Chroogomphus rutilus Regulates Bone Metabolism to Prevent Periodontal Bone Loss during Orthodontic Tooth Movement in Osteoporotic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. CR Component Detection
2.2. Animal Experiments and Agent Administration Protocol
2.3. X-ray and Micro-Computed Tomography (Micro-CT) Analysis
2.4. Intestinal Microflora Analysis
2.5. Non-Targeted Metabolomics Analysis
2.6. Histopathological Analysis and Immunohistochemical Examination
2.7. Statistical Analysis
3. Results
3.1. Main Composition of CR
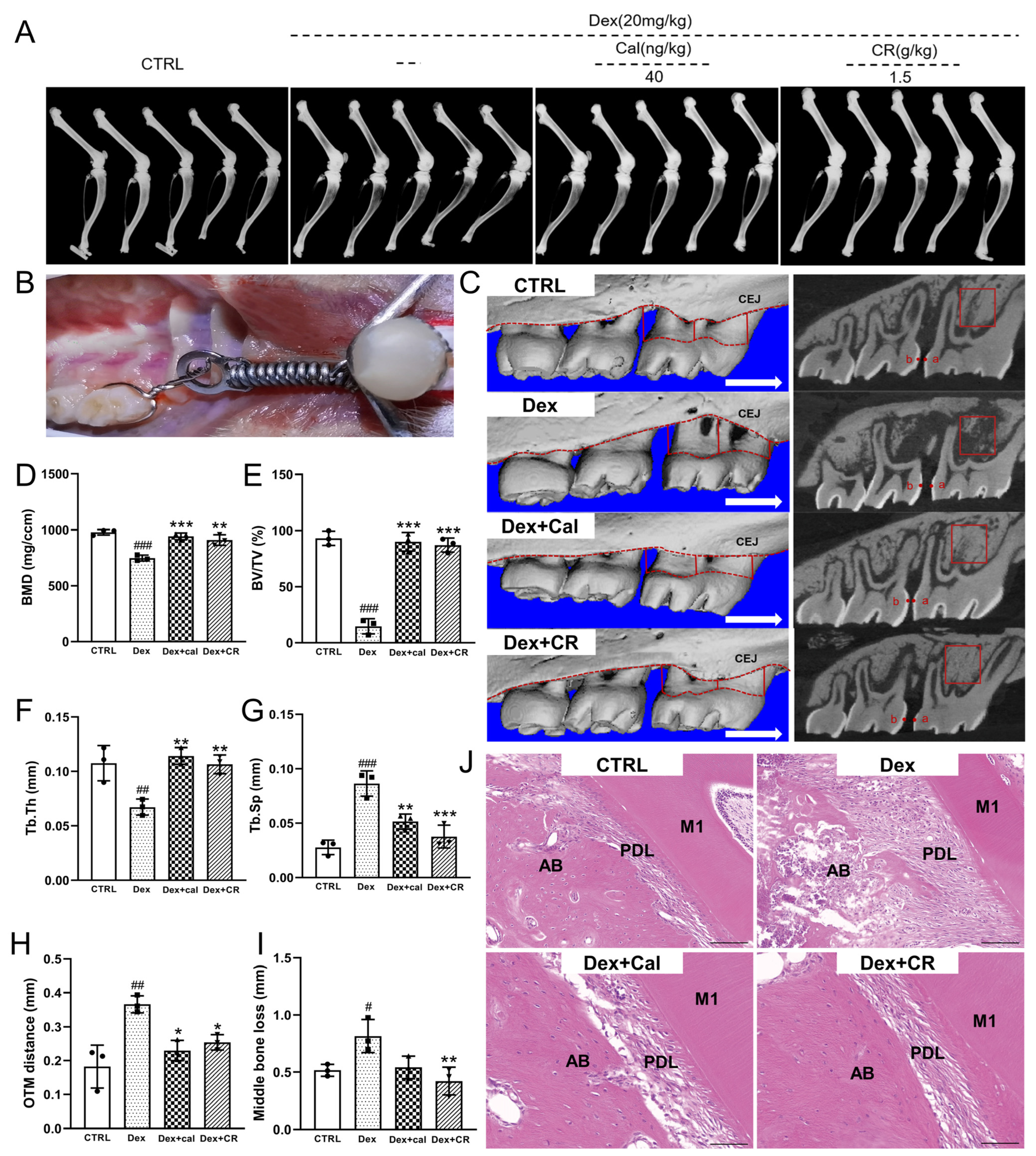
3.2. CR Promoted Bone Remolding in Osteoporotic Alveolar Bone during OTM
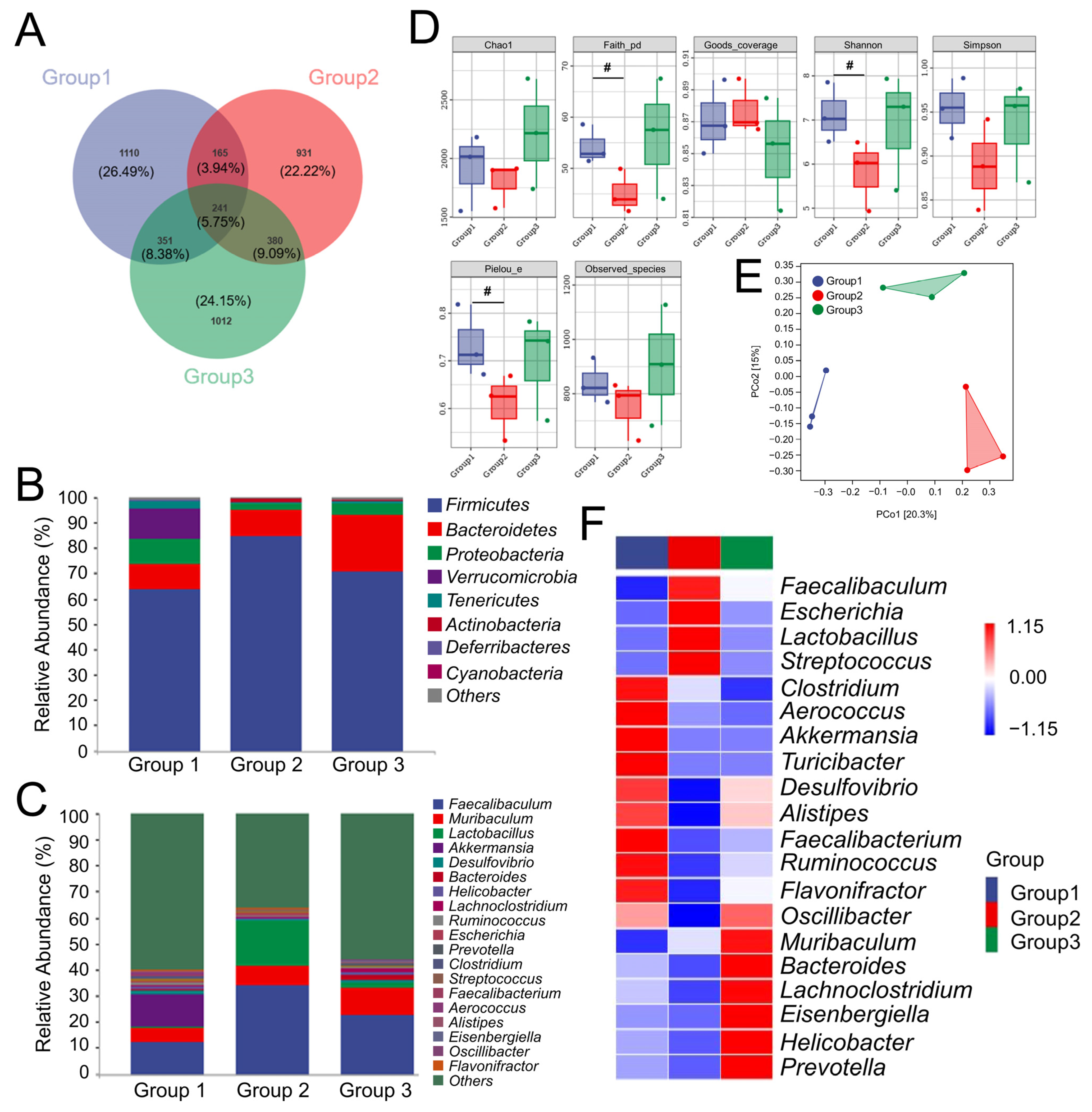
3.3. CR Preserved the Balance of Intestinal Flora in OP Rats
3.4. CR Modified the Metabolite Levels in Serum of OP Rats
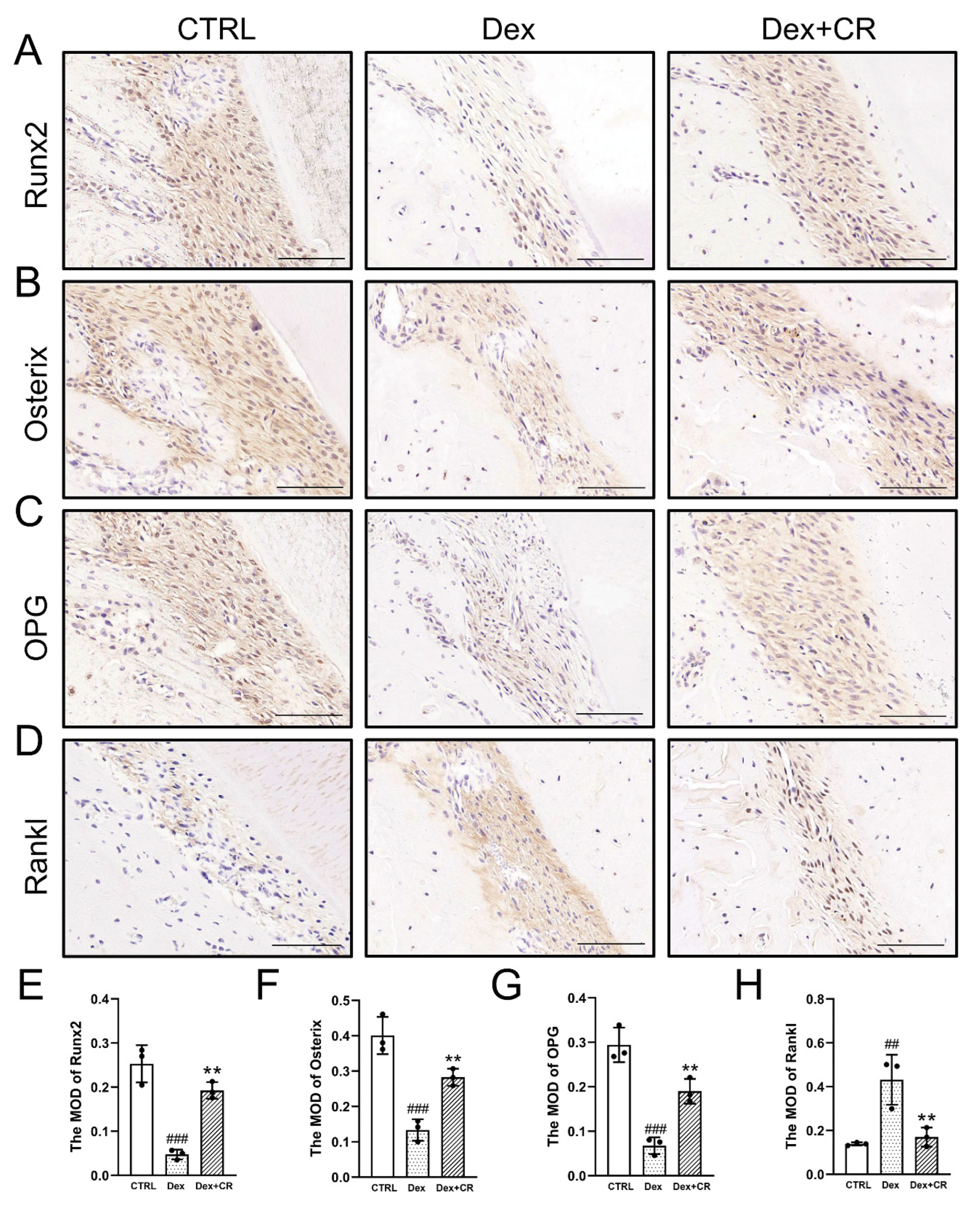
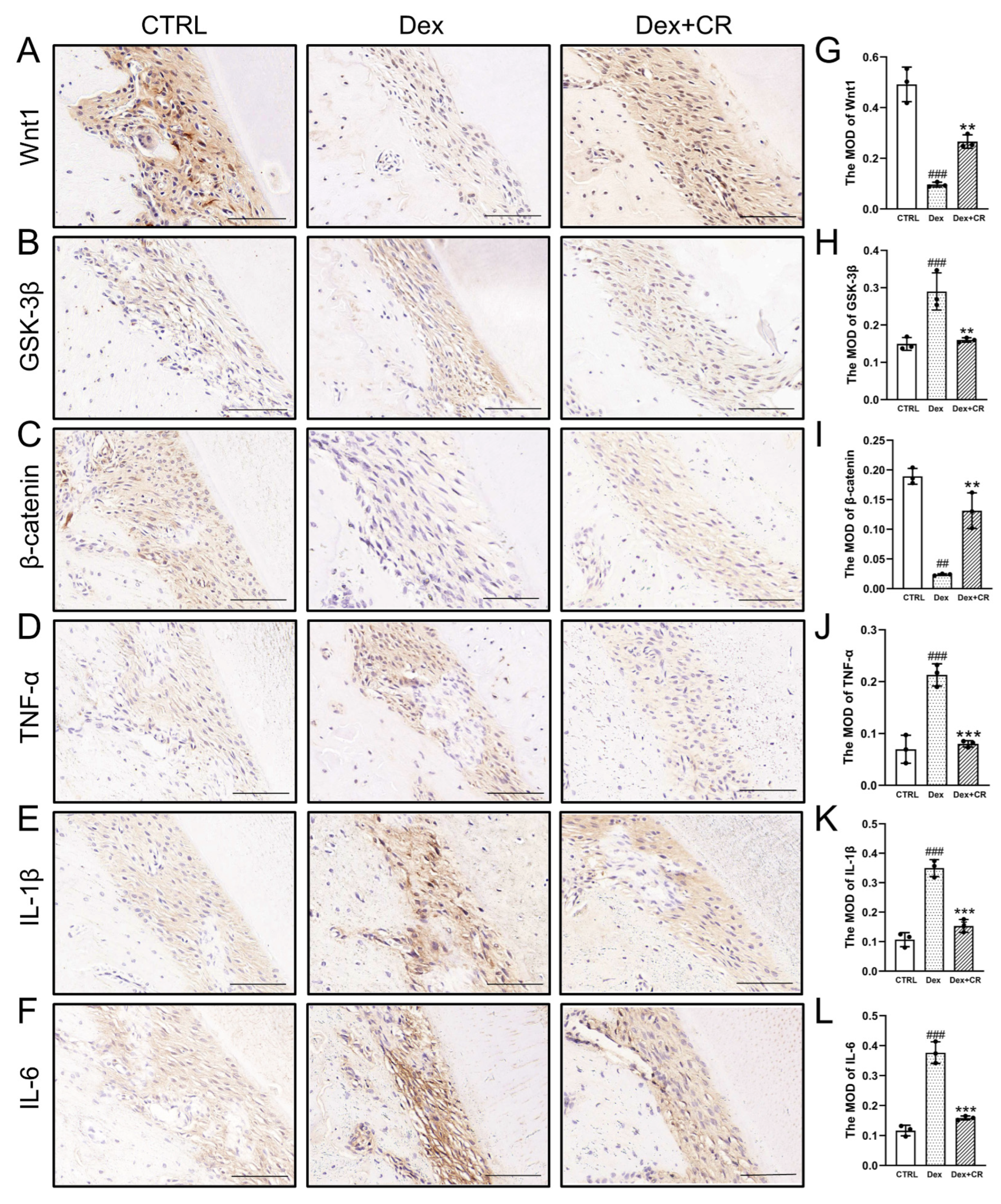
3.5. CR Regulated Wnt/β-Catenin Signaling to Promote Periodontal Bone Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Yin, X.; Chen, J.; Liu, R.; Xiao, X.; Hu, Z.; He, Y.; Zou, S. Lithium chloride promotes osteogenesis and suppresses apoptosis during orthodontic tooth movement in osteoporotic model via regulating autophagy. Bioact. Mater. 2021, 6, 3074–3084. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, C. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontology 2000 2022, 89, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Gao, S.; Zhang, X.; Zhang, T.; Zhang, T.; Tian, T.; Li, S.; Lin, Y.; Cai, X. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioact. Mater. 2021, 6, 1676–1688. [Google Scholar] [CrossRef] [PubMed]
