adolescent Athletes
5. Differences between Adolescent and Adult Athletes
6. Nutrition Knowledge of Adolescents
7. Recovery, Adaptation, and Fatigue during the Training Process
8. Injury Risk in Adolescent Athletes
9. Relationship between Sleep, Nutrition, and Injury Risk in Adolescent Athletes
10. Limitations
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shapiro, C.M. Sleep and the Athlete. Br. J. Sports Med. 1981, 15, 51–55. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allada, R.; Siegel, J.M. Unearthing the Phylogenetic Roots of Sleep. Curr. Biol. 2008, 18, R670–R679. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Sleep and the Elite Athlete. Sports Sci. Exch. 2013, 26, 1–4. [Google Scholar]
- Fullagar, H.H.K.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and Athletic Performance: The Effects of Sleep Loss on Exercise Performance, and Physiological and Cognitive Responses to Exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Chokroverty, S. (Ed.) Overview of Normal Sleep. In Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects; Springer: New York, NY, USA, 2017; pp. 5–27. ISBN 978-1-4939-6578-6. [Google Scholar]
- Hobson, J.A. Sleep Is of the Brain, by the Brain and for the Brain. Nature 2005, 437, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- VanHelder, T.; Symons, J.D.; Radomski, M.W. Effects of Sleep Deprivation and Exercise on Glucose Tolerance. Aviat. Space Environ. Med. 1993, 64, 487–492. [Google Scholar]
- Penev, P.D. Sleep Deprivation and Energy Metabolism: To Sleep, Perchance to Eat? Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.C.-P.; Shui, G.; Cazenave-Gassiot, A.; Wenk, M.R.; Gooley, J.J. Changes in Plasma Lipids during Exposure to Total Sleep Deprivation. Sleep 2015, 38, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Trinder, J.; Waloszek, J.; Woods, M.J.; Jordan, A.S. Sleep and Cardiovascular Regulation. Pflüg. Arch. Eur. J. Physiol. 2012, 463, 161–168. [Google Scholar] [CrossRef]
- Vaara, J.; Kyröläinen, H.; Koivu, M.; Tulppo, M.; Finni, T. The Effect of 60-h Sleep Deprivation on Cardiovascular Regulation and Body Temperature. Eur. J. Appl. Physiol. 2009, 105, 439–444. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Cauter, E.V. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse Metabolic and Cardiovascular Consequences of Circadian Misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of Sleep Debt on Metabolic and Endocrine Function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Santos, R.V.T.; Tufik, S.; De Mello, M.T. Exercise, Sleep and Cytokines: Is There a Relation? Sleep Med. Rev. 2007, 11, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Benington, J.H. The Role of Sleep in Memory Consolidation and Brain Plasticity: Dream or Reality? Neuroscientist 2006, 12, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Halson, S.L.; Weakley, J.; Hawley, J.A. Sleep, Circadian Biology and Skeletal Muscle Interactions: Implications for Metabolic Health. Sleep Med. Rev. 2022, 66, 101700. [Google Scholar] [CrossRef] [PubMed]
- Adam, K.; Oswald, I. Sleep Is for Tissue Restoration. J. R. Coll. Physicians 1977, 11, 376. [Google Scholar]
- Malhotra, R.K. Sleep, Recovery, and Performance in Sports. Neurol. Clin. 2017, 35, 547–557. [Google Scholar] [CrossRef]
- Kölling, S.; Duffield, R.; Erlacher, D.; Venter, R.; Halson, S.L. Sleep-Related Issues for Recovery and Performance in Athletes. Int. J. Sports Physiol. Perform. 2019, 14, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Charest, J.; Grandner, M.A. Sleep and Athletic Performance: Impacts on Physical Performance, Mental Performance, Injury Risk and Recovery, and Mental Health. Sleep Med. Clin. 2020, 15, 41–57. [Google Scholar] [CrossRef]
- Doherty, R.; Madigan, S.M.; Nevill, A.; Warrington, G.; Ellis, J.G. The Sleep and Recovery Practices of Athletes. Nutrients 2021, 13, 1330. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the Athlete: Narrative Review and 2021 Expert Consensus Recommendations. Br. J. Sports Med. 2021, 55, 356–368. [Google Scholar] [CrossRef]
- McCarley, R.W. Neurobiology of REM and NREM Sleep. Sleep Med. 2007, 8, 302–330. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2012; Volume 176, p. 2012. [Google Scholar]
- Halson, S.L.; Juliff, L.E. Sleep, Sport, and the Brain. Prog. Brain Res. 2017, 234, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Dement, W.C. Chapter 2—Normal Human Sleep: An Overview. In Principles and Practice of Sleep Medicine, 4th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2005; pp. 13–23. ISBN 978-0-7216-0797-9. [Google Scholar]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. Clues to the Functions of Mammalian Sleep. Nature 2005, 437, 1264–1271. [Google Scholar] [CrossRef]
- Stickgold, R. Sleep-Dependent Memory Consolidation. Nature 2005, 437, 1272–1278. [Google Scholar] [CrossRef]
- Sriraam, N.; Padma Shri, T.K.; Maheshwari, U. Recognition of Wake-Sleep Stage 1 Multichannel Eeg Patterns Using Spectral Entropy Features for Drowsiness Detection. Australas. Phys. Eng. Sci. Med. 2016, 39, 797–806. [Google Scholar] [CrossRef]
