Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
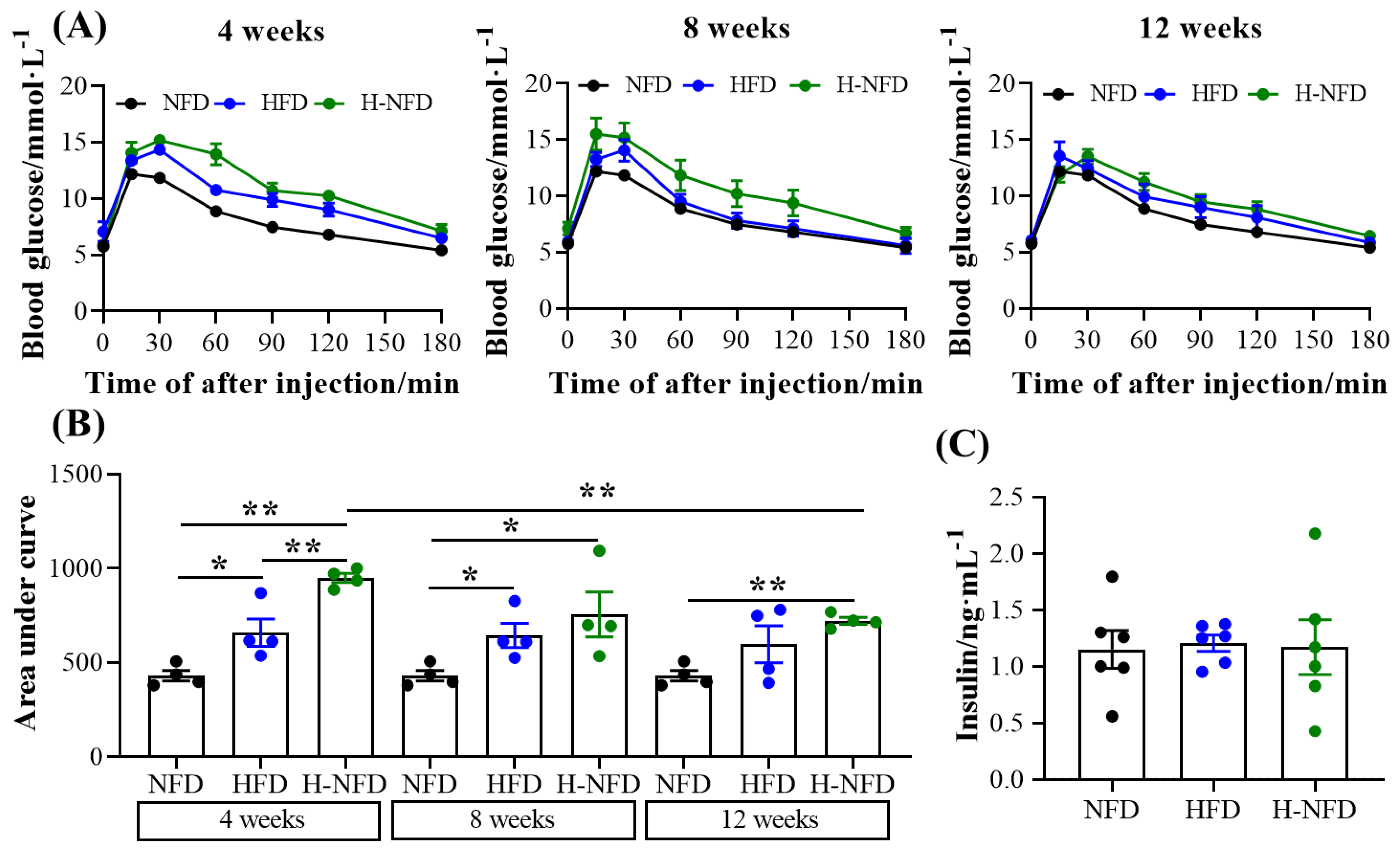
2.2. Intraperitoneal Glucose Tolerance Test
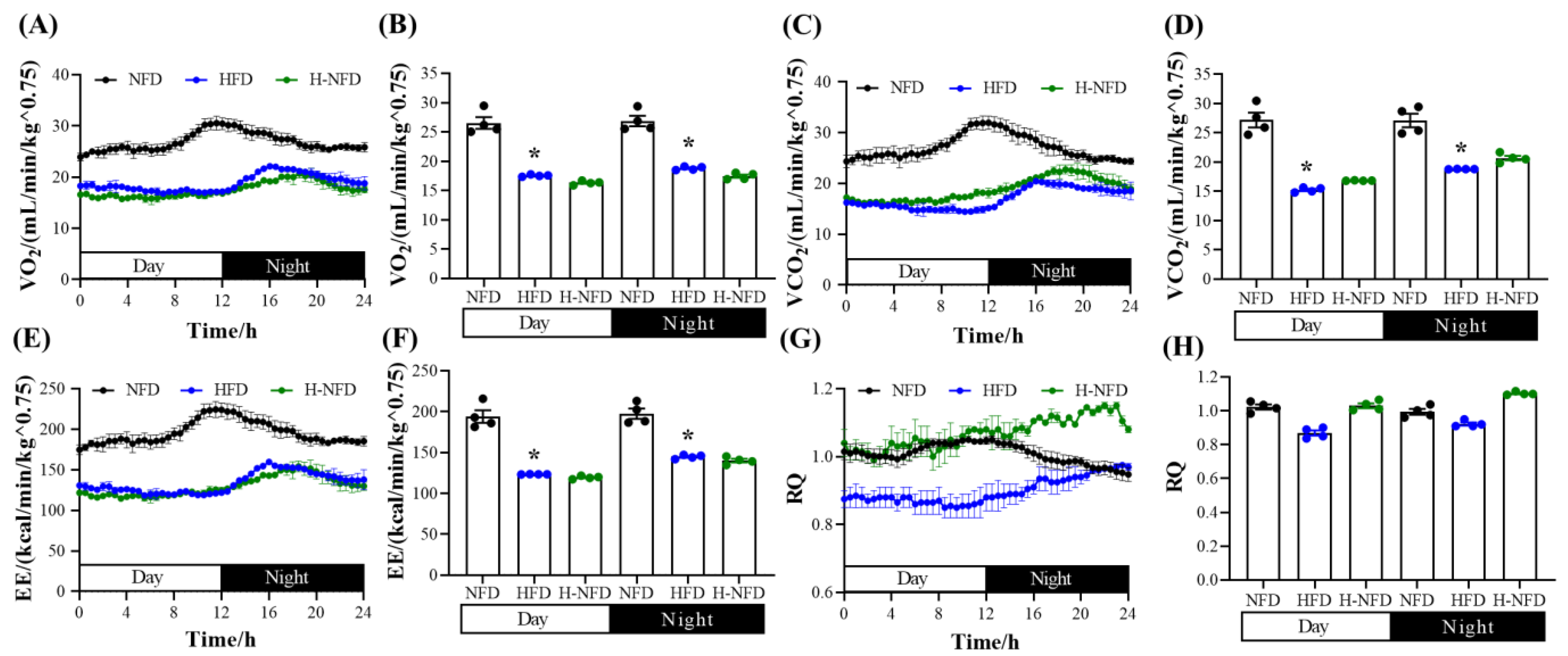
2.3. Assessment of Energy Metabolism
2.4. Isolation of the Stromal Vascular Fraction (SVF)
2.5. Flow Cytometry Sorting
2.6. Histological Analysis
2.7. Oil Red O Staining
2.8. Protein Expression Analysis
2.9. Gene Expression Analysis
2.10. Biochemical Analysis
2.11. Statistical Analysis
3. Results
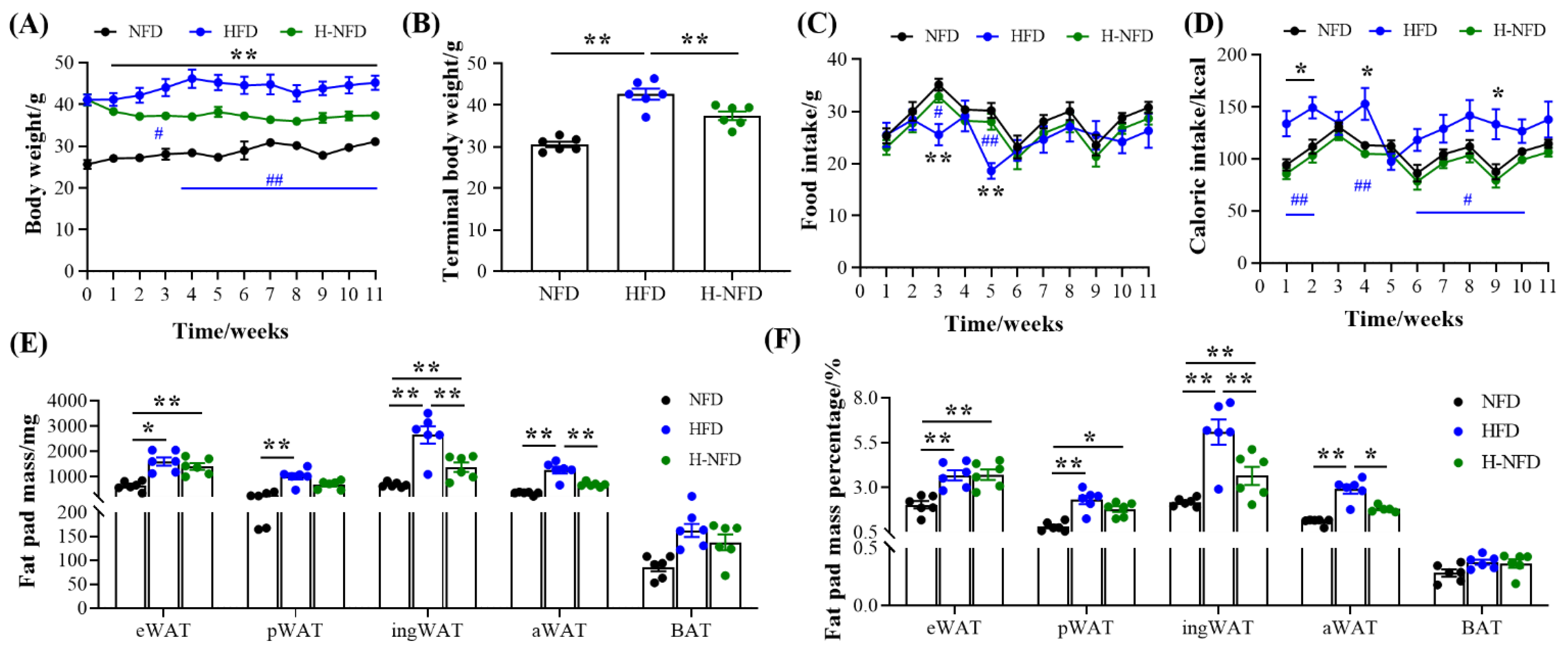
3.1. Change to a NFD Immediately Alleviates HFD-Induced Body Weight and Fat Gain
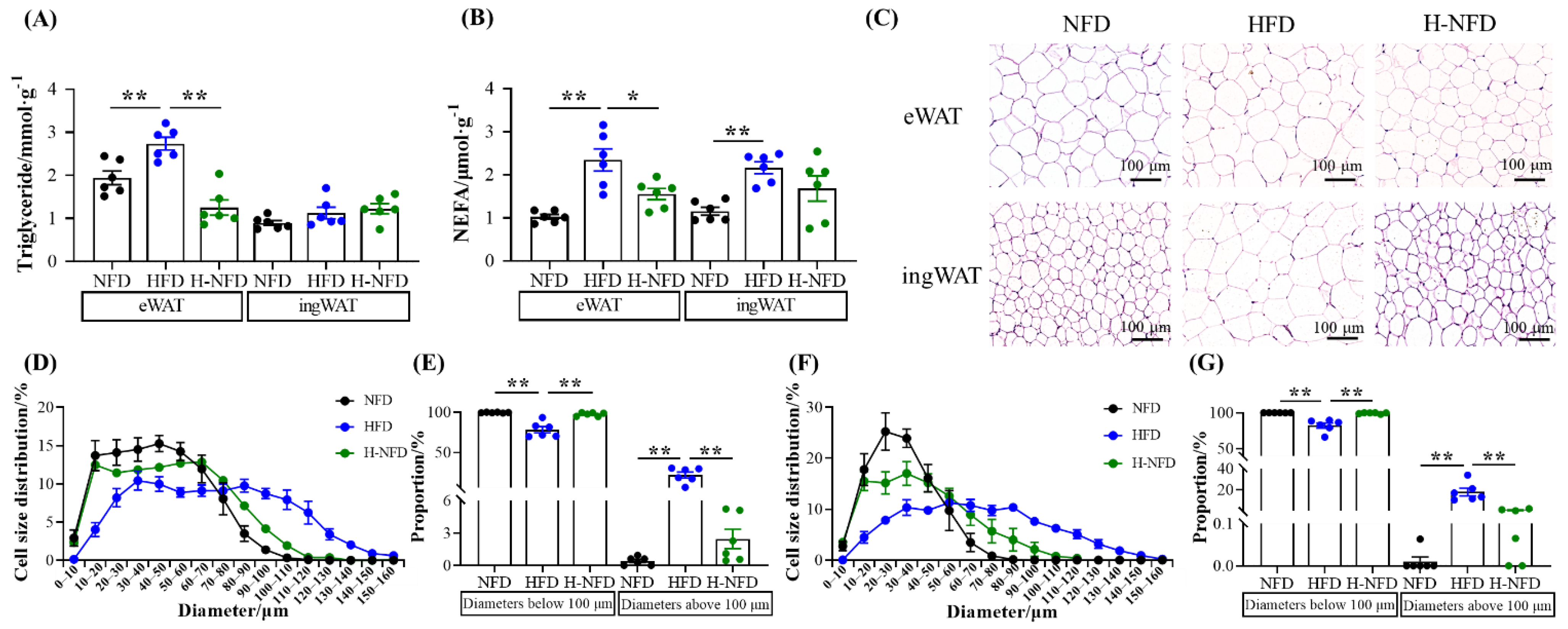
3.2. Change to NFD Decreases Adipocyte Size
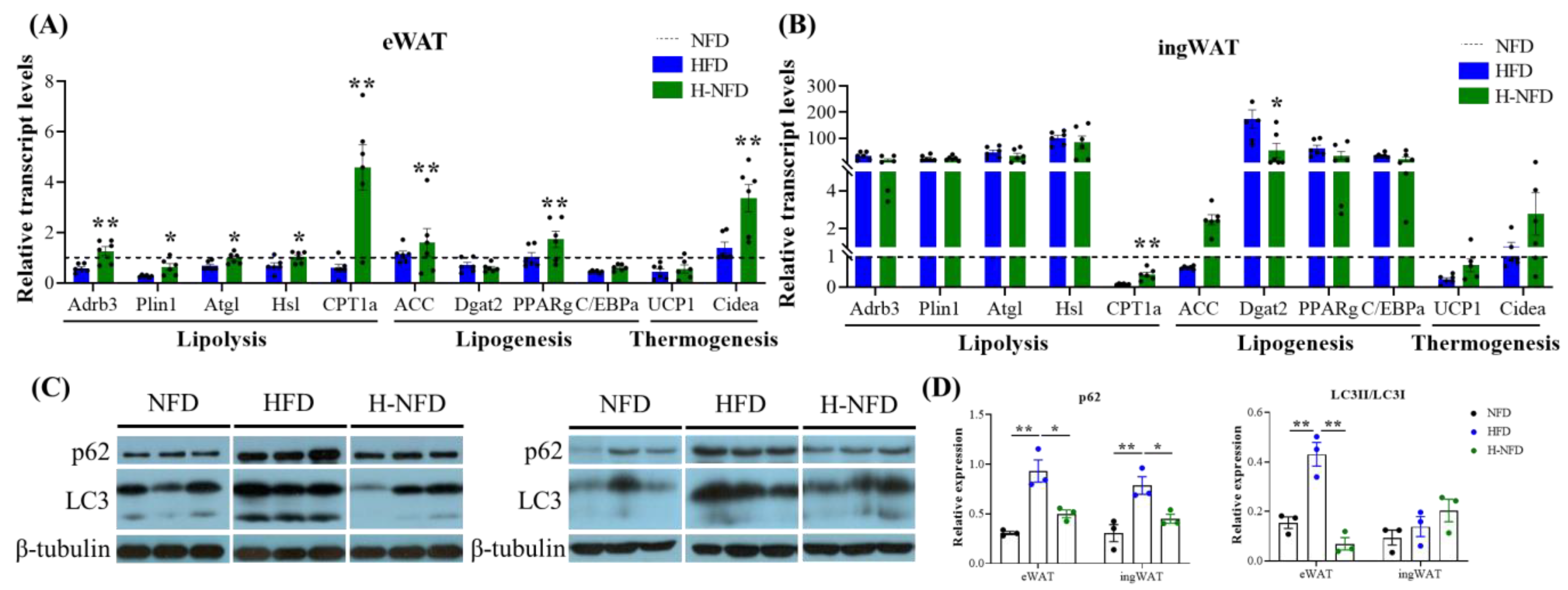
3.3. Change to NFD Enhances Lipolysis in Adipose Tissue
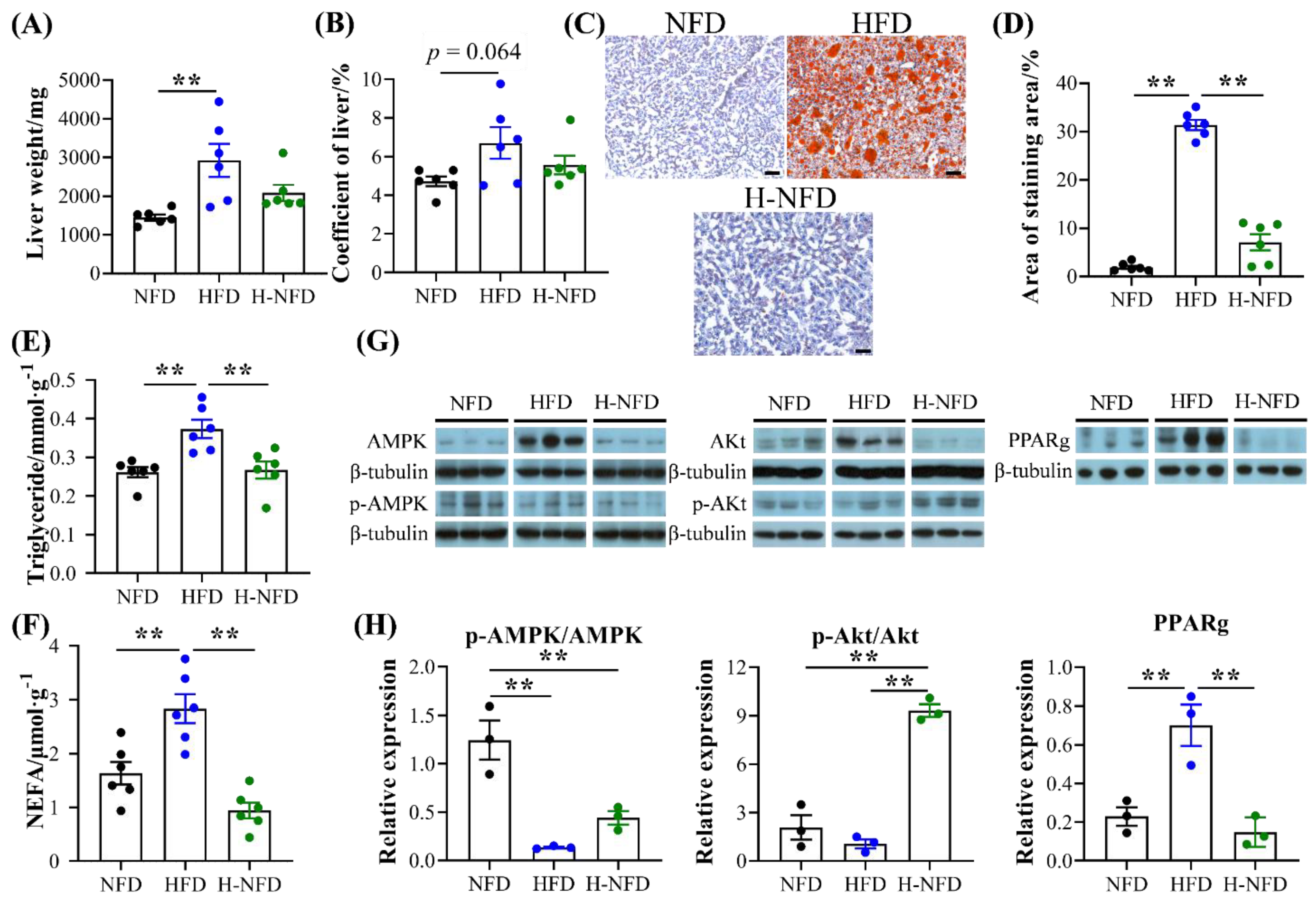
3.4. Change to a NFD Reduces Ectopic Fat Deposition in the Liver and Worsened Glucose Homeostasis
3.5. Change to a NFD Partially Reverse the Microenvironment of Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontana, L. Calorie restriction and cardiometabolic health. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bastías-Pérez, M.; Serra, D.; Herrero, L. Dietary options for rodents in the study of obesity. Nutrients 2020, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, F.; Giacco, A.; Petito, G.; De Matteis, R.; Senese, R.; Lombardi, A.; De Lange, P.; Moreno, M.; Goglia, F.; Lanni, A.; et al. Altered mitochondrial quality control in rats with metabolic dysfunction-associated fatty liver disease (MAFLD) induced by high-fat feeding. Genes 