Seasonal Serum 25(OH) Vitamin D Level and Reproductive or Immune Markers in Reproductive-Aged Women with Infertility: A Cross-Sectional Observational Study in East Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Design and Participants
2.2.1. Measurement of 25(OH) Vitamin D
2.2.2. Th1/Th2 Ratio Analysis
2.3. Ethical Statement
2.4. Statistical Analyses
3. Results
3.1. Population Characteristics
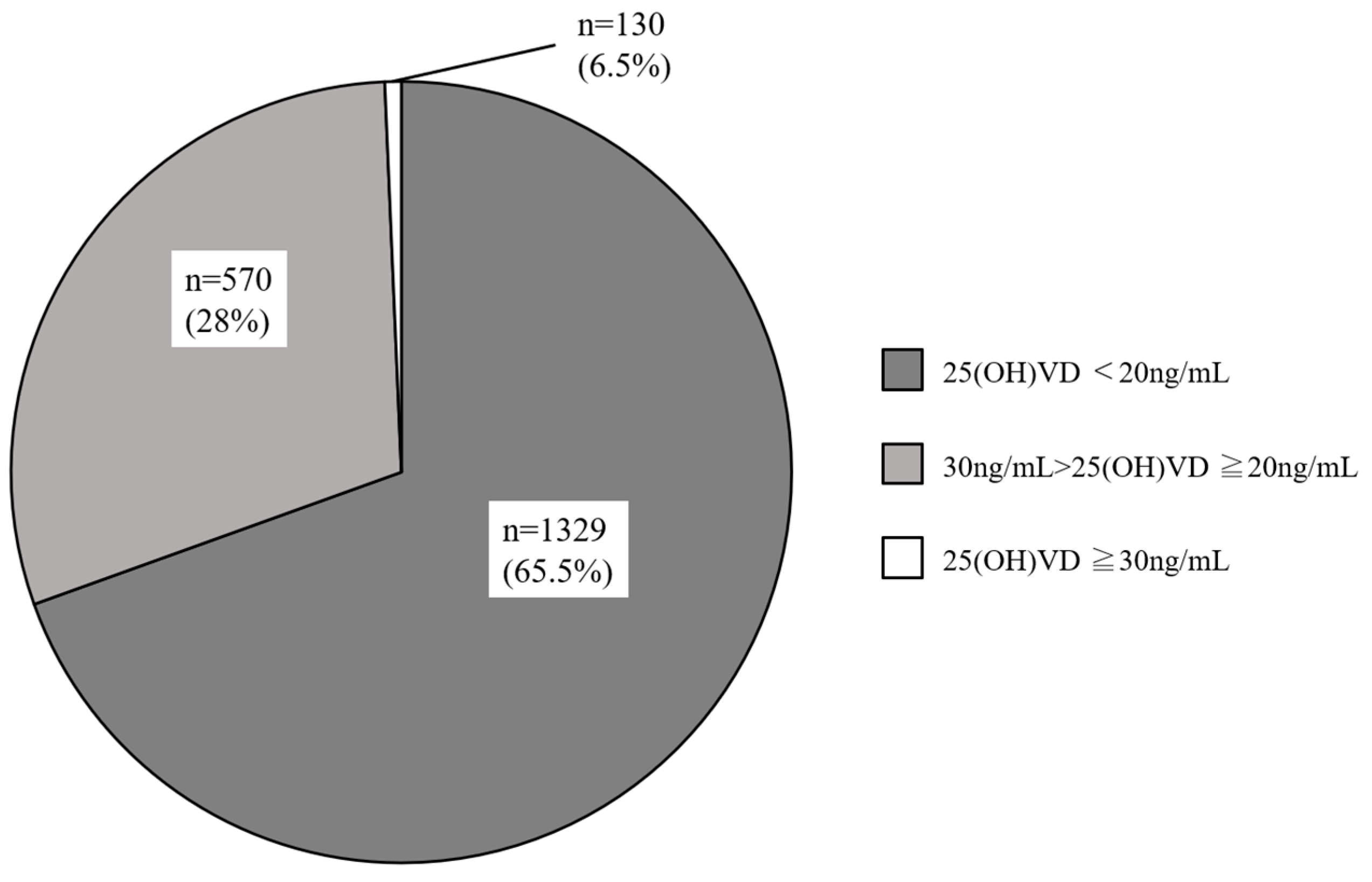
3.2. Serum 25(OH)D Levels in Reproductive-Aged Women with Infertility
3.2.1. Serum 25(OH)D Levels and Reproductive Parameters
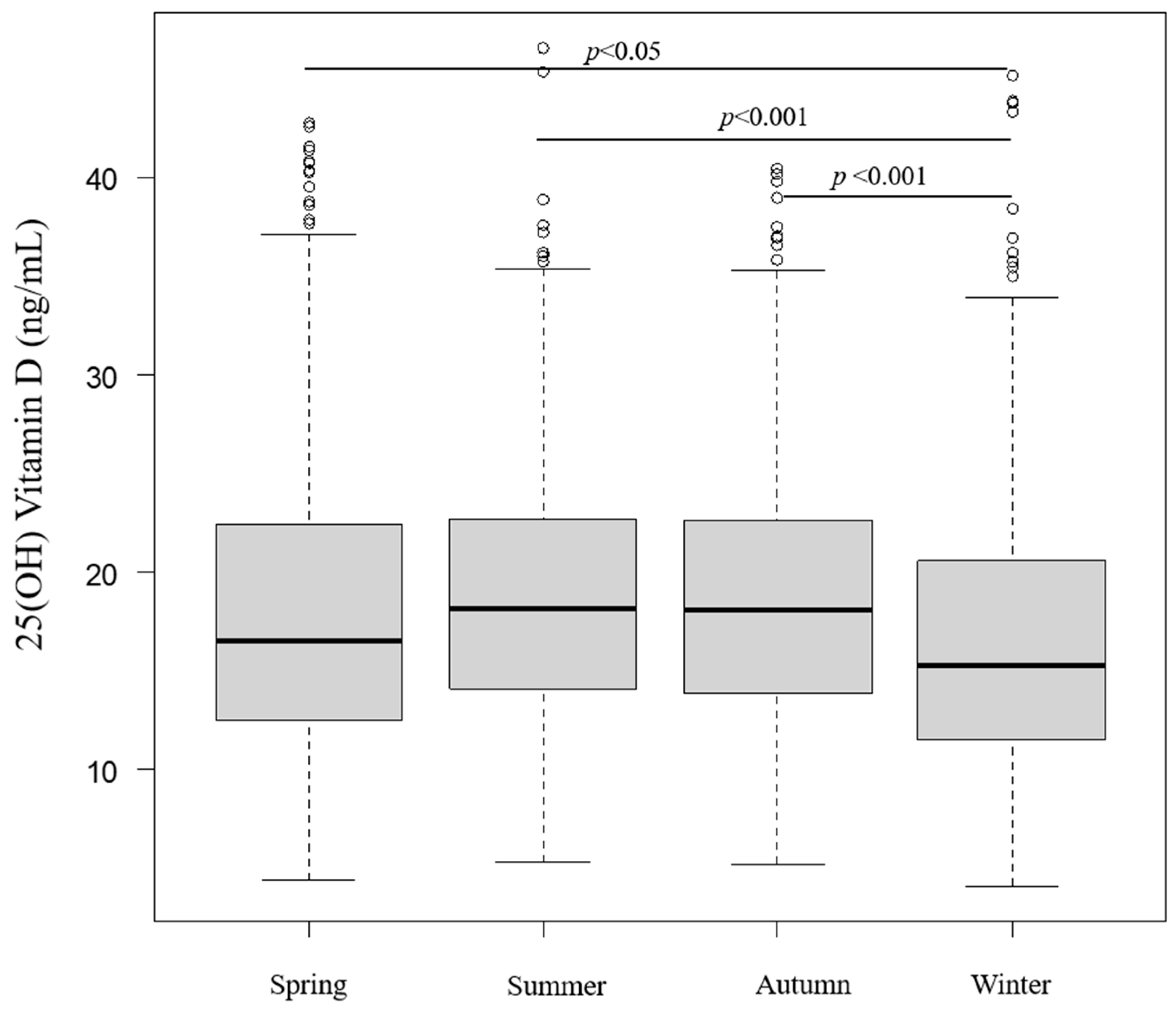
3.2.2. Seasonal and Monthly Serum 25(OH)D Levels
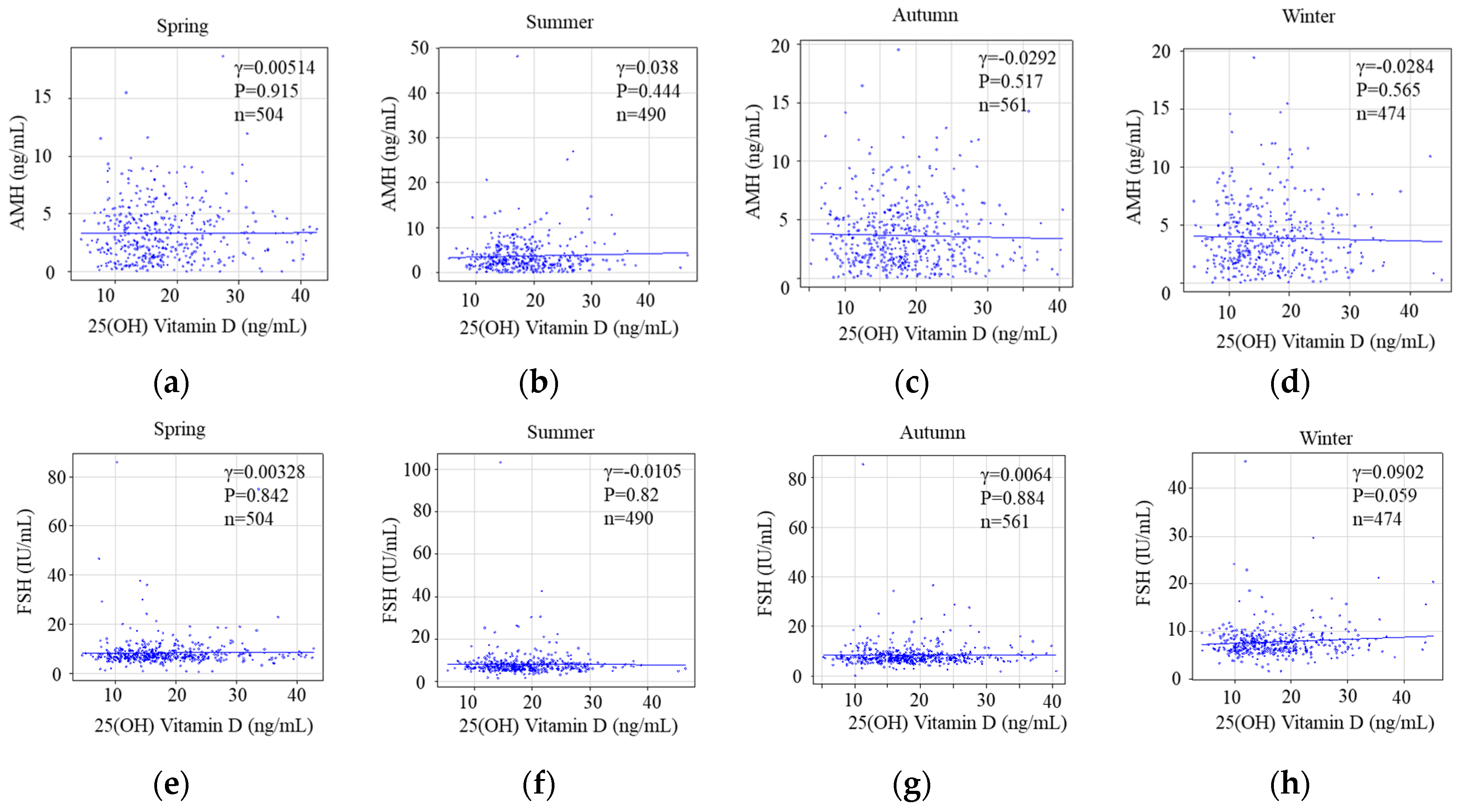
3.2.3. Seasonal Status of Serum 25(OH)D Levels and Reproductive Markers Categorized as Ovarian Reserve
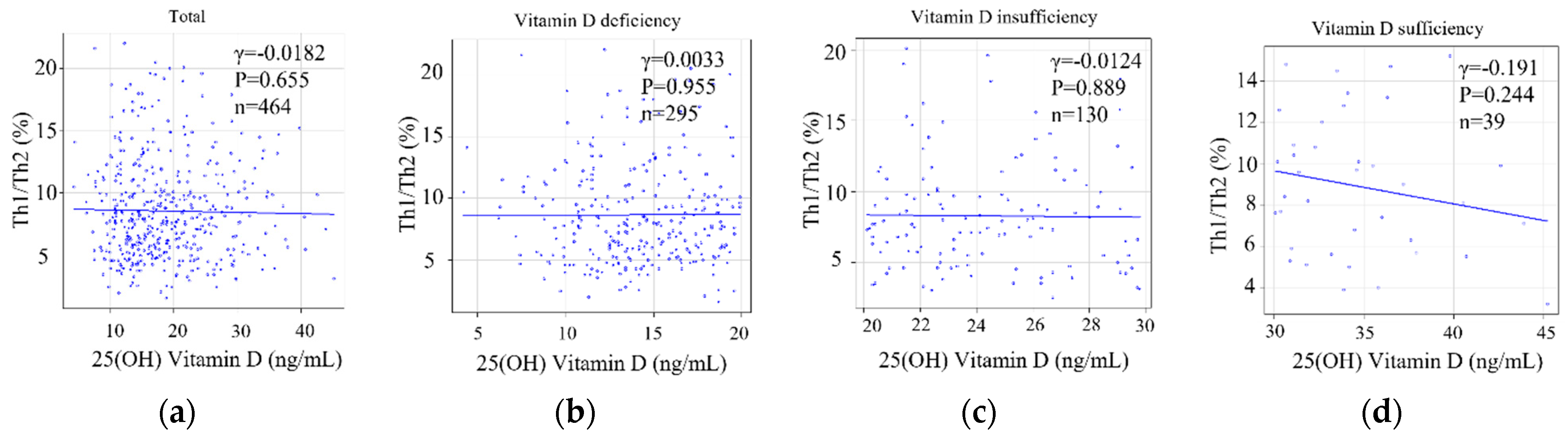
3.2.4. Serum 25(OH)D Levels or the Degree of Deviation of Serum 25(OH)D Levels and the Status of Helper T-Cell Immunity as an Implantation Marker
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Souberbielle, J.-C.; Body, J.-J.; Lappe, J.M.; Plebani, M.; Shoenfeld, Y.; Wang, T.J.; Bischoff-Ferrari, H.A.; Cavalier, E.; Ebeling, P.R.; Fardellone, P. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun. Rev. 2010, 9, 709–715. [Google Scholar] [CrossRef]
- Luk, J.; Torrealday, S.; Neal Perry, G.; Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 2012, 27, 3015–3027. [Google Scholar] [CrossRef]
- Jukic, A.M.; Steiner, A.Z.; Baird, D.D. Association between serum 25-hydroxyvitamin D and ovarian reserve in premenopausal women. Menopause 2015, 22, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.A.; Houghton, L.A.; Jones, G.T.; van Rij, A.M.; Morgan, K.; McLennan, I.S. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J. Clin. Endocrinol. Metab. 2012, 97, 2450–2455. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, F.A.; Grzechocinska, B.; Wielgos, M. The Role of Vitamin D in Reproductive Health—A Trojan Horse or the Golden Fleece? Nutrients 2015, 7, 4139–4153. [Google Scholar] [CrossRef] [PubMed]
- Butts, S.F.; Seifer, D.B.; Koelper, N.; Senapati, S.; Sammel, M.D.; Hoofnagle, A.N.; Kelly, A.; Krawetz, S.A.; Santoro, N.; Zhang, H.; et al. Vitamin D Deficiency Is Associated With Poor Ovarian Stimulation Outcome in PCOS but Not Unexplained Infertility. J. Clin. Endocrinol. Metab. 2019, 104, 369–378. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, S.; Wang, Y.; Wang, P.; Qu, D.; Liu, M.; Ma, W.; Li, Y. Vitamin D improves in-vitro fertilization outcomes in infertile women with polycystic ovary syndrome and insulin resistance. Minerva Med. 2019, 110, 199–208. [Google Scholar] [CrossRef]
- Ota, K.; Dambaeva, S.; Han, A.-R.; Beaman, K.; Gilman-Sachs, A.; Kwak-Kim, J. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum. Reprod. 2014, 29, 208–219. [Google Scholar] [CrossRef]
