Abstract
Dietary practices of masters athletes (MAs) may promote healthy ageing; however, they are poorly understood. The aims of this systematic review were to synthesise the literature on the dietary intakes of MAs and undertake comparisons between younger (35–50 years) and older (>50 years) MAs and the general population. A search was conducted across seven databases to identify relevant publications for screening and data extraction. Averages for energy intake (EI), macronutrients, and micronutrients were compared with data from the 2011–2012 Australian Health Survey (general population). Twenty-six studies (n = 2819) were included. Energy intake was higher for older (8908 kJ/d versus 7792 kJ/d) but not younger MAs (9073 kJ/d versus 8872 kJ/d) versus the general population. Younger versus older male MAs had higher energy and macronutrient intakes. Energy intake for older was comparable to younger female MAs (7819 kJ/d versus 7485 kJ/d), but older had higher protein, lower carbohydrate, and higher micronutrient intakes. Micronutrient intake was higher in MAs than the general population. Similar EIs for older MAs and younger general population may indicate potential for a higher-quality diet. Younger female MAs may restrict or misreport EI, requiring further investigation. There is a need for more comprehensive assessments of dietary intake in MAs to ascertain diet quality in relation to health.
1. Introduction
A variety of physiological changes occur with ageing, including the loss of lean tissue [1] and subsequent declines in resting metabolic rate and physical capacity [2,3]. Without an increase in physical activity, this may result in an altered energy balance due to reduced energy expenditure [4] and energy intake [3] to remain weight-stable. As a result of a lower energy ‘budget’ [5], older individuals are at greater risk of nutritional inadequacy, often exacerbated by poor dietary practices. In the 2011–2012 Australian Health Survey (AHS), Australians aged 51 years and over consumed at least 30% of their energy intake from discretionary sources [6]. Suboptimal dietary intakes, including the consumption of energy-dense, nutrient-poor foods, are a leading risk factor for noncommunicable diseases [7]. This is reflected in the rising prevalence of nutrition-related chronic diseases with increasing age, particularly after 55 years, including cardiovascular diseases, sarcopenia, osteoporosis, and diabetes [8].
Masters athletes (MAs) are a unique group of older adults whose lifestyle behaviours may reduce the risk or burden of chronic disease and/or optimise athletic performance. When compared to the general Australian population, MAs have a lower prevalence of hypertension, type 2 diabetes mellitus, hyperlipidaemia, cancers, and osteoporosis [9]. MAs are a heterogeneous group of physically active individuals, typically aged 35 years or over, or as defined by sports-specific age cut-offs. They exceed population guidelines in their levels of physical activity; additionally, some may engage in systematic training, and some may compete [5,9,10].
To date, much of the existing literature focuses on dietary intakes of younger elite athletes. Little is known about the dietary practices of MAs. Limited evidence suggests that they may consume more nutritionally complete, higher-energy diets to a greater extent than their less active peers [11,12,13,14,15]. Expending more energy through physical activity allows MAs to consume greater quantities of food to meet nutritional requirements, particularly those that increase with age, such as protein and calcium [16,17]. Investigating the dietary practices of MAs can help to inform on strategies to support their health and performance. These strategies may potentially be translated more broadly to the general population.
While dietary studies on MAs do exist, they are often limited to pre-, within-competition, or recovery nutrition strategies, which are unlikely to represent an athlete’s usual diet [12,18,19,20,21]. The extent of dietary analysis may also be limited in the number of nutrients or food groups examined [6,17,22]. Additionally, studies in MAs generally focus on the effects of physical activity and have not been designed to determine or assess nutritional adequacy. This paper has two distinct aims: (1) to synthesise the available evidence on dietary intakes of MAs and (2) to undertake comparative assessments between MAs, the general population, and dietary recommendations. The findings will provide a greater insight into the adequacy of their dietary practices, highlighting their risk profiles compared to the general population. Where applicable, MA dietary strategies may then be utilised in health promotion strategies to encourage healthy ageing in the general population.
2. Materials and Methods
The protocol for this systematic review was developed and registered on the Open Science Platform https://osf.io/9kzvx/ (accessed on 11 May 2023). The conduct of this systematic review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [23].
Studies were eligible for inclusion in the review if they addressed dietary intake in MAs. The Australian Masters Games, World Masters Games, World Masters Athletics, and Pan Pacific Games were used for MA age cut-off by sport. Criteria for exclusion were (1) animal studies, (2) non-English papers, (3) not a primary study design (poster, review article, editorial, or nutrition recommendation articles), (4) wrong age, (5) wrong population (retired/former MA if not currently fitting the description of an MA, self-defined professional/elite and Olympic athletes, or athletes with disabilities), (6) non-dietary-related (drug use/doping; nutrition habits/knowledge only), (7) not a full-text article (conference abstracts), (8) age not mentioned, (9) unable to separate by age, (10) inadequate dietary data available (alcohol, hydration status, supplements only, and dietary intervention studies without baseline intake), and (11) diets that failed to capture normal/usual intake (e.g., Ramadan, COVID-19, specific competition diets, eating disorders, rapid weight-loss diets, or studies that exclusively examine pre/post-meal recovery).
A comprehensive search strategy was developed with an academic librarian. Subject headings and keyword search terms were formed for the two domains of “masters athletes” and “dietary intake”. Keywords were combined using the Boolean operator “OR”, and domains were combined with “AND”. Due to the limited research available within the two domains, broad search terms were used to maximise the search results relevant to the population of this study. A literature search was conducted in August 2021 and updated in June 2022. The search was conducted in seven electronic databases: Medline (via Ovid), Embase (via Ovid), CINAHL (via EBSCO), Web of Science, SPORTDiscus (via EBSCO), Scopus, and AUSPORT. In addition, handsearching was carried out to identify key articles that may have been missed during the database search. An example of the search strategy conducted in MEDLINE is provided in Supplementary Material Table S1.
