Intestinal Microbiota and Metabolomics Reveal the Role of Auricularia delicate in Regulating Colitis-Associated Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experimental Protocol
2.2. Histopathological Examination
2.3. Intestinal Microbiota Analysis
2.4. Metabolomics Analysis
2.5. The Analysis of Inflammatory Cytokines in Tumor Tissue
2.6. Western Blotting
2.7. Statistical Analysis
3. Results
3.1. ADe Inhibited the Tumor in CAC Mice
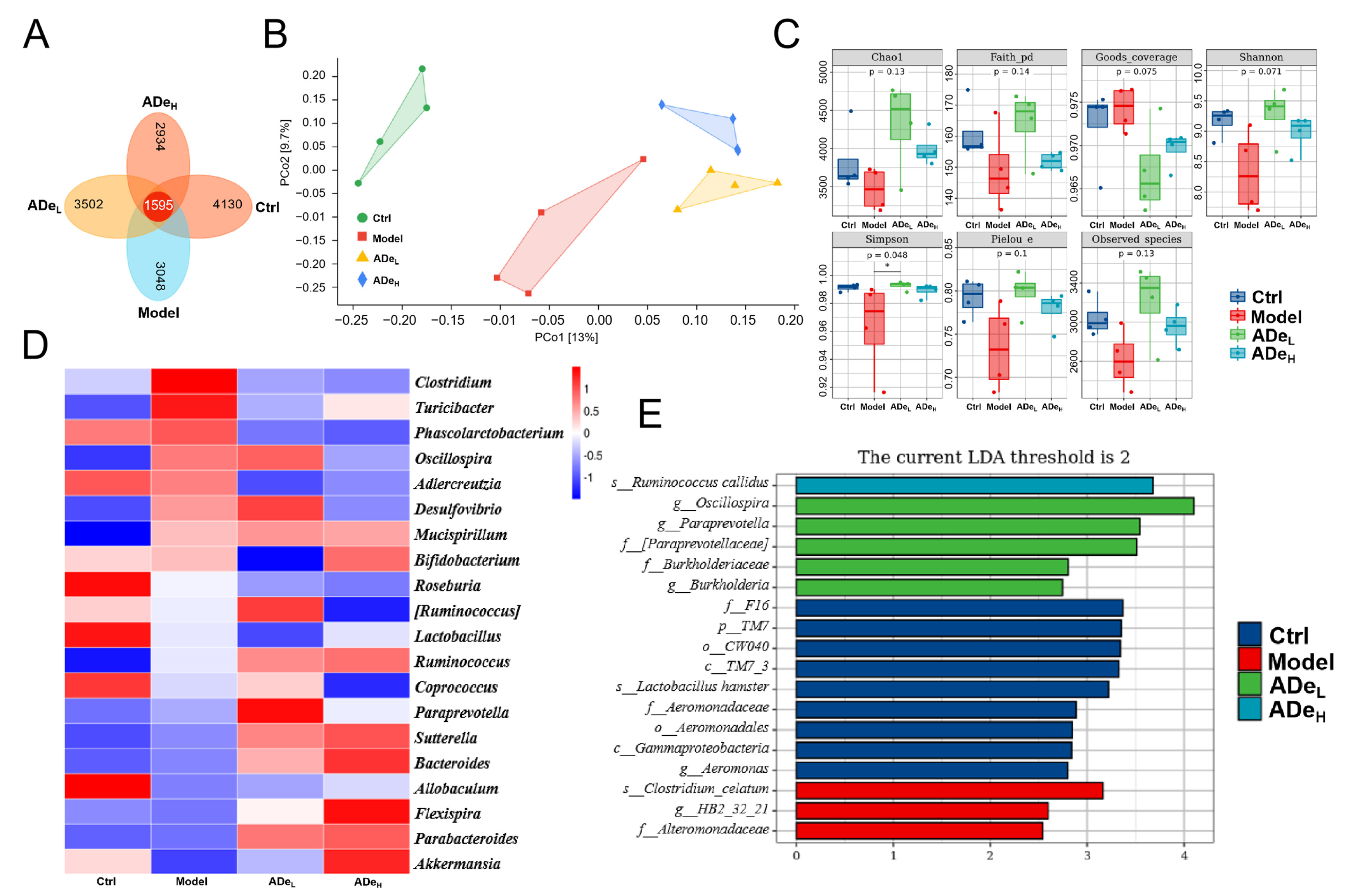
3.2. ADe Regulated Intestinal Microbiota in CAC Mice
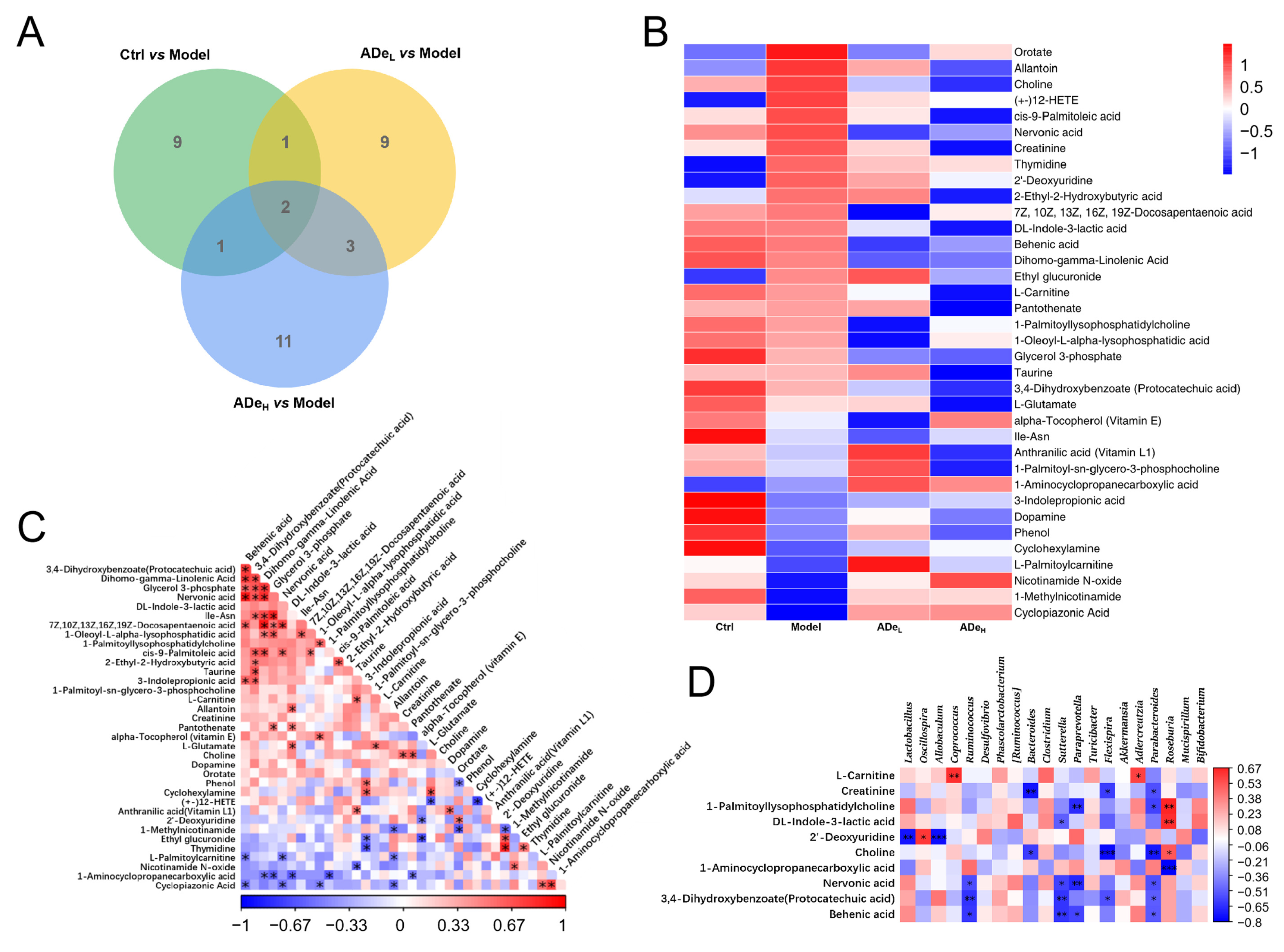
3.3. ADe Regulated Serum Metabolism in CAC Mice
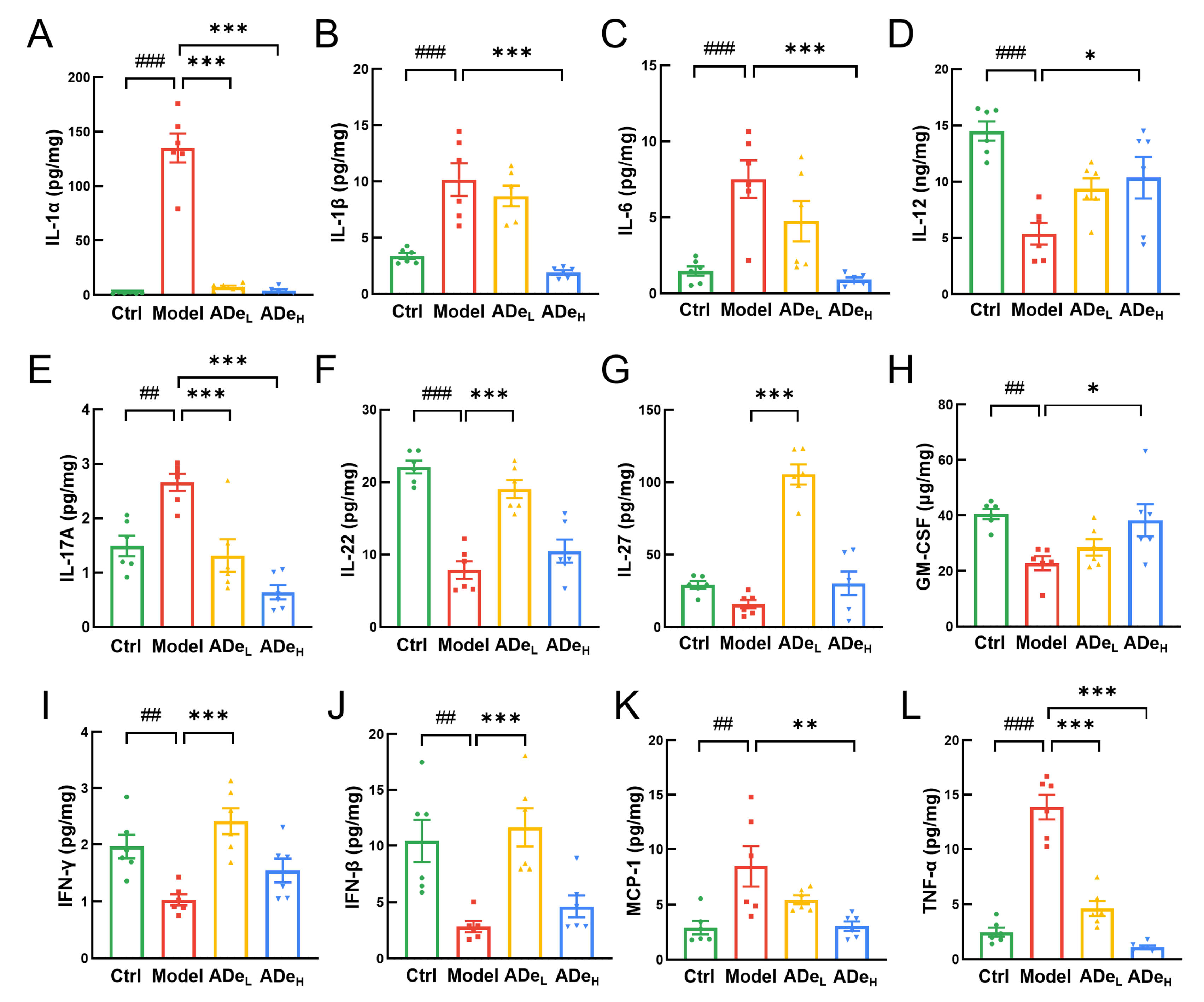
3.4. ADe Suppressed the Inflammation and NF-κB Signaling in CAC Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.E.; Hahn, A.; Hart, A.; Kahl, A.; Charlton, M.; Kapadia, M.R.; Hrabe, J.E.; Cromwell, J.W.; Hassan, I.; Gribovskaja-Rupp, I. Male sex, ostomy, infection, and intravenous fluids are associated with increased risk of postoperative ileus in elective colorectal surgery. Surgery 2021, 170, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, T.; Lee, Y.; Hong, D.; Dionne, J.; Doumouras, A.; Parpia, S.; Bhandari, M.; Eskicioglu, C. A history of bariatric surgery before surgery for colorectal cancer may improve short-term postoperative outcomes: Analysis of the national inpatient sample 2015–2019. Surgery 2023, 174, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.H.; Lu, Z.J.; Sheng, J.Q.; Song, Y.N.; Jiang, W.L.; Liu, F.; Zheng, P. 5-fluorouracil attenuates dextran sodium sulfate-induced acute colitis in mice. Mol. Med. Rep. 2016, 13, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, L.; Zhao, J.J.; Mi, X.F.; Wang, O.J.; Yu, M. Effects of oxaliplatin on inflammation and intestinal floras in rats with colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10542–10549. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Donald, E.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Gastrointestinal dysfunction and enteric neurotoxicity following treatment with anticancer chemotherapeutic agent 5-fluorouracil. Neurogastroenterol. Motil. 