Adherence to Mediterranean Diet and Food Neophobia Occurrence in Children: A Study Carried out in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection Procedure
2.3. The Questionnaire
2.4. Statical Analysis
3. Results
3.1. Characteristics of the Sample
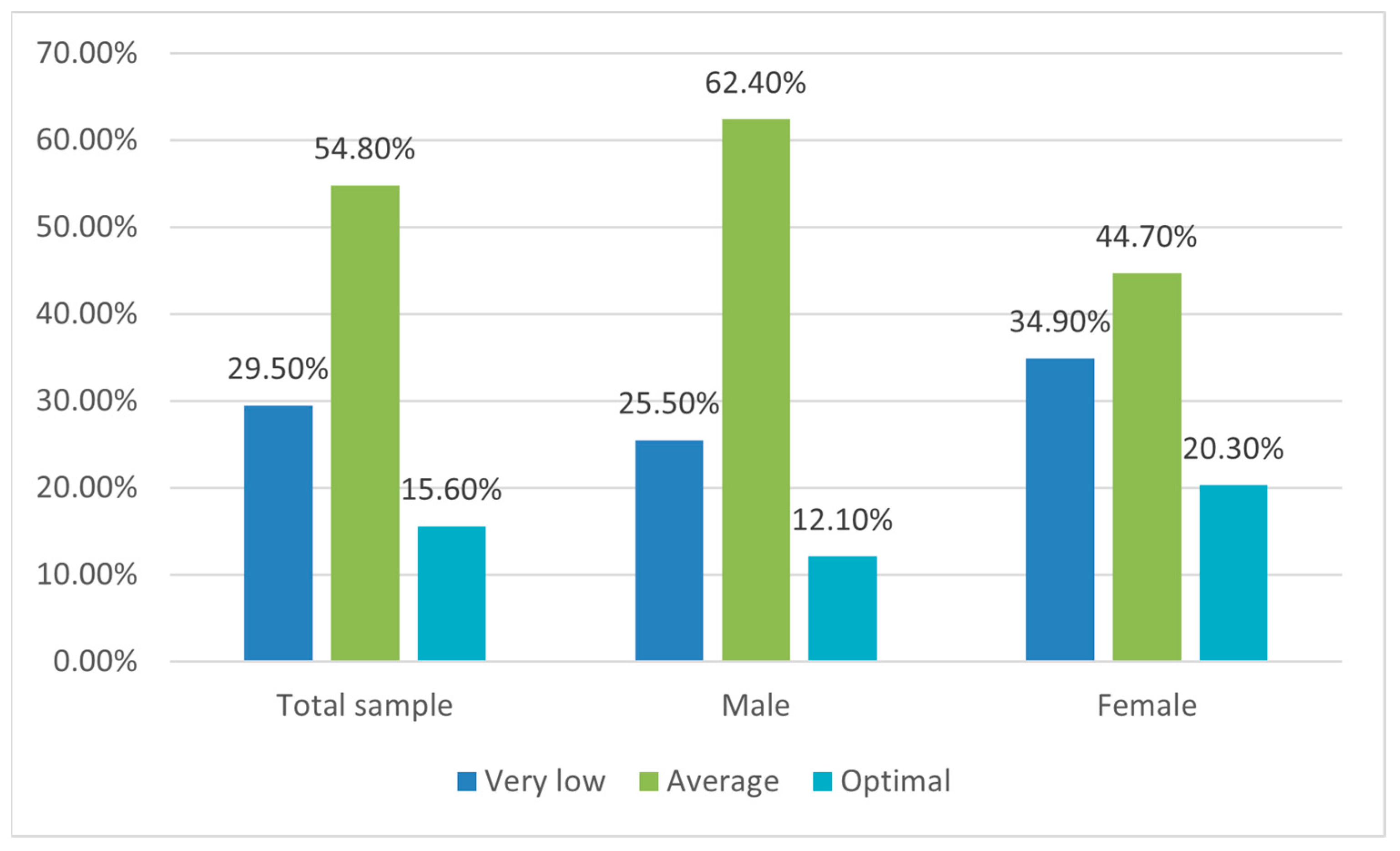
3.2. The Adherence to Mediterranean Diet Assessment (KIDMED Test)
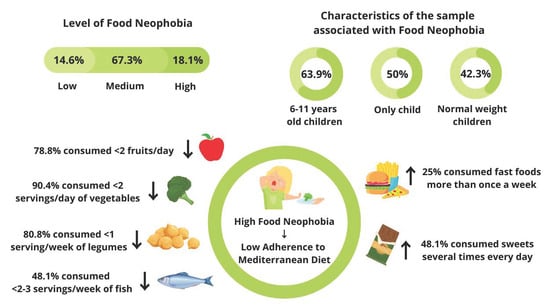
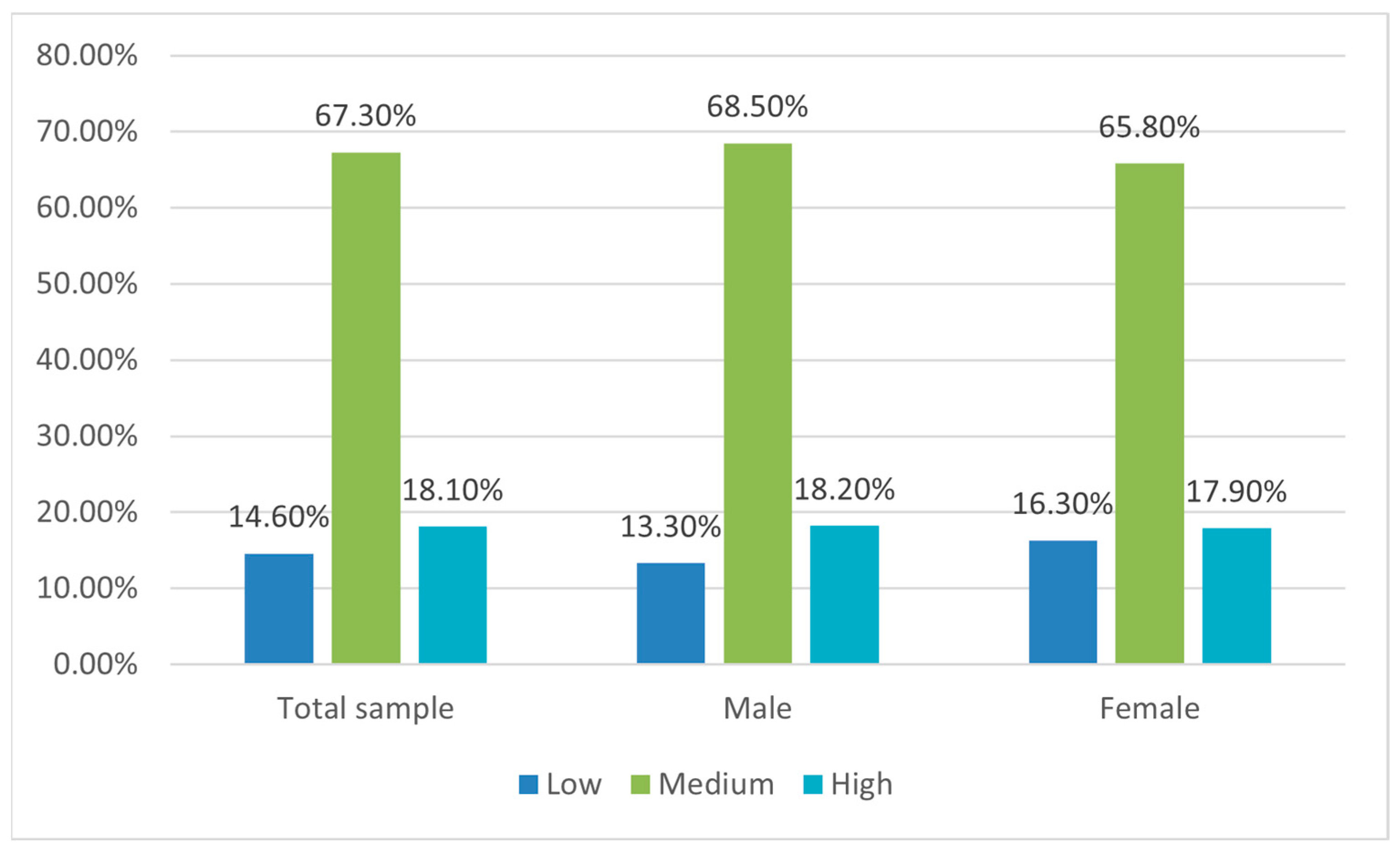
3.3. Food Neophobia Assessment
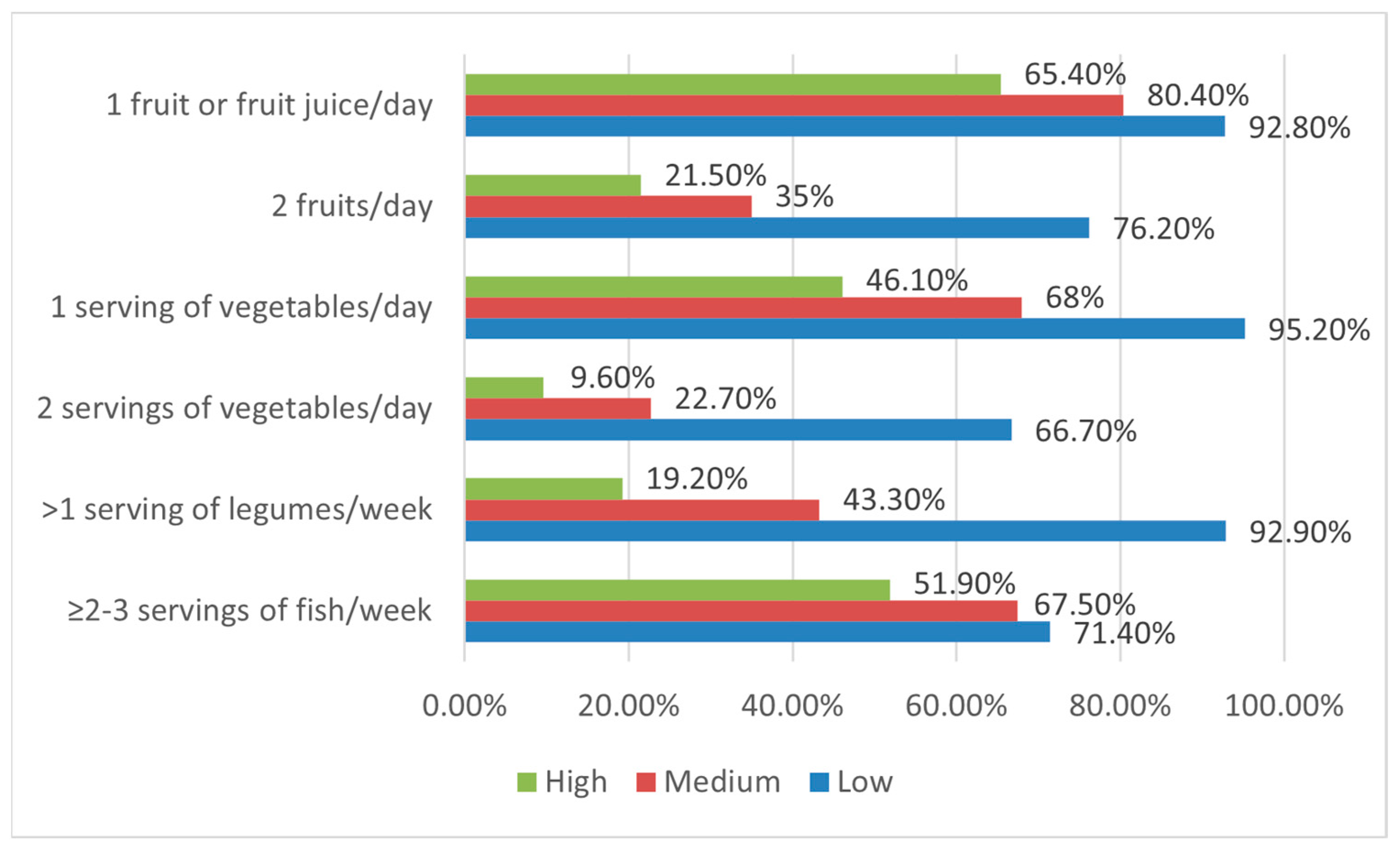
3.4. The Relationship between Adherence to the Mediterranean Diet and Food Neophobia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Tadeo, A.; Patiño-Villena, B.; González-Martínez-La-Cuesta, E.; Urquídez-Romero, R.; Ros-Berruezo, G. Food Neophobia, Mediterranean Diet Adherence and Acceptance of Healthy Foods Prepared in Gastronomic Workshops by Spanish Students. Nutr. Hosp. 2018, 35, 642–649. [Google Scholar] [PubMed]
- Dovey, T.M.; Staples, P.A.; Gibson, E.L.; Halford, J.C.G. Food Neophobia and ‘Picky/Fussy’ Eating in Children: A Review. Appetite 2008, 50, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Karaağaç, Y.; Bellikci-Koyu, E. A Narrative Review on Food Neophobia throughout the Lifespan: Relationships with Dietary Behaviours and Interventions to Reduce It. Br. J. Nutr. 2023, 130, 793–826. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.C.; An, R.; Lee, S.-Y.; Donovan, S.M. Correlates of Picky Eating and Food Neophobia in Young Children: A Systematic Review and Meta-Analysis. Nutr. Rev. 2017, 75, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, S.R.; Prescott, J.; Worch, T. Food Neophobia Modulates Importance of Food Choice Motives: Replication, Extension, and Behavioural Validation. Food Qual. Prefer. 2022, 97, 104439. [Google Scholar] [CrossRef]
- Agnoli, C.; Krogh, V.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Frasca, G.; et al. A Priori-Defined Dietary Patterns Are Associated with Reduced Risk of Stroke in a Large Italian Cohort. J. Nutr. 2011, 141, 1552–1558. [Google Scholar] [CrossRef]
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean Diet and Cardiovascular Risk Factors: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 593–610. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing Evidence on Benefits of Adherence to the Mediterranean Diet on Health: An Updated Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; Vecchia, C.L.; Bamia, C. Mediterranean Diet and Its Components in Relation to All-Cause Mortality: Meta-Analysis. Brit. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.L.; García-Vigara, A.; Hidalgo-Mora, J.J.; García-Pérez, M.-Á.; Tarín, J.; Cano, A. Mediterranean Diet and Health: A Systematic Review of Epidemiological Studies and Intervention Trials. Maturitas 2020, 136, 25–37. [Google Scholar] [CrossRef]
