A Ropy Exopolysaccharide-Producing Strain Bifidobacterium pseudocatenulatum Bi-OTA128 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Culture Suspension
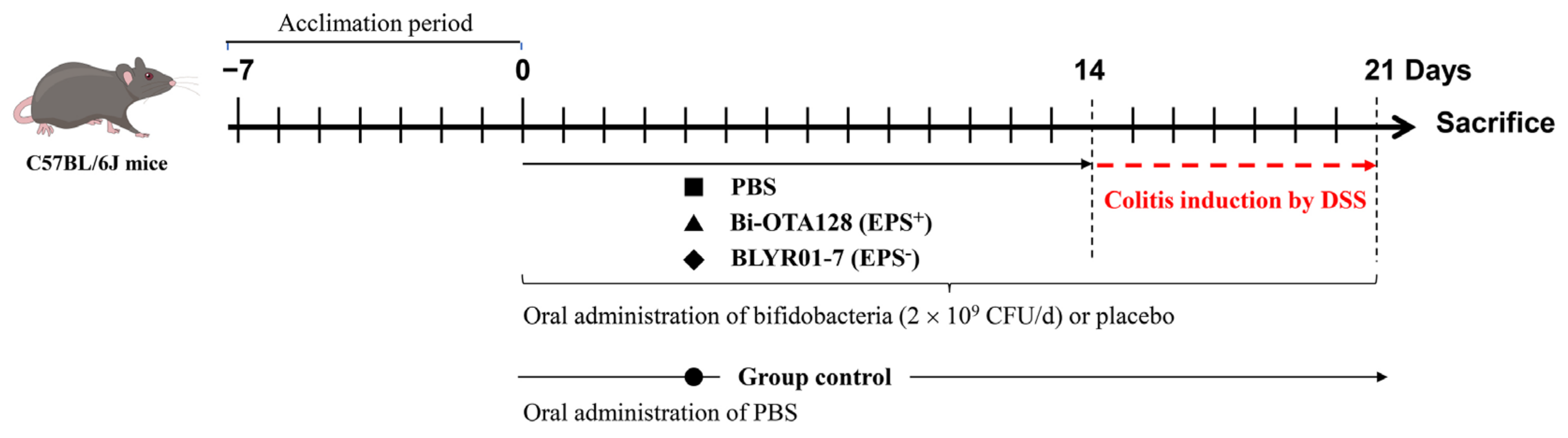
2.2. Animals and Colitis Induced by DSS
2.3. Colitis Assessment and Histopathology Analysis
2.4. Determination of Biochemical Indices in Colon
2.5. Quantitative Real-Time PCR Analysis
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
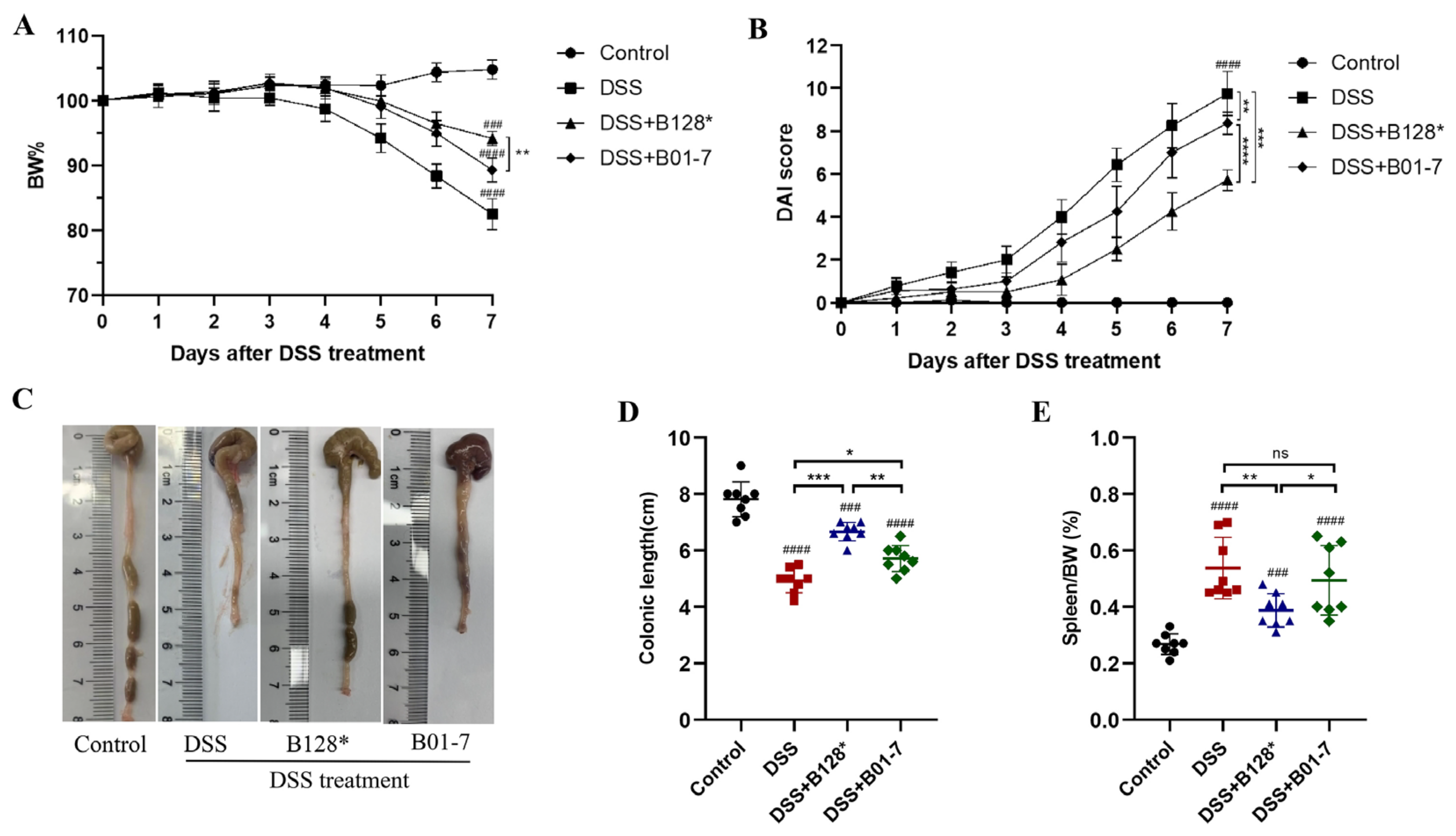
3.1. B. pseudocatenulatum Intervention Improved Colitis Symptoms
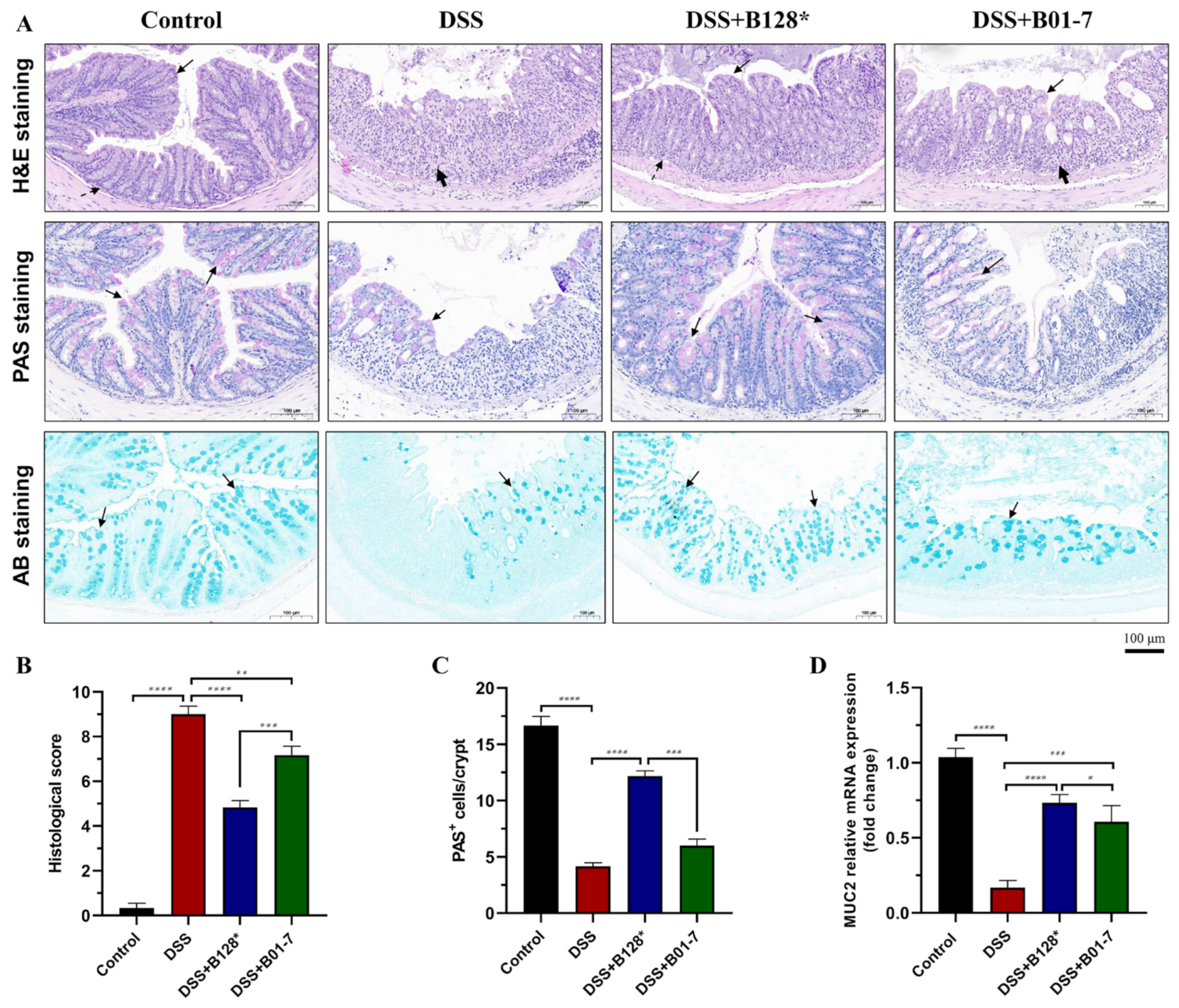
3.2. B. pseudocatenulatum Alleviated Colon Damage and Recovered Mucous Layer
3.3. B. pseudocatenulatum Attenuated Oxidative Stress in Colon Tissues
3.4. B. pseudocatenulatum Improved Gut Barrier Integrity and Enhanced Tight Junctions Expression
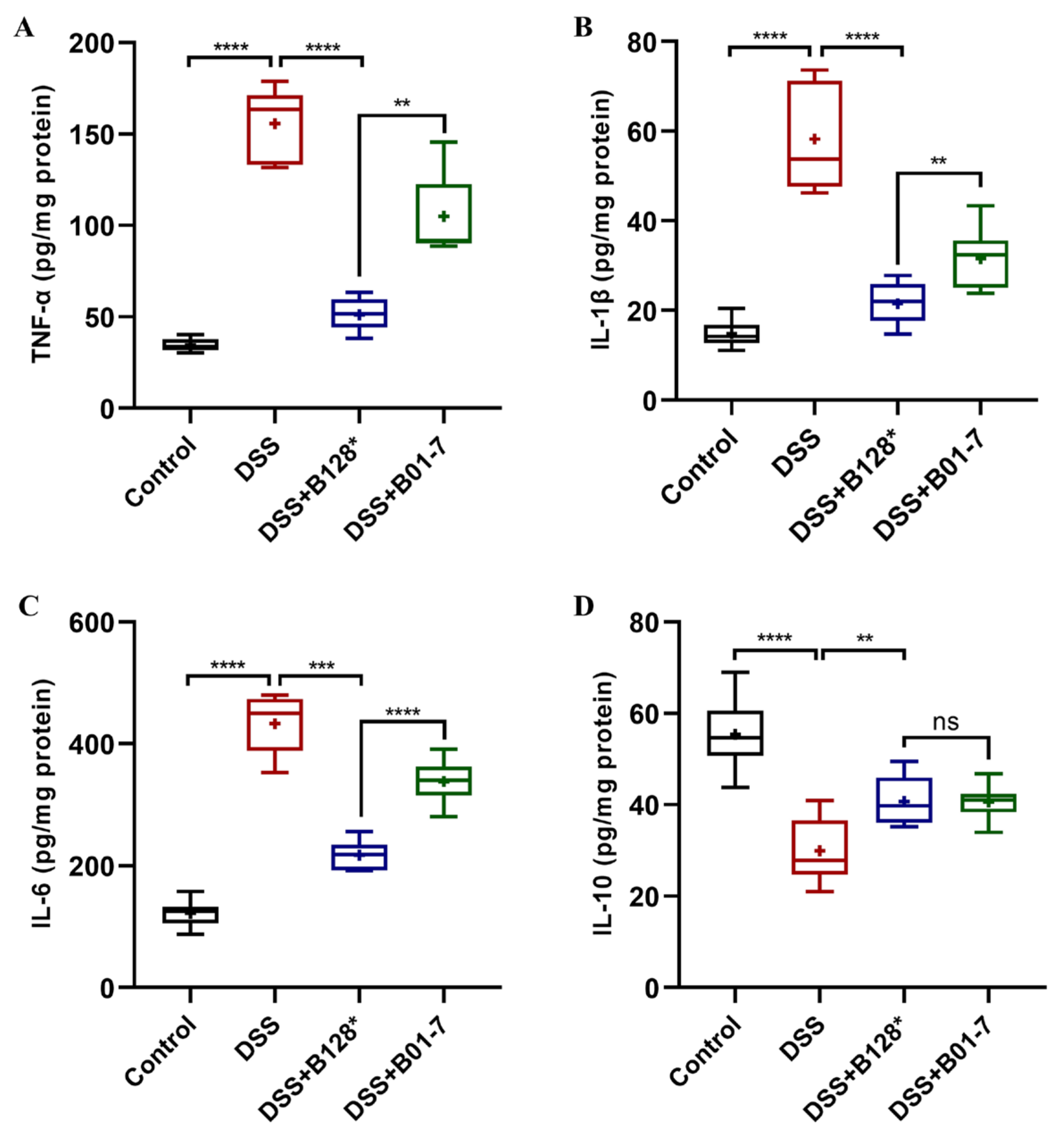
3.5. B. pseudocatenulatum Regulated Inflammatory Response in Colon Tissues
3.6. B. pseudocatenulatum Modulated Gut Microbiota
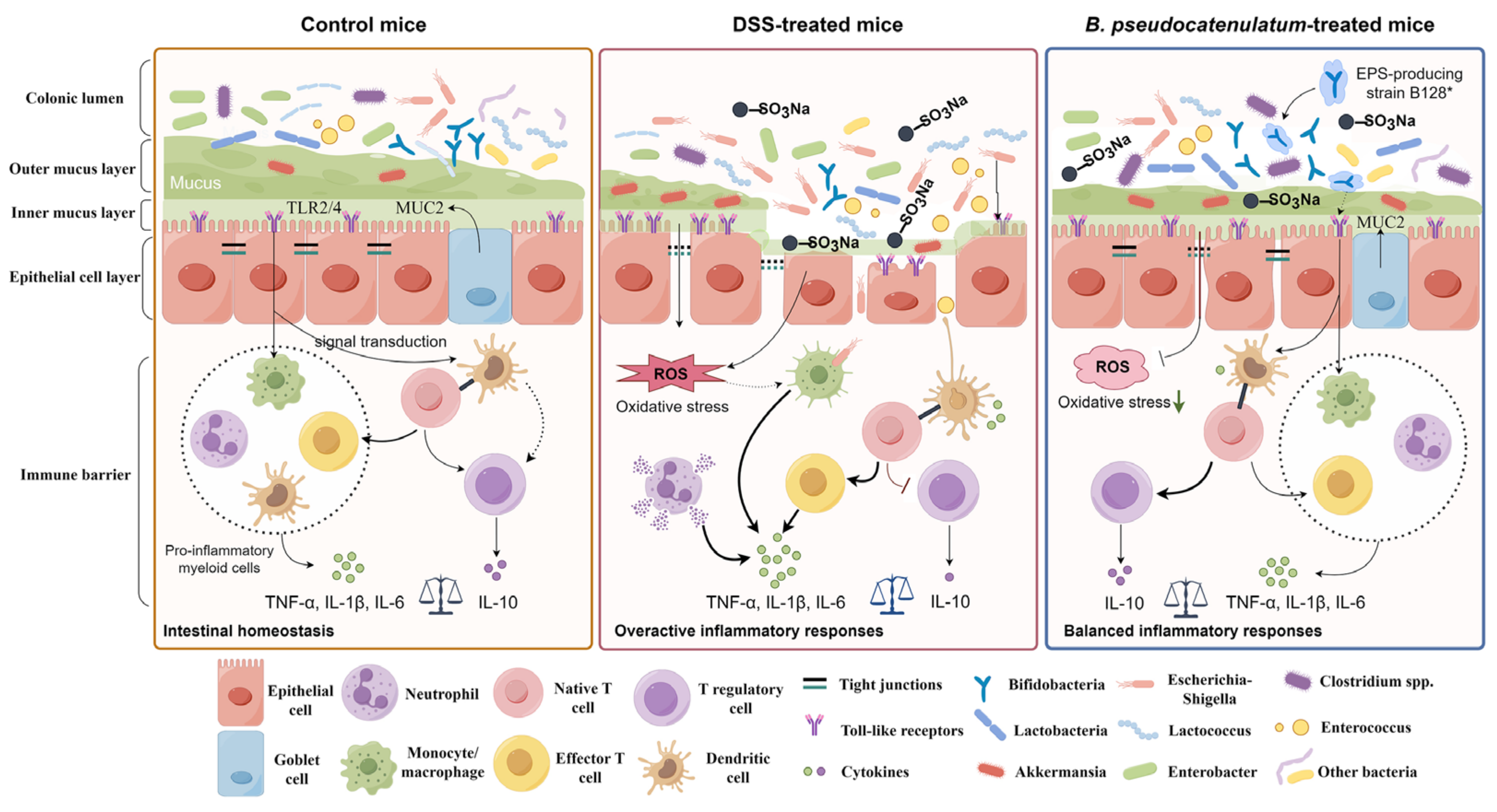
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Tang, Y. Oxidative Stress, inflammation, gut Dysbiosis: What can polyphenols do in inflammatory bowel disease? Antioxidants 2023, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: Systematic review. Gastroenterology 2022, 162, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Moon, W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver 2019, 13, 604–616. [Google Scholar] [CrossRef]