2016, 28, 1861–1875. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Stavely, R.; Timpani, C.; Petersen, A.C.; Abalo, R.; Bornstein, J.C.; Rybalka, E.; Nurgali, K. Oxaliplatin-induced enteric neuronal loss and intestinal dysfunction is prevented by co-treatment with bgp-15. Br. J. Pharmacol. 2018, 175, 656–677. [Google Scholar] [CrossRef]
- Kuraishy, A.; Karin, M.; Grivennikov, S.I. Tumor promotion via injury- and death-induced inflammation. Immunity 2011, 35, 467–477. [Google Scholar] [CrossRef]
- Waldner, M.J.; Neurath, M.F. Mechanisms of immune signaling in colitis-associated cancer. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 6–16. [Google Scholar] [CrossRef]
- Bradley, J.R. Tnf-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Faustman, D.; Davis, M. Tnf receptor 2 pathway: Drug target for autoimmune diseases. Nat. Rev. Drug Discov. 2010, 9, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Zhao, T.T.; Wu, D.C.; Li, J.J.; Wang, M.; Sun, Y.Y.; Hou, S.C. Colorectal cancer in ulcerative colitis: Mechanisms, surveillance and chemoprevention. Curr. Oncol. 2022, 29, 6091–6114. [Google Scholar] [CrossRef] [PubMed]
- Popov, J.; Caputi, V.; Nandeesha, N.; Rodriguez, D.A.; Pai, N.K. Microbiota-immune interactions in ulcerative colitis and colitis associated cancer and emerging microbiota-based therapies. Int. J. Mol. Sci. 2021, 22, 11365. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Pardoll, D.M. Perspective: Alpha-bugs, their microbial partners, and the link to colon cancer. J. Infect. Dis. 2011, 203, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018, 25, 79. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Nie, Y.W.; Xie, X.Q.; Zhou, L.X.; Guan, Q.J.; Ren, Y.L.; Mao, Y.; Shi, J.S.; Xu, Z.H.; Geng, Y. Desulfovibrio fairfieldensis-derived outer membrane vesicles damage epithelial barrier and induce inflammation and pyroptosis in macrophages. Cells 2023, 12, 89. [Google Scholar] [CrossRef]