- van der Burgh, A.C.; de Keyser, C.E.; Zillikens, M.C.; Stricker, B.H. The Effects of Osteoporotic and Non-osteoporotic Medications on Fracture Risk and Bone Mineral Density. Drugs 2021, 81, 1831–1858. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Gu, J.; Xu, S.; Zhang, C.; Wang, J.; Wang, S.; Xu, J. Dietary nitrate improves jaw bone remodelling in zoledronate-treated mice. Cell Prolif. 2023, 56, e13395. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, C.; Miyazawa, K.; Tabuchi, M.; Hirano, M.; Mizuno, M.; Yoshizako, M.; Torii, Y.; Asano, Y.; Sato, T.; Kawatani, M.; et al. Alteration of tooth movement by reveromycin A in osteoprotegerin-deficient mice. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Jia, L.; Tu, Y.; Jia, X.; Du, Q.; Zheng, X.; Yuan, Q.; Zheng, L.; Zhou, X.; Xu, X. Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif. 2021, 54, e13075. [Google Scholar] [CrossRef]
- Qi, W.; Yan, Y.; Wang, P.; Lei, W. The co-effect of Cordyceps sinensis and strontium on osteoporosis in ovariectomized osteopenic rats. Biol. Trace Elem. Res. 2011, 141, 216–223. [Google Scholar] [CrossRef]
- Kerezoudi, E.N.; Mitsou, E.K.; Gioti, K.; Terzi, E.; Avgousti, I.; Panagiotou, A.; Koutrotsios, G.; Zervakis, G.I.; Mountzouris, K.C.; Tenta, R.; et al. Fermentation of Pleurotus ostreatus and Ganoderma lucidum mushrooms and their extracts by the gut microbiota of healthy and osteopenic women: Potential prebiotic effect and impact of mushroom fermentation products on human osteoblasts. Food Funct. 2021, 12, 1529–1546. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.K.; Kim, Y.K.; Jung, E.Y.; Park, C.S.; Woo, M.J.; Lee, S.H.; Kim, J.S.; Suh, H.J. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J. Ethnopharmacol. 2011, 133, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, M.; Lü, J.; Li, J.; Zhang, K.; Zhi, H.; Zhang, H.; Sun, J. Antioxidant, Anti-inflammatory and Cytotoxic Activities of Polyphenols Extracted from Chroogomphus rutilus. Chem. Biodivers. 