- Ota, K.; Dambaeva, S.; Kim, M.W.I.; Han, A.R.; Fukui, A.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur. J. Immunol. 2015, 45, 3188–3199. [Google Scholar] [CrossRef]
- Rajaei, S.; Mirahmadian, M.; Jeddi-Tehrani, M.; Tavakoli, M.; Zonoobi, M.; Dabbagh, A.; Zarnani, A.H. Effect of 1,25(OH)2 vitamin D3 on cytokine production by endometrial cells of women with repeated implantation failure. Gynecol. Endocrinol. 2012, 28, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 1995, 53, 599–602. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.; Cippitelli, M.; Cocciolo, M.G.; Mazzeo, D.; Di Lucia, P.; Lang, R.; Sinigaglia, F.; Panina-Bordignon, P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J. Clin. Investig. 1998, 101, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Hayes, C.E.; DeLuca, H.F. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 7861–7864. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Humpal-Winter, J.; DeLuca, H.F. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 2000, 377, 135–138. [Google Scholar] [CrossRef]
- Das, M.; Tomar, N.; Sreenivas, V.; Gupta, N.; Goswami, R. Effect of vitamin D supplementation on cathelicidin, IFN-γ, IL-4 and Th1/Th2 transcription factors in young healthy females. Eur. J. Clin. Nutr. 2014, 68, 338–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwak-Kim, J.; Skariah, A.; Wu, L.; Salazar, D.; Sung, N.; Ota, K. Humoral and cellular autoimmunity in women with recurrent pregnancy losses and repeated implantation failures: A possible role of vitamin D. Autoimmun. Rev. 2016, 15, 943–947. [Google Scholar] [CrossRef]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta, M.; Farhat, R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 713–718. [Google Scholar] [CrossRef]
- Ng, S.C.; Gilman-Sachs, A.; Thaker, P.; Beaman, K.D.; Beer, A.E.; Kwak-Kim, J. Expression of intracellular Th1 and Th2 cytokines in women with recurrent spontaneous abortion, implantation failures after IVF/ET or normal pregnancy. Am. J. Reprod. Immunol. 2002, 48, 77–86. [Google Scholar] [CrossRef]
- Mekinian, A.; Cohen, J.; Alijotas-Reig, J.; Carbillon, L.; Nicaise-Roland, P.; Kayem, G.; Daraï, E.; Fain, O.; Bornes, M. Unexplained Recurrent Miscarriage and Recurrent Implantation Failure: Is There a Place for Immunomodulation? Am. J. Reprod. Immunol. 2016, 76, 8–28. [Google Scholar] [CrossRef]
- Kwak-Kim, J.Y.H.; Chung-Bang, H.S.; Ng, S.C.; Ntrivalas, E.I.; Mangubat, C.P.; Beaman, K.D.; Beer, A.E.; Gilman-Sachs, A. Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Hum. Reprod. 2003, 18, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Odukoya, O.A.; Ajjan, R.A.; Li, T.C.; Weetman, A.P.; Cooke, I.D. The role of T-helper cytokines in human reproduction. Fertil. Steril. 