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.T.; Huang, Y.W.; Hou, C.Y.; Wang, J.J.; Wu, C.C.; Hsieh, S.L. D-Limonene promotes anti-obesity in 3T3-L1 adipocytes and high-calorie diet-induced obese rats by activating the ampk signaling pathway. Nutrients 2023, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bak, J.; Go, Y.; Park, J.; Park, M.; Lee, H.J.; Kim, H. Scytosiphon lomentaria extract ameliorates obesity and modulates gut microbiota in high-fat-diet-fed mice. Nutrients 2023, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.X.; Flay, K.J.; Huang, X.N.; Hu, X.S.; Chen, F.; Li, C.B.; Yang, D.A. Polysaccharides from Platycodon grandiflorus attenuates high-fat diet induced obesity in mice through targeting gut microbiota. Biomed. Pharmacother. 2023, 166, 15. [Google Scholar] [CrossRef]
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and ageing: Two sides of the same coin. Obes. Rev. 2020, 21, 21. [Google Scholar] [CrossRef]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.C.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of weight loss among named diet programs in overweight and obese adults a meta-analysis. JAMA-J. Am. Med. Assoc. 2014, 312, 923–933. [Google Scholar] [CrossRef]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef]
- Vatarescu, M.; Bechor, S.; Haim, Y.; Pecht, T.; Tarnovscki, T.; Slutsky, N.; Nov, O.; Shapiro, H.; Shemesh, A.; Porgador, A.; et al. Adipose tissue supports normalization of macrophage and liver lipid handling in obesity reversal. J. Endocrinol. 2017, 233, 293–305. [Google Scholar] [CrossRef]
