Body Mass Index (BMI) Is the Strongest Predictor of Systemic Hypertension and Cardiac Mass in a Cohort of Children
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HTN | hypertension |
| CV | cardiovascular |
| RF | risk factor |
| LV | left ventricular |
| LVH | left ventricular hypertrophy |
| BP | blood pressure |
| BMI | body mass index |
| CKD | chronic kidney disease |
| ED | endocrine disorders |
| OSAS | obstructive sleep apnea syndrome |
| CVD | cardiovascular diseases |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| IVSD | interventricular septal width at end-diastole |
| LVEDD | left ventricle end-diastole diameter |
| LVESD | left ventricle end-systole diameter |
| PWD | posterior wall thickness at end-diastole |
| LA | left atrium volume |
| FS | fractional shortening |
| LVMI | left ventricular mass index |
| RWT | relative wall thickness |
| EF | ejection fraction |
| EDV | end-diastole volume |
| ESV | end-systole volume |
| SD | standard deviations |
| T0 | first evaluation at enrollment time |
| T1 | first follow-up |
| T2 | second follow-up |
| SGA | small for gestational age |
| LGA | large for gestational age |
References
- Nadruz, W. Myocardial remodeling in hypertension. J. Hum. Hypertens. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Sarnecki, J.; Obrycki, Ł.; Feber, J.; Chełstowska, S.; Jurkiewicz, E.; Litwin, M. Isolated systolic hypertension is associated with increased left ventricular mass index and aortic stiffness in adolescents: A cardiac magnetic resonance study. J. Hypertens. 2022, 40, 985–995. [Google Scholar] [CrossRef]
- Cheng, H.; Xi, B.; Liu, J.; Yan, Y.; Mi, J. Performance of different adiposity measures for predicting left ventricular remodeling in Chinese hypertensive youth. Sci. Rep. 2021, 11, 21943. [Google Scholar] [CrossRef]
- Daniels, S.R.; Loggie, J.M.; Khoury, P.; Kimball, T.R. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation 1998, 97, 1907–1911. [Google Scholar] [CrossRef]
- Hanevold, C.; Waller, J.; Daniels, S.; Portman, R.; Sorof, J.; International Pediatric Hypertension Association. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: A collaborative study of the International Pediatric Hypertension Association. Pediatrics 2004, 113, 328–333. [Google Scholar] [CrossRef]