- Balamurugan, R.; Rajendiran, E.; George, S.; Samuel, G.V.; Ramakrishna, B.S. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, desulfovibrio and enterococcus faecalis in the feces of patients with colorectal cancer. J. Gastroenterol. Hepatol. 2008, 23, 1298–1303. [Google Scholar] [CrossRef]
- Cui, Y.L.; Zhang, L.S.; Wang, X.; Yi, Y.L.; Shan, Y.Y.; Liu, B.F.; Zhou, Y.; Lu, X. Roles of intestinal parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, B.W.; Peng, B.; Wang, J.; Hu, Y.Z.; Wang, R.C.; Wang, S. Different dose of sucrose consumption divergently influences gut microbiota and ppar-gamma/mapk/nf-kappa b pathway in dss-induced colitis mice. Nutrients 2022, 14, 2765. [Google Scholar] [CrossRef]
- Wu, M.N.; Li, J.M.; An, Y.Y.; Li, P.Z.; Xiong, W.C.; Li, J.S.; Yan, D.; Wang, M.Y.; Zhong, G.S. Chitooligosaccharides prevents the development of colitis-associated colorectal cancer by modulating the intestinal microbiota and mycobiota. Front. Microbiol. 2019, 10, 2101. [Google Scholar] [CrossRef]
- Sawin, E.A.; De Wolfe, T.J.; Aktas, B.; Stroup, B.M.; Murali, S.G.; Steele, J.L.; Ney, D.M. Glycomacropeptide is a prebiotic that reduces desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am. J. Physiol. Gastroint. Liver Physiol. 2015, 309, G590–G601. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jacob, M.S.; Zhang, B.; Xu, A. Research progress on auricularia delicata. J. Adv. Biol. Biotechnol. 2020, 23, 8–32. [Google Scholar] [CrossRef]
- Wangkheirakpam, S.D.; Joshi, D.D.; Leishangthem, G.D.; Biswas, D.; Deb, L. Hepatoprotective effect of auricularia delicata (agaricomycetes) from india in rats: Biochemical and histopathological studies and antimicrobial activity. Int. J. Med. Mushrooms 2018, 20, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.W.; Teng, X.; Zhang, S.S.; Liu, T.T.; Li, X.; Wang, D.W. Structural characteristics, rheological properties, and antioxidant activity of novel polysaccharides from “deer tripe mushroom”. J. Food Qual. 2021, 2021, 6593293. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhai, S.Y.; Wang, R.C.; Kong, F.E.; Yang, A.H.; Wang, C.Y.; Yu, H.; Li, Y.; Wang, D. Anti-obesity effect of auricularia delicate involves intestinal-microbiota-mediated oxidative stress regulation in high-fat-diet-fed mice. Nutrients 2023, 15, 872. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Maysarah, N.M.; Ei-Sherbiny, M.; Al-Gayyar, M.M. Renal protective effects of thymoquinone against sodium nitrite-induced chronic toxicity in rats: Impact on inflammation and apoptosis. Life Sci. 2017, 180, 1–8. [Google Scholar] [CrossRef]
- Qu, Y.D.; Yang, H.X.; Li, S.Y.; Li, L.Z.; Li, Y.; Wang, D. The involvement of th1 cell differentiation in the anti-tumor effect of purified polysaccharide from sanghuangporus vaninii in colorectal cancer via multi-omics analysis. Int. J. Biol. Macromol. 2023, 237, 123927. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, J.; Liu, Z.J.; Ma, X.T.; Feng, Y.X.; Teng, L.R.; Li, Y.; Wang, D. Anti-obesity effects of grifola frondosa through the modulation of lipid metabolism via ceramide in mice fed a high-fat diet. Food Funct. 2021, 12, 6725–6739. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Li, L.; Wang, W.; Tan, H.-Y.; Qu, Y.; Wang, D. The involvement of gut microbiota in the anti-tumor effect of carnosic acid via il-17 suppression in colorectal cancer. Chem. Biol. Interact. 2022, 365, 110080. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Hao, J.; Liu, Z.J.; Li, Z.G.; Teng, L.R.; Wang, D. Inonotus hispidus protects against hyperlipidemia by inhibiting oxidative stress and inflammation through nrf2/nf-κb signaling in high fat diet fed mice. Nutrients 2022, 14, 3477. [Google Scholar] [CrossRef]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological mechanisms in inflammation-associated colon carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef] [PubMed]
- Du, R.H.; Zhou, Y.; Xia, M.L.; Lu, M.; Ding, J.H.; Hu, G. Alpha-synuclein disrupts the anti-inflammatory role of drd2 via interfering beta-arrestin2-tab1 interaction in astrocytes. J. Neuroinflamm. 2018, 15, 258. [Google Scholar] [CrossRef] [PubMed]