- Makris, A.; Foster, G.D. Dietary approaches to the treatment of obesity. Psychiatr. Clin. N. Am. 2011, 34, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Barnosky, A.R.; Hoddy, K.K.; Unterman, T.G.; Varady, K.A. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: A review of human findings. Transl. Res. 2014, 164, 302–311. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Dyck, J.R. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta 2011, 1812, 1477–1489. [Google Scholar] [CrossRef]
- Stern, J.S.; Gades, M.D.; Wheeldon, C.M.; Borchers, A.T. Calorie restriction in obesity: Prevention of kidney disease in rodents. J. Nutr. 2001, 131, 913S–917S. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.J.; de Cabo, R.; Lakatta, E.G.; Talan, M.I. Effects of calorie restriction on cardioprotection and cardiovascular health. J. Mol. Cell. Cardiol. 2011, 51, 263–271. [Google Scholar] [CrossRef]
- Qin, W.; Zhao, W.; Ho, L.; Wang, J.; Walsh, K.; Gandy, S.; Pasinetti, G.M. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann. N. Y. Acad. Sci. 2008, 1147, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, O.; Youm, Y.; Shchukina, I.; Ryu, S.; Sidorov, S.; Ravussin, A.; Nguyen, K.; Aladyeva, E.; Predeus, A.N.; Smith, S.R.; et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 2022, 375, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Dixit, S.; Saulsberry, A.C.; May, J.M.; Harrison, F.E. Reversal of high fat diet-induced obesity improves glucose tolerance, inflammatory response, beta-amyloid accumulation and cognitive decline in the APP/PSEN1 mouse model of Alzheimer’s disease. Neurobiol. Dis. 2017, 100, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sobesky, J.L.; Barrientos, R.M.; De May, H.S.; Thompson, B.M.; Weber, M.D.; Watkins, L.R.; Maier, S.F. High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1 beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav. Immun. 