- McNiece, K.L.; Gupta-Malhotra, M.; Samuels, J.; Bell, C.; Garcia, K.; Poffenbarger, T.; Sorof, J.M.; Portman, R.J. National High Blood Pressure Education Program Working Group. Left ventricular hypertrophy in hypertensive adolescents: Analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension 2007, 50, 392–395. [Google Scholar] [CrossRef]
- Brady, T.M.; Fivush, B.; Flynn, J.T.; Parekh, R. Ability of blood pressure to predict left ventricular hypertrophy in children with primary hypertension. J. Pediatr. 2008, 152, 73–78.e1. [Google Scholar] [CrossRef]
- Li, Y.; Gu, H.; Sinha, M.D.; Chowienczyk, P. Hemodynamic Characterization of Primary Hypertension in Children and Adolescents. J. Am. Heart Assoc. 2020, 9, e015097. [Google Scholar] [CrossRef]
- Haley, J.E.; Woodly, S.A.; Daniels, S.R.; Falkner, B.; Ferguson, M.A.; Flynn, J.T.; Hanevold, C.D.; Hooper, S.R.; Ingelfinger, J.R.; Khoury, P.R.; et al. Association of Blood Pressure-Related Increase in Vascular Stiffness on Other Measures of Target Organ Damage in Youth. Hypertension 2022, 79, 2042–2050. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation 2008, 117, 3171–3180. [Google Scholar] [CrossRef]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114 (Suppl. S2), 555–576. [Google Scholar]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, Y.; Yu, J.; Zha, M.; Zhu, Y.; Rahimi, K.; Rudan, I. Global Prevalence of Hypertension in Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Yaniv, G.; Levine, H.; Leiba, A.; Goldberger, N.; Derazne, E.; Shor, D.B.-A.; Tzur, D.; Afek, A.; Shamiss, A.; et al. Body-Mass Index in 2.3 Million Adolescents and Cardiovascular Death in Adulthood. N. Engl. J. Med. 2016, 374, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- Urbina, E.M.; Khoury, P.R.; McCoy, C.; Daniels, S.R.; Kimball, T.R.; Dolan, L.M. Cardiac and vascular consequences of pre-hypertension in youth. J. Clin. Hypertens. 2011, 13, 332–342. [Google Scholar] [CrossRef]
- Mead, E.; Brown, T.; Rees, K.; Azevedo, L.B.; Whittaker, V.; Jones, D.; Olajide, J.; Mainardi, G.M.; Corpeleijn, E.; O’Malley, C.; et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev. 2017, 6, CD012651. [Google Scholar] [CrossRef]