2020, 17, e1900479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Zhao, L.; Zhao, J.; Qi, Z.; Wang, L. A Primary Study of the Antioxidant, Hypoglycemic, Hypolipidemic, and Antitumor Activities of Ethanol Extract of Brown Slimecap Mushroom, Chroogomphus rutilus (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zhao, Y.; Wen, C.; Cheng, S.; Lin, S. Effects of electron beam irradiation on physicochemical properties of corn flour and improvement of the gelatinization inhibition. Food Chem. 2017, 233, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Teng, S.; Wang, X.; Li, S.; Zhang, Y.; Wang, D. The Antidiabetic and Antinephritic Activities of Tuber melanosporum via Modulation of Nrf2-Mediated Oxidative Stress in the db/db Mouse. Oxid. Med. Cell Longev. 2018, 2018, 7453865. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.W.; Lai, Y.; Yang, F.C. Enhanced production of triterpenoid in submerged cultures of Antrodia cinnamomea with the addition of citrus peel extract. Bioprocess Biosyst. Eng. 2014, 37, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, S.X.; Wang, S.M.; Liang, S.W. Investigation into the anti-thrombosis effect and contents of total saponins and flavonoids in the bioactive fraction of Naodesheng prescription. J. Ethnopharmacol. 2012, 144, 208–212. [Google Scholar] [CrossRef]
- Santos, W.P.C.; Ribeiro, N.M.; Santos, D.; Korn, M.G.A.; Lopes, M.V. Bioaccessibility assessment of toxic and essential elements in produced pulses, Bahia, Brazil. Food Chem. 2018, 240, 112–122. [Google Scholar] [CrossRef]
- Ajanal, M.; Gundkalle, M.B.; Nayak, S.U. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Anc. Sci. Life 2012, 31, 198–201. [Google Scholar] [CrossRef]