- Azzam, A. Is the World Converging to a ‘Western Diet’? Public Health Nutr. 2021, 24, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Montaño, Z.; Smith, J.D.; Dishion, T.J.; Shaw, D.S.; Wilson, M.N. Longitudinal Relations between Observed Parenting Behaviors and Dietary Quality of Meals from Ages 2 to 5. Appetite 2015, 87, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.d.O.; Gomes, D.R.; Mattos, M.P. Factors associated with food neophobia in children: Systematic review. Rev. Paul. Pediatr. 2021, 39, e2020089. [Google Scholar] [CrossRef]
- Cabrera, S.G.; Fernández, N.H.; Hernández, C.R.; Nissensohn, M.; Román-Viñas, B.; Serra-Majem, L. Kidmed test; prevalence of low adherence to the mediterranean diet in children and young; A Systematic Review. Nutr. Hosp. 2015, 32, 2390–2399. [Google Scholar] [CrossRef]
- Vilarnau, C.; Stracker, D.M.; Funtikov, A.; da Silva, R.; Estruch, R.; Bach-Faig, A. Worldwide Adherence to Mediterranean Diet between 1960 and 2011. Eur. J. Clin. Nutr. 2019, 72, 83–91. [Google Scholar] [CrossRef] [PubMed]
- EpiCentro. Indagine Nazionale 2019: I Dati Nazionali. Available online: https://www.epicentro.iss.it/okkioallasalute/indagine-2019-dati (accessed on 29 July 2022).