- Venter, R.E. Role of Sleep in Performance and Recovery of Athletes: A Review Article. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2012, 34, 167–184. [Google Scholar]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth with Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Sassin, J.F.; Parker, D.C.; Mace, J.W.; Gotlin, R.W.; Johnson, L.C.; Rossman, L.G. Human Growth Hormone Release: Relation to Slow-Wave Sleep and Sleep-Waking Cycles. Science 1969, 165, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J. Sleep Health: Can We Define It? Does It Matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Matricciani, L.; Bin, Y.S.; Lallukka, T.; Kronholm, E.; Wake, M.; Paquet, C.; Dumuid, D.; Olds, T. Rethinking the Sleep-Health Link. Sleep Health 2018, 4, 339–348. [Google Scholar] [CrossRef]
- Benítez, I.; Roure, N.; Pinilla, L.; Sapiña-Beltran, E.; Buysse, D.J.; Barbé, F.; de Batlle, J. Validation of the Satisfaction, Alertness, Timing, Efficiency and Duration (SATED) Questionnaire for Sleep Health Measurement. Ann. Am. Thorac. Soc. 2020, 17, 338–343. [Google Scholar] [CrossRef]
- Halson, S.L. Nutrition, Sleep and Recovery. Eur. J. Sport Sci. 2008, 8, 119–126. [Google Scholar] [CrossRef]
- Samuels, C. Sleep, Recovery, and Performance: The New Frontier in High-Performance Athletics. Neurol. Clin. 2008, 26, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Samuels, C.; James, L.; Lawson, D.; Meeuwisse, W. The Athlete Sleep Screening Questionnaire: A New Tool for Assessing and Managing Sleep in Elite Athletes. Br. J. Sports Med. 2016, 50, 418–422. [Google Scholar] [CrossRef] [PubMed]
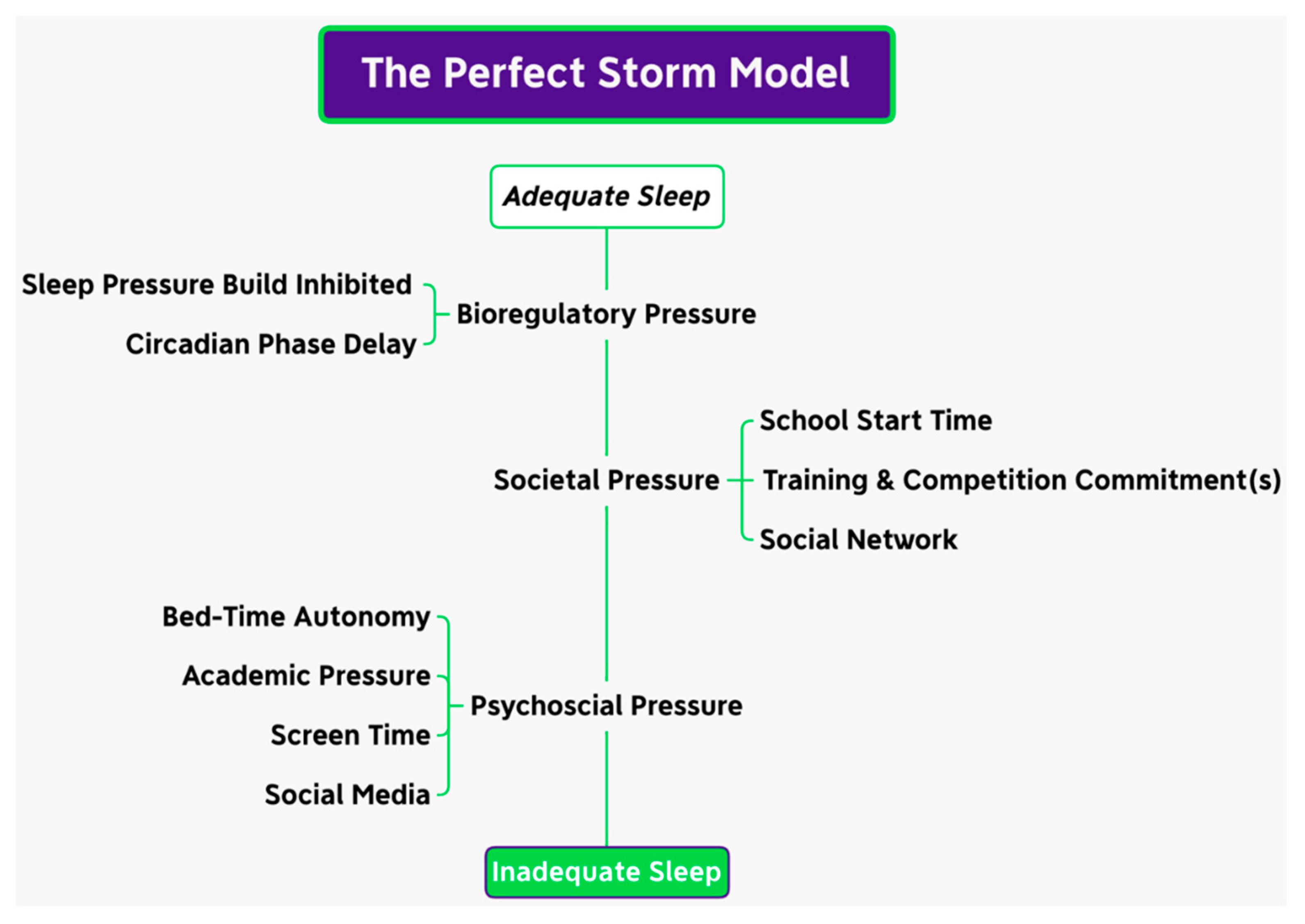
- Carskadon, M.A. Sleep in Adolescents: The Perfect Storm. Pediatr. Clin. N. Am. 2011, 58, 637–647. [Google Scholar] [CrossRef]
- Crowley, S.J.; Wolfson, A.R.; Tarokh, L.; Carskadon, M.A. An Update on Adolescent Sleep: New Evidence Informing the Perfect Storm Model. J. Adolesc. 2018, 67, 55–65. [Google Scholar] [CrossRef]
- Ong, J.L.; Lo, J.C.; Gooley, J.J.; Chee, M.W.L. EEG Changes Accompanying Successive Cycles of Sleep Restriction with and without Naps in Adolescents. Sleep 2017, 40, zsx030. [Google Scholar] [CrossRef]
- Skein, M.; Duffield, R.; Minett, G.M.; Snape, A.; Murphy, A. The Effect of Overnight Sleep Deprivation after Competitive Rugby League Matches on Postmatch Physiological and Perceptual Recovery. Int. J. Sports Physiol. Perform. 2013, 8, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Milewski, M.D.; Skaggs, D.L.; Bishop, G.A.; Pace, J.L.; Ibrahim, D.A.; Wren, T.A.L.; Barzdukas, A. Chronic Lack of Sleep Is Associated with Increased Sports Injuries in Adolescent Athletes. J. Pediatr. Orthop. 2014, 34, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Katz, E.S.; Kheirandish-Gozal, L.; et al. National Sleep Foundation’s Sleep Time Duration Recommendations: Methodology and Results Summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Copenhaver, E.A.; Diamond, A.B. The Value of Sleep on Athletic Performance, Injury, and Recovery in the Young Athlete. Pediatr. Ann. 2017, 46, e106–e111. [Google Scholar] [CrossRef] [PubMed]
- Coel, R.A.; Pujalte, G.G.A.; Applewhite, A.I.; Zaslow, T.; Cooper, G.; Ton, A.N.; Benjamin, H.J. Sleep and the Young Athlete. Sports Health 2023, 15, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating Clocks Shape Circadian Homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef] [PubMed]
- Kryger, M.H.; Roth, T.; Dement, W.C. (Eds.) Principles and Practice of Sleep Medicine, 6th ed.; Elsevier: Philadelphia, PA, USA, 2017; ISBN 978-0-323-24288-2. [Google Scholar]
- Lassi, G.; Tucci, V. Genomic Imprinting and the Control of Sleep in Mammals. Curr. Opin. Behav. Sci. 2019, 25, 77–82. [Google Scholar] [CrossRef]
- Kurien, P.; Ptáček, L.J.; Fu, Y.-H. Chapter 11—The Genetic Regulation of Human Sleep-Wake Rhythms and Patterns. In Handbook of Behavioral Neuroscience; Dringenberg, H.C., Ed.; Handbook of Sleep Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 169–180. [Google Scholar]
- Borbély, A.A. A Two Process Model of Sleep Regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The Two-Process Model of Sleep Regulation: A Reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Rosenthal, L.; Day, R.; Gerhardstein, R.; Meixner, R.; Roth, T.; Guido, P.; Fortier, J. Sleepiness/Alertness among Healthy Evening and Morning Type Individuals. Sleep Med. 2001, 2, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Karan, M.; Bai, S.; Almeida, D.M.; Irwin, M.R.; McCreath, H.; Fuligni, A.J. Sleep-Wake Timings in Adolescence: Chronotype Development and Associations with Adjustment. J. Youth Adolesc. 2021, 50, 628–640. [Google Scholar] [CrossRef]
- Kuula, L.; Pesonen, A.-K.; Merikanto, I.; Gradisar, M.; Lahti, J.; Heinonen, K.; Kajantie, E.; Räikkönen, K. Development of Late Circadian Preference: Sleep Timing From Childhood to Late Adolescence. J. Pediatr. 2018, 194, 182–189.e1. [Google Scholar] [CrossRef] [PubMed]
- Hagenauer, M.H.; Lee, T.M. The Neuroendocrine Control of the Circadian System: Adolescent Chronotype. Front. Neuroendocrinol. 2012, 33, 211–229. [Google Scholar] [CrossRef]
- Fischer, D.; Lombardi, D.A.; Marucci-Wellman, H.; Roenneberg, T. Chronotypes in the US—Influence of Age and Sex. PLoS ONE 2017, 12, e0178782. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Vieira, C.; Acebo, C. Association between Puberty and Delayed Phase Preference. Sleep 1993, 16, 258–262. [Google Scholar] [CrossRef]
- Jenni, O.G.; Achermann, P.; Carskadon, M.A. Homeostatic Sleep Regulation in Adolescents. Sleep 2005, 28, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Weydahl, A. Chronotype, Physical Activity, and Sport Performance: A Systematic Review. Sports Med. 2017, 47, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Sleep Monitoring in Athletes: Motivation, Methods, Miscalculations and Why It Matters. Sports Med. 2019, 49, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.; Lastella, M.; Halson, S.L.; Roach, G.D. How Much Sleep Does an Elite Athlete Need? Int. J. Sports Physiol. Perform. 2021, 16, 1746–1757. [Google Scholar] [CrossRef]