- Yang, H.; Han, N.; Luo, Z.; Xu, J.; Guo, L.; Liu, Y. D-mannose alleviated alveolar bone loss in mice with experimental periodontitis via regulating the anti-inflammatory effect of amino acids. J. Periodontol. 2023, 94, 542–553. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, J.; Liu, Z.; Ma, X.; Feng, Y.; Teng, L.; Li, Y.; Wang, D. Anti-obesity effects of Grifola frondosa through the modulation of lipid metabolism via ceramide in mice fed a high-fat diet. Food Funct. 2021, 12, 6725–6739. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Y.; Zhu, Y.; Jin, X.; Li, L.; Wang, C.; Zhou, Y.; Li, Y.; Wang, D.; Hu, M. Structural characterization and anti-osteoporosis effects of polysaccharide purified from Eucommia ulmoides Oliver cortex based on its modulation on bone metabolism. Carbohydr. Polym. 2023, 306, 120601. [Google Scholar] [CrossRef] [PubMed]
- Baker-LePain, J.C.; Nakamura, M.C.; Lane, N.E. Effects of inflammation on bone: An update. Curr. Opin. Rheumatol. 2011, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qin, W.; Lin, H.; Liu, Y.; Tian, Y.; Zhao, X.; Ding, T.; Wang, Y.; Mao, T.; Li, J.; et al. Inhibitory effect of polysaccharides extracted from Changbai Mountain Ganoderma lucidum on periodontal inflammation. Heliyon 2023, 9, e13205. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cao, Z.; Tickner, J.; Qiu, H.; Wang, C.; Chen, K.; Wang, Z.; Guo, C.; Dong, S.; Xu, J. Poria cocos polysaccharide attenuates RANKL-induced osteoclastogenesis by suppressing NFATc1 activity and phosphorylation of ERK and STAT3. Arch. Biochem. Biophys. 2018, 647, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Jia, L.; Mo, L.; Yuan, S.; Zheng, X.; He, J.; Chen, V.; Guo, Q.; Zheng, L.; Yuan, Q.; et al. Berberine Ameliorates Periodontal Bone Loss by Regulating Gut Microbiota. J. Dent. Res. 2019, 98, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yamashita, T.; Kishino, S.; Watanabe, H.; Sasaki, K.; Sasaki, D.; Tabata, T.; Sugiyama, Y.; Kitamura, N.; Saito, Y.; et al. A possible beneficial effect of Bacteroides on faecal lipopolysaccharide activity and cardiovascular diseases. Sci. Rep. 2020, 10, 13009. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Gregoire, B.R.; Shen, C. A High-Fat Diet Decreases Bone Mass in Growing Mice with Systemic Chronic Inflammation Induced by Low-Dose, Slow-Release Lipopolysaccharide Pellets. J. Nutr. 2017, 147, 1909–1916. [Google Scholar] [CrossRef]
- Li, J.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, W.; Zhang, L.; Chen, L.; Zhang, Q.; Zhang, Y.; He, S.; Li, J. Gougunao tea polysaccharides ameliorate high-fat diet-induced hyperlipidemia and modulate gut microbiota. Food Funct. 2022, 14, 703–719. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin Alleviates Obesity-Associated Metabolic Syndrome by Regulating the Composition of the Gut Microbiome. Front. Microbiol. 2021, 12, 805827. [Google Scholar] [CrossRef] [PubMed]
- Abdallah Ismail, N.; Ragab, S.H.; Abd Elbaky, A.; Shoeib, A.R.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch. Med. Sci. 2011, 7, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016, 12, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, Y.; Shi, X.; Gao, H.; Wang, Y.; Lin, Z. Phosphocreatine-modified chitosan porous scaffolds promote mineralization and osteogenesis in vitro and in vivo. Appl. Mater. Today 2018, 12, 21–33. [Google Scholar] [CrossRef]
- Jiang, C.; Li, D.; Chen, L.; Liu, Y.; Zhao, Y.; Mei, G.; Tang, Y.; Yang, Y.; Yao, P.; Gao, C. Quercetin ameliorated cardiac injury via reducing inflammatory actions and the glycerophospholipid metabolism dysregulation in a diabetic cardiomyopathy mouse model. Food Funct. 2022, 13, 7847–7856. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, H.; Li, Y.; Song, L. Lipid metabolism within the bone micro-environment is closely associated with bone metabolism in physiological and pathophysiological stages. Lipids Health Dis. 2022, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, Y.; Jian, L.; Zhang, J.; Liang, S.; Xiao, S.; Liu, B.; Wang, H. Linoelaidic