- Roccaldo, R.; Censi, L.; D’Addezio, L.; Toti, E.; Martone, D.; D’Addesa, D.; Cernigliaro, A. ZOOM8 Study group Adherence to the Mediterranean Diet in Italian School Children (The ZOOM8 Study). Int. J. Food Sci. Nutr. 2014, 65, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.G.; Worsley, A. A Population-Based Study of Preschoolers’ Food Neophobia and Its Associations with Food Preferences. J. Nutr. Educ. Behav. 2008, 40, 11–19. [Google Scholar] [CrossRef]
- Falciglia, G.A.; Couch, S.C.; Gribble, L.S.; Pabst, S.M.; Frank, R. Food Neophobia in Childhood Affects Dietary Variety. J. Am. Diet. Assoc. 2000, 100, 1474–1481. [Google Scholar] [CrossRef]
- dos Anjos, L.A.; Vieira, D.A.d.S.; Siqueira, B.N.F.; Voci, S.M.; Botelho, A.J.; da Silva, D.G. Low Adherence to Traditional Dietary Pattern and Food Preferences of Low-Income Preschool Children with Food Neophobia. Public Health Nutr. 2021, 24, 2859–2866. [Google Scholar] [CrossRef]
- Brown, C.L.; Schaaf, E.B.V.; Cohen, G.M.; Irby, M.B.; Skelton, J.A. Association of Picky Eating and Food Neophobia with Weight: A Systematic Review. Child. Obes. 2016, 12, 247–262. [Google Scholar] [CrossRef]
- Perry, R.A.; Mallan, K.M.; Koo, J.; Mauch, C.E.; Daniels, L.A.; Magarey, A.M. Food Neophobia and Its Association with Diet Quality and Weight in Children Aged 24 Months: A Cross Sectional Study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Laureati, M.; Bertoli, S.; Bergamaschi, V.; Leone, A.; Lewandowski, L.; Giussani, B.; Battezzati, A.; Pagliarini, E. Food Neophobia and Liking for Fruits and Vegetables Are Not Related to Italian Children’s Overweight. Food Qual. Pref. 2015, 40, 125–131. [Google Scholar] [CrossRef]
- Finistrella, V.; Manco, M.; Ferrara, A.; Rustico, C.; Presaghi, F.; Morino, G. Cross-Sectional Exploration of Maternal Reports of Food Neophobia and Pickiness in Preschooler-Mother Dyads. J. Am. Coll. Nutr. 2012, 31, 152–159. [Google Scholar] [CrossRef]
- Di Nucci, A.; Scognamiglio, U.; Grant, F.; Rossi, L. The Impact of COVID-19 Pandemic on Food Habits and Neophobia in Children in the Framework of the Family Context and Parents’ Behaviors: A Study in an Italian Central Region. Front. Nutr. 2022, 9, 1070388. [Google Scholar] [CrossRef] [PubMed]
- Cooke, L.J.; Haworth, C.M.; Wardle, J. Genetic and Environmental Influences on Children’s Food Neophobia. Am. J. Clin. Nutr. 2007, 86, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Lafraire, J.; Rioux, C.; Giboreau, A.; Picard, D. Food Rejections in Children: Cognitive and Social/Environmental Factors Involved in Food Neophobia and Picky/Fussy Eating Behavior. Appetite 2016, 96, 347–357. [Google Scholar] [CrossRef]
- Kutbi, H.A.; Alhatmi, A.A.; Alsulami, M.H.; Alghamdi, S.S.; Albagar, S.M.; Mumena, W.A.; Mosli, R.H. Food Neophobia and Pickiness among Children and Associations with Socioenvironmental and Cognitive Factors. Appetite 2019, 142, 104373. [Google Scholar] [CrossRef]
- Kaar, J.L.; Shapiro, A.L.B.; Fell, D.M.; Johnson, S.L. Parental Feeding Practices, Food Neophobia, and Child Food Preferences: What Combination of Factors Results in Children Eating a Variety of Foods? Food Qual. Pref. 2016, 50, 57–64. [Google Scholar] [CrossRef]
- Wolstenholme, H.; Kelly, C.; Hennessy, M.; Heary, C. Childhood Fussy/Picky Eating Behaviours: A Systematic Review and Synthesis of Qualitative Studies. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 2. [Google Scholar] [CrossRef]
- Clayton, D.A. Socially Facilitated Behavior. Quart. Rev. Biol. 1978, 53, 373–392. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Cardinal, T.M.; Jankowski, M.; Kaciroti, N.; Gelman, S.A. Children’s Use of Adult Testimony to Guide Food Selection. Appetite 2008, 51, 302–310. [Google Scholar] [CrossRef]
- Bandura, A. Self-Efficacy: Toward a Unifying Theory of Behavioral Change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Litterbach, E.V.; Campbell, K.J.; Spence, A.C. Family Meals with Young Children: An Online Study of Family Mealtime Characteristics, among Australian Families with Children Aged Six Months to Six Years. BMC Public Health 2017, 17, 111. [Google Scholar] [CrossRef]
- Sharps, M.A.; Coulthard, H.; Salvy, S.J.; Ryan, S.; Fallon, V. The Influence of Experimental Confederate Peers on Children’s Food Intake: A Systematic Review and Meta-Analysis. Appetite 2022, 169, 105863. [Google Scholar] [CrossRef]