Endnote X9.3.1 citation management software (Thomson Reuters, Philadelphia, PA, USA) was used to download citations and abstracts of studies retrieved from the database search and remove duplicate articles. An online screening and data extraction tool, Covidence (Veritas Health Innovation, Melbourne, Australia), was used to further exclude duplicates and screen articles based on the eligibility criteria. Titles and abstracts were independently reviewed by two reviewers (SG and GS) during the first stage of screening. The full text was retrieved for all eligible articles and screened independently. Discrepancies were resolved by third reviewers (JG and WSS).
Data from eligible studies were independently extracted by two researchers (SG and GS) with discrepancies discussed following extraction and resolved. For RCTs, pre-intervention dietary data were extracted as they were representative of the usual diet. Two longitudinal studies assessed both baseline and follow-up dietary intake [24,25], but only baseline data were included in this review to reflect usual intake. Supplements were included. Energy intake data reported as kilocalories (kcal) were multiplied by 4.184 to convert to kilojoules (kJ) for consistency. Only studies with data reported in the specified units—kilojoules per day (kJ/day), grams per day (g/day), grams per kilogram of body weight per day (g/kg BW/day), percent energy (%E), and milligrams per day (mg/day)—were included in the calculation of each value.
Quality and risk of bias was assessed independently by two reviewers (SG and GS) using the Academy of Nutrition and Dietetics Evidence Analysis Manual Quality Criteria Checklist: Primary Research ratings [26]. Any discrepancies were discussed and resolved with third reviewers (JG and WSS).
The participant and study characteristics and dietary intakes of MAs were summarised in a tabular form. Dietary intake data were grouped into energy, the intake of macronutrients, protein (g; g/kg BW; %E), total fat (g; g/kg BW; %E), carbohydrate (g; g/kg BW; %E), and alcohol (g/day; %E), key micronutrients (mg) for athletes (calcium, magnesium, iron, zinc, and sodium), and food or beverage items/food. These five micronutrients were selected for comparison as they have been associated with chronic disease [27] and/or higher prevalence of inadequate intakes [28,29]. The results for each variable were tabulated in Microsoft Excel, according to age, younger (35–50 years) and older (>50 years), and gender (combined data (male and female), male only, female only, and unable to separate by gender). The cut-off of 50 years was deliberately chosen to match the cut-off at middle age for reported nutrients in the AHS data and the marked change in metabolic conditions that occurs around that age for men and women [8,30,31]. MAs were separated into ‘younger’ and ‘older’ age groups based on the mean age (where provided), or the mean was calculated using the age range provided. For the studies where genders were unable to be separated, the results were only included in the combined data category, which included both males and females. Where a range was reported, the mean was taken. The mean for each variable was calculated and the count depended on the number of studies reporting the variable. For example, energy intake of younger MAs (combined data) included eight studies to calculate the mean, while five studies were used to calculate %E for fat.
MA dietary intake data were compared with the latest 2011–2012 Australian population dietary data from adults aged 31–50 years and over 50 years [6]. Data were averaged across the 51–70 years and 71 years and over age groups to yield a single value for comparison. The percentage difference was calculated for the categories of combined data, males only, and females only using the formula % difference = 100 × (value A − value B)/(value A + value B)/2, where A is the MA data. An arbitrary 10% difference cut-off was chosen to highlight differences between MAs and the general population from the 2011–2012 AHS for discussion.
3. Results
3.1. Study Selection
The initial search identified a total of 10,693 articles. Following title and abstract screening and exclusion at the full-text stage, 19 articles remained for inclusion. Handsearching provided an additional six articles. The search was repeated in June 2022 and identified a total of 11,692 articles. Duplicates were removed using automation tools and manually identified during title and abstract screening. After applying the exclusion criteria, three articles remained. In addition to the studies identified from the initial search, a total of 26 articles met the inclusion criteria. This process is summarised in Figure 1.
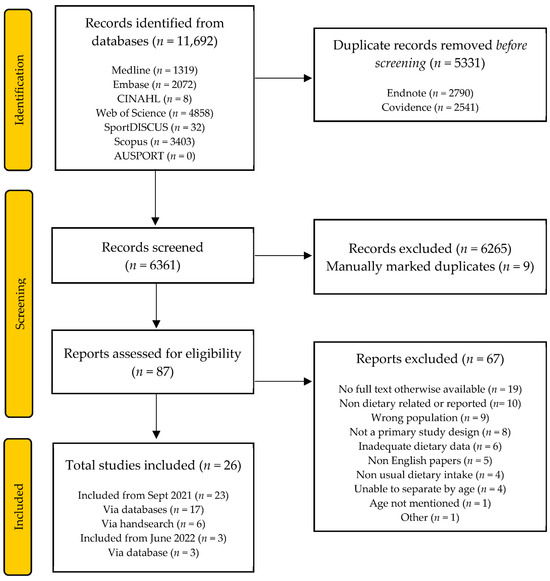
Figure 1.
PRISMA flow diagram of record identification and study selection for a systematic review of the dietary intake of masters athletes.
3.2. Participants and Study Characteristics
Participant and study characteristics of the 26 included studies [2,11,12,13,14,15,24,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50], including quality ratings, are summarised in Table 1. Of these, seventeen were cross-sectional [2,11,12,13,14,15,32,33,34,37,38,42,43,44,46,47,48], three were RCTs [35,40,41], two longitudinal [24,45], two case studies [39,50], one was a validation study [36], and one a before-and-after study [49]. Three studies were published in the 1980s [11,14,37], two in the 1990s [12,13], five between 2000 and 2010 [2,15,24,32,45], and the remaining published after 2010 [33,34,35,36,38,39,40,41,42,43,44,46,47,48,49]. A total of 2819 participants were included. Twelve studies were male-only [2,12,13,15,37,38,39,40,41,43,45,49], four studies female-only [24,32,35,50], and the remaining nine included both males and females [11,14,33,34,36,42,44,46,47,48]. Eleven studies were conducted across European countries: Italy [33,34,45], the United Kingdom [39,40,41], France [12], the Netherlands [46], Poland [43], Finland [15], and Denmark [38]; eleven studies were conducted in the USA [2,11,13,14,24,32,35,37,47,49,50], two in Canada [36,44], one in South Africa [42]; one included participants from 21 countries [48]. Nineteen studies were on competitive athletes [2,13,15,24,32,33,34,35,36,37,38,39,40,42,43,45,47,49,51], two on recreational athletes [41,44], and five did not report participation level [11,12,14,46,50]. Dietary assessment methods included food records (n = 16) [2,11,12,13,14,15,24,32,33,35,39,40,41,42,45,47], food frequency questionnaires (FFQs) (n = 8) [24,36,37,38,43,44,46,49], and questionnaires on dietary behaviours [48] and diet history [34].