acid enhances adipogenic differentiation in adipose tissue-derived stromal cells through suppression of Wnt/β-catenin signaling pathway in vitro. Prostaglandins Leukot. Essent. Fat. Acids 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, J.; Bian, Q. Small molecules for mesenchymal stem cell fate determination. World J. Stem Cells 2019, 11, 1084–1103. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.J.; Park, J.C.; Hong, J.; Yang, W. Zanthoxylum piperitum reversed alveolar bone loss of periodontitis via regulation of bone remodeling-related factors. J. Ethnopharmacol. 2017, 195, 137–142. [Google Scholar] [CrossRef]
- Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Dalle Carbonare, L.; Mottes, M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells 2019, 8, 742. [Google Scholar] [CrossRef]
- Harmer, D.; Falank, C.; Reagan, M.R. Interleukin-6 Interweaves the Bone Marrow Microenvironment, Bone Loss, and Multiple Myeloma. Front. Endocrinol. 2019, 9, 788. [Google Scholar] [CrossRef] [PubMed]
- Shi, E.; Bai, L.; Mao, L.; Wang, H.; Yang, X.; Wang, Y.; Zhang, M.; Li, C.; Wang, Y. Self-assembled nanoparticles containing photosensitizer and polycationic brush for synergistic photothermal and photodynamic therapy against periodontitis. J. Nanobiotechnol. 2021, 19, 413. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Zheng, L.; Xu, X.; Lin, Y.; Lin, J.; Wu, J.; Tan, J. Dysregulation of the miR-146a-Smad4 axis impairs osteogenesis of bone mesenchymal stem cells under inflammation. Bone Res. 2017, 5, 17037. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Shi, H.; Yang, W. Osteoprotective Effect of Cimiracemate in Glucocorticoid-Induced Osteoporosis by Osteoprotegerin/Receptor Activator of Nuclear Factor κ B/Receptor Activator of Nuclear Factor Kappa-Β Ligand Signaling. Pharmacology 2019, 103, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Schluessel, S.; Hartmann, E.S.; Koehler, M.I.; Beck, F.; Redeker, J.I.; Saller, M.M.; Akova, E.; Krebs, S.; Holzapfel, B.M.; Mayer-Wagner, S. Dental and Orthopaedic Implant Loosening: Overlap in Gene Expression Regulation. Front. Immunol. 2022, 13, 820843. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Shanbhag, S.; Rashad, A.; Nymark, E.H.; Suliman, S.; de Lange Davies, C.; Stavropoulos, A.; Bolstad, A.I.; Mustafa, K. Spheroid Coculture of Human Gingiva-Derived Progenitor Cells with Endothelial Cells in Modified Platelet Lysate Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 739225. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Lu, M.K.; Jen, C.I.; Chao, C.H.; Hsu, Y.C.; Ng, L.T. SPS, a sulfated galactoglucan of Laetiporus sulphureus, exhibited anti-inflammatory activities. Int. J. Biol. Macromol. 2023, 226, 1236–1247. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Ge, J.C.; Wang, L.; Peng, D.Y.; Yu, N.J.; Zhang, Y.; Jiang, Y.H.; Luo, J.P.; Chen, W.D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef]

| Compounds | Contents (%) | Compounds | Contents (%) | |
|---|---|---|---|---|
| General nutrients | Total sugar | 35.00 | Total triterpenoids | 1.94 |
| Reducing sugar | 0.69 | Total phenol | 1.02 | |
| Crude protein | 14.40 | Total saponins | 0.30 | |
| Total ash | 7.30 | Total alkaloids | 1.69 | |
| Crude fat | 6.10 | Total sterol | 0.32 | |
| Total flavonoids | 0.51 | |||
| Amino acids | Aspartic acid (Asp) | 0.83 | Threonine (Thr) | 0.44 |
| Serine (Ser) | 0.48 | Glutamic acid (Glu) | 1.36 | |
| Glycine (Gly) | 0.40 | Alanine (Ala) | 0.52 | |
| Cystine (Gys) | 0.041 | Valine (Val) | 0.45 | |
| Methionine (Met) | 0.14 | Isoleucine (IIe) | 0.33 | |
| Leucine (Leu) | 0.69 | Tyrosine (Tyr) | 0.23 | |
| Phenylalanine (Phe) | 0.38 | Lysine (Lys) | 0.48 | |