2014, 42, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005, 126, 913–922. [Google Scholar] [CrossRef]
- Jolly, C.A. Dietary restriction and immune function. J. Nutr. 2004, 134, 1853–1856. [Google Scholar] [CrossRef]
- Clifton, P.M. Dietary treatment for obesity. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 672–681. [Google Scholar] [CrossRef]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Schlienger, J.L.; Colette, C.; Bonnet, F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2021, 47, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.L.; Wang, H.; Yang, Y.; Zhang, Y.L.; Lai, H.J.; Cheng, Y.L.; Yu, H.M.; Feng, N.; Huang, R.; Liu, S.; et al. High-protein diet prevents fat mass increase after dieting by counteracting Lactobacillus-enhanced lipid absorption. Nat. Metab. 2022, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Tav, S.I.; Rothschild, D.; Eijer, M.T.M.; Levy, M.; Moresi, C.; Dohnalova, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Sanz, C.; Gautier, J.F.; Hanaire, H. Physical exercise for the prevention and treatment of type 2 diabetes. Diabetes Metab. 2010, 36, 346–351. [Google Scholar] [CrossRef]
- Hensrud, D.D. Dietary treatment and long-term weight loss and maintenance in type 2 diabetes. Obes. Res. 2001, 9 (Suppl. S4), 348S–353S. [Google Scholar] [CrossRef] [PubMed]