Table 1.
Participant and study characteristics of the included studies.
Results of baseline energy, nutrient, food and beverage items, or food groups are presented in Table 2. Seventeen studies reported energy in kJ/day [2,11,12,13,14,15,32,33,34,35,42,44,45,46,47,49,50]. The range of energy intake in kJ/day was larger for male athletes (range = 6686–14,535 kJ/day) [2,11,12,13,14,15,42,44,45,47,49] compared to female athletes (range = 5073–9983 kJ/day) [11,14,32,35,42,44,47,50]. Eighteen studies reported protein intake in g/day, %E, or g/kg/BW [2,11,12,13,14,15,32,34,35,39,40,41,42,44,45,47,49,50]. Protein intake for males ranged from 57 to 131 g/day [11,12,13,14,44,45,47,49], 13 to 18%E [2,11,13,14,15,39,45,49], and 1.0 to 2.0 g/kg/BW [2,13,14,15,39,40,41,42,47,49]. Protein intake for females ranged from 74 to 104 g/day [11,14,32,35,44,47], 14 to 20%E [11,14,32], and 1.2 to 1.3 g/kg/BW [14,42,47,50]. Eighteen studies reported fat intake in g/day, %E, or g/kg/BW [2,11,12,13,14,15,32,34,35,39,40,41,42,44,45,47,49,50]. Fat intake for males ranged from 51 to 134 g/day [11,12,13,14,44,45,47,49], 22 to 41%E [2,11,13,14,15,39,42,45,49], and 1.0 to 9.0 g/kg BW [2,15,39,40,41,47,49]. Fat intake for females ranged from 61 to 111 g/day [11,14,32,35,44,47], 28 to 41%E [11,14,32,42], and 0.7 to 1.2 g/kg/BW [47,50]. Twenty studies reported carbohydrate intake in g/day, %E, and g/kg/BW [2,11,12,13,14,15,32,34,35,36,39,40,41,42,44,45,47,49,50]. Carbohydrate intake for males ranged from 221 to 350 g/day [11,12,13,14,44,45,47,49], 40 to 61 %E [2,11,13,14,15,39,45,49], and 3.0 to 5.3 g/kg BW [2,15,39,40,41,42,47,49]. Carbohydrate intake for females ranged from 183 to 292 g/day [11,14,32,35,44,47], 40 to 55%E [11,14,32], and 3.5 to 4.1 g/kg BW [42,47,50]. Eight studies reported alcohol consumption in g/day, %E, kJ/day, % consumers, mL, glasses, bottles, or drinks [2,11,13,37,38,44,46,48]. Seven studies reported micronutrients in mg/day [12,14,24,32,40,44,47], including calcium [12,14,24,32,44,47], magnesium [12,14,32], iron [12,14,32,40,44], zinc [14,32,44], and sodium [14,32,44]. Eight studies reported food or beverage items or food groups [14,24,37,38,43,44,46,48].

Table 2.
Energy, nutrient, food or food group intake of masters athletes.
Table 3 shows a comparison between the younger and older cohorts for combined, males, and females, with Figure 2 and Figure 3 showing a comparison of energy and macronutrient intake in these cohorts, respectively. Younger male MAs had a higher overall energy intake compared to older male MAs, with that for females and combined younger and older MA groups being equivalent. Apart from alcohol, the greatest %E differences were for fat and protein in females, with younger MAs having a lower %E protein and greater %E fat. The amounts of macronutrients per kg were very similar across groups except for fat, which was greater in older males, pushing up the combined intake for older MAs. Intake of micronutrients was lower for younger MAs (combined) for magnesium, zinc, and iron and for younger female MAs for all micronutrients except sodium. Calcium intake was higher for younger compared with older male MAs, and the opposite was true for females.

Table 3.
Comparative analysis of younger masters athletes against older masters athletes.
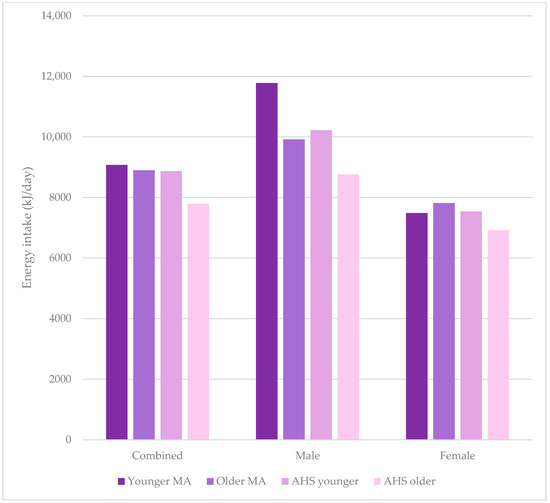
Figure 2.
Energy intake of masters athletes compared to 2011–2012 Australian Health Survey data for all people, males, and females, comparing younger masters athletes, older masters athletes, younger people in the Australian Health Survey, and older people in the Australian Health Survey.
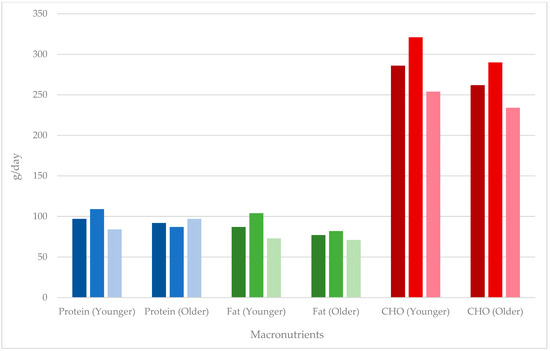
Figure 3.