- Al-Khudairy, L.; Loveman, E.; Colquitt, J.L.; Mead, E.; Johnson, R.E.; Fraser, H.; Olajide, J.; Murphy, M.; Velho, R.M.; O’Malley, C.; et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 2017, 6, CD012691. [Google Scholar] [CrossRef]
- Salam, R.A.; Padhani, Z.A.; Das, J.K.; Shaikh, A.Y.; Hoodbhoy, Z.; Jeelani, S.M.; Lassi, Z.S.; Bhutta, Z.A. Effects of Lifestyle Modification Interventions to Prevent and Manage Child and Adolescent Obesity: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2208. [Google Scholar] [CrossRef]
- Kirk, S.; Ogata, B.; Wichert, E.; Handu, D.; Rozga, M. Treatment of Pediatric Overweight and Obesity: Position of the Academy of Nutrition and Dietetics Based on an Umbrella Review of Systematic Reviews. J. Acad. Nutr. Diet. 2022, 122, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef]
- Leone, A.; Vizzuso, S.; Brambilla, P.; Mameli, C.; Ravella, S.; De Amicis, R.; Battezzati, A.; Zuccotti, G.; Bertoli, S.; Verduci, E. Evaluation of Different Adiposity Indices and Association with Metabolic Syndrome Risk in Obese Children: Is there a Winner? Int. J. Mol. Sci. 2020, 21, 4083. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Gillebert, T.C.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 2015, 28, 727–754. [Google Scholar] [CrossRef]
- Khoury, P.R.; Mitsnefes, M.; Daniels, S.R.; Kimball, T.R. Age-specific reference intervals for indexed left ventricular mass in children. J. Am. Soc. Echocardiogr. 2009, 22, 709–714. [Google Scholar] [CrossRef]
- Chinali, M.; Emma, F.; Esposito, C.; Rinelli, G.; Franceschini, A.; Doyon, A.; Raimondi, F.; Pongiglione, G.; Schaefer, F.; Matteucci, M.C. Left Ventricular Mass Indexing in Infants, Children, and Adolescents: A Simplified Approach for the Identification of Left Ventricular Hypertrophy in Clinical Practice. J. Pediatr. 2016, 170, 193–198. [Google Scholar] [CrossRef]
- Daniels, S.R.; Kimball, T.R.; Morrison, J.A.; Khoury, P.; Witt, S.; Meyer, R.A. Effect of lean body mass, fat mass, blood pressure, and sexual maturation on left ventricular mass in children and adolescents. Statistical, biological, and clinical significance. Circulation 1995, 92, 3249–3254. [Google Scholar] [CrossRef]
