Exploring the Anticancer Potential of Origanum majorana Essential Oil Monoterpenes Alone and in Combination against Non-Small Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cellular Viability
2.3. Colony Growth Assay
2.4. In Ovo Tumor Growth Assay
2.5. Western Blot
2.6. Statistical Analysis
3. Results and Discussions
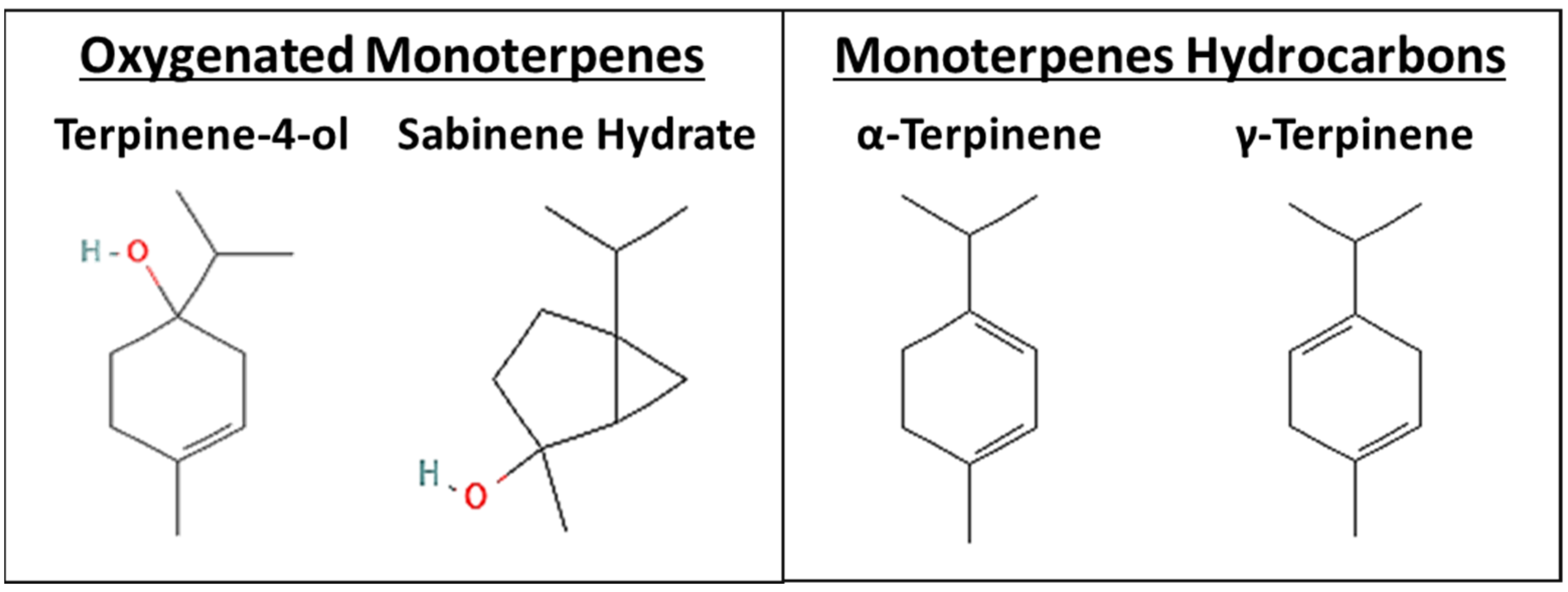
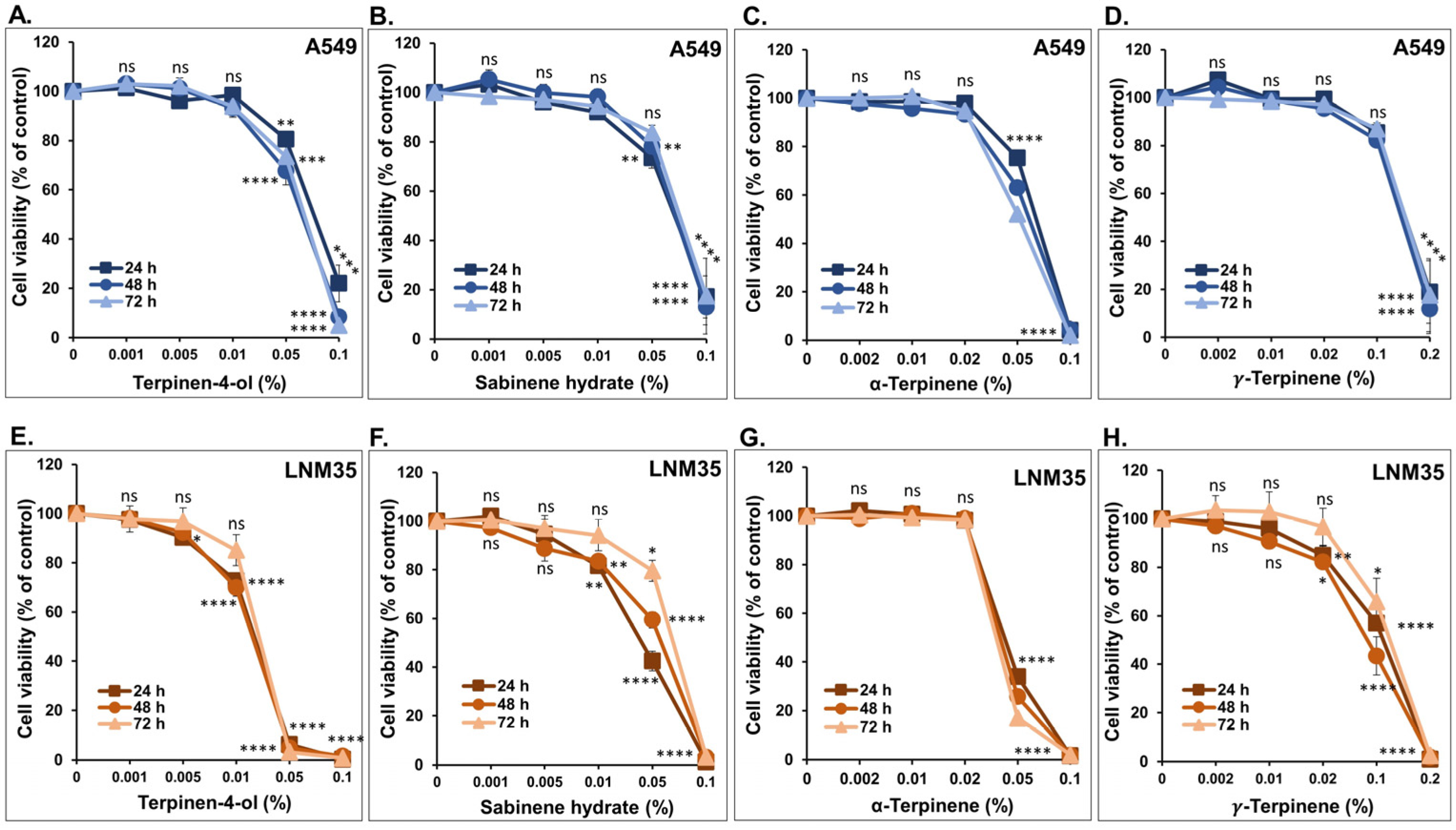
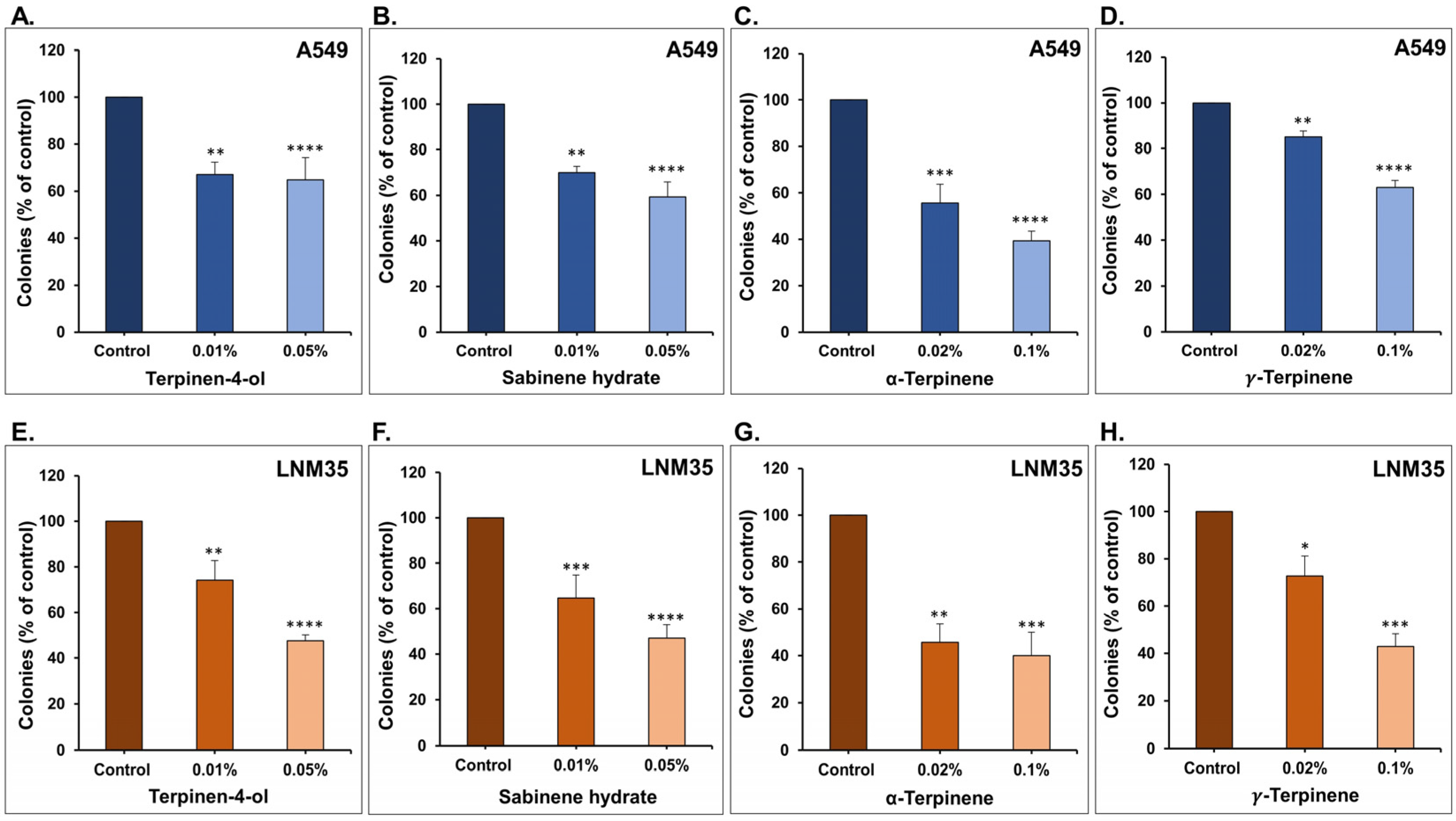
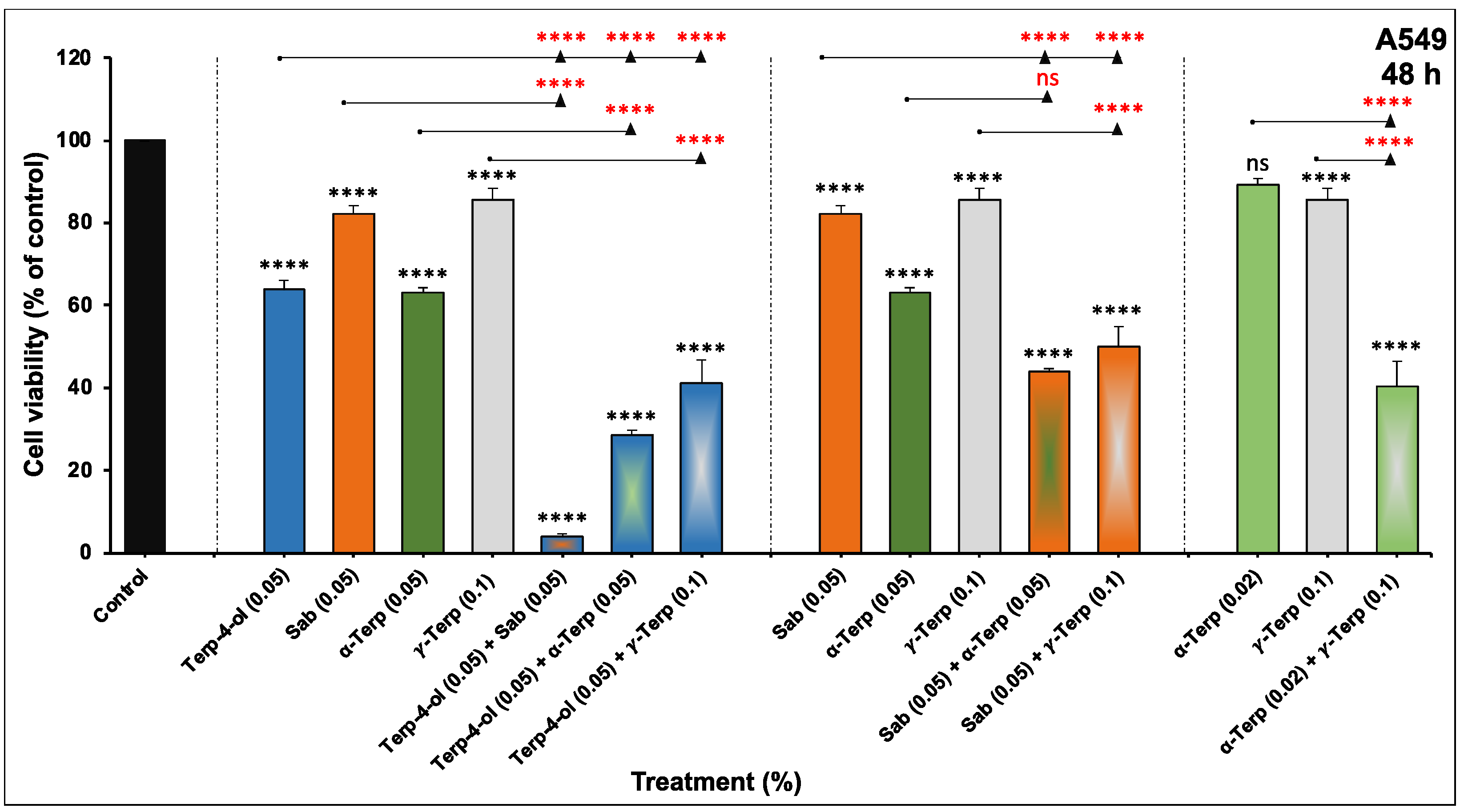
3.1. The Four Monoterpenes Inhibited NSCLC Cellular Viability and Colony Growth In Vitro
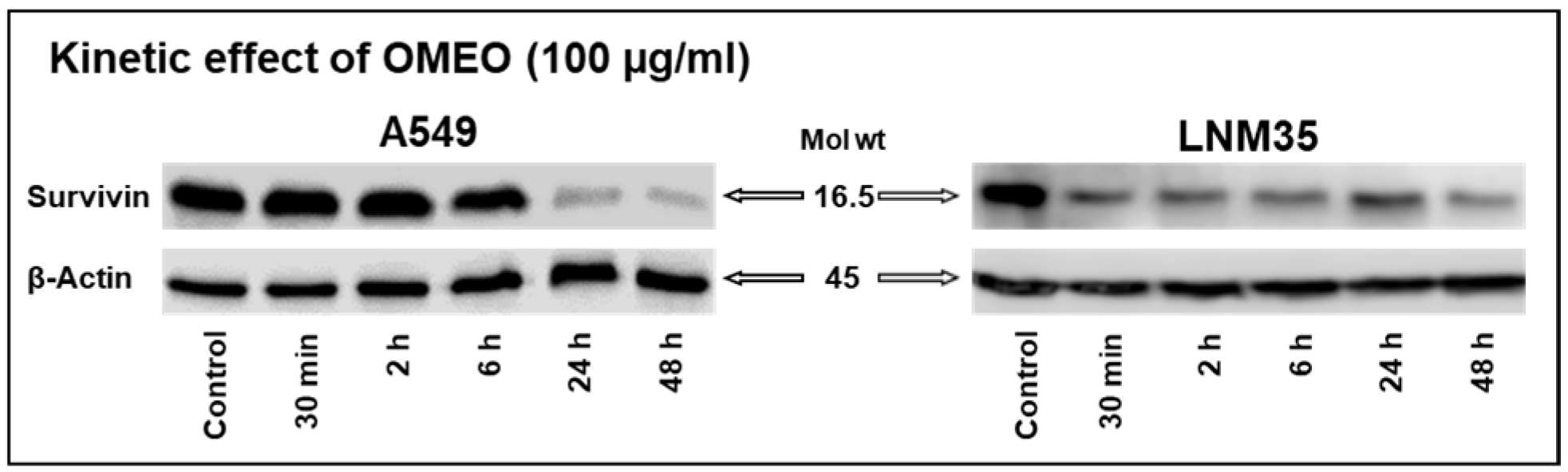
3.2. The Four Monoterpenes Inhibited Survivin Expression In Vitro
3.3. The Anticancer Effect of Monoterpene Combinations on NSCLC In Vitro
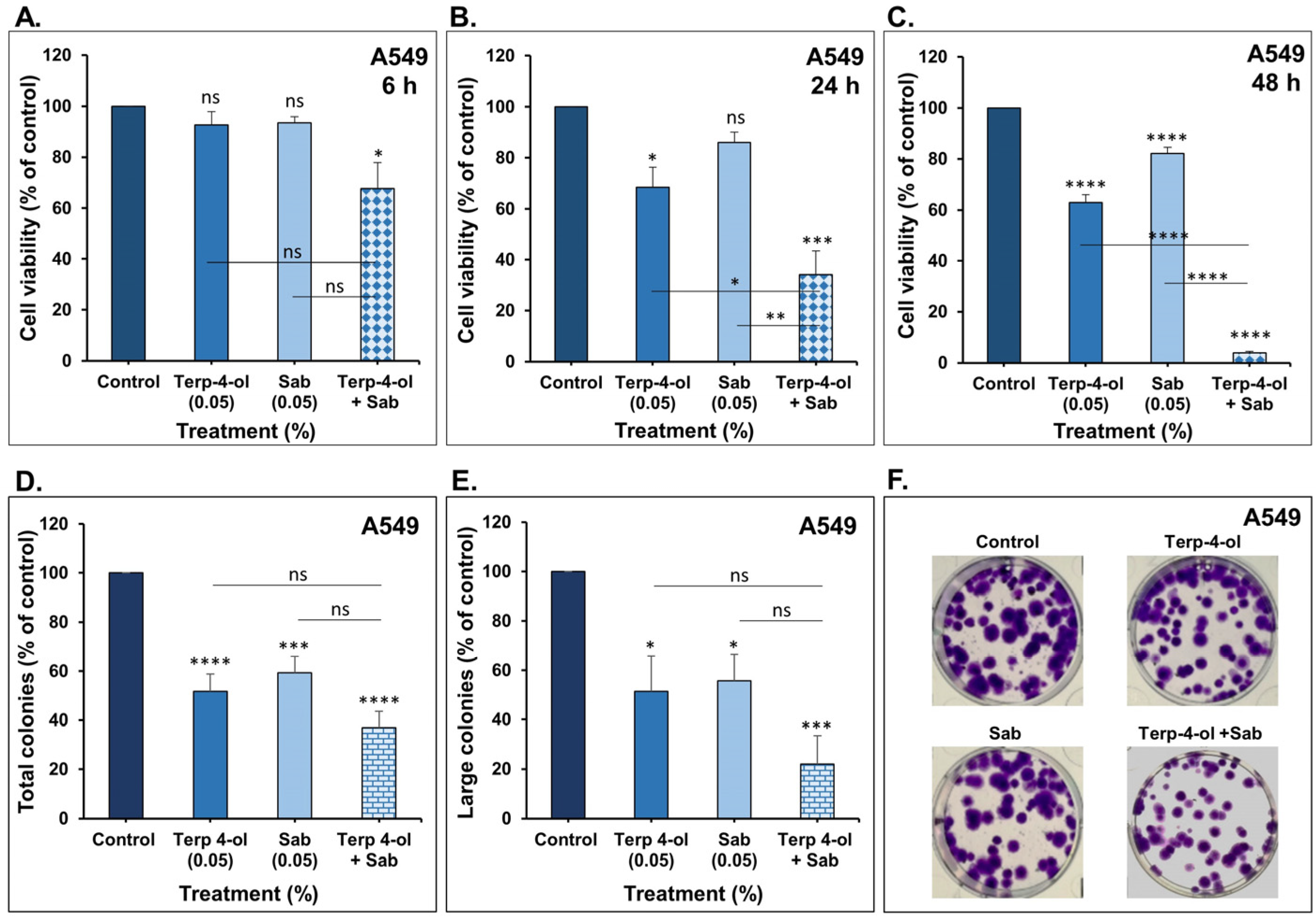
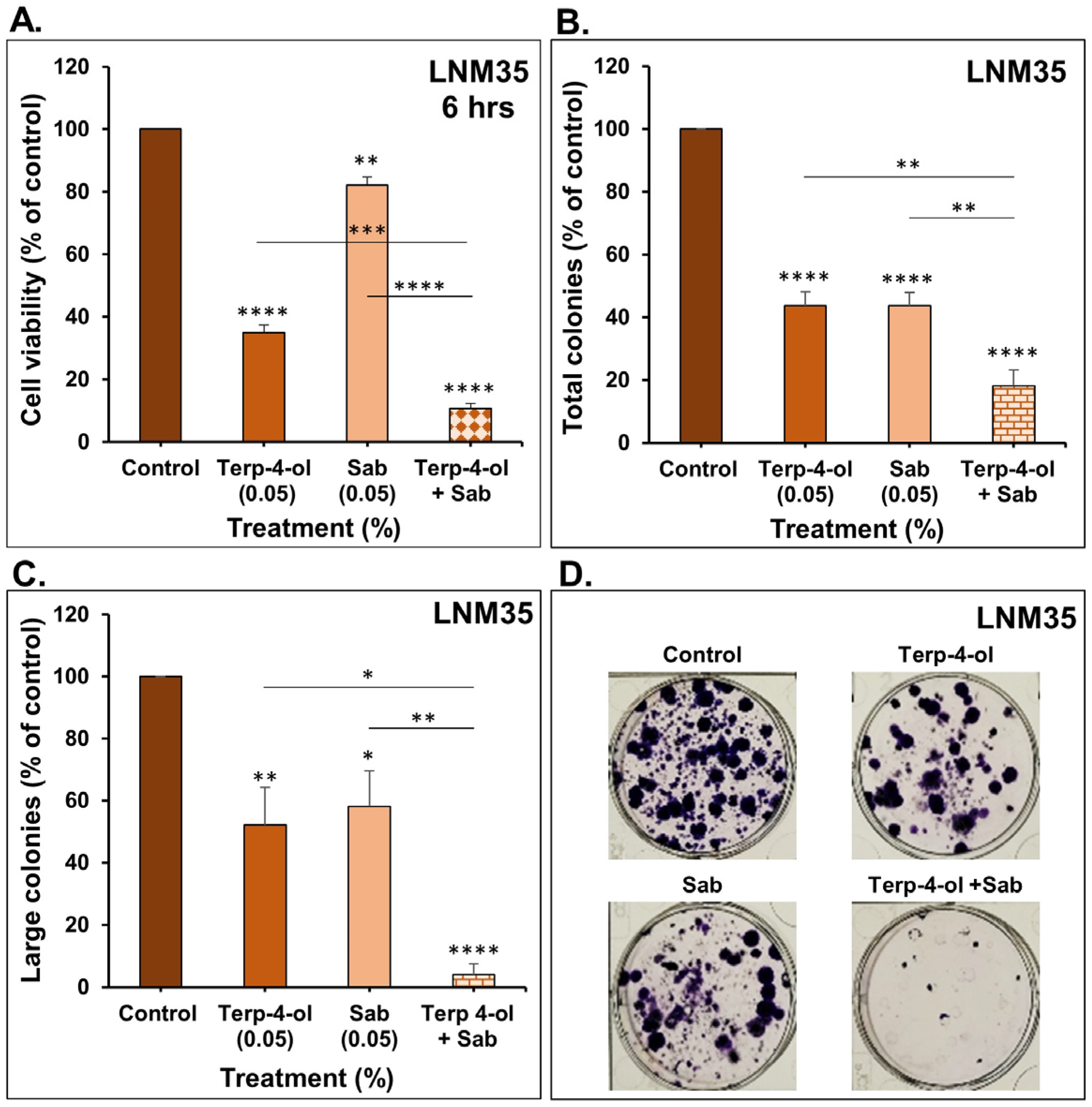
3.3.1. Impact of Terpinen-4-ol in Combination with Sabinene Hydrate on NSCLC Cellular Viability and Colony Growth In Vitro
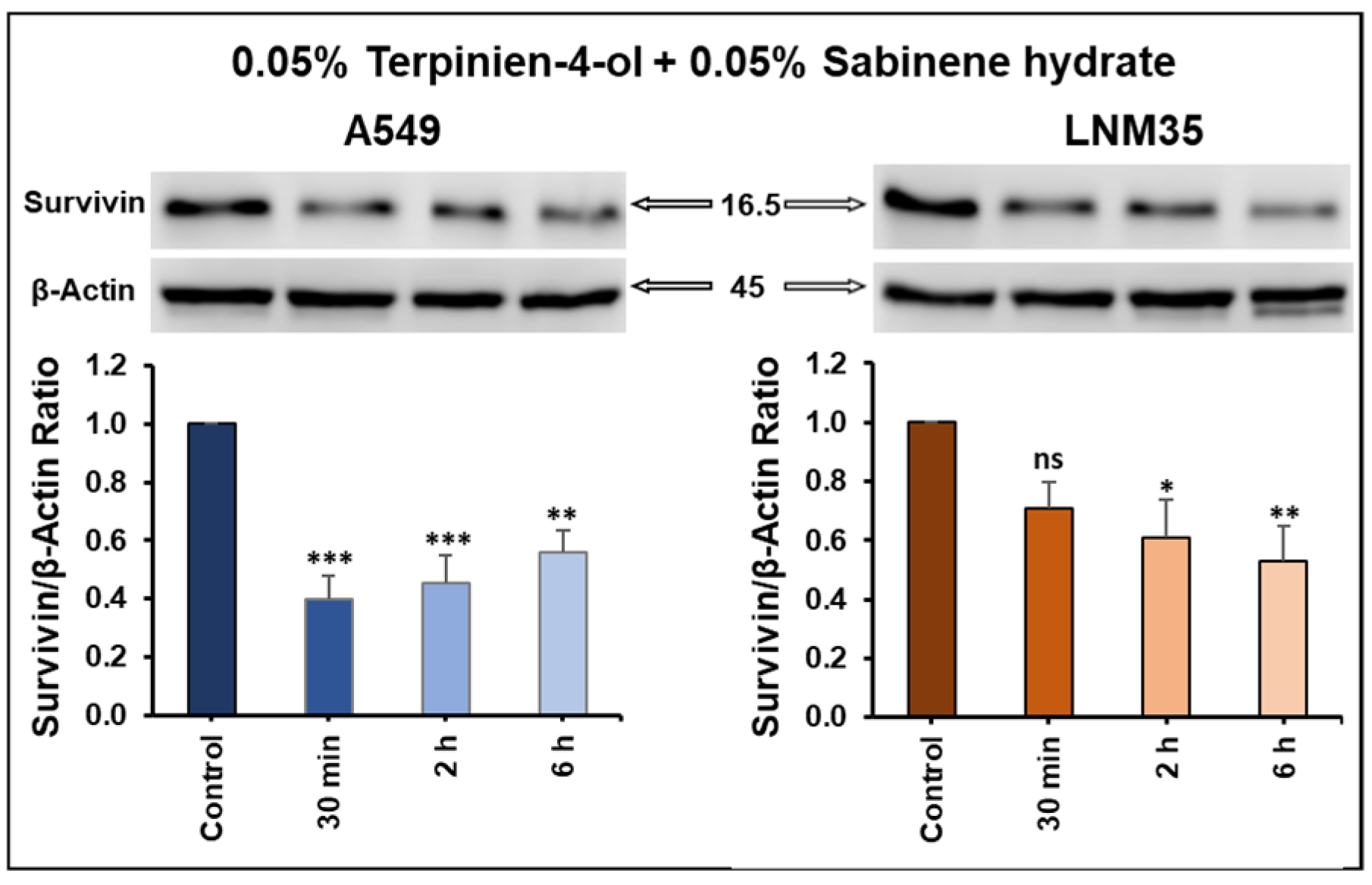