Macronutrient intake of masters athletes. Data presented as combined (males and females), males, and females from left to right (darkest to lightest) for each macronutrient.
Table 4 and Table 5 show the combined averages of nutrients for MAs 35–50 years and >50 years, respectively, with comparison to data from the AHS and percentage differences between the cohorts. There was a greater difference in energy intake for older MAs and younger male MAs compared to the Australian population data. The data indicate that carbohydrate intake is higher for MAs, with carbohydrate g/day and %E being comparatively higher, particularly for older MAs. Calcium, iron, zinc, and sodium intakes were higher in MAs (both younger and older) compared with the population data. Differences were not noted for magnesium in comparisons to population data with younger MAs (combined, male, and female) or older male MAs.

Table 4.
Comparative analysis of masters athletes aged 35 to 50 years against the Australian population data.

Table 5.
Comparative analysis of masters athletes aged > 50 years against the Australian population data.
4. Discussion
To date, research investigating the usual dietary practices of MAs is limited. Nutrition adequacy in relation to health and sporting requirements is uncertain and complicated by the metabolic challenges that may accompany ageing, the presence of chronic disease and/or risk factors, changing physiological systems, increasing prevalence of supplement use with age, and the need for sport-specific information regarding training load and/or energy expenditure. This is the first systematic review to synthesise the available data and provide a summary of the nutritional intakes of MAs. The findings provide a basis for comparison between MAs and the general population to better understand nutritional adequacy. Higher energy intakes in MAs were generally reported in comparison to the Australian national average intakes, as well as higher micronutrient intakes, and they were more likely to meet national dietary guidelines.
Included studies highlight the heterogeneity of MAs (Table 1). Analysis of MA data across sports showed a wide variation in energy intake, ranging from 5073 kJ/day (one female triathlete) [50] to 14,535 kJ/day (average of nine male triathletes) [42]. While individual (e.g., gender and age), sport (including the primary energy system), and contextual (e.g., goals for adaptation and/or body composition and individual training load) factors [53] should be taken into consideration in assessing adequacy, it is possible that some MAs are not consuming enough to meet their health and sporting requirements, compromising performance, recovery, and desired physiological adaptations.
On the other hand, self-reported dietary intake data have known limitations. Indeed, in the current study, younger female MAs reported a similar energy intake to younger females in the AHS and older female MAs (Figure 2, Table 3 and Table 4), where they would be expected to consume more than both of these groups due to their activity and age. This pattern was not observed for males (Figure 2, Table 3 and Table 4), who followed the expected order. The differences between younger MAs and older MAs, younger MAs versus AHS, and older MAs versus AHS was 17%, 14%, and 12%, respectively. In fact, the energy requirement of women aged 30–60 years with active or moderately active lifestyles is suggested to be >8.9 MJ (≥60 kg body weight) [54], whereas younger MAs had an average of 7485 kJ/day. This is suggestive of underconsumption or underreporting of energy intake. While the systematic review of McKenzie et al. (2021) [55] on sex differences in the accuracy of energy intake assessment (versus doubly labelled water) showed that underreporting across dietary assessment methods was similar in males and females; there have been few studies comparing the reporting of male and female athletes specifically. No known studies investigate the accuracy of reporting of MAs. Alternatively, underconsumption may be part of a state of low energy availability (LEA), where energy intake minus exercise energy expenditure and energy intake (per kg of fat free mass) is suboptimal for health [56]. In this case, the lower energy intake may support exercise (although perhaps not at peak) through “compensatory energetic savings in other processes” but results in maladaptive physiological responses [57]. Logue et al. (2019) [58] reported that 17% of women aged 35–44 across a range of sporting levels in endurance activities were at risk of LEA, supporting this possibility in the younger MAs in the current study.
As expected, younger male MAs reported the highest protein intakes (109 g/day) given their higher energy intake. Older female MAs were found to have higher absolute intakes of protein compared to their younger counterparts (97 g/day vs. 84 g/day) (14% difference; Table 3), suggesting that the larger energy intake reported by older female MAs may have facilitated a higher intake of protein. This finding is reassuring because older people require more protein for the maintenance of muscle mass, good health, and functionality [16]. Additionally, for women, having more skeletal muscle mass may also provide some protection against osteoporosis [59]. Turning to athletic requirements, protein and carbohydrates are generally prescribed on a g/kg basis rather than on percentage of energy, and this may fluctuate daily depending on activity. Protein requirements for athletes are likely to be met with 1.2–2.0 g/kg per day, and requirements for older (nonathletic) individuals are suggested to be ≥1.2 g/kg/day [16]. In the current review, protein intakes relative to body weight in MAs were reported in 12 out of 26 studies and ranged from 1.0 g/kg/day [42] to 2.0 g/kg/day [15], indicating that athletic needs are likely to have been met. However, it has additionally been proposed that active older individuals consume protein doses of at least 30 g (even up to 35–40 g [60]), approximately 3–4 h apart [3] throughout the day and, in particular, following muscle-damaging exercise [60]. Relative to body weight, this would equate to ~1.7 g/kg of body mass (for a 70 kg athlete) [3], higher than most values reported for MAs in the current study. This may indicate that some MAs may need to prioritise greater protein intakes in order to stimulate muscle protein synthesis and minimise lean tissue loss. More targeted guidelines for protein intake in MAs are warranted.
Considering the higher energy intakes in MAs compared to the general population, it was expected that macronutrient intakes in MAs would also reflect this. This was seen with respect to protein, where younger MAs and AHS data showed small percentage differences (5% or less) in absolute intakes. However, greater differences (12–18%) were observed when intakes were expressed as a percentage of total energy intake (Table 4). These findings imply higher total energy intakes for MAs and, consequently, a lower percentage of their intake coming from protein. Protein intakes observed in the general population that appear to match those of younger MAs may be attributed to Australia’s meat-eating culture encouraging greater consumption [61,62], resulting in only minor percentage differences when compared to MAs (Table 4). In the comparisons of MAs and AHS data, the largest percentage difference in absolute intakes (26%) was observed between older females (Table 5).