- Goldman, R.L.; Radnitz, C.L.; McGrath, R.E. The Role of Family Variables in Fruit and Vegetable Consumption in Pre-School Children. J. Public Health Res. 2012, 1, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.J.; Mallan, K.M.; Byrne, R.; Magarey, A.; Daniels, L.A. Toddlers’ Food Preferences. The Impact of Novel Food Exposure, Maternal Preferences and Food Neophobia. Appetite 2012, 59, 818–825. [Google Scholar] [CrossRef]
- Kral, T.V.E.; Rauh, E.M. Eating Behaviors of Children in the Context of Their Family Environment. Physiol. Behav. 2010, 100, 567–573. [Google Scholar] [CrossRef]
- Faith, M.S.; Heo, M.; Keller, K.L.; Pietrobelli, A. Child Food Neophobia Is Heritable, Associated with Less Compliant Eating, and Moderates Familial Resemblance for BMI. Obesity 2013, 21, 1650–1655. [Google Scholar] [CrossRef]
- Yong, C.; Kuang, X.; Liu, Y.; Xiang, C.; Xi, Y.; Huo, J.; Liang, J.; Zou, H.; Lin, Q. Parental Food Neophobia, Feeding Practices, and Preschooler’s Food Neophobia: A Cross-Sectional Study in China. Appetite 2023, 185, 106547. [Google Scholar] [CrossRef]
- Marlow, C.S.; Forestell, C.A. The Effect of Parental Food Neophobia on Children’s Fruit and Vegetable Consumption: A Serial Mediation Model. Appetite 2022, 172, 105942. [Google Scholar] [CrossRef]
- Hazley, D.; Stack, M.; Walton, J.; McNulty, B.A.; Kearney, J.M. Food Neophobia across the Life Course: Pooling Data from Five National Cross-Sectional Surveys in Ireland. Appetite 2022, 171, 105941. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, K.; Rönkä, A.; Hujo, M.; Lyytikäinen, A.; Nuutinen, O. Sensory-Based Food Education in Early Childhood Education and Care, Willingness to Choose and Eat Fruit and Vegetables, and the Moderating Role of Maternal Education and Food Neophobia. Public Health Nutr. 2018, 21, 2443–2453. [Google Scholar] [CrossRef]
- Scaglioni, S.; De Cosmi, V.; Ciappolino, V.; Parazzini, F.; Brambilla, P.; Agostoni, C. Factors Influencing Children’s Eating Behaviours. Nutrients 2018, 10, 706. [Google Scholar] [CrossRef]
- Wen, X.; Kong, K.L.; Eiden, R.D.; Sharma, N.N.; Xie, C. Sociodemographic Differences and Infant Dietary Patterns. Pediatrics 2014, 134, e1387–e1398. [Google Scholar] [CrossRef]
- Idelson, P.I.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in Children and Adolescents: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 283–299. [Google Scholar] [CrossRef]
- Maiz, E.; Balluerka, N. Nutritional Status and Mediterranean Diet Quality among Spanish Children and Adolescents with Food Neophobia. Food Qual. Pref. 2016, 52, 133–142. [Google Scholar] [CrossRef]
- Kozioł-Kozakowska, A.; Piórecka, B.; Schlegel-Zawadzka, M. Prevalence of Food Neophobia in Pre-School Children from Southern Poland and Its Association with Eating Habits, Dietary Intake and Anthropometric Parameters: A Cross-Sectional Study. Public Health Nutr. 2018, 21, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Kutbi, H.A.; Asiri, R.M.; Alghamdi, M.A.; Albassami, M.Z.; Mosli, R.H.; Mumena, W.A. Food Neophobia and Its Association with Nutrient Intake among Saudi Children. Food Qual. Pref. 2022, 96, 104372. [Google Scholar] [CrossRef]
- Białek-Dratwa, A.; Kowalski, O. Prevalence of Feeding Problems in Children and Associated Factors—A Cross-Sectional Study among Polish Children Aged 2–7 Years. Nutrients 2023, 15, 3185. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample Size Calculation in Medical Studies. Gastroenterol. Hepatol. Bed. Bench. 2013, 6, 14–17. [Google Scholar]
- Predieri, S.; Sinesio, F.; Monteleone, E.; Spinelli, S.; Cianciabella, M.; Daniele, G.M.; Dinnella, C.; Gasperi, F.; Endrizzi, I.; Torri, L.; et al. Gender, Age, Geographical Area, Food Neophobia and Their Relationships with the Adherence to the Mediterranean Diet: New Insights from a Large Population Cross-Sectional Study. Nutrients 2020, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Human, D.; Fluss, S.S. The World Medical Association-Declaration of Helsinki; World Medical Association: Ferney-Voltaire, France, 2001. [Google Scholar]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C. The Third Italian National Food Consumption Survey, INRAN-SCAI 2005–06—Part 1: Nutrient Intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- de Onis, M. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Pliner, P. Development of Measures of Food Neophobia in Children. Appetite 1994, 23, 147–163. [Google Scholar] [CrossRef]
