Reversal of Conditioned Food Aversion Using a Cognitive Intervention: A Sham-Controlled, Randomized, Parallel Study
Abstract
1. Introduction
2. Material and Methods
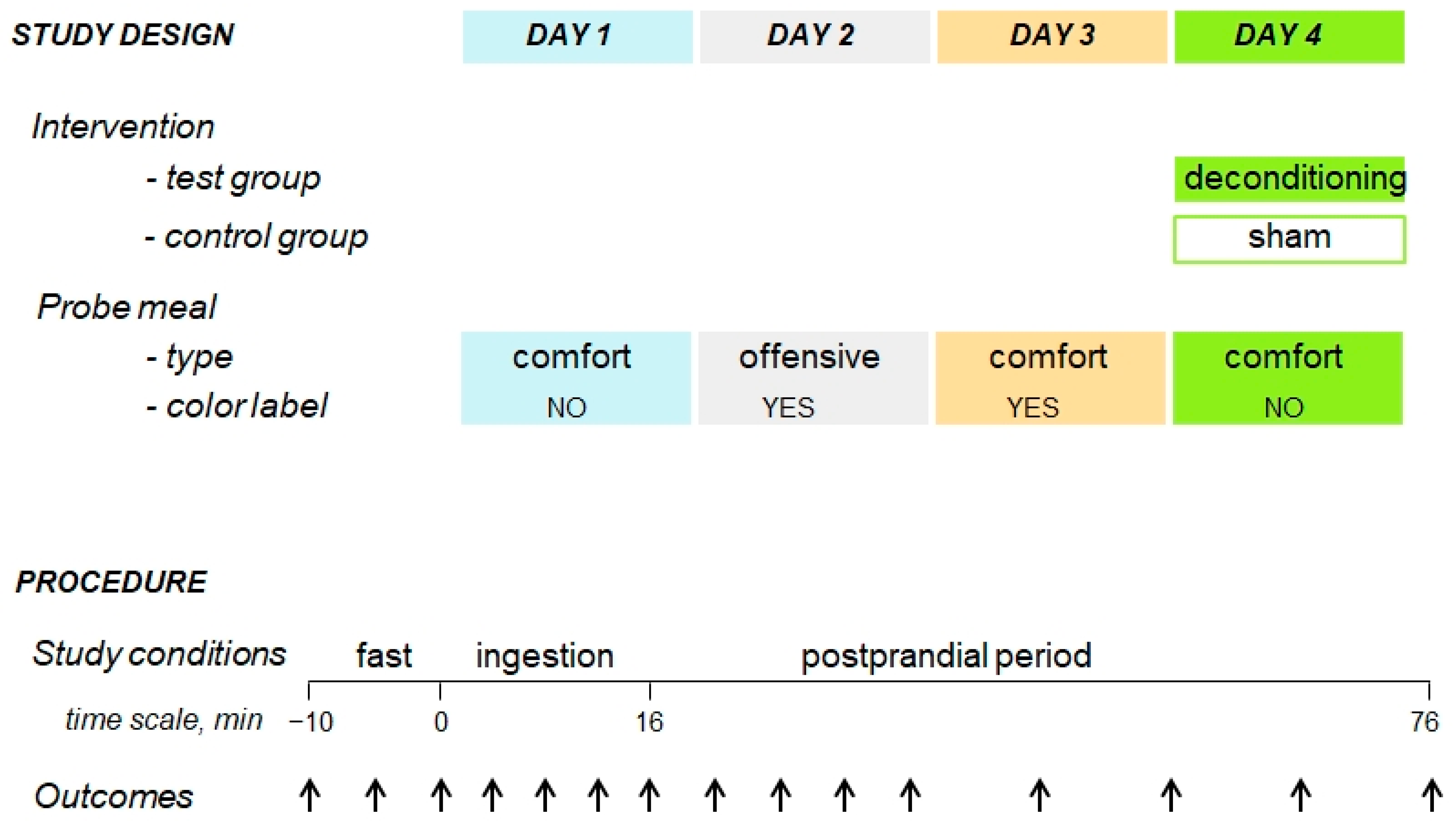
2.1. Experimental Design
2.2. Participants
2.3. General Procedure
2.4. Interventions
2.4.1. Probe Meal
2.4.2. Experimental Paradigm
2.5. Outcome Measures: Hedonic and Homeostatic Sensations
2.6. Statistical Analysis
3. Results
3.1. Demographics and Study Flow
3.2. Original Responses to the Comfort Meal (Study Day 1)
3.3. Offending Meal (Study Day 2 versus Day 1)
3.4. Aversive Conditioning (Study Day 3 versus Day 1)
3.5. Effect of the Cognitive Intervention (Day 4 versus Day 3)
3.6. Results of the Sensitivity Analysis Using Generalized Linear Mixed Modelling (GLMM)
4. Discussion
5. Limitations
6. Conclusions and Inferences
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livovsky, D.M.; Azpiroz, F. Gastrointestinal Contributions to the Postprandial Experience. Nutrients 2021, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.C. Conditioned Taste Aversions. World J. Otorhinolaryngol. Head. Neck Surg. 2018, 4, 92–100. [Google Scholar] [CrossRef]
- Nieto, A.; Livovsky, D.M.; Azpiroz, F. Conditioning by a Previous Experience Impairs the Rewarding Value of a Comfort Meal. Nutrients 2023, 15, 2247. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.J.; Rogers, H.E.; Feldman, S.E. Deconditioning Anxiety by Individualized Inhibitors. J. Behav. Ther. Exp. Psychiatry 1973, 4, 361–363. [Google Scholar] [CrossRef]
- Meulders, A. Fear in the Context of Pain: Lessons Learned from 100 Years of Fear Conditioning Research. Behav. Res. Ther. 2020, 131, 103635. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef]
- Ceunen, E.; Zaman, J.; Weltens, N.; Sarafanova, E.; Arijs, V.; Vlaeyen, J.W.S.; Van Oudenhove, L.; Van Diest, I. Learned Fear of Gastrointestinal Sensations in Healthy Adults. Clin. Gastroenterol. Hepatol. 2016, 14, 1552–1558.e2. [Google Scholar] [CrossRef][Green Version]
- Icenhour, A.; Labrenz, F.; Ritter, C.; Theysohn, N.; Forsting, M.; Bingel, U.; Elsenbruch, S. Learning by Experience? Visceral Pain-Related Neural and Behavioral Responses in a Classical Conditioning Paradigm. Neurogastroenterol. Motil. 2017, 29, e13026. [Google Scholar] [CrossRef]
- Koenen, L.R.; Icenhour, A.; Forkmann, K.; Theysohn, N.; Forsting, M.; Bingel, U.; Elsenbruch, S. From Anticipation to the Experience of Pain: The Importance of Visceral Versus Somatic Pain Modality in Neural and Behavioral Responses to Pain-Predictive Cues. Psychosom. Med. 2018, 80, 826–835. [Google Scholar] [CrossRef]