- Park, B.O.; Kang, J.S.; Paudel, S.; Park, S.G.; Park, B.C.; Han, S.B.; Kwak, Y.S.; Kim, J.H.; Kim, S. Novel gpr43 agonists exert an anti-inflammatory effect in a colitis model. Biomol. Ther. 2022, 30, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the edible mushroom pleurotus pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J. Gastroenterol. 2012, 47, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, F.; van Sinderen, D.; O’Toole, P.W.; Stanton, C. Feeding the microbiota: Transducer of nutrient signals for the host. Gut 2017, 66, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Garcia, L.; Ruiz-Malagon, A.J.; Huertas, F.; Rodriguez-Sojo, M.J.; Molina-Tijeras, J.A.; Diez-Echave, P.; Becerra, P.; Miron, B.; Moron, R.; Rodriguez-Nogales, A.; et al. Administration of intestinal mesenchymal stromal cells reduces colitis-associated cancer in c57bl/6j mice modulating the immune response and gut dysbiosis. Pharmacol. Res. 2023, 195, 106891. [Google Scholar] [CrossRef]
- Yu, Y.N.; Cai, Y.K.; Yang, B.; Xie, S.Y.; Shen, W.J.; Wu, Y.Y.; Sui, Z.Q.; Cai, J.T.; Ni, C.; Ye, J. High-fat diet enhances the liver metastasis potential of colorectal cancer through microbiota dysbiosis. Cancers 2022, 14, 2573. [Google Scholar] [CrossRef]
- Shi, M.X.; Yue, Y.S.; Ma, C.; Dong, L.; Chen, F. Pasteurized akkermansia muciniphila ameliorate the lps-induced intestinal barrier dysfunction via modulating ampk and nf-kappa b through tlr2 in caco-2 cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Liu, T.L.; Gao, Z.H.; Liu, R.B.; Wang, Z.X.; Chen, Y.X.; Cao, J.; Dong, Y.L. Akkermansia muciniphila colonization alleviating high fructose and restraint stress-induced jejunal mucosal barrier disruption. Nutrients 2022, 14, 3164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Tang, L.; Feng, Y.M.; Zhao, S.Y.; Han, M.; Zhang, C.; Yuan, G.H.; Zhu, J.; Cao, S.Y.; Wu, Q.; et al. A purified membrane protein from akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of cd8(+) t cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Wang, K.C.; Wu, W.R.; Wang, Q.; Yang, L.Y.; Bian, X.Y.; Jiang, X.W.; Lv, L.X.; Yan, R.; Xia, J.F.; Han, S.Y.; et al. The negative effect of akkermansia muciniphila-mediated post-antibiotic reconstitution of the gut microbiota on the development of colitis-associated colorectal cancer in mice. Front. Microbiol. 