Carbohydrates are essential to optimise athletic performance. Depleted carbohydrate stores can hinder performance, so it would be desirable for MAs to achieve the recommended daily fuelling requirements. For younger athletes, this ranges between 3 to 5 g/kg/day and 8 to 12 g/kg/day depending on the activities undertaken [53]. In this review, ten studies [2,15,36,39,40,41,42,47,49,50] reported a g/kg amount, and eight [36,39,40,41,42,47,49,50] reported the type of sport, with all of these being endurance activities. For these activities, recommendations would be 5–10 g/kg/day for days with moderate- to high-intensity activity. In most studies, intakes in MAs fell short of these recommendations, with the highest relative intake reported being 5.4 g/kg BW/day for younger male triathlete MAs [36]. Despite this, similarities between younger MAs and older MAs were noted with percentage differences of 10% (or less) (Table 3). Larger differences were noted for male MAs for protein and fat, contributing to the difference in energy intake between these groups (Table 3). Table 4 and Table 5 highlight greater absolute carbohydrate intakes between both younger MAs and older MAs in comparison to age-matched AHS data (22% and 26% differences, respectively), which may result from the larger energy budgets available in MAs.
As seen in Table 3, larger percentage differences were noted in fat intakes between younger MAs and older MAs when expressed relative to body weight; however, the findings are skewed by a single study suggesting intakes of 9.0 g/kg BW/day [2] and, likely, a misreported value. In comparisons to the general population, MAs reported higher intake of fat (Table 4 and Table 5). The largest percentage differences were observed with absolute fat intakes when comparing younger male MAs and older female MAs to age-matched AHS data (19% and 18%, respectively).
Even with greater absolute intakes of carbohydrates and fats demonstrated in MAs, consideration of type and quality is important in the context of chronic disease development. Diets with a lower glycaemic load have demonstrated improved glycaemic control [63], reducing the risk of impaired glucose tolerance or the development of insulin resistance, both of which are major risk factors for type 2 diabetes. Such diets are typically characterised by higher intakes of fruits and vegetables, corresponding with increased total dietary fibre, and these positive dietary patterns are evident in MAs [36,46,48]. Similarly, the types of fat consumed differ in their metabolic impacts. Saturated and trans-fatty acids can contribute to unfavourable blood lipid profiles, increasing the risk of coronary heart disease (CHD), while unsaturated fatty acids aid in lowering cholesterol and contribute to a reduced risk of CHD [17]. One study reported a lower consumption of high saturated-fat foods compared to inactive controls in younger MAs [37]. It is also possible that higher fat intakes in MAs may reflect the popularity of low-carbohydrate, high-fat diets claimed to induce ‘glycogen-sparing’ to enhance performance [64]. It should be noted, though, that these diets have been shown to impair carbohydrate utilisation and the ability to sustain high-intensity performance [65]. The studies within this review do not report on the specific types consumed or dietary patterns, therefore precluding such conclusions from being drawn.
As energy needs often reduce with ageing [3], greater intakes provide MAs with a larger ‘budget’ with which to achieve nutritional adequacy. Studies show that MAs may adopt healthier lifestyles and have more nutritionally complete, higher-energy diets compared to their sedentary counterparts [11,12,13,14,15]. This may further contribute to a reduced risk of lean tissue loss, sarcopenia, and functional decline in combination with the benefits of ongoing exercise [3,10]. This is in contrast to the prevalence of poor dietary habits observed in older Australians, with adults aged 51 years and older found to consume at least 30% of energy intake from discretionary sources [6].
Eight [12,14,24,32,40,44,47,50] out of 26 studies included the five key micronutrients selected for comparison in the data analysis, however, were not comprehensively reported across these. The interpretation of the findings is therefore unlikely to be representative across all MAs or of the usual dietary intakes. It is interesting to note that some of the largest percentage differences in the present review were observed in the micronutrient comparisons, with a difference of 125% between older female MAs’ iron intakes and those of their general population counterparts (Table 4). This may be explained by the known skewed distribution of iron intakes [66] and supplement use among MAs [32,67]; however, the use of supplementation to achieve recommended intakes should be cautioned against. Female runners included in a similar study [24] showed higher intakes of calcium from supplementation compared to dietary calcium, suggesting that diet alone was inadequate. The importance of dietary calcium is reflected in the changing nutrient recommendations across life stages to protect and maintain bone health. There is also some emerging evidence to suggest an association between calcium supplementation and an increased risk of cardiovascular disease [68]. In the present review, sodium was only reported in three studies [14,32,44], where MAs demonstrated higher intakes compared to the general population. Reducing sodium for risk-factor management is important with ageing, with the prevalence of hypertension reported to be three times higher among Australians aged 45–54 years than 35–44 years [25]. While hypertension prevalence is lower among MAs [9], intakes above the suggested dietary target (SDT) (2000 mg/day) may have negative impacts on the future risk or current management of the condition. Higher intakes may relate to rehydration and electrolyte replacement strategies [53]; however, this cannot be confirmed given the lack of detail on supplementation or exercise-specific nutrition strategies.
The strengths of this review include the systematic nature of the search and the extensiveness of the data extraction. The majority of the literature was published within recent years, reflecting contemporary consumption patterns [69]. Additionally, both genders were represented, providing a more inclusive dietary assessment. However, this review has a few limitations. Differences between MAs and AHS data may have been influenced by differences in the dietary methodologies used. The majority (16 out of 26) of MA studies used FR and some used FFQ (8 out of 26 studies), while the AHS data were collected with interviewer-administered 24 h recalls [6]. However, in a systematic review of the validity of dietary assessment methods compared to doubly labelled water, the majority of studies reported significant underreporting, and FR, FFQ, and 24 h recall produced underestimations to a similar degree (11–41%, 5–42%, and 8–30%, respectively) [70]. Additionally, there was inconsistency in the data reported across the studies. For example, variation in units of measurement provided some challenges in analysis; however, the calculation of combined average intake values enabled some degree of comparison. An average value was also calculated for Australian population data across the 51–70 and 71 and over age groups to allow for comparisons with older MAs because studies did not separate MA results by age and age-matched comparisons were not possible. For MA data, the calculation of male- or female-only results excluded data from mixed-cohort studies, where data for each gender could not be separated out, which reduced their pool size. Endurance sports were disproportionately represented among the studies in this review, and comparisons between competitive and noncompetitive MAs could not be undertaken as many studies failed to specify the level of competition for their athletes. As masters-level competitions often do not have qualification requirements, ‘competitive’ MAs may not be substantially different from recreational MAs, and they may be similarly unaware of sport-specific dietary practices.