- Agagunduz, D.; Gencer Bingol, F.; Celik, E.; Cemali, O.; Ozenir, C.; Ozogul, F.; Capasso, R. Recent developments in the probiotics as live biotherapeutic products (LBPs) as modulators of gut brain axis related neurological conditions. J. Transl. Med. 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Ladda, B.; Jantararussamee, C.; Pradidarcheep, W.; Kasorn, A.; Matsathit, U.; Taweechotipatr, M. Anti-inflammatory and gut microbiota modulating effects of probiotic Lactobacillus paracasei MSMC39-1 on dextran sulfate sodium-induced colitis in rats. Nutrients 2023, 15, 1388. [Google Scholar] [CrossRef]
- Biagioli, M.; Capobianco, D.; Carino, A.; Marchiano, S.; Fiorucci, C.; Ricci, P.; Distrutti, E.; Fiorucci, S. Divergent effectiveness of multispecies probiotic preparations on intestinal microbiota structure depends on metabolic properties. Nutrients 2019, 11, 325. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Eibl, G. Nutritional support and probiotics as a potential treatment of IBD. Curr. Drug. Targets 2020, 21, 1417–1427. [Google Scholar] [CrossRef]
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- De Greef, E.; Vandenplas, Y.; Hauser, B.; Devreker, T.; Veereman-Wauters, G. Probiotics and IBD. Acta Gastroenterol. Belg. 2013, 76, 15–19. [Google Scholar] [PubMed]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.M.; Heo, W.; Cho, H.T.; Lee, D.H.; Kim, J.H.; Rhee, M.S.; Park, T.S.; Kim, Y.K.; Lee, J.H.; Kim, Y.J. Effects of orally-administered Bifidobacterium animalis subsp. lactis strain BB12 on dextran sodium sulfate-induced colitis in mice. J. Microbiol. Biotechnol. 2018, 28, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, B.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium pseudocatenulatum ameliorates DSS-induced colitis by maintaining intestinal mechanical barrier, blocking proinflammatory cytokines, inhibiting TLR4/NF-kappaB signaling, and altering gut microbiota. J. Agric. Food Chem. 2021, 69, 1496–1512. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.Y.; Tan, Y.; Zhang, H. Heat-killed Bifidobacterium bifidum B1628 may alleviate dextran sulfate sodium-induced colitis in mice, and the anti-inflammatory effect is associated with gut microbiota modulation. Nutrients 2022, 14, 5233. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, C.; Zhao, Z.; Zhai, Z.; Hao, Y. Anti-inflammatory effect of Bifidobacterium animalis subsp. lactis A6 on DSS-induced colitis in mice. J. Appl. Microbiol. 