- Barger, J.L. An adipocentric perspective of resveratrol as a calorie restriction mimetic. Ann. N. Y. Acad. Sci. 2013, 1290, 122–129. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. GTTs and ITTs in mice: Simple tests, complex answers. Nat. Metab. 2021, 3, 883–886. [Google Scholar] [CrossRef]
- Bargut, T.C.; Silva-e-Silva, A.C.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.U.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Hinder, L.M.; O’Brien, P.D.; Hayes, J.M.; Backus, C.; Solway, A.P.; Sims-Robinson, C.; Feldman, E.L. Dietary reversal of neuropathy in a murine model of prediabetes and metabolic syndrome. Dis. Model. Mech. 2017, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta-Mol. Basis Dis. 2014, 1842, 358–369. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 18, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 41. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Despres, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Beller, M.; Bulankina, A.V.; Hsiao, H.H.; Urlaub, H.; Jackle, H.; Kuhnlein, R.P. Perilipin-dependent control of lipid droplet structure and fat storage in drosophila. Cell Metab. 2010, 12, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human brown adipocyte thermogenesis is driven by beta 2-ar stimulation. Cell Metab. 2020, 32, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, H.; Perfield, J.W.; Obin, M.S.; Greenberg, A.S. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J. Cell. Biochem. 2008, 10, 1430–1436. [Google Scholar] [CrossRef]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.L.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Toh, S.Y.; Chen, Z.M.; Guo, K.; Ng, C.P.; Ponniah, S.; Lin, S.C.; Hong, W.J.; Li, P. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 2003, 35, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Z.; Zhou, L.K.; Chen, C.; Gong, J.Y.; Xu, L.; Ye, J.; Li, D.; Li, P. Cidea controls lipid droplet fusion and lipid storage in brown and white adipose tissue. Sci. China Life Sci. 2014, 57, 107–116. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.G.; Jin, S.K. Autophagy in adipose tissue biology. Pharmacol. Res. 2012, 66, 505–512. [Google Scholar] [CrossRef]
- Stienstra, R.; Haim, Y.; Riahi, Y.; Netea, M.; Rudich, A.; Leibowitz, G. Autophagy in adipose tissue and the beta cell: Implications for obesity and diabetes. Diabetologia 2014, 57, 1505–1516. [Google Scholar] [CrossRef]
- Marcelin, G.; Cunha, C.; Gamblin, C.; Suffee, N.; Rouault, C.; Leclerc, A.; Lacombe, A.; Sokolovska, N.; Gautier, E.L.; Clement, K.; et al. Autophagy inhibition blunts PDGFRA adipose progenitors’ cell-autonomous fibrogenic response to high-fat diet. Autophagy 2020, 16, 2156–2166. [Google Scholar] [CrossRef]
- Haim, Y.; Bluher, M.; Slutsky, N.; Goldstein, N.; Kloting, N.; Harman-Boehm, I.; Kirshtein, B.; Ginsberg, D.; Gericke, M.; Jurado, E.G.; et al. Elevated autophagy gene expression in adipose tissue of obese humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy 2015, 11, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Maixner, N.; Bechor, S.; Vershinin, Z.; Pecht, T.; Goldstein, N.; Haim, Y.; Rudich, A. Transcriptional dysregulation of adipose tissue autophagy in obesity. Physiology 2016, 31, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Ghandriz, R.; Lerman, L.O. Renal cellular autophagy in obesity: Boon or bane? Semin. Nephrol. 