5. Conclusions
The available data indicate that, for the most part, MAs appear to have higher absolute intakes of energy, macronutrients, and included micronutrients compared to the general Australian population. They also compared more favourably, with the exception of sodium, with the Australian dietary recommendations for key nutrients measured. In conjunction with exercise, MA dietary practices may attenuate age-associated physiological declines, with potential improvements in the chronic disease risk profile. More comprehensive assessments of dietary intake to ascertain diet quality in relation to health, with larger sample sizes and addressing various sporting disciplines (endurance-based, power-based, and mixed sports and reported as such) would be beneficial. Studies should adopt a longitudinal approach across all phases of competition and training to account for dietary variations, with further exploration into the rationale behind their dietary practices. These insights will inform future research, sports nutrition guidelines for competitive MAs, and nutrition strategies for healthy ageing among the general population.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu15234973/s1: Table S1: Sample search strategy conducted in MEDLINE.
Author Contributions
Conceptualisation and oversight, J.A.G. and W.A.S.-S.; methodology and initial interpretation, J.A.G., W.A.S.-S., S.G. and G.L.L.S.; initial preparation of results and draft manuscript, S.G. and G.L.L.S.; revision of results following update of search A.J.D.; overall results, interpretation, review, and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank Yulia Ulyannikova (Academic Liaison Librarian, Faculty of Medicine and Health, the University of Sydney) for her assistance in developing the search strategy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanaka, H.; Tarumi, T.; Rittweger, J. Aging and Physiological Lessons from Master Athletes. Compr. Physiol. 2020, 10, 261–296. [Google Scholar] [CrossRef]
- Van Pelt, R.E.; Dinneno, F.A.; Seals, D.R.; Jones, P.P. Age-related decline in RMR in physically active men: Relation to exercise volume and energy intake. Am. J. Physiol. Endocrinol. Metab. 2001, 44, E633–E639. [Google Scholar] [CrossRef]
- Louis, J.; Vercruyssen, F.; Dupuy, O.; Bernard, T. Nutrition for master athletes: Is there a need for specific recommendations? J. Aging Phys. Act. 2019, 28, 489–498. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Insufficient Physical Activity; Australian Institute of Health and Welfare: Paddington, NSW, Australia, 2020.
- Gifford, J.; O’Connor, H. Masters athletes. In Nutrition for Sport, Exercise and Performance: A Practical Guide for Students, Sports Enthusiasts and Professionals; Belski, R., Forsyth, A., Mantzioris, E., Eds.; Allen & Unwin: Crows Nest, NSW, Australia, 2019. [Google Scholar]
- Australian Bureau of Statistics. Australian Health Survey: Nutrition First Results—Foods and Nutrients, 2011–2012. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-nutrition-first-results-foods-and-nutrients/latest-release (accessed on 10 July 2022).
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. National Health Survey: First Results, 2017–2018—Australia. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/chronic-conditions/latest-release (accessed on 2 November 2021).
- Black, D.; Climstein, M.; Gifford, J.; Halar, F.; O’Connor, H.; Prvan, T.; Reaburn, P.; Stuart-Smith, W. Prevalence of chronic conditions in masters games athletes. J. Sci. Med. Sport 2021, 24, S5–S6. [Google Scholar] [CrossRef]
- Geard, D.; Reaburn, P.R.J.; Rebar, A.L.; Dionigi, R.A. Masters Athletes: Exemplars of Successful Aging? J. Aging Phys. Act. 2017, 25, 490–500. [Google Scholar] [CrossRef]
- Blair, S.N.; Ellsworth, N.M.; Haskell, W.L.; Stern, M.P.; Farquhar, J.W.; Wood, P.D. Comparison of nutrient intake in middle-aged men and women runners and controls. Med. Sci. Sports Exerc. 1981, 13, 310–315. [Google Scholar] [CrossRef]
- Chatard, J.C.; Boutet, C.; Tourny, C.; Garcia, S.; Berthouze, S.; Guezennec, C.Y. Nutritional status and physical fitness of elderly sportsmen. Eur. J. Appl. Physiol. 1998, 77, 157–163. [Google Scholar] [CrossRef]
- Hallfrisch, J.; Drinkwater, D.T.; Muller, D.C.; Fleg, J.; Janette; Busby-Whitehead, M.; Andres, R.; Goldberg, A. Physical conditioning status and diet intake in active and sedentary older men. Nutr. Res. 1994, 14, 817–827. [Google Scholar] [CrossRef]
- Nieman, D.C.; Butler, J.V.; Pollett, L.M.; Dietrich, S.J.; Lutz, R.D. Nutrient intake of marathon runners. J. Am. Diet. Assoc. 1989, 89, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, J.; Ojanen, T.; Karavirta, L.; Ahtiainen, J.P.; Häkkinen, K. Muscle mass and strength, body composition and dietary intake in master strength athletes vs untrained men of different ages. J. Sports Med. Phys. Fit. 2008, 48, 190–196. [Google Scholar]
- Bauer, J.M.D.; Biolo, G.M.D.P.; Cederholm, T.M.D.P.; Cesari, M.M.D.P.; Cruz-Jentoft, A.J.M.D.; Morley, J.E.M.B.B.; Phillips, S.P.; Sieber, C.M.D.P.; Stehle, P.M.D.P.; Teta, D.M.D.P.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council; Australian Government Department of Health and Ageing; New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand. Available online: https://www.nrv.gov.au/home (accessed on 11 October 2023).