- Masihy, M.; Monrroy, H.; Borghi, G.; Pribic, T.; Galan, C.; Nieto, A.; Accarino, A.; Azpiroz, F. Influence of Eating Schedule on the Postprandial Response: Gender Differences. Nutrients 2019, 11, E401. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Baños, R.M.; Cebolla, A.; Moragrega, I.; Van Strien, T.; Fernández-Aranda, F.; Agüera, Z.; de la Torre, R.; Casanueva, F.F.; Fernández-Real, J.M.; Fernández-García, J.C.; et al. Relationship between Eating Styles and Temperament in an Anorexia Nervosa, Healthy Control, and Morbid Obesity Female Sample. Appetite 2014, 76, 76–83. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Arthurs, J.; Reilly, S. Conditioned Taste Aversions: From Poisons to Pain to Drugs of Abuse. Psychon. Bull. Rev. 2017, 24, 335–351. [Google Scholar] [CrossRef]
- Pribic, T.; Vilaseca, H.; Nieto, A.; Hernandez, L.; Malagelada, C.; Accarino, A.; Roca, J.; Azpiroz, F. Education of the Postprandial Experience by a Sensory-Cognitive Intervention. Neurogastroenterol. Motil. 2018, 30, e13197. [Google Scholar] [CrossRef]
- Park, B.-K.; Cho, M.-S. Taste Education Reduces Food Neophobia and Increases Willingness to Try Novel Foods in School Children. Nutr. Res. Pract. 2016, 10, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hoppu, U.; Prinz, M.; Ojansivu, P.; Laaksonen, O.; Sandell, M.A. Impact of Sensory-Based Food Education in Kindergarten on Willingness to Eat Vegetables and Berries. Food Nutr. Res. 2015, 59, 28795. [Google Scholar] [CrossRef]
- Pribic, T.; Vilaseca, H.; Nieto, A.; Hernandez, L.; Monrroy, H.; Malagelada, C.; Accarino, A.; Roca, J.; Azpiroz, F. Meal Composition Influences Postprandial Sensations Independently of Valence and Gustation. Neurogastroenterol. Motil. 2018, 30, e13337. [Google Scholar] [CrossRef]
- Ramos, R.; Wu, C.-H.; Turrigiano, G.G. Strong Aversive Conditioning Triggers a Long-Lasting Generalized Aversion. Front. Cell Neurosci. 2022, 16, 854315. [Google Scholar] [CrossRef] [PubMed]
- Hadamitzky, M.; Bösche, K.; Engler, A.; Schedlowski, M.; Engler, H. Extinction of Conditioned Taste Aversion Is Related to the Aversion Strength and Associated with C-Fos Expression in the Insular Cortex. Neuroscience 2015, 303, 34–41. [Google Scholar] [CrossRef]
- Bouton, M.E.; Maren, S.; McNally, G.P. Behavioral and Neurobiological Mechanisms of Pavlovian and Instrumental Extinction Learning. Physiol. Rev. 2021, 101, 611–681. [Google Scholar] [CrossRef]



| Dependent Variable: | Digestive Well-Being | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | −0.169 ± 0.226 | −0.832 * ± 0.435 | −0.311 ± 0.317 | 1.949 *** ± 0.394 |
| Time | 0.031 *** ± 0.01 | −0.033 *** ± 0.009 | −0.009 *** ± 0.003 | −0.011 * ± 0.006 |
