Knockdown of Placental Major Facilitator Superfamily Domain Containing 2a in Pregnant Mice Reduces Fetal Brain Growth and Phospholipid Docosahexaenoic Acid Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Lentiviral Vectors
2.2. Animals
2.3. Gene Expression Analysis
2.4. Placental and Brain Lipid Extraction and Phospholipid Analysis
2.5. Statistics
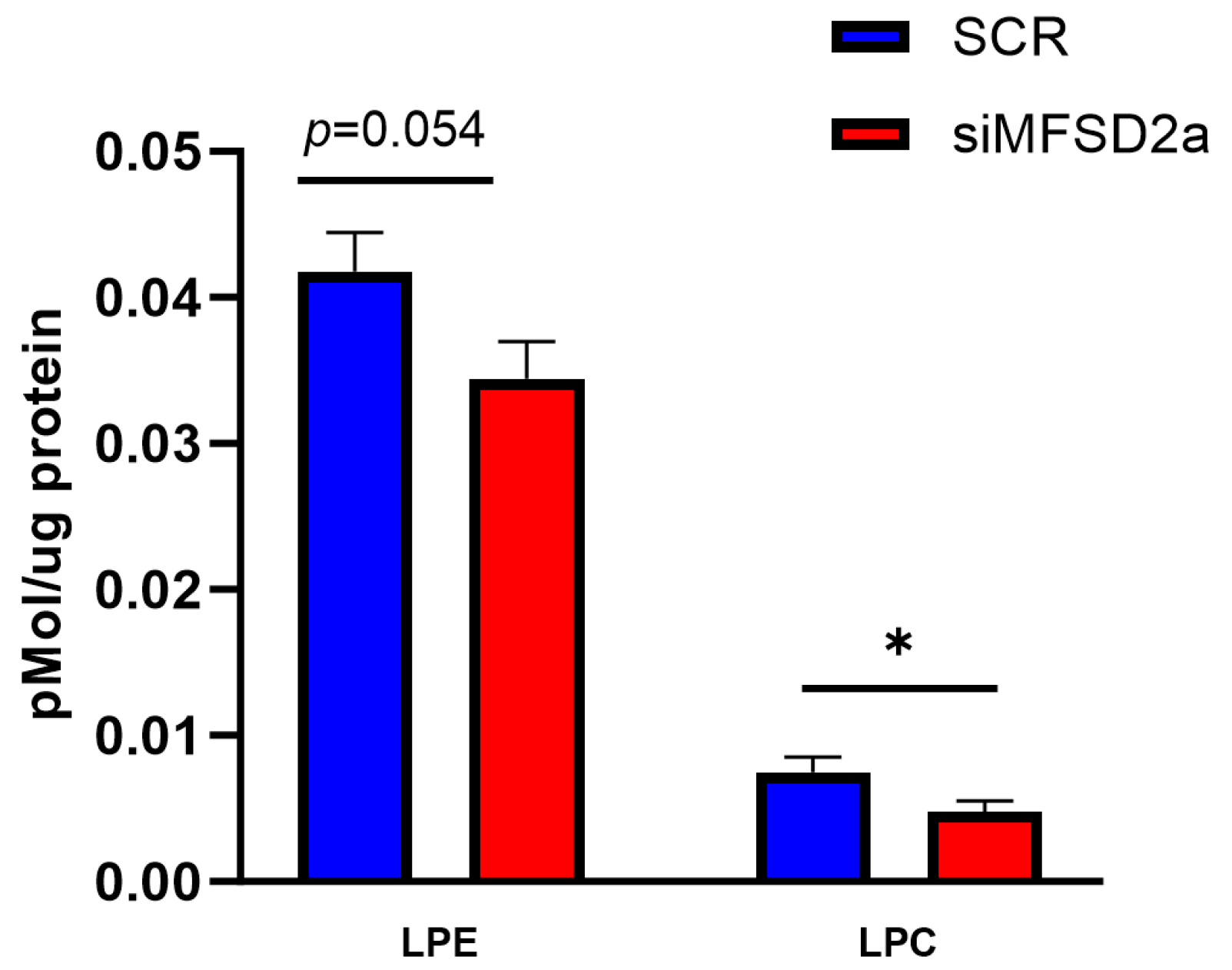
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenberg, J.A.; Bell, S.J.; Ausdal, W.V. Omega-3 Fatty Acid supplementation during pregnancy. Rev. Obstet. Gynecol. 2008, 1, 162–169. [Google Scholar] [PubMed]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Hazels Mitmesser, S. Maternal Omega-3 Nutrition, Placental Transfer and Fetal Brain Development in Gestational Diabetes and Preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Mallick, R.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment. Nutrients 2021, 13, 2061. [Google Scholar] [CrossRef] [PubMed]
- Betsholtz, C. Lipid transport and human brain development. Nat. Genet. 2015, 47, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Giusto, N.; Pasquaré, S.; Salvador, G.; Castagnet, P.; Roque, M.; de Boschero, M.I. Lipid metabolism in vertebrate retinal rod outer segments. Prog. Lipid. Res. 2000, 39, 315–391. [Google Scholar] [CrossRef]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Hashimoto, A.; Takemoto, Y.; Okuyama, H.; Nomura, M.; Kitajima, R.; Togashi, T.; Tamai, Y. Effect of the dietary alpha-linolenate/linoleate balance on lipid compositions and learning ability of rats. II. Discrimination process, extinction process, and glycolipid compositions. J. Lipid Res. 1988, 29, 1013–1021. [Google Scholar] [CrossRef]
- Ahmad, A.; Moriguchi, T.; Salem, N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr. Neurol. 2002, 26, 210–218. [Google Scholar] [CrossRef]