2021, 41, 349–357. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Li, W.H.; Chen, H.; Du, L.; Liu, D.; Wang, X.H.; Xu, T.; Liu, L.; Chen, Q. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy 2019, 15, 1882–1898. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, M.; Funai, K.; Boudina, S. Autophagy in adipose tissue physiology and pathophysiology. Antioxid. Redox Signal. 2019, 31, 487–501. [Google Scholar] [CrossRef]
- Nunez, C.E.; Rodrigues, V.S.; Gomes, F.S.; De Moura, R.F.; Victorio, S.C.; Bombassaro, B.; Chaim, E.A.; Pareja, J.C.; Geloneze, B.; Velloso, L.A.; et al. Defective regulation of adipose tissue autophagy in obesity. Int. J. Obes. 2013, 37, 1473–1480. [Google Scholar] [CrossRef]
- Nahmgoong, H.; Jeon, Y.G.; Park, E.S.; Ha Choi, Y.; Han, S.M.; Park, J.; Ji, Y.; Sohn, J.H.; Han, J.S.; Kim, Y.Y.; et al. Distinct properties of adipose stem cell subpopulations determine fat depot-specific characteristics. Cell Metab. 2022, 34, 458–472. [Google Scholar] [CrossRef]
- Stefkovich, M.; Traynor, S.; Cheng, L.; Merrick, D.; Seale, P. Dpp4+ interstitial progenitor cells contribute to basal and high fat diet-induced adipogenesis. Mol. Metab. 2021, 54, 11. [Google Scholar] [CrossRef]

| Diet | Company | Catalog | Cal Density (kcal/g) | Fat (%) | Protein (%) | Carbohydrate (%) | |
|---|---|---|---|---|---|---|---|
| NFD | Research Diets | AIN-93G | 3.72 | 11 | 34 | 55 | |
| soybean oil/2.8% | |||||||
| HFD | Research Diets | D12492 | 5.24 | 60 | 20 | 20 | |
| soybean oil/3.23% | Lard/31.66% | ||||||
| Gene Name | Forward (5′- > 3′) | Reverse (5′- > 3′) |