| Group×time | 0.015 ± 0.014 | 0.005 ± 0.014 | 0.001 ± 0.006 | 0.056 *** ± 0.015 |
| Constant | 1.813 *** ± 0.174 | 0.411 ± 0.34 | 0.156 ± 0.244 | −0.222 ± 0.337 |
| Observations | 180 | 180 | 180 | 180 |
| Log likelihood | −367.151 | −356.12 | −294.939 | −325.659 |
| Akaike inf. crit. | 746.302 | 724.24 | 601.878 | 663.318 |
| Bayesian inf. crit. | 765.46 | 743.398 | 621.036 | 682.476 |
| Dependent Variable: | Mood | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | 0.017 ± 0.689 | −1.092 ± 0.859 | −0.496 ± 0.341 | 0.373 ± 0.755 |
| Time | −0.005 ± 0.007 | −0.019 * ± 0.011 | −0.005 ± 0.004 | −0.010 *** ± 0.003 |
| Group×time | 0.006 ± 0.007 | 0.011 ± 0.013 | −0.003 ± 0.007 | 0.021 ** ± 0.009 |
| Constant | 3.622 *** ± 0.536 | 3.506 *** ± 0.513 | 3.044 *** ± 0.205 | 2.830 *** ± 0.441 |
| Observations | 180 | 180 | 180 | 180 |
| Log likelihood | −163.442 | −254.835 | −164.307 | −184.011 |
| Akaike inf. crit. | 338.884 | 521.67 | 340.614 | 380.023 |
| Bayesian inf. crit. | 358.042 | 540.827 | 359.772 | 399.18 |
| Dependent Variable: | Satiety | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | −0.278 ± 0.478 | −0.348 ± 0.301 | −0.452 * ± 0.261 | −1.477 ** ± 0.641 |
| Time | 0.096 *** ± 0.007 | 0.122 *** ± 0.007 | 0.106 *** ± 0.005 | 0.107 *** ± 0.007 |
| Group×time | −0.033 * ± 0.019 | −0.025 * ± 0.015 | −0.015 ± 0.012 | −0.045 *** ± 0.016 |
| Constant | −0.162 ± 0.362 | −0.026 ± 0.122 | 0.740 *** ± 0.149 | 0.787 *** ± 0.185 |
| Observations | 180 | 180 | 180 | 180 |
| Log likelihood | −450.093 | −457.832 | −455.353 | −444.794 |
| Akaike inf. crit. | 912.186 | 927.664 | 922.707 | 901.589 |
| Bayesian inf. crit. | 931.344 | 946.822 | 941.865 | 920.746 |
| Dependent Variable: | Bloating/Fullness | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | −0.349 ± 0.298 | 0.083 ± 0.314 | −0.234 ± 0.329 | −1.153 *** ± 0.245 |
| Time | 0.022 ** ± 0.01 | 0.063 *** ± 0.019 | 0.048 *** ± 0.014 | 0.046 *** ± 0.011 |
| Group×time | −0.016 ± 0.01 | −0.03 ± 0.027 | −0.025 ± 0.016 | −0.022 * ± 0.012 |
| Constant | 1.144 *** ± 0.272 | 1.480 *** ± 0.232 | 1.487 *** ± 0.264 | 1.595 *** ± 0.163 |
| Observations | 180 | 180 | 180 | 180 |
| Log likelihood | −275.225 | −374.271 | −337.335 | −300.091 |
| Akaike inf. crit. | 562.45 | 760.542 | 686.669 | 612.183 |
| Bayesian inf. crit. | 581.608 | 779.7 | 705.827 | 631.34 |
| Dependent Variable: | Discomfort/Pain | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | −0.093 ± 0.061 | 0.896 ** ± 0.395 | −0.112 ± 0.192 | −0.287 ± 0.181 |
| Time | 0.01 ± 0.007 | 0.048 *** ± 0.015 | 0.020 * ± 0.012 | 0.016 * ± 0.01 |
| Group×time | −0.01 ± 0.007 | −0.016 ± 0.024 | −0.008 ± 0.016 | −0.016 * ± 0.01 |