- Coti Bertrand, P.; O’Kusky, J.R.; Innis, S.M. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J. Nutr. 2006, 136, 1570–1575. [Google Scholar] [CrossRef]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef]
- Colombo, J.; Gustafson, K.M.; Gajewski, B.J.; Shaddy, D.J.; Kerling, E.H.; Thodosoff, J.M.; Doty, T.; Brez, C.C.; Carlson, S.E. Prenatal DHA supplementation and infant attention. Pediatr. Res. 2016, 80, 656–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 2008, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev. 2018, 11, CD003402. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Treyvaud, K.; Yelland, L.N.; Anderson, P.J.; Smithers, L.G.; McPhee, A.J.; Makrides, M. Seven-Year Follow-up of Children Born to Women in a Randomized Trial of Prenatal DHA Supplementation. JAMA 2017, 317, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, Y.; Wei, L.; Zhang, H.; Zhang, J.; Zhou, X.; Zhu, S.; Du, Y.; Su, R.; Fang, C.; et al. DHA supplementation and pregnancy complications. J. Transl. Med. 2023, 21, 394. [Google Scholar] [CrossRef]
- Leveille, P.; Rouxel, C.; Plourde, M. Diabetic pregnancy, maternal and fetal docosahexaenoic acid: A review of existing evidence. J. Matern. Fetal Neonatal. Med. 2018, 31, 1358–1363. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Sengottuvel, V.; Hota, M.; Oh, J.; Galam, D.L.; Wong, B.H.; Wenk, M.R.; Ghosh, S.; Torta, F.; Silver, D.L. Deficiency in the omega-3 lysolipid transporter Mfsd2a leads to aberrant oligodendrocyte lineage development and hypomyelination. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef]
- Vaughan, O.R.; Maksym, K.; Silva, E.; Barentsen, K.; Anthony, R.V.; Brown, T.L.; Hillman, S.L.; Spencer, R.; David, A.L.; Rosario, F.J.; et al. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin. Sci. 2021, 135, 2049–2066. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Okada, Y.; Ueshin, Y.; Isotani, A.; Saito-Fujita, T.; Nakashima, H.; Kimura, K.; Mizoguchi, A.; Oh-Hora, M.; Mori, Y.; Ogata, M.; et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat. Biotechnol. 2007, 25, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Cater, R.J.; Chua, G.L.; Erramilli, S.K.; Keener, J.E.; Choy, B.C.; Tokarz, P.; Chin, C.F.; Quek, D.Q.Y.; Kloss, B.; Pepe, J.G.; et al. Structural basis of omega-3 fatty acid transport across the blood-brain barrier. Nature 2021, 595, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lei, H.-T.; Lai, L.T.F.; Gallenito, M.J.; Mu, X.; Matthies, D.; Gonen, T. Lipid flipping in the omega-3 fatty-acid transporter. Nat. Commun. 2023, 14, 2571. [Google Scholar] [CrossRef] [PubMed]
- Alakbarzade, V.; Hameed, A.; Quek, D.Q.Y.; Chioza, B.A.; Baple, E.L.; Cazenave-Gassiot, A.; Nguyen, L.N.; Wenk, M.R.; Ahmad, A.Q.; Sreekantan-Nair, A.; et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat. Genet. 2015, 47, 814–817. [Google Scholar] [CrossRef]
- Huang, B.; Li, X. The Role of Mfsd2a in Nervous System Diseases. Front. Neurosci. 2021, 15, 730534. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Hassam, A.G.; Williams, G. Essential fatty acids and fetal brain growth. Lancet 1976, 307, 452–453. [Google Scholar] [CrossRef]
- Lewis, R.M.; Hanson, M.A.; Burdge, G.C. Umbilical venous-arterial plasma composition differences suggest differential incorporation of fatty acids in NEFA and cholesteryl ester pools. Br. J. Nutr. 2011, 106, 463–467. [Google Scholar] [CrossRef]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Blanco, J.E.; Pagán, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 1908S–1913S. [Google Scholar] [CrossRef]
- Gil-Sanchez, A.; Koletzko, B.; Larque, E. Current understanding of placental fatty acid transport. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 265–272. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K. Transport mechanisms for long-chain polyunsaturated fatty acids in the human placenta. Am. J. Clin. Nutr. 2000, 71, 315S–322S. [Google Scholar] [CrossRef]