- Zucchini, S.; Fabi, M.; Maltoni, G.; Zioutas, M.; Trevisani, V.; Di Natale, V.; Cassio, A.; Pession, A. Adolescents with severe obesity show a higher cardiovascular (CV) risk than those with type 1 diabetes: A study with skin advanced glycation end products and intima media thickness evaluation. Acta Diabetol. 2020, 57, 1297–1305. [Google Scholar] [CrossRef]
- Pruette, C.S.; Fivush, B.A.; Flynn, J.T.; Brady, T.M. Effects of obesity and race on left ventricular geometry in hypertensive children. Pediatr. Nephrol. 2013, 28, 2015–2022. [Google Scholar] [CrossRef]
- Litwin, M.; Niemirska, A.; Sladowska-Kozlowska, J.; Wierzbicka, A.; Janas, R.; Wawer, Z.T.; Wisniewski, A.; Feber, J. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr. Nephrol. 2010, 25, 2489–2499. [Google Scholar] [CrossRef]
- Yang, L.; Yang, L.; Zhang, Y.; Xi, B. Prevalence of Target Organ Damage in Chinese Hypertensive Children and Adolescents. Front. Pediatr. 2018, 6, 333. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, S.; Salvi, P.; Nava, E.; Tassistro, E.; Giussani, M.; Desimone, I.; Orlando, A.; Battaglino, M.; Lieti, G.; Montemerlo, M.; et al. Blood Pressure and Body Weight Have Different Effects on Pulse Wave Velocity and Cardiac Mass in Children. J. Clin. Med. 2020, 9, 2954. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.A.; Omran, J.; Mehra, A.; Ardhanari, S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog. Cardiovasc. Dis. 2014, 56, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [PubMed]
- de Simone, G.; Izzo, R.; De Luca, N.; Gerdts, E. Left ventricular geometry in obesity: Is it what we expect? Nutr. Metab. Cardiovasc. Dis. 2013, 23, 905–912. [Google Scholar] [CrossRef]
- Liang, J.H.; Zhao, Y.; Chen, Y.C.; Huang, S.; Zhang, S.X.; Jiang, N.; Kakaer, A.; Chen, Y.-J. Development and Validation of a Nomogram-Based Prognostic Model to Predict High Blood Pressure in Children and Adolescents-Findings From 342,736 Individuals in China. Front. Cardiovasc. Med. 2022, 9, 884508. [Google Scholar] [CrossRef]