2022, 133, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.M.; Guo, H.X.; Cai, J.W.; Meng, X.C. Bifidobacterium breve alleviates DSS-induced colitis in mice by maintaining the mucosal and epithelial barriers and modulating gut microbes. Nutrients 2022, 14, 3671. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Algieri, F.; Rodriguez-Nogales, A.; Vezza, T.; Martinez-Camblor, P.; Margolles, A.; Ruas-Madiedo, P.; Galvez, J. Effect of a ropy exopolysaccharide-producing Bifidobacterium animalis subsp. lactis strain orally administered on DSS-induced colitis mice model. Front. Microbiol. 2016, 7, 868. [Google Scholar] [CrossRef]
- Yan, S.; Yang, B.; Zhao, J.; Zhao, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W. A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct. 2019, 10, 1595–1608. [Google Scholar] [CrossRef]
- Hidalgo-Cantabrana, C.; Lopez, P.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Suarez, A.; Margolles, A.; Ruas-Madiedo, P. Immune modulation capability of exopolysaccharides synthesized by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob. Proteins 2012, 4, 227–237. [Google Scholar] [CrossRef]
- Laino, J.; Villena, J.; Kanmani, P.; Kitazawa, H. Immunoregulatory effects triggered by lactic acid bacteria exopolysaccharides: New insights into molecular interactions with host cells. Microorganisms 