- Cunha, L.A.; Costa, J.A.; Marques, E.A.; Brito, J.; Lastella, M.; Figueiredo, P. The Impact of Sleep Interventions on Athletic Performance: A Systematic Review. Sports Med. Open 2023, 9, 58. [Google Scholar] [CrossRef]
- Lo, H.; Leung, J.; Chau, K.Y.; Lam, M.H.S.; Lee, K.Y.; Ho, A. Factors Affecting Sleep Quality among Adolescent Athletes. Sports Nutr. Ther. 2017, 2, 2. [Google Scholar] [CrossRef]
- Patel, A.R.; Hsu, A.; Perez, I.A.; Wren, T.A.L.; Edison, B.R. Assessing the Effects of Sleep on Neurocognitive Performance and Injury Rate in Adolescent Athletes Using Actigraphy. Res. Sports Med. 2020, 28, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Tarokh, L.; Short, M.; Crowley, S.J.; Fontanellaz-Castiglione, C.E.G.; Carskadon, M.A. Sleep and Circadian Rhythms in Adolescence. Curr. Sleep Med. Rep. 2019, 5, 181–192. [Google Scholar] [CrossRef]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Malow, B.A.; Maski, K.; Nichols, C.; Quan, S.F.; et al. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2016, 12, 785–786. [Google Scholar] [CrossRef]
- Crowley, S.J.; Reen, E.V.; LeBourgeois, M.K.; Acebo, C.; Tarokh, L.; Seifer, R.; Barker, D.H.; Carskadon, M.A. A Longitudinal Assessment of Sleep Timing, Circadian Phase, and Phase Angle of Entrainment across Human Adolescence. PLoS ONE 2014, 9, e112199. [Google Scholar] [CrossRef] [PubMed]
- Skeldon, A.C.; Derks, G.; Dijk, D.-J. Modelling Changes in Sleep Timing and Duration across the Lifespan: Changes in Circadian Rhythmicity or Sleep Homeostasis? Sleep Med. Rev. 2016, 28, 96–107. [Google Scholar] [CrossRef]
- Deboer, T. Sleep Homeostasis and the Circadian Clock: Do the Circadian Pacemaker and the Sleep Homeostat Influence Each Other’s Functioning? Neurobiol. Sleep Circadian Rhythms 2018, 5, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Tarokh, L.; Saletin, J.M.; Carskadon, M.A. Sleep in Adolescence: Physiology, Cognition and Mental Health. Neurosci. Biobehav. Rev. 2016, 70, 182–188. [Google Scholar] [CrossRef]
- Carskadon, M.A. Sleep and Circadian Rhythms in Children and Adolescents: Relevance for Athletic Performance of Young People. Clin. Sports Med. 2005, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.G. Sleep Duration and Injury-Related Risk Behaviors Among High School Students—United States, 2007–2013. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 337–341. [Google Scholar] [CrossRef]
- Yang, C.-K.; Kim, J.K.; Patel, S.R.; Lee, J.-H. Age-Related Changes in Sleep/Wake Patterns among Korean Teenagers. Pediatrics 2005, 115, 250–256. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef]
- Mary, A.C.; Harvey, K.; Duke, P.; Thomas, F.A.; Iris, F.L.; William, C.D. Pubertal Changes in Daytime Sleepiness. Sleep 1980, 2, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping Hours: What Is the Ideal Number and How Does Age Impact This? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef]
- Kelley, P.; Lockley, S.W.; Foster, R.G.; Kelley, J. Synchronizing Education to Adolescent Biology: ‘Let Teens Sleep, Start School Later’. Learn. Media Technol. 2015, 40, 210–226. [Google Scholar] [CrossRef]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in Adolescent Growth and Development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cumming, S.P.; Lloyd, R.S.; Oliver, J.L.; Eisenmann, J.C.; Malina, R.M. Bio-Banding in Sport: Applications to Competition, Talent Identification, and Strength and Conditioning of Youth Athletes. Strength Cond. J. 2017, 39, 34–47. [Google Scholar] [CrossRef]
- Bergeron, M.F.; Mountjoy, M.; Armstrong, N.; Chia, M.; Côté, J.; Emery, C.A.; Faigenbaum, A.; Hall, G.; Kriemler, S.; Léglise, M. International Olympic Committee Consensus Statement on Youth Athletic Development. Br. J. Sports Med. 2015, 49, 843–851. [Google Scholar] [CrossRef]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity; Human Kinetics: Champaign, IL, USA, 2004; ISBN 0-88011-882-2. [Google Scholar]
- Armstrong, N.; Van Mechelen, W. Oxford Textbook of Children’s Sport and Exercise Medicine; Oxford University Press: Oxford, UK, 2017; ISBN 0-19-875767-0. [Google Scholar]
- Malina, R.M.; Rogol, A.D.; Cumming, S.P.; Coelho e Silva, M.J.; Figueiredo, A.J. Biological Maturation of Youth Athletes: Assessment and Implications. Br. J. Sports Med. 2015, 49, 852–859. [Google Scholar] [CrossRef]
- Baxter-Jones, A.D.; Faulkner, R.A.; Forwood, M.R.; Mirwald, R.L.; Bailey, D.A. Bone Mineral Accrual from 8 to 30 Years of Age: An Estimation of Peak Bone Mass. J. Bone Miner. Res. 2011, 26, 1729–1739. [Google Scholar] [CrossRef]
- Rodriguez-Negro, J.; Llodio, I.; Castillo, D.; Romaratezabala, E.; Yanci, J. Changes in Selected Locomotor Skills of Young Runners after One Athletic Season: Influence of Sex and Age. Int. J. Sports Sci. Coach. 2021, 16, 1152–1161. [Google Scholar] [CrossRef]
- Malina, R.M.; Rogol, A.D. Sport Training and the Growth and Pubertal Maturation of Young Athletes. Pediatr. Endocrinol. Rev. PER 2011, 9, 441–455. [Google Scholar]