- Prieto-Sánchez, M.T.; Ruiz-Palacios, M.; Blanco-Carnero, J.E.; Pagan, A.; Hellmuth, C.; Uhl, O.; Peissner, W.; Ruiz-Alcaraz, A.J.; Parrilla, J.J.; Koletzko, B.; et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin. Nutr. 2017, 36, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.L.; Barner, K.; Madi, L.; Armstrong, M.; Manke, J.; Uhlson, C.; Jansson, T.; Ferchaud-Roucher, V. Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158861. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.G.; Legette, L.L.; Ikonte, C.J.; Mitmesser, S.H. Choline and DHA in Maternal and Infant Nutrition: Synergistic Implications in Brain and Eye Health. Nutrients 2019, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Ferchaud-Roucher, V.; Barner, K.; Jansson, T.; Powell, T.L. Maternal obesity results in decreased syncytiotrophoblast synthesis of palmitoleic acid, a fatty acid with anti-inflammatory and insulin-sensitizing properties. FASEB J. 2019, 33, 6643–6654. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B. New appreciation for an old pathway: The Lands Cycle moves into new arenas in health and disease. Biochem. Soc. Trans. 2022, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Quek, D.Q.; Nguyen, L.N.; Fan, H.; Silver, D.L. Structural Insights into the Transport Mechanism of the Human Sodium-dependent Lysophosphatidylcholine Transporter MFSD2A. J. Biol. Chem. 2016, 291, 9383–9394. [Google Scholar] [CrossRef]
- Lacombe, R.J.S.; Chouinard-Watkins, R.; Bazinet, R.P. Brain docosahexaenoic acid uptake and metabolism. Mol. Asp. Med. 2018, 64, 109–134. [Google Scholar] [CrossRef]