- Laureati, M.; Spinelli, S.; Monteleone, E.; Dinnella, C.; Prescott, J.; Cattaneo, C.; Proserpio, C.; De Toffoli, A.; Gasperi, F.; Endrizzi, I.; et al. Associations between Food Neophobia and Responsiveness to “Warning” Chemosensory Sensations in Food Products in a Large Population Sample. Food Qual. Pref. 2018, 68, 113–124. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, Youth and the Mediterranean Diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in Children and Adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- Hinton, P.; McMurray, I.; Brownlow, C. SPSS Explained; Routledge: London, UK, 2004; ISBN 978-0-203-64259-7. [Google Scholar]
- Laureati, M.; Cattaneo, C.; Bergamaschi, V.; Proserpio, C.; Pagliarini, E. School Children Preferences for Fish Formulations: The Impact of Child and Parental Food Neophobia. J. Sens. Stud. 2016, 31, 408–415. [Google Scholar] [CrossRef]
- Białek-Dratwa, A.; Szczepańska, E.; Szymańska, D.; Grajek, M.; Krupa-Kotara, K.; Kowalski, O. Neophobia—A Natural Developmental Stage or Feeding Difficulties for Children? Nutrients 2022, 14, 1521. [Google Scholar] [CrossRef]
- Heyman, M.B.; Abrams, S.A.; Section on Gastroenterology, Hepatology, and Nutrition; Committee on Nutrition; Heitlinger, L.A.; Cabana, M.D.; Gilger, M.A.; Gugig, R.; Hill, I.D.; Lightdale, J.R.; et al. Fruit juice in infants, children, and adolescents: Current Recommendations. Pediatrics 2017, 139, e20170967. [Google Scholar] [CrossRef] [PubMed]
- Salvy, S.-J.; Kieffer, E.; Epstein, L.H. Effects of Social Context on Overweight and Normal-Weight Children’s Food Selection. Eat. Behav. 2008, 9, 190–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ragelienė, T.; Grønhøj, A. The Influence of Peers’ and Siblings’ on Children’s and Adolescents’ Healthy Eating Behavior. A Systematic Literature Review. Appetite 2020, 148, 104592. [Google Scholar] [CrossRef]
- Łoboś, P.; Januszewicz, A. Food Neophobia in Children. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 150–154. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, P.C.; Vasconcelos, I.A.L.; Zandonadi, R.P.; Nakano, E.Y.; Raposo, A.; Han, H.; Araya-Castillo, L.; Ariza-Montes, A.; Botelho, R.B.A. Food Neophobia among Brazilian Children: Prevalence and Questionnaire Score Development. Sustainability 2022, 14, 975. [Google Scholar] [CrossRef]
- Cassells, E.L.; Magarey, A.M.; Daniels, L.A.; Mallan, K.M. The Influence of Maternal Infant Feeding Practices and Beliefs on the Expression of Food Neophobia in Toddlers. Appetite 2014, 82, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Moding, K.J.; Stifter, C.A. Temperamental Approach/Withdrawal and Food Neophobia in Early Childhood: Concurrent and Longitudinal Associations. Appetite 2016, 107, 654–662. [Google Scholar] [CrossRef]
- Cooke, L.J.; Chambers, L.C.; Añez, E.V.; Wardle, J. Facilitating or Undermining? The Effect of Reward on Food Acceptance. A Narrative Review. Appetite 2011, 57, 493–497. [Google Scholar] [CrossRef]
- Bante, H.; Elliott, M.; Harrod, A.; Haire-Joshu, D. The Use of Inappropriate Feeding Practices by Rural Parents and Their Effect on Preschoolers’ Fruit and Vegetable Preferences and Intake. J. Nutr. Educ. Behav. 2008, 40, 28–33. [Google Scholar] [CrossRef]
- Galloway, A.T.; Fiorito, L.M.; Francis, L.A.; Birch, L.L. “Finish Your Soup”: Counterproductive Effects of Pressuring Children to Eat on Intake and Affect. Appetite 2006, 46, 318–323. [Google Scholar] [CrossRef]
- Tan, C.C.; Holub, S.C. Maternal Feeding Practices Associated with Food Neophobia. Appetite 2012, 59, 483–487. [Google Scholar] [CrossRef]
- Johnson, S.L.; Davies, P.L.; Boles, R.E.; Gavin, W.J.; Bellows, L.L. Young Children’s Food Neophobia Characteristics and Sensory Behaviors Are Related to Their Food Intake. J. Nutr. 2015, 145, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Saarijärvi, M.; Bratt, E.-L. When Face-to-Face Interviews Are Not Possible: Tips and Tricks for Video, Telephone, Online Chat, and Email Interviews in Qualitative Research. Eur. J. Cardiovasc. Nurs. 2021, 20, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Peasgood, T.; Bourke, M.; Devlin, N.; Rowen, D.; Yang, Y.; Dalziel, K. Randomised Comparison of Online Interviews versus Face-to-Face Interviews to Value Health States. Soc. Sci. Med. 2023, 323, 115818. [Google Scholar] [CrossRef] [PubMed]