- McBurnie, A.; Dos’Santos, T.; Johnson, D.; Leng, E. Training Management of the Elite Adolescent Soccer Player throughout Maturation. Sports 2021, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, N.; Schley, S.; Cumming, S.P.; Myer, G.D.; Saffel, H.; Hartwig, T.; Gabbett, T.J. Developmental Training Model for the Sport Specialized Youth Athlete: A Dynamic Strategy for Individualizing Load-Response During Maturation. Sports Health 2022, 14, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, B. Youth Athlete Development and Nutrition. Sports Med. 2021, 51, 3–12. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Afifi, R.A.; Bearinger, L.H.; Blakemore, S.-J.; Dick, B.; Ezeh, A.C.; Patton, G.C. Adolescence: A Foundation for Future Health. Lancet 2012, 379, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Meeus, W. Adolescent Psychosocial Development: A Review of Longitudinal Models and Research. Dev. Psychol. 2016, 52, 1969–1993. [Google Scholar] [CrossRef]
- Hannon, M.P.; Parker, L.J.F.; Carney, D.J.; Mckeown, J.; Speakman, J.R.; Hambly, C.; Drust, B.; Unnithan, V.B.; Close, G.L.; Morton, J.P. Energy Requirements of Male Academy Soccer Players from the English Premier League. Med. Sci. Sports Exerc. 2021, 53, 200–210. [Google Scholar] [CrossRef]
- Hannon, M.P.; Close, G.L.; Morton, J.P. Energy and Macronutrient Considerations for Young Athletes. Strength Cond. J. 2020, 42, 109–119. [Google Scholar] [CrossRef]
- Berg, E.K. Performance Nutrition for the Adolescent Athlete: A Realistic Approach. Clin. J. Sport Med. 2019, 29, 345–352. [Google Scholar] [CrossRef]
- Aerenhouts, D.; Deriemaeker, P.; Hebbelinck, M.; Clarys, P. Energy and Macronutrient Intake in Adolescent Sprint Athletes: A Follow-up Study. J. Sports Sci. 2011, 29, 73–82. [Google Scholar] [CrossRef]
- Jeukendrup, A.; Gleeson, M. Sport Nutrition; Human Kinetics: Champaign, IL, USA, 2018; ISBN 1-4925-2903-6. [Google Scholar]
- Benardot, D. Advanced Sports Nutrition, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2021; ISBN 978-1-4925-9311-9. [Google Scholar]
- Spano, M.A.; Kruskall, L.J.; Thomas, D.T. Nutrition for Sport, Exercise, and Health; Human Kinetics: Champaign, IL, USA, 2018; ISBN 978-1-4504-1487-6. [Google Scholar]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.P.; Unnithan, V.; Morton, J.P.; Close, G.L. Nutritional Strategies to Support Young Athletes. In Strength and Conditioning for Young Athletes; Routledge: Oxford, UK, 2019; pp. 300–335. [Google Scholar]
- Pontzer, H.; Yamada, Y.; Sagayama, H.; Ainslie, P.N.; Andersen, L.F.; Anderson, L.J.; Arab, L.; Baddou, I.; Bedu-Addo, K.; Blaak, E.E. Daily Energy Expenditure through the Human Life Course. Science 2021, 373, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.W.; Tseh, W.; Caputo, J.L.; Keefer, D.J.; Craig, I.S.; Griffith, K.B.; Akins, M.B.; Griffith, G.E.; Krahenbuhl, G.S.; Martin, P.E. Longitudinal Stratification of Gait Economy in Young Boys and Girls: The Locomotion Energy and Growth Study. Eur. J. Appl. Physiol. 2004, 91, 30–34. [Google Scholar] [PubMed]
- Morgan, D.W. Locomotor Economy. In Paediatric Exercise Science and Medicine; Armstrong, N., Van Mechelen, W., Eds.; Oxford Medical Publications; Oxford University Press: Oxford, UK; New York, NY, USA, 2008; pp. 283–295. ISBN 978-0-19-923248-2. [Google Scholar]
- Eriksson, O.; Saltin, B. Muscle Metabolism during Exercise in Boys Aged 11 to 16 Years Compared to Adults. Acta Paediatr. Belg. 1974, 28, 257–265. [Google Scholar] [PubMed]
- Stephens, B.R.; Cole, A.S.; Mahon, A.D. The Influence of Biological Maturation on Fat and Carbohydrate Metabolism during Exercise in Males. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 166–179. [Google Scholar] [CrossRef]
- Eriksson, B.O.; Gollnick, P.D.; Saltin, B. Muscle Metabolism and Enzyme Activities after Training in Boys 11–13 Years Old. Acta Physiol. Scand. 1973, 87, 485–497. [Google Scholar] [CrossRef]
- Timmons, B.W.; Bar-Or, O.; Riddell, M.C. Oxidation Rate of Exogenous Carbohydrate during Exercise Is Higher in Boys than in Men. J. Appl. Physiol. 2003, 94, 278–284. [Google Scholar] [CrossRef]
- Timmons, B.W.; Bar-Or, O.; Riddell, M.C. Energy Substrate Utilization during Prolonged Exercise with and without Carbohydrate Intake in Preadolescent and Adolescent Girls. J. Appl. Physiol. 2007, 103, 995–1000. [Google Scholar] [CrossRef]
- Timmons, B.W.; Bar-Or, O.; Riddell, M.C. Influence of Age and Pubertal Status on Substrate Utilization during Exercise with and without Carbohydrate Intake in Healthy Boys. Appl. Physiol. Nutr. Metab. 2007, 32, 416–425. [Google Scholar] [CrossRef]
- MacDougall, J.; Roche, P.; Bar-Or, O.; Moroz, J. Maximal Aerobic Capacity of Canadian Schoolchildren: Prediction Based on Age-Related Oxygen Cost of Running. Int. J. Sports Med. 1983, 4, 194–198. [Google Scholar] [CrossRef]
- Roche, D.M.; Rowland, T.; Garrard, M.; Marwood, S.; Unnithan, V. Skin Microvascular Reactivity in Trained Adolescents. Eur. J. Appl. Physiol. 2010, 108, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Bar-Or, O.; MacDougall, J.D. Thermoregulatory Responses of Pre-, Mid-, and Late-Pubertal Boys to Exercise in Dry Heat. Med. Sci. Sports Exerc. 1992, 24, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.A.; Baker, L.B.; Chow, M.; Kenney, W.L. Two Percent Dehydration Impairs and Six Percent Carbohydrate Drink Improves Boys Basketball Skills. Med. Sci. Sports Exerc. 2006, 38, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Dyerberg, J.; Elwood, P.; Hermansen, K.; Hu, F.B.; Jakobsen, M.U.; Kok, F.J.; Krauss, R.M.; Lecerf, J.M.; LeGrand, P. The Role of Reducing Intakes of Saturated Fat in the Prevention of Cardiovascular Disease: Where Does the Evidence Stand in 2010? Am. J. Clin. Nutr. 2011, 93, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Carl, R.L.; Johnson, M.D.; Martin, T.J.; LaBella, C.R.; Brooks, M.A.; Diamond, A.; Hennrikus, W.; LaBotz, M.; Logan, K.; Loud, K.J. Promotion of Healthy Weight-Control Practices in Young Athletes. Pediatrics 2017, 140, e20171871. [Google Scholar] [CrossRef] [PubMed]