- Rodriguez-Ayllon, M.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Migueles, J.H.; Mora-Gonzalez, J.; Henriksson, P.; Martin-Matillas, M.; Mena-Molina, A.; Molina-Garcia, P.; Estevez-Lopez, F.; et al. Physical fitness and psychological health in overweight/obese children: A cross-sectional study from the activebrains project. J. Sci. Med. Sport 2018, 21, 179–184. [Google Scholar] [CrossRef]
- Yu, H.J.; Li, F.; Hu, Y.F.; Li, C.F.; Yang, X.H.; Yuan, S.; Huang, Y.; Tang, B.W.; Gong, J.; He, Q.Q. Associations of physical activity and fruit and vegetable intake with well-being and depressive symptoms among obese schoolchildren in wuhan, china: A cross-sectional study. BMC Public Health 2018, 18, 986. [Google Scholar] [CrossRef]
- Ha, N.T.; Trang, D.T.H.; Ha, L.T.T. Is obesity associated with decreased health-related quality of life in school-age children?—Results from a survey in Vietnam. AIMS Public Health 2018, 5, 338–351. [Google Scholar] [PubMed]
- Yu, H.J.; Li, F.; Hu, Y.F.; Li, C.F.; Yuan, S.; Song, Y.; Zheng, M.; Gong, J.; He, Q.Q. Improving the Metabolic and Mental Health of Children with Obesity: A School-Based Nutrition Education and Physical Activity Intervention in Wuhan, China. Nutrients 2020, 12, 194. [Google Scholar] [CrossRef] [PubMed]
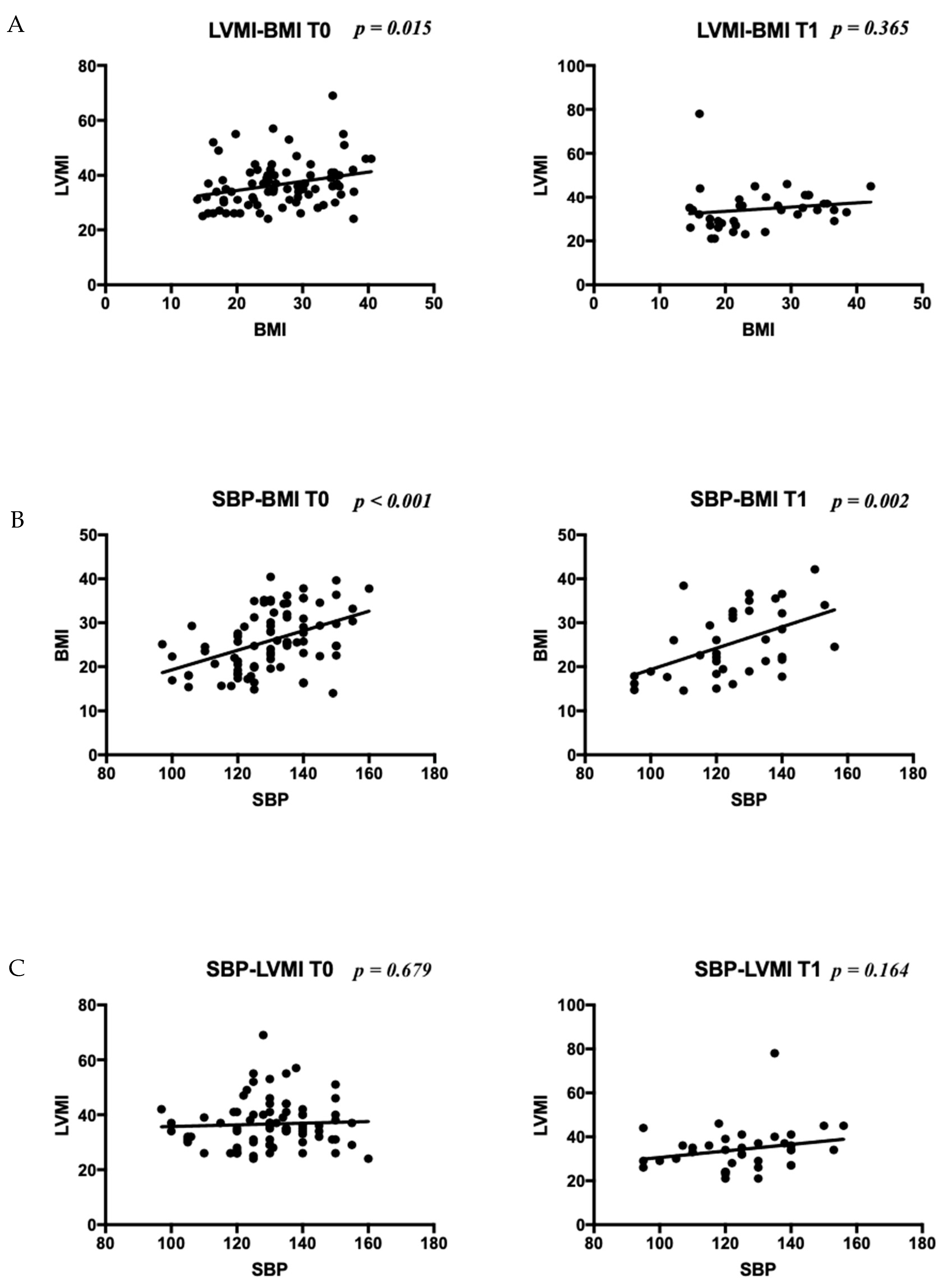

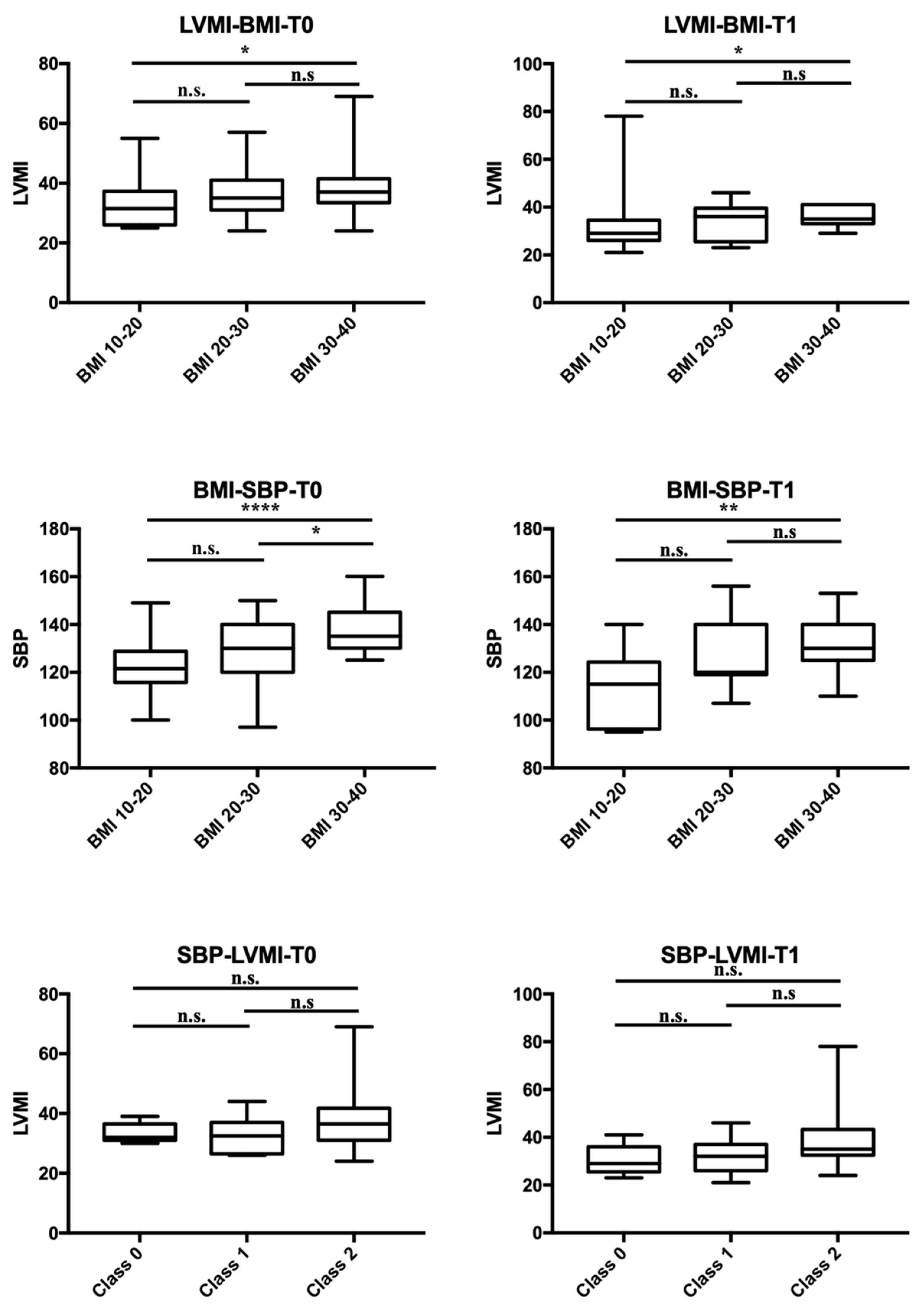
| Patients, n (boys n, %) | 92 (50, 54.3%) | |||
| Mean age (years) ± SD | 11.4 ± 3 | |||
| Etiology, n (%) | ||||
| Overweight/obesity | 51 (55) | |||
| CKD | 27 (29.3) | |||
| OSAS/snoring | 9 (9.8) | |||
| Primary hypertension | 5 (5.4) | |||
| BMI, n (%) | ||||
| Normal weight | 37 (40.2) | |||
| Overweight | 10 (10.9) | |||
| Obese | 45 (48.9) | |||
| BP classification, n (%) | ||||
| Normal | 10 (10.9) | |||
| High-normal | 12 (13) | |||
| Hypertension | 70 (76.1) | |||
| BP classification according to diagnosis, n (%) | CKD | ED with normal weight | ED with overweight | ED obeses |
| Normal | 3 (17.6) | 3 (15) | 0 | 1 (2.6) |
| High-normal | 7 (41.2) | 8 (40) | 1 (25) | 3 (7.9) |
| Hypertension | 7 (41.2) | 9 (45) | 3 (75) | 34 (89.5) |
| Cardiac morphology, n (%) | ||||
| Normal | 57 (61.9) | |||
| Remodeling | 9 (9.8) | |||
| Eccentric hypertrophy | 17 (18.5) | |||
| Concentric hypertrophy | 9 (9.8) | |||
| Ventricular function, n (%) | ||||
| Normal | 74 (80.4) | |||
| Systolic dysfunction | 12 (13) | |||