- Dixon, D.; Hegedus, C. An Analysis of the Nutritional Practices of Club Level Cyclists before and during a Cyclocross Race. Int. J. Sports Sci. 2014, 4, 7–13. [Google Scholar]
- Lavoué, C.; Siracusa, J.; Chalchat, É.; Bourrilhon, C.; Charlot, K. Analysis of food and fluid intake in elite ultra-endurance runners during a 24-h world championship. J. Int. Soc. Sports Nutr. 2020, 17, 36. [Google Scholar] [CrossRef]
- García-Rovés, P.M.; Terrados, N.; Fernández, S.; Patterson, A.M. Comparison of dietary intake and eating behavior of professional road cyclists during training and competition. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Methenitis, S.; Mouratidis, A.; Manga, K.; Chalari, E.; Feidantsis, K.; Arnaoutis, G.; Arailoudi-Alexiadou, X.; Skepastianos, P.; Hatzitolios, A.; Mourouglakis, A.; et al. The importance of protein intake in master marathon runners. Nutrition 2021, 86, 111154. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Br. Med. J.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Beshgetoor, D.; Nichols, J.F.; Rego, I. Effect of Training Mode and Calcium Intake on Bone Mineral Density in Female Master Cyclists, Runners, and Non-Athletes. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 290–301. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Hypertension and Measured High Blood Pressure. 2017–2018. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/hypertension-and-measured-high-blood-pressure/2017-18 (accessed on 10 November 2023).
- Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Available online: https://www.andeal.org/vault/2440/web/files/2016_April_EA_Manual.pdf (accessed on 10 October 2021).
- National Health and Medical Research Council. Micronutrients & Dietary Fibre. Available online: https://www.nrv.gov.au/node/41 (accessed on 14 November 2021).
- Volpe, S.L. Micronutrient Requirements for Athletes. Clin. Sports Med. 2007, 26, 119–130. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Health Survey: Usual Nutrient Intakes; Australian Bureau of Statistics: Sydney, NSW, Australia, 2015.
- Australian Bureau of Statistics. Arthritis and Osteoporosis. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/arthritis-and-osteoporosis/2017-18 (accessed on 24 November 2023).
- Australian Bureau of Statistics. Heart, Stroke and Vascular Disease. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/heart-stroke-and-vascular-disease/latest-release (accessed on 24 November 2023).
- Beshgetoor, D.; Nichols, J.F. Dietary intake and supplement use in female master cyclists and runners. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 166–172. [Google Scholar] [CrossRef]
- Condello, G.; Capranica, L.; Migliaccio, S.; Forte, R.; Di Baldassarre, A.; Pesce, C. Energy Balance and Active Lifestyle: Potential Mediators of Health and Quality of Life Perception in Aging. Nutrients 2019, 11, 2122. [Google Scholar] [CrossRef]
- Di Girolamo, F.G.; Situlin, R.; Fiotti, N.; Tence, M.; De Colle, P.; Mearelli, F.; Minetto, M.A.; Ghigo, E.; Pagani, M.; Lucini, D.; et al. A higher protein intake is associated with improved muscle strength in elite senior athletes. Nutrition 2017, 42, 82–86. [Google Scholar] [CrossRef]
- Glenn, J.M.; Gray, M.; Stewart, R.; Moyen, N.E.; Kavouras, S.A.; DiBrezzo, R.; Turner, R.; Baum, J. Incremental effects of 28 days of beta-alanine supplementation on high-intensity cycling performance and blood lactate in masters female cyclists. Amino Acids 2015, 47, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Carbonneau, É.; Talbot, D.; Lemieux, S.; Lamarche, B. Development and validation of a dietary screener for carbohydrate intake in endurance athletes. J. Int. Soc. Sports Nutr. 2018, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Hartung, G.H.; Foreyt, J.P.; Mitchell, R.E.; Vlasek, I.; Gotto, A.M. Relation of Diet to High-Density-Lipoprotein Cholesterol in Middle-Aged Marathon Runners, Joggers, and Inactive Men. N. Engl. J. Med. 1980, 302, 357–361. [Google Scholar] [CrossRef]
- Hjerrild, J.N.; Wobbe, A.; Stausholm, M.B.; Larsen, A.E.; Josefsen, C.O.; Malmgaard-Clausen, N.M.; Dela, F.; Kjaer, M.; Magnusson, S.P.; Hansen, M.; et al. Effects of Long-Term Physical Activity and Diet on Skin Glycation and Achilles Tendon Structure. Nutrients 2019, 11, 1409. [Google Scholar] [CrossRef]
- Louis, J.; Tiollier, E.; Lamb, A.; Bontemps, B.; Areta, J.; Bernard, T. Retraining and Nutritional Strategy of an Endurance Master Athlete Following Hip Arthroplasty: A Case Study. Front. Sports Act. Living 2020, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, F.; Seijo, M.; Larumbe-Zabala, E.; Ashrafi, N.; Christides, T.; Karsten, B.; Nielsen, B.V. Effects of Supplementation with Beef or Whey Protein Versus Carbohydrate in Master Triathletes. J. Am. Coll. Nutr. 2017, 36, 593–601. [Google Scholar] [CrossRef]
- Naclerio, F.; Larumbe-Zabala, E.; Seijo, M.; Ashrafi, N.; Nielsen, B.V.; Earnest, C.P. Effects of Protein Versus Carbohydrate Supplementation on Markers of Immune Response in Master Triathletes: A Randomized Controlled Trial. J. Am. Coll. Nutr. 2019, 38, 395–404. [Google Scholar] [CrossRef]
- Potgieter, S.; Labadarios, D.; Labuschagne, I. Body composition, dietary intake and supplement use among triathletes residing in the Western Cape. S. Afr. J. Sports Med. 2011, 23, 74. [Google Scholar] [CrossRef][Green Version]