3.3.2. Terpinen-4-ol in Combination with Sabinene Hydrate Decreased Survivin Expression
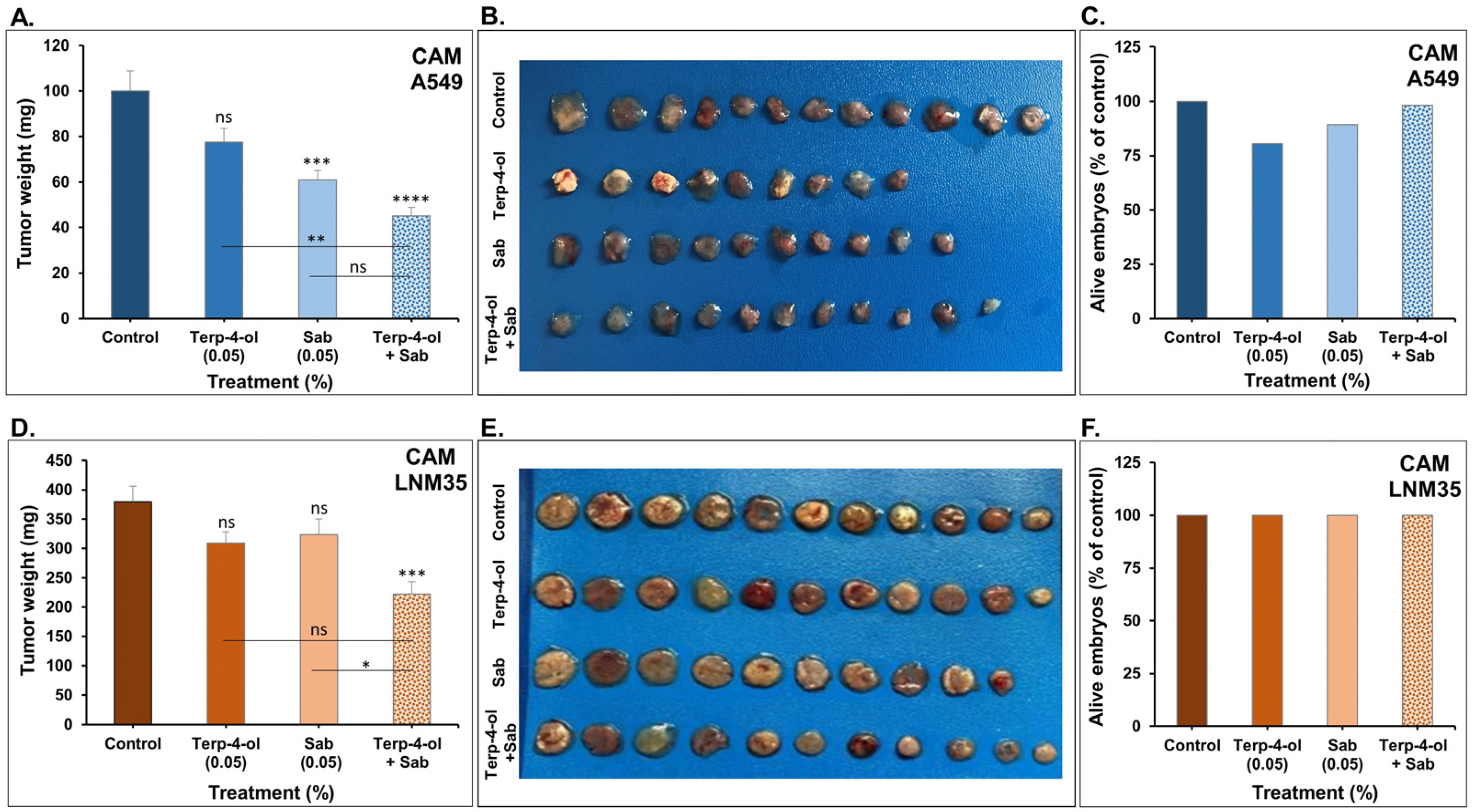
3.3.3. Impact of Terpinen-4-ol in Combination with Sabinene Hydrate on NSCLC Tumors In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Cordero, R.; Devine, W.P. Targeted Therapy and Checkpoint Immunotherapy in Lung Cancer. Surg. Pathol. Clin. 2020, 13, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 1 January 2023).
- Oliver, A.L. Lung Cancer: Epidemiology and Screening. Surg. Clin. N. Am. 2022, 102, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the Use of Targeted Therapies in Non-Small Cell Lung Cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. -Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Arafat, K.; Sulaiman, S.; Al-Azawi, A.M.; Yasin, J.; Sugathan, S.; Nemmar, A.; Karam, S.; Attoub, S. Origanum Majorana Essential Oil Decreases Lung Tumor Growth and Metastasis in Vitro and in Vivo. Biomed. Pharmacother. 2022, 155, 113762. [Google Scholar] [CrossRef]