2000, 73, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, Y.; Kuroda, K.; Nakagawa, K.; Ochiai, A.; Ozaki, R.; Murakami, K.; Jinushi, M.; Matsumoto, A.; Sugiyama, R.; Takeda, S. Vitamin D Regulates Maternal T-Helper Cytokine Production in Infertile Women. Nutrients 2018, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Baylin, A.; Levy, P.D. Vitamin D deficiency and insufficiency among US adults: Prevalence, predictors and clinical implications. Br. J. Nutr. 2018, 119, 928–936. [Google Scholar] [CrossRef]
- Herrick, K.A.; Storandt, R.J.; Afful, J.; Pfeiffer, C.M.; Schleicher, R.L.; Gahche, J.J.; Potischman, N. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 2019, 110, 150–157. [Google Scholar] [CrossRef]
- Liu, W.; Hu, J.; Fang, Y.; Wang, P.; Lu, Y.; Shen, N. Vitamin D status in Mainland of China: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 101017. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Wang, R.; Li, M.; Yun, C.; Li, W.; Yang, Y.; Piao, J.; Yang, X.; Yang, L. Vitamin D nutritional status and its related factors for Chinese children and adolescents in 2010–2012. Nutrients 2017, 9, 1024. [Google Scholar] [CrossRef]
- Nakamura, K.; Kitamura, K.; Takachi, R.; Saito, T.; Kobayashi, R.; Oshiki, R.; Watanabe, Y.; Tsugane, S.; Sasaki, A.; Yamazaki, O. Impact of demographic, environmental, and lifestyle factors on vitamin D sufficiency in 9084 Japanese adults. Bone 2015, 74, 10–17. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Morita, M.; Yamada, H.; Tanaka, S.; Kawaguchi, H.; Nakamura, K.; Akune, T. Profiles of vitamin D insufficiency and deficiency in Japanese men and women: Association with biological, environmental, and nutritional factors and coexisting disorders: The ROAD study. Osteoporos. Int. 2013, 24, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Ban Frangež, H.; Vrtačnik-Bokal, E.; D’Anna, R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar] [PubMed]
- Evers, J.L.H. Female subfertility. Lancet 2002, 360, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, R.; Ozono, K.; Fukumoto, S.; Inoue, D.; Yamauchi, M.; Minagawa, M.; Michigami, T.; Takeuchi, Y.; Matsumoto, T.; Sugimoto, T. Assessment criteria for vitamin D deficiency/insufficiency in Japan: Proposal by an expert panel supported by the Research Program of Intractable Diseases, Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society [Opinion]. J. Bone Min. Metab. 2017, 35, 1–5. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kwak-Kim, J.; Ota, K.; Kuroda, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am. J. Reprod. Immunol. 2015, 73, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell–mediated tissue damage. Nat. Med. 2007, 13, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Akter, S.; Eguchi, M.; Kurotani, K.; Kochi, T.; Kashino, I.; Ito, R.; Kuwahara, K.; Tsuruoka, H.; Kabe, I.; Mizoue, T. Serum 25-hydroxyvitamin D and metabolic syndrome in a Japanese working population: The Furukawa Nutrition and Health Study. Nutrition 2017, 36, 26–32. [Google Scholar] [CrossRef]