- Torun, B. Energy Requirements of Children and Adolescents. Public Health Nutr. 2005, 8, 968–993. [Google Scholar] [CrossRef]
- World Health Organization. Measuring Change in Nutritional Status; World Health Organization: Geneva, Switzerland, 1982; ISBN 92-4-154166-0. [Google Scholar]
- Silva, A.M.; Santos, D.A.; Matias, C.N.; Minderico, C.S.; Schoeller, D.A.; Sardinha, L.B. Total Energy Expenditure Assessment in Elite Junior Basketball Players: A Validation Study Using Doubly Labeled Water. J. Strength Cond. Res. 2013, 27, 1920–1927. [Google Scholar] [CrossRef]
- Hannon, M.P.; Carney, D.J.; Floyd, S.; Parker, L.J.F.; McKeown, J.; Drust, B.; Unnithan, V.B.; Close, G.L.; Morton, J.P. Cross-Sectional Comparison of Body Composition and Resting Metabolic Rate in Premier League Academy Soccer Players: Implications for Growth and Maturation. J. Sports Sci. 2020, 38, 1326–1334. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy Availability in Athletes. J. Sports Sci. 2011, 29, S7–S15. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- Kudret Saribay, A.; Kirbaş, Ş. Determination of Nutrition Knowledge of Adolescents Engaged in Sports. Univers. J. Educ. Res. 2019, 7, 40–47. [Google Scholar] [CrossRef]
- Smith, J.W.; Holmes, M.E.; McAllister, M.J. Nutritional Considerations for Performance in Young Athletes. J. Sports Med. 2015, 2015, e734649. [Google Scholar] [CrossRef] [PubMed]
- Spronk, I.; Heaney, S.E.; Prvan, T.; O’Connor, H.T. Relationship between General Nutrition Knowledge and Dietary Quality in Elite Athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; de Mendonça, C.R.; de Souza Rosa, L.P.; Silveira, E.A. Determinants of Eating Patterns and Nutrient Intake among Adolescent Athletes: A Systematic Review. Nutr. J. 2017, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.P.; Rushton, B.D. Nutritional Knowledge of Youth Academy Athletes. BMC Nutr. 2020, 6, 35. [Google Scholar] [CrossRef]
- Tam, R.; Gifford, J.A.; Beck, K.L. Recent Developments in the Assessment of Nutrition Knowledge in Athletes. Curr. Nutr. Rep. 2022, 11, 241–252. [Google Scholar] [CrossRef]
- Vázquez-Espino, K.; Rodas-Font, G.; Farran-Codina, A. Sport Nutrition Knowledge, Attitudes, Sources of Information, and Dietary Habits of Sport-Team Athletes. Nutrients 2022, 14, 1345. [Google Scholar] [CrossRef]
- Janiczak, A.; Devlin, B.L.; Forsyth, A.; Trakman, G.L. A Systematic Review Update of Athletes’ Nutrition Knowledge and Association with Dietary Intake. Br. J. Nutr. 2022, 128, 1156–1169. [Google Scholar] [CrossRef]
- Wardle, J.; Parmenter, K.; Waller, J. Nutrition Knowledge and Food Intake. Appetite 2000, 34, 269–275. [Google Scholar] [CrossRef]
- Wansink, B.; Westgren, R.E.; Cheney, M.M. Hierarchy of Nutritional Knowledge That Relates to the Consumption of a Functional Food. Nutrition 2005, 21, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Heaney, S.; O’Connor, H.; Michael, S.; Gifford, J.; Naughton, G. Nutrition Knowledge in Athletes: A Systematic Review. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Spronk, I.; Kullen, C.; Burdon, C.; O’Connor, H. Relationship between Nutrition Knowledge and Dietary Intake. Br. J. Nutr. 2014, 111, 1713–1726. [Google Scholar] [CrossRef] [PubMed]
- Rosi, A.; Ferraris, C.; Guglielmetti, M.; Meroni, E.; Charron, M.; Menta, R.; Manini, F.; Di Gioia, V.; Martini, D.; Erba, D. Validation of a General and Sports Nutrition Knowledge Questionnaire in Italian Early Adolescents. Nutrients 2020, 12, 3121. [Google Scholar] [CrossRef] [PubMed]
- Foo, W.L.; Faghy, M.A.; Sparks, A.; Newbury, J.W.; Gough, L.A. The Effects of a Nutrition Education Intervention on Sports Nutrition Knowledge during a Competitive Season in Highly Trained Adolescent Swimmers. Nutrients 2021, 13, 2713. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.; Crowcroft, S.; Kempton, T. Developing Athlete Monitoring Systems: Theoretical Basis and Practical Applications. In Sport, Recovery and Performance: Interdisciplinary Insights; Routledge: Abingdon, UK, 2017. [Google Scholar]
- Matveev, L.P.; Zdornyj, A.P. Fundamentals of Sports Training; Progress Publishers: Moscow, Russia, 1981. [Google Scholar]
- Halson, S.L.; Jeukendrup, A.E. Does Overtraining Exist? An Analysis of Overreaching and Overtraining Research. Sports Med. 2004, 34, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. The Stress of Life; McGraw-Hill: New York, NY, USA, 1956. [Google Scholar]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef]
- French, D.N.; Torres Ronda, L. National Strength & Conditioning Association’s Essential of Sport Science; French, D.N., Ed.; Human Kinetics: Champaign, IL, USA, 2021; ISBN 978-1-4925-9335-5. [Google Scholar]
- Enoka, R.M.; Duchateau, J. Muscle Fatigue: What, Why and How It Influences Muscle Function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Ryan, S.; Kempton, T.; Impellizzeri, F.M.; Coutts, A.J. Training Monitoring in Professional Australian Football: Theoretical Basis and Recommendations for Coaches and Scientists. Sci. Med. Footb. 2019, 4, 52–58. [Google Scholar] [CrossRef]
- Harre, D. Principles of Sports Training: Introduction to the Theory and Methods of Training; Harre, D., Ed.; Sportverlag: Berlin, Germany, 1982; ISBN 978-0-9896198-1-3. [Google Scholar]