- Amor, G.; Caputo, L.; la Storia, A.; de Feo, V.; Mauriello, G.; Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia Herba-Alba and Origanum Majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef]
- Athamneh, K.; Alneyadi, A.; Alsamri, H.; Alrashedi, A.; Palakott, A.; El-Tarabily, K.A.; Eid, A.H.; Dhaheri, Y.A.; Iratni, R. Origanum Majorana Essential Oil Triggers P38 Mapk-Mediated Protective Autophagy, Apoptosis, and Caspase-Dependent Cleavage of P70S6K in Colorectal Cancer Cells. Biomolecules 2020, 10, 412. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Mighri, H.; Aouni, M.; Gharsallah, N.; Kadri, A. Chemical Composition and in Vitro Evaluation of Antioxidant, Antimicrobial, Cytotoxicity and Anti-Acetylcholinesterase Properties of Tunisian Origanum majorana L. Essential Oil. Microb. Pathog. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- Shapira, S.; Pleban, S.; Kazanov, D.; Tirosh, P.; Arber, N. Terpinen-4-Ol: A Novel and Promising Therapeutic Agent for Human Gastrointestinal Cancers. PLoS ONE 2016, 11, e0156540. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Chen, Y.-J.; Chen, J.J.W.; Shieh, J.-J.; Huang, C.-H.; Lin, P.-S.; Chang, G.-C.; Chang, J.-T.; Lin, C.-C. Terpinen-4-Ol Induces Apoptosis in Human Nonsmall Cell Lung Cancer in Vitro and in Vivo. Evid.-Based Complement. Altern. Med. 2012, 2012, 818261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wu, J.L.; Dan-Wei, M.; Jiao, L.; Zhang, D.Y. Anticancer Effects of Chenopodium ambrosiodes L. Essential Oil on Human Breast Cancer MCF-7 Cells in Vitro. Trop. J. Pharm. Res. 2015, 14, 1813–1820. [Google Scholar] [CrossRef][Green Version]
- Ferraz, R.P.C.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.P.; Da Silva, T.B.; Machado, W.J.; Prata, A.P.N.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; et al. Cytotoxic Effect of Leaf Essential Oil of Lippia Gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Murata, S.; Ito, H.; Iwasaki, K.; Villareal, M.O.; Zheng, Y.W.; Matsui, H.; Isoda, H.; Ohkohchi, N. Terpinen-4-Ol Inhibits Colorectal Cancer Growth via Reactive Oxygen