2016, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, S.; Guidesi, E.; Zonenschain, D.; Sagheddu, V.; Lee, S.; Lim, C.Y.; Elli, M. Isolation and characterization of new probiotic strains from Chinese babies. J. Clin. Gastroenterol. 2018, 52, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, W.; Zhang, Y.; Zhang, D.; Qiu, B.; Wang, X.; Liu, J.; Liu, L. Therapeutic and prebiotic effects of five different native starches on dextran sulfate sodium-induced mice model of colonic colitis. Mol. Nutr. Food Res. 2021, 65, e2000922. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted selection of biomarkers by linear discriminant analysis effect size (LEfSe) in microbiome data. J. Vis. Exp. 2022, 183, e61715. [Google Scholar]
- Amer, M.; Nadeem, M.; Nazir, S.U.R.; Fakhar, M.; Abid, F.; Ain, Q.U.; Asif, E. Probiotics and their use in inflammatory bowel disease. Altern. Ther. Health Med. 2018, 24, 16–23. [Google Scholar]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Chen, S.J.; Liu, X.W.; Liu, J.P.; Yang, X.Y.; Lu, F.G. Ulcerative colitis as a polymicrobial infection characterized by sustained broken mucus barrier. World J. Gastroenterol. 2014, 20, 9468–9475. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [PubMed]