- Ratajczak, J.; Czerniak, U.; Wieliński, D.; Ciekot-Sołtysiak, M.; Zieliński, J.; Gronek, P.; Demuth, A. Pro-Healthy Diet Properties and Its Determinants among Aging Masters Athletes. Int. J. Environ. Res. Public Health 2021, 18, 7614. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Bertrand, L.; Deprez, D.; Ko, J.; Zello, G.A.; Chilibeck, P.D. The impact of the COVID-19 pandemic on the diet, training habits and fitness of Masters cyclists. Nutr. Health 2021, 28, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Sullo, A.; Brizzi, G.; Meninno, V.; Mercadante, F.; Cardinale, P. Morphofunctional modification induced by a training protocol in the elderly. Acta Med. 2004, 47, 25–28. [Google Scholar]
- Van der Avoort, C.M.; Ten Haaf, D.S.M.; De Vries, J.H.M.; Verdijk, L.B.; Van Loon, L.J.C.; Eijsvogels, T.M.H.; Hopman, M.T.E. Higher levels of physical activity are associated with greater fruit and vegetable intake in older adults. J. Nutr. Health Aging 2021, 25, 230–241. [Google Scholar] [CrossRef]
- Viner, R.T.; Harris, M.; Berning, J.R.; Meyer, N.L. Energy Availability and Dietary Patterns of Adult Male and Female Competitive Cyclists with Lower Than Expected Bone Mineral Density. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Croteau, K.A.; Eduljee, N.; Murphy, L. Global Region Comparisons in Health, Lifestyle Behaviors, and Well-Being of International Master’s World Cup Field Hockey Players: 195. Med. Sci. Sports Exerc. 2021, 53, 60. [Google Scholar] [CrossRef]
- Waldman, H.S.; Heatherly, A.J.; Killen, L.G.; Hollingsworth, A.; Koh, Y.; O’Neal, E.K. A 3-Week, Low-Carbohydrate, High-Fat Diet Improves Multiple Serum Inflammatory Markers in Endurance-Trained Males. J. Strength Cond. Res. 2020, 36, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.J.; Seijo, M.; Larumbe-Zabala, E.; Ashrafi, N.; Christides, T.; Karsten, B.; Nielsen, B.V.; Naclerio, F. Case studies: Effects of beef, whey and carbohydrate supplementation in female master triathletes. J. Hum. Sport Exerc. 2019, 14, 170–184. [Google Scholar] [CrossRef]
- Croteau, K.; Eduljee, N.B.; Murphy, L. Health, lifestyle behaviours, and well-being of International Masters field hockey athletes. Int. Sports Stud. 2021, 43, 6–26. [Google Scholar] [CrossRef]
- Priego Quesada, J.I.; Kerr, Z.Y.; Bertucci, W.M.; Carpes, F.P. The categorization of amateur cyclists as research participants: Findings from an observational study. J. Sports Sci. 2018, 36, 2018–2024. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics; Dietitians of Canada; American College of Sports Medicine. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Food and Agriculture Organization of the United Nations; United Nations University. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Rome, 17–24 October 2001; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- McKenzie, B.L.; Coyle, D.H.; Santos, J.A.; Burrows, T.; Rosewarne, E.; Peters, S.A.E.; Carcel, C.; Jaacks, L.M.; Norton, R.; Collins, C.E.; et al. Investigating sex differences in the accuracy of dietary assessment methods to measure energy intake in adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 113, 1241–1255. [Google Scholar] [CrossRef] [PubMed]
- Margo, M.; Kathryn, E.A.; David, M.B.; Louise, M.B.; Naama, C.; Anthony, C.H.; Ida Aliisa, H.; Anna, M.; Anne Marte, P.; Trent, S.; et al. 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 2023, 57, 1073. [Google Scholar] [CrossRef]
- Dolan, E.; Koehler, K.; Areta, J.; Longman, D.P.; Pontzer, H. Energy constraint and compensation: Insights from endurance athletes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 285, 111500. [Google Scholar] [CrossRef]
- Logue, D.M.; Madigan, S.M.; Heinen, M.; McDonnell, S.-J.; Delahunt, E.; Corish, C.A. Screening for risk of low energy availability in athletic and recreationally active females in Ireland. Eur. J. Sport Sci. 2019, 19, 112–122. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Doering, T.M.; Reaburn, P.R.; Phillips, S.M.; Jenkins, D.G. Postexercise Dietary Protein Strategies to Maximize Skeletal Muscle Repair and Remodeling in Masters Endurance Athletes: A Review. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 168–178. [Google Scholar] [CrossRef]
- Khara, T.; Riedy, C.; Ruby, M.B. The Evolution of Urban Australian Meat-Eating Practices. Front. Sustain. Food Syst. 2021, 5, 624288. [Google Scholar] [CrossRef]
- Whitton, C.; Bogueva, D.; Marinova, D.; Phillips, C.J.C. Are We Approaching Peak Meat Consumption? Analysis of Meat Consumption from 2000 to 2019 in 35 Countries and Its Relationship to Gross Domestic Product. Animals 2021, 11, 3466. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Chang, C.-K.; Borer, K.; Lin, P.-J. Low-Carbohydrate-High-Fat Diet: Can it Help Exercise Performance? J. Hum. Kinet. 2017, 56, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Milman, N.T. Dietary Iron Intake in Women of Reproductive Age in Europe: A Review of 49 Studies from 29 Countries in the Period 1993–2015. J. Nutr. Metab. 2019, 2019, 7631306. [Google Scholar] [CrossRef]
- Harnett, J.; Climstein, M.; Walsh, J.; Gifford, J. The Use of Medications and Dietary Supplements by Masters Athletes—A Review. Curr. Nutr. Rep. 2022, 11, 253–262. [Google Scholar] [CrossRef]
- Myung, S.-K.; Kim, H.-B.; Lee, Y.-J.; Choi, Y.-J.; Oh, S.-W. Calcium Supplements and Risk of Cardiovascular Disease: A Meta-Analysis of Clinical Trials. Nutrients 2021, 13, 368. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, H. Food demand in Australia: Trends and issues 2018. In Australian Bureau of Agricultural and Resource Economics and Sciences; Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2018. [Google Scholar]
- Burrows, T.L.; Ho, Y.Y.; Rollo, M.E.; Collins, C.E. Validity of Dietary Assessment Methods When Compared to the Method of Doubly Labeled Water: A Systematic Review in Adults. Front. Endocrinol. 2019, 10, 850. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).