Species. Oncol. Lett. 2017, 14, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Tian, R.; Pan, R.; Sun, B.; Xiao, C.; Chen, Y.; Zeng, Z.; Lei, S. Terpinen-4-Ol Inhibits the Proliferation and Mobility of Pancreatic Cancer Cells by Downregulating Rho-Associated Coiled-Coil Containing Protein Kinase 2. Bioengineered 2022, 13, 8643–8656. [Google Scholar] [CrossRef] [PubMed]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’Aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor Effect of Melaleuca Alternifolia Essential Oil and Its Main Component Terpinen-4-Ol in Combination with Target Therapy in Melanoma Models. Cell Death Discov. 2021, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Mariano, F.; Costa, I.; Calcabrini, A.; Molinari, A. Tea Tree Oil and Terpinen-4-Ol Induce Cytoskeletal Reorganization of Human Melanoma Cells. Planta Medica Int. Open 2022, 9, e34–e53. [Google Scholar] [CrossRef]
- Mubarak, E.E.; Ali, L.Z.; Ahmed, I.F.A.; Ahmed, A.B.A.; Taha, R.M. Essential Oil Compositions and Cytotoxicity from Various Organs of Eucalyptus Camaldulensis. Int. J. Agric. Biol. 2015, 17, 320–326. [Google Scholar]