2022, 13, 932047. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.Y.; Zhang, H.; Ding, L.K.; Xi, Y.; Yan, M.; Sun, C.; Wu, L.; Hu, H. Anti-inflammatory effects of nab and napc in acinetobacter baumannii-stimulated thp-1 cells via tlr-2/nf-kappa b/ros/nlrp3 pathway. Acta Pharm. 2022, 72, 615–628. [Google Scholar] [CrossRef]
- Taghizadeh, K.; McFaline, J.L.; Pang, B.; Sullivan, M.; Dong, M.; Plummer, E.; Dedon, P.C. Quantification of DNA damage products resulting from deamination, oxidation and reaction with products of lipid peroxidation by liquid chromatography isotope dilution tandem mass spectrometry. Nat. Protoc. 2008, 3, 1287–1298. [Google Scholar] [CrossRef]
- Vesely, K.R.; Hyde, D.M.; Stovall, M.Y.; Harkema, J.R.; Green, J.F.; Schelegle, E.S. Capsaicin-sensitive c-fiber-mediated protective responses in ozone inhalation in rats. J. Appl. Physiol. 1999, 86, 951–962. [Google Scholar] [CrossRef]
- Okayasu, I.; Kuroiwa, H.; Shinkawa, K.; Hayashi, K.; Sato, S.; Iwata, N.; Tano, G.; Sekizaki, R.; Umeda, K.; Ohnishi, H. Significant increase in prostaglandin e-major urinary metabolite with physical exercise suggesting muscle inflammation. All Life 2023, 16, 2167868. [Google Scholar] [CrossRef]
- Hung, N.D.; Sok, D.E.; Kim, M.R. Prevention of 1-palmitoyl lysophosphatidylcholine-induced inflammation by polyunsaturated acyl lysophosphatidylcholine. Inflamm. Res. 2012, 61, 473–483. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Zhong, Z.Y.; Stubelius, A.; Sweeney, S.R.; Booshehri, L.M.; Antonucci, L.; Liu-Bryan, R.; Lodi, A.; Terkeltaub, R.; Lacal, J.C.; et al. Choline uptake and metabolism modulate macrophage il-1 beta and il-18 production. Cell Metab. 2019, 29, 1350–1362.e7. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The intestinal microbiota in colorectal cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.A.; Salzman, A.L.; Kane, C.D.; Fischer, J.E.; Hasselgren, P.O. Il-6 production in human intestinal epithelial cells following stimulation with il-1 beta is associated with activation of the transcription factor nf-kappa b. J. Surg. Res. 1997, 69, 139–144. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Han, G.C.; Wang, R.X.; Xiao, H.; Hou, C.M.; Guo, R.F.; Dou, Y.; Shen, B.F.; Li, Y.; et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (il-1)/il-6 axis. Mucosal Immunol. 2014, 7, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, Y.Y.; Wang, Y.; Han, G.C.; Wang, R.X.; Xiao, H.; Li, X.Y.; Hou, C.M.; Ma, Y.F.; Sheng, D.S.; et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal il-1 beta/il-17a axis. Mucosal Immunol. 2015, 8, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Yuan, C.X. Il-17a promotes the neuroinflammation and cognitive function in sevoflurane anesthetized aged rats via activation of nf-b signaling pathway. BMC Anesthesiol. 2018, 18, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Wang, W.X.; Tian, J.; Yin, K.; Tang, X.Y.; Ma, J.; Xu, H.X.; Wang, S.J. Il-17a produced by peritoneal macrophages promote the accumulation and function of granulocytic myeloid-derived suppressor cells in the development of colitis-associated cancer. Tumor Biol. 2016, 37, 15883–15891. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, M.; Nagaishi, T.; Kanai, T.; Nagano, K.; Oshima, S.; Nemoto, Y.; Yoshioka, A.; Totsuka, T.; Okamoto, R.; Nakamura, T.; et al. Signaling pathway via tnf-alpha/nf-kappa b in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am. J. Physiol.-Gastroint. Liver Physiol. 2009, 296, G850–G859. [Google Scholar] [CrossRef]
- Li, Y.W.; Yang, C.Q.; Xiao, Y.L.; Li, J.; Xie, C.X.; Zhang, S.H.; Yu, Q.; Wang, H.L.; Lu, W.M.; Chen, M.H. The-a2518g polymorphism in the mcp-1 gene and inflammatory bowel disease risk: A meta-analysis. J. Dig. Dis. 2015, 16, 177–185. [Google Scholar] [CrossRef]
- Taube, C.; Tertilt, C.; Gyulveszi, G.; Dehzad, N.; Kreymborg, K.; Schneeweiss, K.; Michel, E.; Reuter, S.; Renauld, J.C.; Arnold-Schild, D.; et al. Il-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS ONE 2011, 6, e21799. [Google Scholar] [CrossRef]
- Xu, Y.H.; Hunt, N.H.; Bao, S.S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008, 18, 1220–1229. [Google Scholar] [CrossRef]