- Tsugawa, N.; Uenishi, K.; Ishida, H.; Ozaki, R.; Takase, T.; Minekami, T.; Uchino, Y.; Kamao, M.; Okano, T. Association between vitamin D status and serum parathyroid hormone concentration and calcaneal stiffness in Japanese adolescents: Sex differences in susceptibility to vitamin D deficiency. J. Bone Miner. Metab. 2016, 34, 464–474. [Google Scholar] [CrossRef]
- Shibata, M.; Suzuki, A.; Sekiya, T.; Sekiguchi, S.; Asano, S.; Udagawa, Y.; Itoh, M. High prevalence of hypovitaminosis D in pregnant Japanese women with threatened premature delivery. J. Bone Miner. Metab. 2011, 29, 615–620. [Google Scholar] [CrossRef]
- Shiraishi, M.; Haruna, M.; Matsuzaki, M.; Murayama, R. Demographic and lifestyle factors associated with vitamin D status in pregnant Japanese women. J. Nutr. Sci. Vitaminol. 2014, 60, 420–428. [Google Scholar] [CrossRef][Green Version]
- Shiraishi, M.; Haruna, M.; Matsuzaki, M.; Murayama, R.; Kitanaka, S.; Sasaki, S. Validity of a self-administered diet history questionnaire for estimating vitamin D intakes of J apanese pregnant women. Matern. Child Nutr. 2015, 11, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Yonetani, N.; Kaji, T.; Hichijo, A.; Nakayama, S.; Maeda, K.; Irahara, M. Effect of prolonged hospitalization for threatened preterm labor on maternal and fetal vitamin D levels. J. Obstet. Gynaecol. Res. 2018, 44, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, K.T.; Nakayama, T.; Adachi, Y.; Hamazaki, K.; Onishi, K.; Konishi, Y.; Kawanishi, Y.; Go, T.; Sato, K.; Kurozawa, Y. High frequency of vitamin D deficiency in current pregnant Japanese women associated with UV avoidance and hypo-vitamin D diet. PLoS ONE 2019, 14, e0213264. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, E.; Kaji, T.; Nakayama, S.; Yoshida, A.; Yonetani, N.; Maeda, K.; Yasui, T.; Irahara, M. Seasonal variation of serum 25 (OH) vitamin D levels in maternal and umbilical cord blood in Japanese women. J. Med. Investig. 2019, 66, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Yoshikata, H.; Tsugawa, N.; Watanabe, Y.; Tsuburai, T.; Chaki, O.; Hirahara, F.; Miyagi, E.; Sakakibara, H.; Uenishi, K.; Okano, T. 25-Hydroxyvitamin D profiles and maternal bone mass during pregnancy and lactation in Japanese women. J. Bone Miner. Metab. 2020, 38, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, N.; Nishida, K.; Sairenchi, T.; Umesawa, M.; Noguchi, R.; Someya, K.; Kobashi, G. Changes in vitamin D status considering hemodilution factors in Japanese pregnant women according to trimester: A longitudinal survey. PLoS ONE 2020, 15, e0239954. [Google Scholar] [CrossRef]