|---|---|---|
| ACC | GATGAACCATCTCCGTTGGC | CCCAATTATGAATCGGGAGTGC |
| Adrb3 | GGCCCTCTCTAGTTCCCAG | TAGCCATCAAACCTGTTGAGC |
| Atgl | CTGAGAATCACCATTCCCACATC | CACAGCATGTAAGGGGGAGA |
| C/EBPa | CAAGAACAGCAACGAGTACCG | GTCACTGGTCAACTCCAGCAC |
| Cidea | TGCTCTTCTGTATCGCCCAGT | GCCGTGTTAAGGAATCTGCTG |
| CPT1a | CACTGCAGCTCGCACATTAC | CCAGCACAAAGTTGCAGGAC |
| Dgat2 | GCGCTACTTCCGAGACTACTT | GGGCCTTATGCCAGGAAACT |
| GAPDH | TGTGTCCGTCGTGGATCTGA | CCTGCTTCACCACCTTCTTGA |
| Hsl | TCCTCAGAGACCTCCGACTG | ACACACTCCTGCGCATAGAC |
| Plin1 | CAAGCACCTCTGACAAGGTTC | GTTGGCGGCATATTCTGCTG |
| PPARg | GGAAGACCACTCGCATTCCTT | TCGCACTTTGGTATTCTTGGAG |
| UCP1 | GCTTTGCCTCACTCAGGATTGG | CCAATGAACACTGCCACACCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, T.; Fang, B.; Wu, F.; Liu, Y.; Cheng, L.; Li, Y.; Wang, R.; Zhu, L. Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice. Nutrients 2023, 15, 4978. https://doi.org/10.3390/nu15234978
Ji T, Fang B, Wu F, Liu Y, Cheng L, Li Y, Wang R, Zhu L. Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice. Nutrients. 2023; 15(23):4978. https://doi.org/10.3390/nu15234978
Chicago/Turabian StyleJi, Tengteng, Bing Fang, Fang Wu, Yaqiong Liu, Le Cheng, Yixuan Li, Ran Wang, and Longjiao Zhu. 2023. "Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice" Nutrients 15, no. 23: 4978. https://doi.org/10.3390/nu15234978
APA StyleJi, T., Fang, B., Wu, F., Liu, Y., Cheng, L., Li, Y., Wang, R., & Zhu, L. (2023). Diet Change Improves Obesity and Lipid Deposition in High-Fat Diet-Induced Mice. Nutrients, 15(23), 4978. https://doi.org/10.3390/nu15234978