| Diastolic dysfunction | 3 (3.3) | |||
| Impaired relaxation | 3 (3.3) | |||
| CKD T0 | ED T0 | p Value T0 | CKD T1 | ED T1 | p Value T1 | |
|---|---|---|---|---|---|---|
| SBP (mmHg), mean ± SD | 119.68 ± 16.63 | 133.11 ± 12.29 | <0.001 a | 113.5 ± 15.99 | 130.06 ± 14.12 | 0.010 a |
| DBP (mmHg), mean ± SD | 69.5 ± 11.2 | 72.4 ± 9.8 | n.s | 65.2 ± 10.8 | 72 ± 10.5 | 0.038 a |
| BP categories, n (%) | ||||||
| (1) Normal BP | 3/17 (17.6%) | 1/44 (2.3%) | 0.029 b | 4/10 (40%) | 3/15 (20%) | n.s. |
| (2) High Normal BP | 7/17 (41.2%) | 6/44 (13.6%) | 0.018 b | 4/10 (40%) | 6/15 (40%) | n.s. |
| (3) HTN | 7/17 (41.2%) | 37/44 (84.1%) | <0.001 b | 2/10 (20%) | 6/15 (40%) | n.s. |
| BMI, mean ± SD | 19.53 ± 4.42 | 30.98 ± 5.23 | <0.001 a | 19.26 ± 5.47 | 32.75 ± 4.53 | <0.001 a |
| BMI categories, n (%) | ||||||
| (1) Normal weight | 15/17(88.2%) | 5/44 (11.4%) | <0.001 b | 9/10 (90%) | 0 | - |
| (2) Overweight | 1/17 (5.9%) | 3/44 (6.8%) | n.s. | 0 | 0 | - |
| (3) Obesity | 1/17 (5.9%) | 36/44 (81.8%) | <0.001 a | 1/10 (10%) | 15/15 (100%) | - |
| Echocardiographic findings | ||||||
| LVMI (g/h2.7), mean ± SD | 38.35 ± 18.05 | 37.18 ± 10.94 | n.s. | 33.18 ± 16.06 | 36.70 ±4.66 | n.s. |
| LVMI (g/h2.16), mean ± SD | 40.24 ± 9.90 | 45.92 ± 11.69 | n.s. | 34.5 ±10.16 | 46.11 ± 5.97 | <0.001 a |
| RWT, mean ± SD | 0.37 ± 0.04 | 0.39 ± 0.11 | n.s. | 0.36 ± 0.08 | 0.38 ± 0.04 | n.s. |
| FS%, mean ± SD | 39.35 ± 4.02 | 39.04 ± 4.38 | n.s. | 36.2 ± 3.49 | 38.63 ± 4.38 | n.s. |
| LVH (Khoury et al. [27]), % | 17.6% | 33.3% | n.s. | 9.1% | 29.4% | n.s. |
| LVH (Chinali et al. [28]), % | 35.3% | 45.2% | n.s. | 20% | 52.9% | 0.039 b |
| RWT > 0.42, % | 11.8% | 21.4% | n.s. | 18.2% | 17.6% | n.s. |
| FS% < 35%, % | 10% | 14% | n.s. | 18.2% | 3/17 (17.6%) | n.s. |
| E/A < 1,% | 5% | 4.7% | n.s. | 9.1% | 0 | - |
| Normal LV geometry, % | 76.4% | 55.9% | n.s. | 72.7% | 58.8% | n.s. |
| Concentric hypertrophy, % | 0% | 11.6% | - | 9.1% | 5.9% | n.s. |
| Eccentric hypertrophy, % | 11.8% | 20.9% | n.s. | 0 | 23.5% | - |
| Concentric remodeling, % | 11.8% | 11.6% | n.s. | 18.2% | 11.8% | n.s. |
| Parameters at T0 | Normal Weight | Overweight/Obese | p Value |
|---|---|---|---|