| Histidine (His) | 0.23 | Arginine (Arg) | 0.52 | |
| Proline (Pro) | 0.41 | |||
| Compounds | Contents (mg/kg) | Compounds | Contents (mg/kg) | |
| Minerals | Calcium (Ca) | 237 | Iron (Fe) | 328 |
| Zinc (Zn) | 29.9 | Potassium (K) | 2.66 × 104 | |
| Sodium (Na) | 25.7 | Manganese (Mn) | 12.50 | |
| Heavy metals | Lead (Pb) | 0.504 | Arsenic (As) | 0.144 |
| Mercury (Hg) | UD | Copper (Cu) | 5.12 | |
| Cadmium (Cd) | UD | Chromium (Cr) | 3.25 | |
| Nucleotides | Cytidylic acid | 207.44 | Uracil nucleotide | 1252.29 |
| Guanine nucleotide | 55.31 | Hypoxanthine nucleotide | 116.61 | |
| Adenine nucleotide | 2.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhu, Y.; Jin, X.; Zhang, Y.; Song, J.; Wu, Z.; Li, Y.; Yi, J.; Wang, D.; Hu, M. Chroogomphus rutilus Regulates Bone Metabolism to Prevent Periodontal Bone Loss during Orthodontic Tooth Movement in Osteoporotic Rats. Nutrients 2023, 15, 4906. https://doi.org/10.3390/nu15234906
Zhou Y, Zhu Y, Jin X, Zhang Y, Song J, Wu Z, Li Y, Yi J, Wang D, Hu M. Chroogomphus rutilus Regulates Bone Metabolism to Prevent Periodontal Bone Loss during Orthodontic Tooth Movement in Osteoporotic Rats. Nutrients. 2023; 15(23):4906. https://doi.org/10.3390/nu15234906
Chicago/Turabian StyleZhou, Ying, Yanfeng Zhu, Xinghui Jin, Yongfeng Zhang, Jiyu Song, Zhina Wu, Yutong Li, Jingzheng Yi, Di Wang, and Min Hu. 2023. "Chroogomphus rutilus Regulates Bone Metabolism to Prevent Periodontal Bone Loss during Orthodontic Tooth Movement in Osteoporotic Rats" Nutrients 15, no. 23: 4906. https://doi.org/10.3390/nu15234906
APA StyleZhou, Y., Zhu, Y., Jin, X., Zhang, Y., Song, J., Wu, Z., Li, Y., Yi, J., Wang, D., & Hu, M. (2023). Chroogomphus rutilus Regulates Bone Metabolism to Prevent Periodontal Bone Loss during Orthodontic Tooth Movement in Osteoporotic Rats. Nutrients, 15(23), 4906. https://doi.org/10.3390/nu15234906