- Fitsiou, E.; Anestopoulos, I.; Chlichlia, K.; Galanis, A.; Kourkoutas, I.; Panayiotidis, M.I.; Pappa, A. Antioxidant and Antiproliferative Properties of the Essential Oils of Satureja Thymbra and Satureja Parnassica and Their Major Constituents. Anticancer. Res. 2016, 36, 5757–5763. [Google Scholar] [CrossRef]
- Cao, W.; Li, Y.; Zeng, Z.; Lei, S. Terpinen-4-Ol Induces Ferroptosis of Glioma Cells via Downregulating JUN Proto-Oncogene. Molecules 2023, 28, 4643. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.A.; Pitts, T.P.; Tandberg, K.R.; Winder, P.L.; Wright, A.E. Discovery of Survivin Inhibitors Part 1: Screening the Harbor Branch Pure Compound Library. Mar. Drugs 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Albadari, N.; Li, W. Survivin Small Molecules Inhibitors: Recent Advances and Challenges. Molecules 2023, 28, 1376. [Google Scholar] [CrossRef] [PubMed]
- Mull, A.N.; Klar, A.; Navara, C.S. Differential Localization and High Expression of SURVIVIN Splice Variants in Human Embryonic Stem Cells but Not in Differentiated Cells Implicate a Role for SURVIVIN in Pluripotency. Stem. Cell Res. 2014, 12, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Wehinger, S.; Castillo Bennett, J.; Valenzuela, M.; Owen, G.I.; Quest, A.F.G. The Twisted Survivin Connection to Angiogenesis. Mol. Cancer 2015, 14, 198. [Google Scholar] [CrossRef]
- Humphry, N.J.; Wheatley, S.P. Survivin Inhibits Excessive Autophagy in Cancer Cells but Does so Independently of Its Interaction with LC3. Biol. Open 2018, 7, bio037374. [Google Scholar] [CrossRef]
- Huang, W.; Mao, Y.; Zhan, Y.; Huang, J.; Wang, X.; Luo, P.; Li, L.; Mo, D.; Liu, Q.; Xu, H.; et al. Prognostic Implications of Survivin and Lung Resistance Protein in Advanced Non-Small Cell Lung Cancer Treated with Platinum-Based Chemotherapy. Oncol. Lett. 2016, 11, 723–730. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafat, K.; Al-Azawi, A.M.; Sulaiman, S.; Attoub, S. Exploring the Anticancer Potential of Origanum majorana Essential Oil Monoterpenes Alone and in Combination against Non-Small Cell Lung Cancer. Nutrients 2023, 15, 5010. https://doi.org/10.3390/nu15235010
Arafat K, Al-Azawi AM, Sulaiman S, Attoub S. Exploring the Anticancer Potential of Origanum majorana Essential Oil Monoterpenes Alone and in Combination against Non-Small Cell Lung Cancer. Nutrients. 2023; 15(23):5010. https://doi.org/10.3390/nu15235010
Chicago/Turabian StyleArafat, Kholoud, Aya Mudhafar Al-Azawi, Shahrazad Sulaiman, and Samir Attoub. 2023. "Exploring the Anticancer Potential of Origanum majorana Essential Oil Monoterpenes Alone and in Combination against Non-Small Cell Lung Cancer" Nutrients 15, no. 23: 5010. https://doi.org/10.3390/nu15235010
APA StyleArafat, K., Al-Azawi, A. M., Sulaiman, S., & Attoub, S. (2023). Exploring the Anticancer Potential of Origanum majorana Essential Oil Monoterpenes Alone and in Combination against Non-Small Cell Lung Cancer. Nutrients, 15(23), 5010. https://doi.org/10.3390/nu15235010