- Bompa, T.O. Theory and Methodology of Training; Kendall Hunt Publishing: Dubuque, IA, USA, 1983; pp. 91–97. [Google Scholar]
- Kukushkin, G. The System of Physical Education in the USSR; Raduga Publishers: Moscow, Russia, 1983. [Google Scholar]
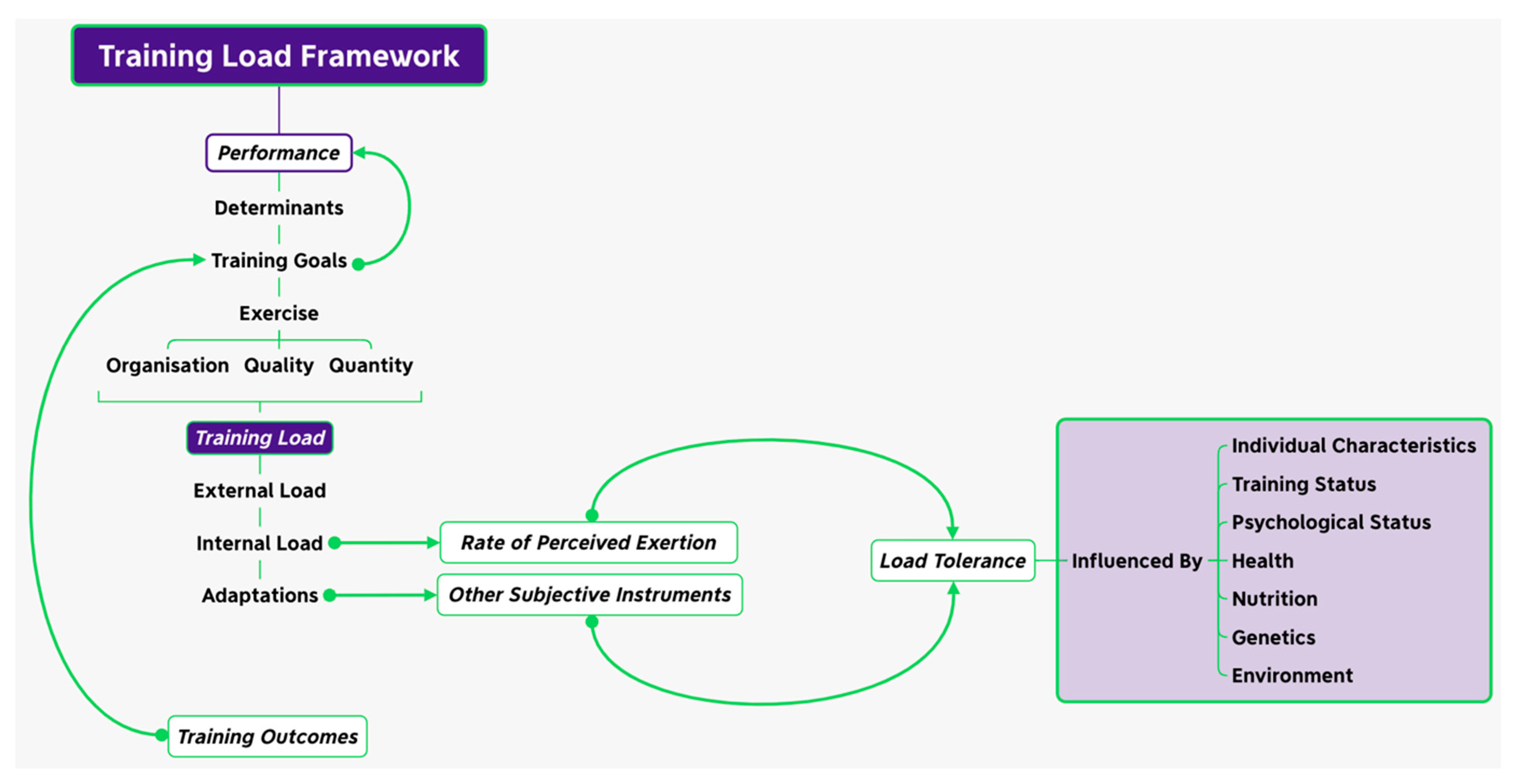
- Drew, M.K.; Finch, C.F. The Relationship between Training Load and Injury, Illness and Soreness: A Systematic and Literature Review. Sports Med. 2016, 46, 861–883. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, F.M.; Marcora, S.M.; Coutts, A.J. Internal and External Training Load: 15 Years On. Int. J. Sports Physiol. Perform. 2019, 14, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Kalkhoven, J.T.; Watsford, M.L.; Coutts, A.J.; Edwards, W.B.; Impellizzeri, F.M. Training Load and Injury: Causal Pathways and Future Directions. Sports Med. 2021, 51, 1137–1150. [Google Scholar] [CrossRef]
- Schwellnus, M.; Soligard, T.; Alonso, J.-M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R. How Much Is Too Much? (Part 2) International Olympic Committee Consensus Statement on Load in Sport and Risk of Illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Soligard, T.; Schwellnus, M.; Alonso, J.-M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R. How Much Is Too Much? (Part 1) International Olympic Committee Consensus Statement on Load in Sport and Risk of Injury. Br. J. Sports Med. 2016, 50, 1030–1041. [Google Scholar] [CrossRef]
- Smith, D.J. A Framework for Understanding the Training Process Leading to Elite Performance. Sports Med. 2003, 33, 1103–1126. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T.; Timmons, J.A. Genomics and Genetics in the Biology of Adaptation to Exercise. Compr. Physiol. 2011, 1, 1603–1648. [Google Scholar] [CrossRef]
- Mann, T.N.; Lamberts, R.P.; Lambert, M.I. High Responders and Low Responders: Factors Associated with Individual Variation in Response to Standardized Training. Sports Med. 2014, 44, 1113–1124. [Google Scholar] [CrossRef]
- Vellers, H.L.; Kleeberger, S.R.; Lightfoot, J.T. Inter-Individual Variation in Adaptations to Endurance and Resistance Exercise Training: Genetic Approaches towards Understanding a Complex Phenotype. Mamm. Genome 2018, 29, 48–62. [Google Scholar] [CrossRef]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New Strategies in Sport Nutrition to Increase Exercise Performance. Free Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Von Rosen, P.; Frohm, A.; Kottorp, A.; Fridén, C.; Heijne, A. Too Little Sleep and an Unhealthy Diet Could Increase the Risk of Sustaining a New Injury in Adolescent Elite Athletes. Scand. J. Med. Sci. Sports 2017, 27, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Von Rosen, P.; Frohm, A.; Kottorp, A.; Fridén, C.; Heijne, A. Multiple Factors Explain Injury Risk in Adolescent Elite Athletes: Applying a Biopsychosocial Perspective. Scand. J. Med. Sci. Sports 2017, 27, 2059–2069. [Google Scholar] [CrossRef]
- Doherty, R.; Madigan, S.; Warrington, G.; Ellis, J. Sleep and Nutrition Interactions: Implications for Athletes. Nutrients 2019, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, E.; Morgulev, E.; Lidor, R.; Meckel, Y. Estimation of Injury Costs: Financial Damage of English Premier League Teams’ Underachievement Due to Injuries. BMJ Open Sport Exerc. Med. 2020, 6, e000675. [Google Scholar] [CrossRef] [PubMed]
- Walia, B.; Boudreaux, C.J. The Cost of Players’ Injuries to Professional Sports Leagues and Other Sports Organizations. Manag. Financ. 2020, 47, 779–788. [Google Scholar] [CrossRef]
- McCall, A.; Fanchini, M.; Coutts, A.J. Prediction: The Modern-Day Sport-Science and Sports-Medicine “Quest for the Holy Grail”. Int. J. Sports Physiol. Perform. 2017, 12, 704–706. [Google Scholar] [CrossRef]
- Cook, J.D.; Charest, J. Sleep and Performance in Professional Athletes. Curr. Sleep Med. Rep. 2023, 9, 56–81. [Google Scholar] [CrossRef]
- Boccia, G.; Moisè, P.; Franceschi, A.; Trova, F.; Panero, D.; La Torre, A.; Rainoldi, A.; Schena, F.; Cardinale, M. Career Performance Trajectories in Track and Field Jumping Events from Youth to Senior Success: The Importance of Learning and Development. PLoS ONE 2017, 12, e0170744. [Google Scholar]
- Raysmith, B.P.; Drew, M.K. Performance Success or Failure Is Influenced by Weeks Lost to Injury and Illness in Elite Australian Track and Field Athletes: A 5-Year Prospective Study. J. Sci. Med. Sport 2016, 19, 778–783. [Google Scholar] [CrossRef]
- Fridén, C.; Ekenros, L.; Von Rosen, P. Previous Injury, Sex and Well-Being Are Associated with Injury Profiles in 422 Adolescent Elite Athletes of Age 15–16 Years: A 20-Week Longitudinal Study. BMJ Open Sport Exerc. Med. 2023, 9, e001485. [Google Scholar] [CrossRef] [PubMed]
- Malina, R.M. Early Sport Specialization: Roots, Effectiveness, Risks. Curr. Sports Med. Rep. 2010, 9, 364–371. [Google Scholar] [CrossRef] [PubMed]