- Yamade, I.; Inoue, T.; Hamada, H.; Sudou, S.; Otsubo, M.; Sawada, M.; Nakayama, T.; Hatayama, H. Ineffectiveness of antenatal guidance intervention for vitamin D insufficiency and deficiency in pregnant women in Kyoto, Japan. J. Obs. Gynaecol. Res. 2021, 47, 3540–3550. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef]
- Hilger, J.; Friedel, A.; Herr, R.; Rausch, T.; Roos, F.; Wahl, D.A.; Pierroz, D.D.; Weber, P.; Hoffmann, K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014, 111, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, J.; Iki, M.; Sato, Y.; Kajita, E.; Nishino, H.; Akiba, T.; Matsumoto, T.; Kagamimori, S. Total 25-hydroxyvitamin D levels predict fracture risk: Results from the 15-year follow-up of the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos. Int. 2017, 28, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nashimoto, M.; Tsuchiya, Y.; Obata, A.; Miyanishi, K.; Yamamoto, M. Vitamin D insufficiency in Japanese female college students: A preliminary report. Int. J. Vitam. Nutr. Res. 2001, 71, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Kuroda, T.; Tsugawa, N.; Onoe, Y.; Okano, T.; Shiraki, M. Optimal vitamin D intake for preventing serum 25-hydroxyvitamin D insufficiency in young Japanese women. J. Bone Min. Metab. 2018, 36, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Tsuprykov, O.; Chen, X.; Elitok, S.; Krämer, B.K.; Hocher, B. Relationship Between Vitamin D and Hormones Important for Human Fertility in Reproductive-Aged Women. Front. Endocrinol. 2021, 12, 666687. [Google Scholar] [CrossRef] [PubMed]
- Horton-French, K.; Dunlop, E.; Lucas, R.M.; Pereira, G.; Black, L.J. Prevalence and predictors of vitamin D deficiency in a nationally representative sample of Australian adolescents and young adults. Eur. J. Clin. Nutr. 2021, 75, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies world report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum. Reprod. 2016, 31, 1588–1609. [Google Scholar] [CrossRef]
- Ishihara, O.; Jwa, S.C.; Kuwahara, A.; Katagiri, Y.; Kuwabara, Y.; Hamatani, T.; Harada, M.; Osuga, Y. Assisted reproductive technology in Japan: A summary report for 2018 by the Ethics Committee of the Japan Society of Obstetrics and Gynecology. Reprod. Med. Biol. 2021, 20, 3–12. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo. Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Asakura, K.; Etoh, N.; Imamura, H.; Michikawa, T.; Nakamura, T.; Takeda, Y.; Mori, S.; Nishiwaki, Y. Vitamin D Status in Japanese Adults: Relationship of Serum 25-Hydroxyvitamin D with Simultaneously Measured Dietary Vitamin D Intake and Ultraviolet Ray Exposure. Nutrients 2020, 12, 743. [Google Scholar] [CrossRef]