| LVMI (g/h2.7), mean ± SD | 38.4 ± 17.6 | 38.9 ± 9.6 | n.s. |
| LVMI (g/h2.16), mean ± SD | 41.3 ± 11.1 | 46.7 ± 11.4 | n.s. |
| RWT, mean ± SD | 0.37 ± 0.03 | 0.38 ± 0.08 | n.s. |
| FS%, mean ± SD | 38.1 ± 4.5 | 39.3 ± 4.3 | n.s. |
| LVH (Khoury et al. [27]), n (%) | 4/21 (19.1%) | 16/46 (34.8%) | n.s. |
| LVH (Chinali et al. [28]), n (%) | 6/21 (28.6%) | 22/46 (47.8%) | n.s. |
| RWT > 0.42, n (%) | 3/21 (14.3%) | 10/46 (21.7%) | n.s. |
| FS% < 35%, n (%) | 5/21 (23.8%) | 5/46 (10.9%) | n.s. |
| E/A < 1, n (%) | 1/21 (4.8%) | 2/46 (4.3%) | n.s. |
| Normal LV geometry, n (%) | 16/21 (76.2%) | 24/46 (52.2%) | 0.026 a |
| Concentric hypertrophy, n (%) | 2/21 (9.5%) | 5/46 (10.8%) | n.s. |
| Eccentric hypertrophy, n (%) | 2/21 (9.5%) | 11/46 (23.9%) | n.s. |
| Concentric remodeling, n (%) | 1/21 (4.8%) | 6/46 (13.1%) | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabi, M.; Meli, M.; Leardini, D.; Andreozzi, L.; Maltoni, G.; Bitelli, M.; Pierantoni, L.; Zarbo, C.; Dondi, A.; Bertulli, C.; et al. Body Mass Index (BMI) Is the Strongest Predictor of Systemic Hypertension and Cardiac Mass in a Cohort of Children. Nutrients 2023, 15, 5079. https://doi.org/10.3390/nu15245079
Fabi M, Meli M, Leardini D, Andreozzi L, Maltoni G, Bitelli M, Pierantoni L, Zarbo C, Dondi A, Bertulli C, et al. Body Mass Index (BMI) Is the Strongest Predictor of Systemic Hypertension and Cardiac Mass in a Cohort of Children. Nutrients. 2023; 15(24):5079. https://doi.org/10.3390/nu15245079
Chicago/Turabian StyleFabi, Marianna, Matteo Meli, Davide Leardini, Laura Andreozzi, Giulio Maltoni, Maria Bitelli, Luca Pierantoni, Chiara Zarbo, Arianna Dondi, Cristina Bertulli, and et al. 2023. "Body Mass Index (BMI) Is the Strongest Predictor of Systemic Hypertension and Cardiac Mass in a Cohort of Children" Nutrients 15, no. 24: 5079. https://doi.org/10.3390/nu15245079
APA StyleFabi, M., Meli, M., Leardini, D., Andreozzi, L., Maltoni, G., Bitelli, M., Pierantoni, L., Zarbo, C., Dondi, A., Bertulli, C., Bernardini, L., Pession, A., & Lanari, M. (2023). Body Mass Index (BMI) Is the Strongest Predictor of Systemic Hypertension and Cardiac Mass in a Cohort of Children. Nutrients, 15(24), 5079. https://doi.org/10.3390/nu15245079