- Sarin, H.V.; Taba, N.; Fischer, K.; Esko, T.; Kanerva, N.; Moilanen, L.; Saltevo, J.; Joensuu, A.; Borodulin, K.; Männistö, S.; et al. Food Neophobia Associates with Poorer Dietary Quality, Metabolic Risk Factors, and Increased Disease Outcome Risk in Population-Based Cohorts in a Metabolomics Study. Am. J. Clin. Nutr. 2019, 110, 233–245. [Google Scholar] [CrossRef]
- Rossi, L.; Ferrari, M.; Martone, D.; Benvenuti, L.; De Santis, A. The Promotions of Sustainable Lunch Meals in School Feeding Programs: The Case of Italy. Nutrients 2021, 13, 1571. [Google Scholar] [CrossRef]

| N | (%) | |
|---|---|---|
| Gender | ||
| Females | 123 | 42.7 |
| Males | 165 | 57.3 |
| Age | ||
| 3–5 years | 83 | 28.8 |
| 6–11 years | 205 | 71.2 |
| Ethnic group | ||
| African | 13 | 4.5 |
| Asiatic | 4 | 1.4 |
| Caucasian | 229 | 79.8 |
| Eurasian | 37 | 12.9 |
| Hispanic | 4 | 1.4 |
| Parents employment | ||
| Both parents employed | 253 | 87.8 |
| ≤1 parent employed | 35 | 12.2 |
| Household income | ||
| Up to 10,000 euros | 26 | 9 |
| Between 10,001 and 25,000 euros | 91 | 31.6 |
| Between 25,001 and 40,000 euros | 103 | 35.8 |
| Between 40,001 and more | 68 | 23.6 |
| Children per family | ||
| 1 | 108 | 37.5 |
| 2 | 145 | 50.3 |
| ≥2 | 35 | 12.2 |
| Body Mass Index (BMI) | ||
| Underweight | 16 | 5.6 |
| Normal weight | 129 | 44.8 |
| Overweight | 60 | 20.8 |
| Obesity | 83 | 28.8 |
| AMD Levels | Very Low (≤3) | Average (4–7) | Optimal (≥8) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Sociodemographic characteristics and BMI | |||||||
| Gender | Males | 42 | 49.4 | 103 * | 65.2 | 20 | 44.4 |
| Females | 43 | 50.6 | 55 | 34.8 | 25 | 55.5 | |
| Household income | <10,000 EUR | 9 | 10.6 | 11 | 6.9 | 6 | 13.3 |
| 10,000–25,000 EUR | 28 | 32.9 | 46 | 29.1 | 17 | 37.8 | |
| 25,000–40,000 EUR | 30 | 35.3 | 62 | 39.2 | 11 | 24.4 | |
| >40,000 EUR | 18 | 21.2 | 39 | 24.7 | 11 | 24.4 | |
| Parents employment | Both parents employed | 68 | 80 | 147 * | 93 | 38 | 84.4 |
| ≤1 parent employed | 17 | 20 | 11 | 7 | 7 | 15.6 | |
| Children per family | 1 | 35 | 41.2 | 65 | 41.1 | 8 | 17.8 |
| 2 | 40 | 47.1 | 73 | 46.2 | 32 * | 71.1 | |
| >2 | 10 | 11.8 | 20 | 10.8 | 5 | 11.1 | |
| Ethnic group | African | 1 | 1.2 | 9 | 5.7 | 3 | 6.7 |
| Asiatic | 2 | 2.3 | 1 | 0.6 | 1 | 2.2 | |
| Caucasian | 69 | 81.2 | 129 | 82.2 | 31 | 68.9 | |
| Eurasian | 11 | 13 | 16 | 10.2 | 10 | 22.2 | |
| Hispanic | 2 | 2.3 | 2 | 1.3 | 0 | 0 | |
| BMI | Underweight | 3 | 3.5 | 9 | 5.7 | 4 | 8.9 |
| Normal weight | 42 | 49.4 | 68 | 43 | 19 | 42.2 | |
| Overweight | 9 | 20 | 40 | 25.3 | 11 | 24.4 | |
| Obesity | 31 | 36.5 | 41 | 26 | 11 | 24.4 | |
| FN Levels | Low | Medium | High | ||||
|---|---|---|---|---|---|---|---|
| n | % | N | % | n | % | ||
| Socio-demographic characteristics and BMI | |||||||
| Gender | Males | 22 | 52.4 | 113 | 58.2 | 30 | 57.7 |
| Females | 20 | 47.6 | 81 | 41.8 | 22 | 42.3 | |
| Household income | <10,000EUR | 2 | 4.8 | 19 | 9.8 | 5 | 9.6 |
| 10,000–25,000 EUR | 8 | 19 | 67 | 34.5 | 16 | 30.8 | |
| 25,000–40,000 EUR | 18 | 42.8 | 68 | 35.1 | 17 | 32.7 | |
| >40,000 EUR | 14 | 33.3 | 40 | 20.6 | 14 | 26.9 | |
| Parents employment | Both parents employed | 40 | 95.2 | 170 | 87.6 | 43 | 82.7 |
| ≤1 parent employed | 2 | 4.8 | 24 | 12.4 | 9 | 17.3 | |
| Children per family | 1 | 7 * | 16.7 | 75 | 38.7 | 26 | 50 |
| 2 | 25 | 59.5 | 98 | 50.5 | 22 | 42.3 | |
| >2 | 10 | 23.8 | 21 | 10.8 | 4 | 7.7 | |
| Ethnic group | African | 2 | 4.8 | 10 | 5.2 | 1 | 1.9 |
| Asiatic | 0 | 0 | 4 | 2.1 | 0 | 0 | |
| Caucasian | 34 | 80.9 | 153 | 79.3 | 42 | 80.8 | |
| Eurasian | 6 | 14.3 | 22 | 11.4 | 9 | 17.3 | |
| Hispanic | 0 | 0 | 4 | 2.1 | 0 | 0 | |
| BMI | Underweight | 0 | 0 | 12 | 6.2 | 4 | 7.7 |
| Normal weight | 26 | 61.9 | 81 | 41.8 | 22 | 42.3 | |
| Overweight | 16 | 38.1 | 36 | 18.5 | 8 | 15.4 | |
| Obesity | 0 * | 0 | 65 | 33.5 | 18 | 34.6 | |
| FN Levels | Low | Medium | High | ||||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| KIDMED total score | Very low | 1 | 2.4 | 57 | 29.4 | 27 * | 51.9 |
| Average | 26 | 61.9 | 109 | 56.2 | 23 | 44.2 | |
| Optimal | 15 | 35.7 | 28 | 14.4 | 2 | 3.8 | |