- Kuwabara, A.; Tsugawa, N.; Mizuno, K.; Ogasawara, H.; Watanabe, Y.; Tanaka, K. A simple questionnaire for the prediction of vitamin D deficiency in Japanese adults (Vitaimn D Deficiency questionnaire for Japanese: VDDQ-J). J. Bone Min. Metab. 2019, 37, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Suzuki, A.; Kotake, M.; Zhang, X.; Nishiwaki-Yasuda, K.; Ishiwata, Y.; Imamura, S.; Nagata, M.; Takamoto, S.; Itoh, M. Seasonal changes of serum 25-hydroxyvitamin D and intact parathyroid hormone levels in a normal Japanese population. J. Bone Miner. Metab. 2005, 23, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Niino, M.; Fukazawa, T.; Miyazaki, Y.; Ura, S.; Takahashi, E.; Minami, N.; Akimoto, S.; Amino, I.; Naganuma, R.; Kikuchi, S. Seasonal fluctuations in serum levels of vitamin D in Japanese patients with multiple sclerosis. J. Neuroimmunol. 2021, 357, 577624. [Google Scholar] [CrossRef] [PubMed]
- Klenk, J.; Rapp, K.; Denkinger, M.D.; Nagel, G.; Nikolaus, T.; Peter, R.; Koenig, W.; Böhm, B.O.; Rothenbacher, D. Seasonality of vitamin D status in older people in Southern Germany: Implications for assessment. Age Ageing 2013, 42, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Heyden, E.L.; Wimalawansa, S.J. Vitamin D: Effects on human reproduction, pregnancy, and fetal well-being. J. Steroid Biochem. Mol. Biol. 2018, 180, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Fichera, M.; Török, P.; Tesarik, J.; Della Corte, L.; Rizzo, G.; Garzon, S.; Carlea, A.; Di Angelo Antonio, S.; Zito, G.; Panella, M.M. Vitamin D, reproductive disorders and assisted reproduction: Evidences and perspectives. Int. J. Food Sci. Nutr. 2020, 71, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Garbose, R.A.; Kim, K.; Kissell, K.; Kuhr, D.L.; Omosigho, U.R.; Perkins, N.J.; Galai, N.; Silver, R.M.; Sjaarda, L.A. Association of preconception serum 25-hydroxyvitamin D concentrations with livebirth and pregnancy loss: A prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 725–732. [Google Scholar] [CrossRef]
- Fung, J.L.; Hartman, T.J.; Schleicher, R.L.; Goldman, M.B. Association of vitamin D intake and serum levels with fertility: Results from the Lifestyle and Fertility Study. Fertil. Steril. 2017, 108, 302–311. [Google Scholar] [CrossRef]
- Somigliana, E.; Paffoni, A.; Lattuada, D.; Colciaghi, B.; Filippi, F.; La Vecchia, I.; Tirelli, A.; Baffero, G.M.; Persico, N.; Viganò, P. Serum levels of 25-hydroxyvitamin D and time to natural pregnancy. Gynecol. Obstet. Investig. 2016, 81, 468–471. [Google Scholar] [CrossRef]
- Møller, U.; Streym, S.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Effects of 25OHD concentrations on chances of pregnancy and pregnancy outcomes: A cohort study in healthy Danish women. Eur. J. Clin. Nutr. 2012, 66, 862–868. [Google Scholar] [CrossRef]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468.e3. [Google Scholar] [CrossRef]