- Goyette, P.; Labbe, C.; Trinh, T.T.; Xavier, R.J.; Rioux, J.D. Molecular pathogenesis of inflammatory bowel disease: Genotypes, phenotypes and personalized medicine. Ann. Med. 2007, 39, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, A.; Foey, A. Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 2017, 9, 1156. [Google Scholar] [CrossRef]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Guan, Z.; Goldfine, H. Lipid diversity in clostridia. BBA-Mol. Cell Biol. Lipids 2021, 1866, 158966. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, Z.; Zhan, P.C.; Lv, A.P.; Li, P.P.; Liu, L.; Li, W.J.; Yang, L.L.; Zhi, X.Y. Comparative genomic analysis and proposal of Clostridium yunnanense sp. nov., Clostridium rhizosphaerae sp. nov., and Clostridium paridis sp. nov., three novel Clostridium sensu stricto endophytes with diverse capabilities of acetic acid and ethanol production. Anaerobe 2023, 79, 102686. [Google Scholar]



| Target Protein | Function | Primer (5′→3′) | Product Size |
|---|---|---|---|
| ZO-1 | Gut barrier integrity | F: GGCCTTGGCCTAGCATACAC | 158 bp |
| R: GTCTTCATTTGACCCTCCCTC | |||
| Occludin | F: TCACTTTTCCTGCGGTGACTT | 136 bp | |
| R: GGGAACGTGGCCGATATAAT | |||
| Claudin-1 | F: AGCTGTGCATGGCCTCTTGT | 128 bp | |
| R: CCAATGTCAATGGCAACACCC | |||
| MUC2 | Mucus layer | F: TGCTGACGAGTGGTTGGTGAAT | 135 bp |
| R: GATGAGGTGGCAGACAGGAGAC | |||