| Constant | 0.093 ± 0.061 | 0.668 ** ± 0.334 | 0.301 * ± 0.163 | 0.287 ± 0.181 |
| Observations | 180 | 180 | 180 | 180 |
| Log likelihood | −85.033 | −357.008 | −247.848 | −162.538 |
| Akaike inf. crit. | 182.066 | 726.016 | 507.697 | 337.076 |
| Bayesian inf. crit. | 201.224 | 745.174 | 526.855 | 356.233 |
| Dependent Variable: | Wanting | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | 0.467 ± 0.482 | −1.167 ± 0.911 | −0.067 ± 0.962 | 3.300 *** ± 0.394 |
| Time | −0.254 *** ± 0.031 | −0.404 *** ± 0.053 | −0.308 *** ± 0.083 | −0.333 *** ± 0.006 |
| Group×time | −0.017 ± 0.037 | −0.013 ± 0.101 | 0.021 ± 0.099 | 0.150 *** ± 0.015 |
| Constant | 4.600 *** ± 0.38 | 4.600 *** ± 0.302 | 2.300 *** ± 0.871 | 1.567 *** ± 0.337 |
| Observations | 60 | 60 | 60 | 60 |
| Log likelihood | −90.783 | −115.857 | −119.504 | −104.623 |
| Akaike inf. crit. | 193.566 | 243.714 | 251.009 | 221.247 |
| Bayesian inf. crit. | 206.132 | 256.28 | 263.575 | 233.813 |
| Dependent Variable: | Wanting | |||
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Group (intervention) | 0.75 ± 0.688 | −0.25 ± 1.24 | −0.167 ± 0.991 | 1.917 *** ± 0.628 |
| Time | −0.146 *** ± 0.054 | −0.321 *** ± 0.061 | −0.221 *** ± 0.048 | −0.283 *** ± 0.063 |
| Group×time | −0.021 ± 0.059 | −0.154 ± 0.104 | −0.075 ± 0.095 | 0.237 *** ± 0.069 |
| Constant | 4.583 *** ± 0.533 | 5.500 *** ± 0.514 | 3.500 *** ± 0.782 | 2.583 *** ± 0.582 |
| Observations | 48 | 48 | 48 | 48 |
| Log likelihood | −70.431 | −87.037 | −82.738 | −68.674 |
| Akaike inf. crit. | 152.863 | 186.074 | 177.475 | 149.347 |
| Bayesian inf. crit. | 164.09 | 197.302 | 188.703 | 160.575 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto, A.; Livovsky, D.M.; Azpiroz, F. Reversal of Conditioned Food Aversion Using a Cognitive Intervention: A Sham-Controlled, Randomized, Parallel Study. Nutrients 2023, 15, 4962. https://doi.org/10.3390/nu15234962
Nieto A, Livovsky DM, Azpiroz F. Reversal of Conditioned Food Aversion Using a Cognitive Intervention: A Sham-Controlled, Randomized, Parallel Study. Nutrients. 2023; 15(23):4962. https://doi.org/10.3390/nu15234962
Chicago/Turabian StyleNieto, Adoracion, Dan M. Livovsky, and Fernando Azpiroz. 2023. "Reversal of Conditioned Food Aversion Using a Cognitive Intervention: A Sham-Controlled, Randomized, Parallel Study" Nutrients 15, no. 23: 4962. https://doi.org/10.3390/nu15234962
APA StyleNieto, A., Livovsky, D. M., & Azpiroz, F. (2023). Reversal of Conditioned Food Aversion Using a Cognitive Intervention: A Sham-Controlled, Randomized, Parallel Study. Nutrients, 15(23), 4962. https://doi.org/10.3390/nu15234962