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The Association between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.N.; Nguyen, L.; Chan, J.; Innes, B.A.; Bulmer, J.N.; Kilby, M.D.; Hewison, M. Effects of 25-Hydroxyvitamin D3 and 1,25-Dihydroxyvitamin D3 on Cytokine Production by Human Decidual Cells1. Biol. Reprod. 2006, 75, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; Craciunas, L.; Vermeulen, N.; Vomstein, K.; Toth, B. Definition, diagnostic and therapeutic options in recurrent implantation failure: An international survey of clinicians and embryologists. Hum. Reprod. 2021, 36, 305–317. [Google Scholar] [CrossRef]
- ESHRE Working Group on Recurrent Implantation Failure; Cimadomo, D.; de los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N.; et al. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [CrossRef] [PubMed]
- he Guideline Group on Unexplained Infertility; Romualdi, D.; Ata, B.; Bhattacharya, S.; Bosch, E.; Costello, M.; Gersak, K.; Homburg, R.; Mincheva, M.; Norman, R.J.; et al. Evidence-based guideline: Unexplained infertility. Hum. Reprod. 2023, 38, 1881–1890. [Google Scholar] [CrossRef]
- Ishihara, J.; Inoue, M.; Kobayashi, M.; Tanaka, S.; Yamamoto, S.; Iso, H.; Tsugane, S. Impact of the revision of a nutrient database on the validity of a self-administered food frequency questionnaire (FFQ). J. Epidemiol. 2006, 16, 107–116. [Google Scholar] [CrossRef]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef]
- Mrozikiewicz, A.E.; Ożarowski, M.; Jędrzejczak, P. Biomolecular Markers of Recurrent Implantation Failure—A Review. Int. J. Mol. Sci. 2021, 22, 10082. [Google Scholar] [CrossRef]



| Means ± SDs or Percentage | Range | |
|---|---|---|
| Age (years) | 34.5 ± 4.5 | 22–47 |
| Gravity | 0.5 ± 0.9 | 0–8 |
| Parity | 0.2 ± 0.5 | 0–4 |
| BMI (kg/m2) | 21.6 ± 3.5 | 14.8–40.9 |
| Duration of infertility (months) | 22.7 ± 18.5 | 0–120 |
| Causal factors of infertility, % (n) | ||
| Reduced ovarian reserve | 20.7 (421) | |
| Ovulation disorder | 24.1 (488) | |
| Uterine factor | 40.8 (827) | |
| Tubal factor | 18.2 (369) | |
| Endometriosis | 11.7 (238) | |
| Male factor | 42.2 (857) | |
| Unexplained | 14.6 (297) | |
| Day 3 serum FSH (IU/mL) | 8.2 ± 5.2 | 0.1–85.9 |
| Day 3 serum LH | 7.4 ± 5.7 | 0.1–61.4 |
| Day 3 serum estrogen (E2) | 50.8 ± 59.8 | 5.0–354 |
| AMH (ng/mL) | 3.6 ± 3.1 | 0.2–48.2 |
| Thyroid-stimulating hormone (TSH) (μIU/mL) | 1.7 ± 1.1 | 0.1–13.5 |
| Free Thyroxine (FT4) (ng/dL) | 1.3 ± 0.2 | 0.5–3.8 |
| Vitamin D serum levels (ng/mL) | 18.2 ± 7.0 | 4.1–46.6 |
| Vitamin D supplement user, % (n) | 10.8 (220) | |
| Current smoker, % (n) | 15.2 (320) | |
| Occupation, % (n) | ||
| Employee | 66.3 (1346) | |
| Part-time | 11.5 (234) | |
| Housewife | 14.5 (295) | |
| Out of work | 2.9 (58) |
| n | Means ± SD (ng/mL) | Median (IQR) (ng/mL) | p-Value | |||
|---|---|---|---|---|---|---|
| Kruskal–Wallis Test | Jonckheere–Terpstra Test | |||||
| Age, years | <25 | 11 | 15.5 ± 5.2 | 15.2 (11.2–18.5) | 0.235 | |