- Gong, W.B.; Liu, P.Z.; Zhao, F.; Liu, J.H.; Hong, Z.W.; Ren, H.J.; Gu, G.S.; Wang, G.F.; Wu, X.W.; Zheng, T.; et al. Sting-mediated syk signaling attenuates tumorigenesis of colitis-associated colorectal cancer through enhancing intestinal epithelium pyroptosis. Inflamm. Bowel Dis. 2022, 28, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.J.; Sun, M.X.; Guo, Y.W.; Tan, S.W.; Wu, X.Y.; Abassa, K.K.; Lin, L.; Liu, H.L.; Jiang, J.; Wei, X.Q. Sodium butyrate protects against lipopolysaccharide-induced liver injury partially via the gpr43/beta-arrestin-2/nf-kappa b network. Gastroenterol. Rep. 2021, 9, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Man, Y.; Gao, C.L.; Zhou, L.P.; Gu, J.L.; Xu, H.W.; Wan, Q.; Long, Y.; Chai, L.; Xu, Y.H.; et al. Short-chain fatty acids ameliorate diabetic nephropathy via gpr43-mediated inhibition of oxidative stress and nf-kappa b signaling. Oxid. Med. Cell. Longev. 2020, 2020, 4074832. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Liu, H.; Yu, J.; Sun, Z.; Jiang, M.; Yu, H.; Wang, C. Intestinal Microbiota and Metabolomics Reveal the Role of Auricularia delicate in Regulating Colitis-Associated Colorectal Cancer. Nutrients 2023, 15, 5011. https://doi.org/10.3390/nu15235011
Li L, Liu H, Yu J, Sun Z, Jiang M, Yu H, Wang C. Intestinal Microbiota and Metabolomics Reveal the Role of Auricularia delicate in Regulating Colitis-Associated Colorectal Cancer. Nutrients. 2023; 15(23):5011. https://doi.org/10.3390/nu15235011
Chicago/Turabian StyleLi, Lanzhou, Honghan Liu, Jinqi Yu, Zhen Sun, Ming Jiang, Han Yu, and Chunyue Wang. 2023. "Intestinal Microbiota and Metabolomics Reveal the Role of Auricularia delicate in Regulating Colitis-Associated Colorectal Cancer" Nutrients 15, no. 23: 5011. https://doi.org/10.3390/nu15235011
APA StyleLi, L., Liu, H., Yu, J., Sun, Z., Jiang, M., Yu, H., & Wang, C. (2023). Intestinal Microbiota and Metabolomics Reveal the Role of Auricularia delicate in Regulating Colitis-Associated Colorectal Cancer. Nutrients, 15(23), 5011. https://doi.org/10.3390/nu15235011