- DiFiori, J.P.; Benjamin, H.J.; Brenner, J.S.; Gregory, A.; Jayanthi, N.; Landry, G.L.; Luke, A. Overuse Injuries and Burnout in Youth Sports: A Position Statement from the American Medical Society for Sports Medicine. Br. J. Sports Med. 2014, 48, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, L.; Bleakley, C. Strategies to Prevent Injury in Adolescent Sport: A Systematic Review. Br. J. Sports Med. 2007, 41, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Baxter-Jones, A.; Grieve, A. Long Term Sport Involvement and Sport Injury Rate in Elite Young Athletes. Arch. Dis. Child. 2005, 90, 525–527. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Räisänen, A.M.; Kokko, S.; Pasanen, K.; Leppänen, M.; Rimpelä, A.; Villberg, J.; Parkkari, J. Prevalence of Adolescent Physical Activity-Related Injuries in Sports, Leisure Time, and School: The National Physical Activity Behaviour Study for Children and Adolescents. BMC Musculoskelet. Disord. 2018, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.; Engebretsen, L. More Data Needed on Injury Risk among Young Elite Athletes. Br. J. Sports Med. 2010, 44, 485–489. [Google Scholar] [CrossRef]
- Von Rosen, P.; Heijne, A.; Frohm, A.; Fridén, C.; Kottorp, A. High Injury Burden in Elite Adolescent Athletes: A 52-Week Prospective Study. J. Athl. Train. 2018, 53, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tibbetts, A.S.; Covassin, T.; Cheng, G.; Nayar, S.; Heiden, E. Epidemiology of Overuse and Acute Injuries among Competitive Collegiate Athletes. J. Athl. Train. 2012, 47, 198–204. [Google Scholar] [CrossRef]
- Bahr, R.; Holme, I. Risk Factors for Sports Injuries—A Methodological Approach. Br. J. Sports Med. 2003, 37, 384–392. [Google Scholar] [CrossRef]
- Bahr, R.; Krosshaug, T. Understanding Injury Mechanisms: A Key Component of Preventing Injuries in Sport. Br. J. Sports Med. 2005, 39, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Frisch, A.; Croisier, J.-L.; Urhausen, A.; Seil, R.; Theisen, D. Injuries, Risk Factors and Prevention Initiatives in Youth Sport. Br. Med. Bull. 2009, 92, 95–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halson, S.L. Sleep in Elite Athletes and Nutritional Interventions to Enhance Sleep. Sports Med. 2014, 44, 13–23. [Google Scholar] [CrossRef]
- Von Rosen, P.; Olofsson, O.; Väsbom, S.; Heijne, A. Correlates of Health in Adolescent Elite Athletes and Adolescents: A Cross-Sectional Study of 1016 Adolescents. Eur. J. Sport Sci. 2019, 19, 707–716. [Google Scholar] [CrossRef]
- Prieto-González, P.; Martínez-Castillo, J.L.; Fernández-Galván, L.M.; Casado, A.; Soporki, S.; Sánchez-Infante, J. Epidemiology of Sports-Related Injuries and Associated Risk Factors in Adolescent Athletes: An Injury Surveillance. Int. J. Environ. Res. Public Health 2021, 18, 4857. [Google Scholar] [CrossRef] [PubMed]
- Viegas, F.; Ocarino, J.M.; de Sousa Freitas, L.; Pinto, M.C.; Facundo, L.A.; Amaral, A.S.; Silva, S.; de Mello, M.T.; Silva, A. The Sleep as a Predictor of Musculoskeletal Injuries in Adolescent Athletes. Sleep Sci. 2022, 15, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Stables, R.G.; Hannon, M.P.; Jacob, A.D.; Topping, O.; Costello, N.B.; Boddy, L.M.; Hambly, C.; Speakman, J.R.; Sodhi, J.S.; Close, G.L.; et al. Daily Energy Requirements of Male Academy Soccer Players Are Greater than Age-Matched Non-Academy Soccer Players: A Doubly Labelled Water Investigation. J. Sports Sci. 2023, 41, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Dwivedi, S.; Milewski, M.D.; Cruz, A.I. Lack of Sleep and Sports Injuries in Adolescents: A Systematic Review and Meta-Analysis. J. Pediatr. Orthop. 2019, 39, e324–e333. [Google Scholar] [CrossRef]
- Stables, R.G.; Hannon, M.P.; Costello, N.B.; McHaffie, S.J.; Sodhi, J.S.; Close, G.L.; Morton, J.P. Acute Fuelling and Recovery Practices of Academy Soccer Players: Implications for Growth, Maturation, and Physical Performance. Sci. Med. Footb. 2022, 1–15. [Google Scholar] [CrossRef]
- McHugh, D.; Mason, L.; Jeffreys, I. Integrating On-Pitch Conditioning in Football with Psychological Outcomes. J. UK Strength Cond. Assoc. 2022, 17–26. [Google Scholar]
- Read, M.; Rietveld, R.; Deigan, D.; Birnie, M.; Mason, L.; Centofanti, A. Periodisation. In Peak Performance for Soccer; Routledge: New York, NY, USA, 2022; pp. 259–288. ISBN 978-1-00-320042-0. [Google Scholar]
- Towlson, C.; Salter, J.; Ade, J.D.; Enright, K.; Harper, L.D.; Page, R.M.; Malone, J.J. Maturity-Associated Considerations for Training Load, Injury Risk, and Physical Performance in Youth Soccer: One Size Does Not Fit All. J. Sport Health Sci. 2021, 10, 403–412. [Google Scholar] [CrossRef]
- Hannon, M.P.; Coleman, N.M.; Parker, L.J.F.; McKeown, J.; Unnithan, V.B.; Close, G.L.; Drust, B.; Morton, J.P. Seasonal Training and Match Load and Micro-Cycle Periodization in Male Premier League Academy Soccer Players. J. Sports Sci. 2021, 39, 1838–1849. [Google Scholar] [CrossRef]
- Marshall, G.J.G.; Turner, A.N. The Importance of Sleep for Athletic Performance. Strength Cond. J. 2016, 38, 61–67. [Google Scholar] [CrossRef]
- McKay, D.; Broderick, C.; Steinbeck, K. The Adolescent Athlete: A Developmental Approach to Injury Risk. Pediatr. Exerc. Sci. 2016, 28, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Wik, E.H.; Martínez-Silván, D.; Farooq, A.; Cardinale, M.; Johnson, A.; Bahr, R. Skeletal Maturation and Growth Rates Are Related to Bone and Growth Plate Injuries in Adolescent Athletics. Scand. J. Med. Sci. Sports 2020, 30, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Cumming, S.P.; Bradley, B.; Williams, S. The Influence of Exposure, Growth and Maturation on Injury Risk in Male Academy Football Players. J. Sports Sci. 2022, 40, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
| Summary of Main Physiological and Metabolic Issues Surrounding Growth and Maturation | Potential Consequences of These Differences in Physiology and Metabolism on Nutritional Recommendations |
|---|---|
| Greater Energy Cost of Movement | |