| 25–29 | 229 | 18.4 ± 7.6 | 16.2 (13.1–20.1) | |||
| 30–34 | 651 | 17.9 ± 6.8 | 16.9 (12.9–21.9) | |||
| 35–39 | 576 | 18.3 ± 6.9 | 17.5 (13.3–22.2) | |||
| ≥40 | 280 | 18.1 ± 7.3 | 16.5 (12.4–22.5) | |||
| BMI (kg/m2) | <18.35 | 285 | 18.4 ± 7.6 | 17.0 (13.2–22.3) | 0.88 | 0.675 |
| ≥18.5–<25 | 1376 | 18.2 ± 7.0 | 17.1 (13.1–22.4) | |||
| ≥25 | 279 | 17.8 ± 6.2 | 16.8 (13.1–21.5) | |||
| AMH (ng/mL) | Q1 (<1.57) | 435 | 18.4 ± 7.0 | 17.5 (13.2–22.5) | 0.336 | 0.195 |
| Q2 (1.57–2.985) | 438 | 17.7 ± 6.8 | 16.6 (12.9–21.7) | |||
| Q3 (2.986–4.9374) | 436 | 18.1 ± 7.1 | 16.8 (13.1–21.5) | |||
| Q4 (≥4.9375) | 437 | 17.5 ± 6.5 | 16.8 (12.9–21.2) | |||
| FSH (IU/mL) | Q1 (<6.3) | 480 | 18.0 ± 6.9 | 1.7 (12.8–21.6) | 0.241 | 0.0902 |
| Q2 (6.3–7.3) | 477 | 17.8 ± 6.9 | 16.6 (12.7–21.9) | |||
| Q3 (7.4–8.7) | 476 | 18.5 ± 7.0 | 17.4 (13.5–22.2) | |||
| Q4 (≥8.8) | 490 | 18.5 ± 7.1 | 17.4 (13.1–23.0) | |||
| TSH (μIU/mL) | <2.5 | 1382 | 18.1 ± 6.9 | 17.1 (0.94–1.72) | 0.981 | 0.8697 |
| 2.5–4.9 | 278 | 18.0 ± 6.8 | 16.8 (2.74–4.88) | |||
| ≥5.0 | 33 | 18.6 ± 9.0 | 16.4 (5.34–7.19) | |||
| Season of blood collection | Spring | 504 | 18.2 ± 7.7 | 16.5 (12.6–22.4) | 0.00000000439 | |
| Summer | 490 | 19.0 ± 6.4 | 18.2 (14.1–22.7) | |||
| Autumn | 561 | 18.7 ± 6.6 | 18.1 (13.9–22.6) | |||
| Winter | 474 | 16.9 ± 7.2 | 15.3 (11.5–20.6) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, K.; Mitsui, J.; Katsumata, S.; Takayanagi, Y.; Nako, Y.; Tajima, M.; Komiya, A.; Takahashi, T.; Kawai, K. Seasonal Serum 25(OH) Vitamin D Level and Reproductive or Immune Markers in Reproductive-Aged Women with Infertility: A Cross-Sectional Observational Study in East Japan. Nutrients 2023, 15, 5059. https://doi.org/10.3390/nu15245059
Ota K, Mitsui J, Katsumata S, Takayanagi Y, Nako Y, Tajima M, Komiya A, Takahashi T, Kawai K. Seasonal Serum 25(OH) Vitamin D Level and Reproductive or Immune Markers in Reproductive-Aged Women with Infertility: A Cross-Sectional Observational Study in East Japan. Nutrients. 2023; 15(24):5059. https://doi.org/10.3390/nu15245059
Chicago/Turabian StyleOta, Kuniaki, Junichiro Mitsui, Shoko Katsumata, Yuko Takayanagi, Yurie Nako, Makiko Tajima, Akira Komiya, Toshifumi Takahashi, and Kiyotaka Kawai. 2023. "Seasonal Serum 25(OH) Vitamin D Level and Reproductive or Immune Markers in Reproductive-Aged Women with Infertility: A Cross-Sectional Observational Study in East Japan" Nutrients 15, no. 24: 5059. https://doi.org/10.3390/nu15245059
APA StyleOta, K., Mitsui, J., Katsumata, S., Takayanagi, Y., Nako, Y., Tajima, M., Komiya, A., Takahashi, T., & Kawai, K. (2023). Seasonal Serum 25(OH) Vitamin D Level and Reproductive or Immune Markers in Reproductive-Aged Women with Infertility: A Cross-Sectional Observational Study in East Japan. Nutrients, 15(24), 5059. https://doi.org/10.3390/nu15245059