| TLR2 | Pattern recognition receptor | F: GACTCTTCACTTAAGCGAGTCT | 102 bp |
| R: AACCTGGCCAAGTTAGTATCTC | |||
| TLR4 | F: GCCATCATTATGAGTGCCAATT | 107 bp | |
| R: AGGGATAAGAACGCTGAGAATT | |||
| β-actin | Reference | F: CCTAAGAGGAGGATGGTCGC | 230 bp |
| R: CTCAACACCTCAACCCCCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, X.; Kou, X.; Zhai, Z.; Hao, Y. A Ropy Exopolysaccharide-Producing Strain Bifidobacterium pseudocatenulatum Bi-OTA128 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2023, 15, 4993. https://doi.org/10.3390/nu15234993
Wang H, Zhang X, Kou X, Zhai Z, Hao Y. A Ropy Exopolysaccharide-Producing Strain Bifidobacterium pseudocatenulatum Bi-OTA128 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients. 2023; 15(23):4993. https://doi.org/10.3390/nu15234993
Chicago/Turabian StyleWang, Hui, Xinyuan Zhang, Xinfang Kou, Zhengyuan Zhai, and Yanling Hao. 2023. "A Ropy Exopolysaccharide-Producing Strain Bifidobacterium pseudocatenulatum Bi-OTA128 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice" Nutrients 15, no. 23: 4993. https://doi.org/10.3390/nu15234993
APA StyleWang, H., Zhang, X., Kou, X., Zhai, Z., & Hao, Y. (2023). A Ropy Exopolysaccharide-Producing Strain Bifidobacterium pseudocatenulatum Bi-OTA128 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients, 15(23), 4993. https://doi.org/10.3390/nu15234993