| Children and adolescents have a higher (relative) energy cost of movement compared with that of adults. This may be due to increased stride frequency, a greater surface area:volume ratio, a more distal distribution of mass in the legs, or greater levels of contraction of the antagonist leg muscles while moving [105,106]. | Increased (relative) energy requirements for physical activity need to be accounted for. |
| Reduced Glycogen Storage Capacity | |
| Children and adolescents have a lower endogenous glycogen storage capacity compared with that of adults [107]. | Reduced emphasis for young athletes to have a carbohydrate load before training/competition. |
| Reduced Glycolytic Capabilities | |
| Children and adolescents have reduced glycolytic capabilities, with full anaerobic capabilities developing towards the end of puberty [108]. As a result, children and adolescents have lower levels of lactate production than those of adults during high-intensity exercise of the same relative intensity [107,109]. | Reduced requirement for the use of buffering agents with young athletes, particularly those in pre- and peri-puberty stages. |
| Higher Rates of Aerobic Metabolism | |
| Higher rates of aerobic metabolism exist in children during exercise. Fat oxidation rates during submaximal exercise (of the same relative intensity) are greater in children and adolescents compared with that in adults. Less mature children have a greater reliance on fat as a fuel compared with more mature adolescents. It has been suggested that these higher fat oxidation rates in children compared with those in adults are the result of lower endogenous carbohydrate stores and reduced glycolytic capabilities [110]. | Young athletes may not require the same relative amount of carbohydrate as adult athletes do; however, there is a lack of evidence to support this. Further research is warranted. |
| Greater Reliance on Exogenous Carbohydrate | |
| Children and adolescents have greater reliance on exogenous carbohydrate as a fuel source. During exercise, exogenous carbohydrate is a greater contributor to total energy supply in children and adolescents compared with adults [110]. Exogenous carbohydrate oxidation rates are higher in less mature boys compared with more mature boys of the same chronological age; however, this is not the case in females [111,112]. | Exogenous carbohydrate should be consumed during moderate-/high intensity exercise lasting longer than −60 min. |
| Thermoregulatory Differences | |
| Children and adolescents have a larger surface area:body mass ratio [113], so, consequently, they gain and lose more heat from the environment through conduction, convection, and radiation. Adolescents who undertake regular exercise do adapt, however, improving their ability to thermoregulate through enhanced peripheral vasodilatation [114]. | Regular consumption of cold flavoured fluids during exercise |
| Reduced Sweating Capacity | |
| Children and adolescents have a lower sweating capacity compared with that of adults and therefore a reduced ability to lose sweat through sweat evaporation. As children mature, so too do their thermoregulation mechanisms (particularly their ability to sweat); however, these are not fully developed until late puberty [115]. | Regular consumption of cold flavoured fluids during exercise. There is no evidence to suggest that fluid requirements in young athletes are less than those of their adult counterparts, despite reduced sweat rates. |
| Growth and Increase in Body Size | |
| Macronutrient requirements are often prescribed relative to body mass (i.e., grams per kilo, g/kg) to account for individual differences in size among young athletes. Although fat mass does not seem to significantly change throughout growth and maturation in young athletes, increases in body mass are primarily derived from an increase in fat-free mass [116]. An increase in stature is the result of skeletal growth and the laying down of bone mineral content (i.e., skeletal tissue). Around 95% of adult bone mineral content is achieved by the end of adolescence, with ~26% of this being accrued at a peak bone mineral content velocity (~12.5 and ~14 years old in girls and boys respectively) [117]. Changes in fat-free mass and stature are significantly influenced by the energy and macronutrient intake of a young athlete during childhood and adolescence [118]. | Increased (relative) energy requirements need to be accounted for during peak weight and height velocity periods. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, L.; Connolly, J.; Devenney, L.E.; Lacey, K.; O’Donovan, J.; Doherty, R. Sleep, Nutrition, and Injury Risk in Adolescent Athletes: A Narrative Review. Nutrients 2023, 15, 5101. https://doi.org/10.3390/nu15245101
Mason L, Connolly J, Devenney LE, Lacey K, O’Donovan J, Doherty R. Sleep, Nutrition, and Injury Risk in Adolescent Athletes: A Narrative Review. Nutrients. 2023; 15(24):5101. https://doi.org/10.3390/nu15245101
Chicago/Turabian StyleMason, Lorcán, James Connolly, Lydia E. Devenney, Karl Lacey, Jim O’Donovan, and Rónán Doherty. 2023. "Sleep, Nutrition, and Injury Risk in Adolescent Athletes: A Narrative Review" Nutrients 15, no. 24: 5101. https://doi.org/10.3390/nu15245101
APA StyleMason, L., Connolly, J., Devenney, L. E., Lacey, K., O’Donovan, J., & Doherty, R. (2023). Sleep, Nutrition, and Injury Risk in Adolescent Athletes: A Narrative Review. Nutrients, 15(24), 5101. https://doi.org/10.3390/nu15245101