| KIDMED test components | |||||||
| Fruit or fruit juice every day | Yes | 39 | 92.9 | 156 | 80.4 | 34 | 65.4 |
| No | 3 | 7.1 | 38 | 19.6 | 18 * | 34.6 | |
| Second fruit every day | Yes | 32 * | 76.2 | 67 | 34.5 | 11 | 21.2 |
| No | 10 | 23.8 | 127 | 65.5 | 41 | 78.8 | |
| Fresh or cooked vegetables regularly once a day | Yes | 40 * | 95.2 | 132 | 68 | 24 | 46.2 |
| No | 2 | 4.8 | 62 | 32 | 28 | 53.8 | |
| Fresh or cooked vegetables more than once a day | Yes | 28 * | 66.7 | 44 | 22.7 | 5 | 9.6 |
| No | 14 | 33.3 | 150 | 77.3 | 47 | 90.4 | |
| Fish at least 2–3 times per week | Yes | 30 | 71.4 | 131 | 67.5 | 27 | 51.9 |
| No | 12 | 28.6 | 63 | 32.5 | 25 * | 48.1 | |
| Fast-food more than once a week | Yes | 1 | 2.4 | 40 | 20.6 | 13 | 25 |
| No | 41* | 97.6 | 154 | 79.4 | 39 | 75 | |
| Legumes more than once a week | Yes | 39 * | 92.9 | 84 | 43.3 | 10 | 19.2 |
| No | 3 | 7.1 | 110 | 56.7 | 42 | 80.8 | |
| Cereals or grains (bread, etc.) for breakfast | Yes | 13 | 31 | 35 | 18 | 6 | 11.5 |
| No | 29 | 69 | 159 | 82 | 46 | 88.5 | |
| Nuts at least 2–3 times per week | Yes | 7 | 16.7 | 56 | 28.9 | 10 | 19.2 |
| No | 35 | 83.3 | 138 | 71.1 | 42 | 80.8 | |
| Skips breakfast | Yes | 2 | 4.8 | 22 | 11.3 | 10 | 19.2 |
| No | 40 | 95.2 | 172 | 88.7 | 42 | 80.8 | |
| Dairy products for breakfast (yogurt, milk, etc.) | Yes | 40 | 95.2 | 180 | 92.8 | 44 | 84.6 |
| No | 2 | 4.8 | 14 | 7.2 | 8 | 15.4 | |
| Commercially baked goods or pastries for breakfast | Yes | 36 | 85.7 | 156 | 80.4 | 43 | 82.7 |
| No | 6 | 14.3 | 38 | 19.6 | 9 | 17.3 | |
| Sweets and candy several times every day | Yes | 11 | 26.2 | 79 | 40.7 | 25 | 48.1 |
| No | 31 | 73.8 | 115 | 59.3 | 27 | 51.9 | |
| Variable | Category | Estimation | p-Value |
|---|---|---|---|
| FN Continuous score | Intercept | 8.61 | 0.000 |
| β | −0.09 | 0.000 | |
| FN levels | Low | 6.93 | 0.000 |
| Medium | 4.92 | 0.000 | |
| High | 3.54 | 0.000 |
| Variable | Category | Estimation | p-Value |
|---|---|---|---|
| Intercept | 9.85 | 0.000 | |
| FN continuous score | Β | −0.09 | 0.000 |
| Gender | Males | 0.22 | 0.390 |
| Household income | 10,000–25,000 EUR | −0.52 | 0.447 |
| 25,000–40,000 EUR | −1.25 | 0.080 | |
| >40,000 EUR | −0.82 | 0.269 | |
| Parents employment | Both parents employed | 1.40 | 0.025 |
| Children per family | 2 | 0.42 | 0.143 |
| >2 | −0.39 | 0.378 | |
| Ethnic group | Asiatic | −0.20 | 0.864 |
| Caucasian | −1.27 | 0.049 | |
| Eurasian | −0.92 | 0.188 | |
| Hispanic | −2.74 | 0.026 | |
| Body Mass Index (BMI) | Normal weight | −0.97 | 0.075 |
| Overweight | −0.67 | 0.249 | |
| Obesity | −0.63 | 0.279 | |
| Variable | Category | Estimation | p-Value |
|---|---|---|---|
| FN levels | Low | 7.10 | 0.000 |
| Medium | 4.94 | 0.000 | |
| High | 3.69 | 0.000 | |
| Gender | Males | 0.22 | 0.418 |
| Household income | 10,000–25,000 EUR | −0.79 | 0.150 |
| 25,000–40,000 EUR | 0.38 | 0.345 | |
| >40,000 EUR | 0.34 | 0.212 | |
| Parents employment | Both parents employed | 1.43 | 0.032 |
| Children per family | 2 | −0.10 | 0.759 |
| >2 | −0.44 | 0.076 | |
| Ethnic group | Asiatic | −0.42 | 0.739 |
| Caucasian | −1.29 | 0.062 | |
| Eurasian | −0.95 | 0.203 | |
| Hispanic | −2.69 | 0.041 | |
| Body Mass Index (BMI) | Normal weight | −0.58 | 0.170 |
| Overweight | 0.64 | 0.070 | |
| Obesity | −0.34 | 0.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nucci, A.; Pilloni, S.; Scognamiglio, U.; Rossi, L. Adherence to Mediterranean Diet and Food Neophobia Occurrence in Children: A Study Carried out in Italy. Nutrients 2023, 15, 5078. https://doi.org/10.3390/nu15245078
Di Nucci A, Pilloni S, Scognamiglio U, Rossi L. Adherence to Mediterranean Diet and Food Neophobia Occurrence in Children: A Study Carried out in Italy. Nutrients. 2023; 15(24):5078. https://doi.org/10.3390/nu15245078
Chicago/Turabian StyleDi Nucci, Annalisa, Simone Pilloni, Umberto Scognamiglio, and Laura Rossi. 2023. "Adherence to Mediterranean Diet and Food Neophobia Occurrence in Children: A Study Carried out in Italy" Nutrients 15, no. 24: 5078. https://doi.org/10.3390/nu15245078
APA StyleDi Nucci, A., Pilloni, S., Scognamiglio, U., & Rossi, L. (2023). Adherence to Mediterranean Diet and Food Neophobia Occurrence in Children: A Study Carried out in Italy. Nutrients, 15(24), 5078. https://doi.org/10.3390/nu15245078