- Wijendran, V.; Bendel, R.B.; Couch, S.C.; Philipson, E.H.; Cheruku, S.; Lammi-Keefe, C.J. Fetal erythrocyte phospholipid polyunsaturated fatty acids are altered in pregnancy complicated with gestational diabetes mellitus. Lipids 2000, 35, 927–931. [Google Scholar] [CrossRef]
- Wadhwani, N.; Patil, V.; Pisal, H.; Joshi, A.; Mehendale, S.; Gupte, S.; Wagh, G.; Joshi, S. Altered maternal proportions of long chain polyunsaturated fatty acids and their transport leads to disturbed fetal stores in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 21–30. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Mehendale, S.S.; Yadav, H.R.; Joshi, S.R. Reduced placental docosahexaenoic acid levels associated with increased levels of sFlt-1 in preeclampsia. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 51–55. [Google Scholar] [CrossRef]
- Toufaily, C.; Vargas, A.; Lemire, M.; Lafond, J.; Rassart, E.; Barbeau, B. MFSD2a, the Syncytin-2 receptor, is important for trophoblast fusion. Placenta 2013, 34, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Nelson, S.M.; Macdonald-Wallis, C.; Lawlor, D.A. Associations of existing diabetes, gestational diabetes, and glycosuria with offspring IQ and educational attainment: The Avon Longitudinal Study of Parents and Children. Exp. Diabetes Res. 2012, 2012, 963735. [Google Scholar] [CrossRef] [PubMed]
- Figueiró-Filho, E.A.; Mak, L.E.; Reynolds, J.N.; Stroman, P.W.; Smith, G.N.; Forkert, N.D.; Paolozza, A.; Rätsep, M.T.; Croy, B.A. Neurological function in children born to preeclamptic and hypertensive mothers—A systematic review. Pregnancy Hypertens 2017, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Eser Ocak, P.; Ocak, U.; Sherchan, P.; Zhang, J.H.; Tang, J. Insights into major facilitator superfamily domain-containing protein-2a (Mfsd2a) in physiology and pathophysiology. What do we know so far? J. Neurosci. Res. 2020, 98, 29–41. [Google Scholar] [CrossRef]

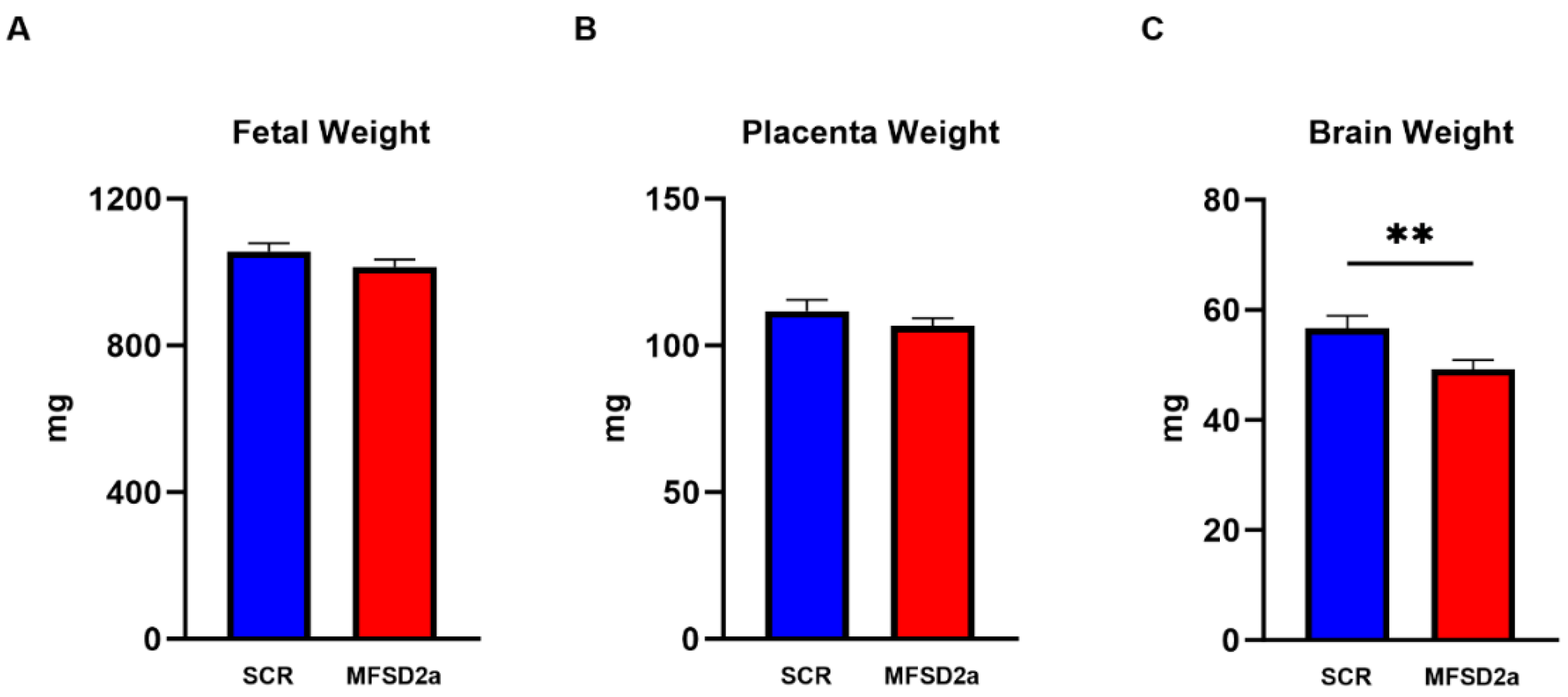
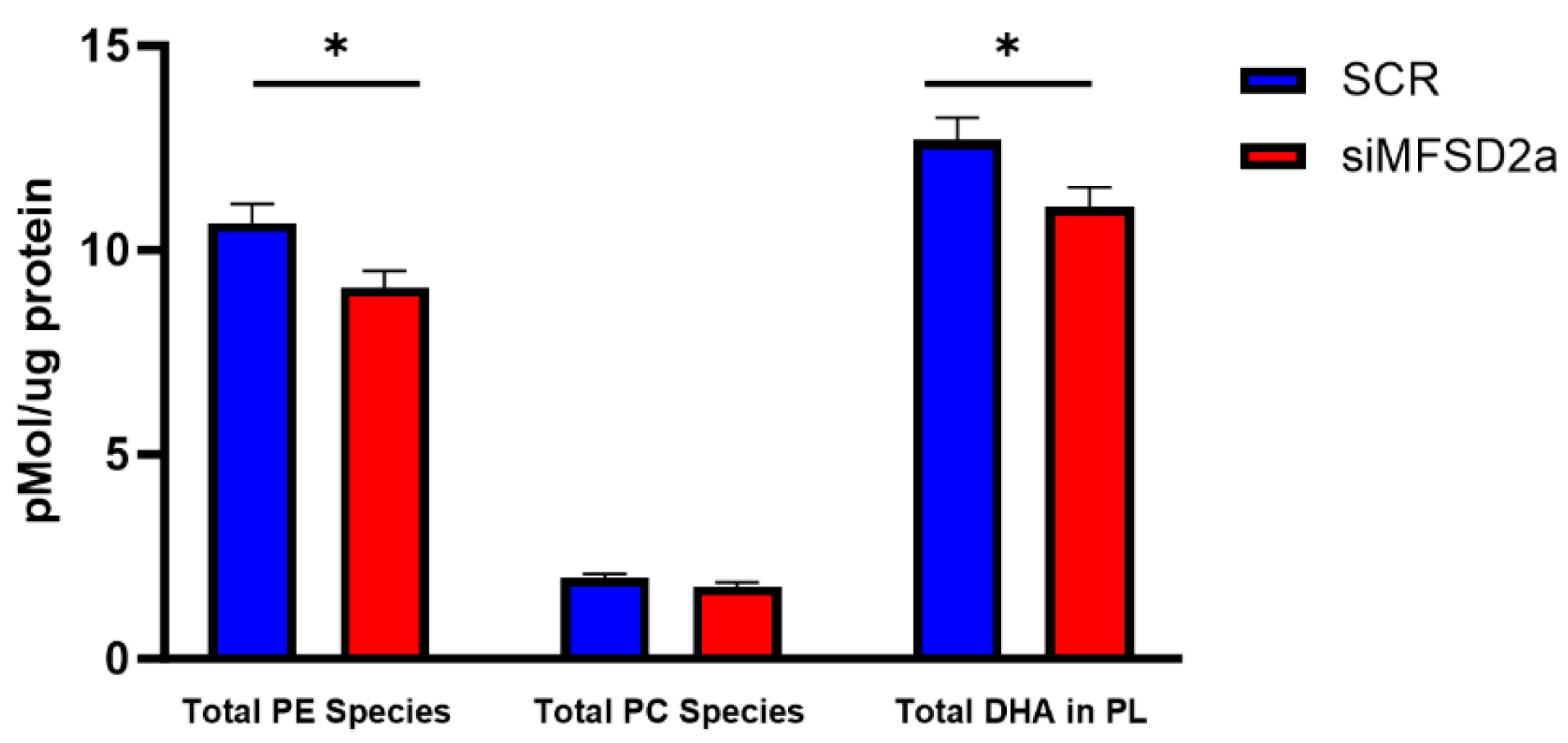
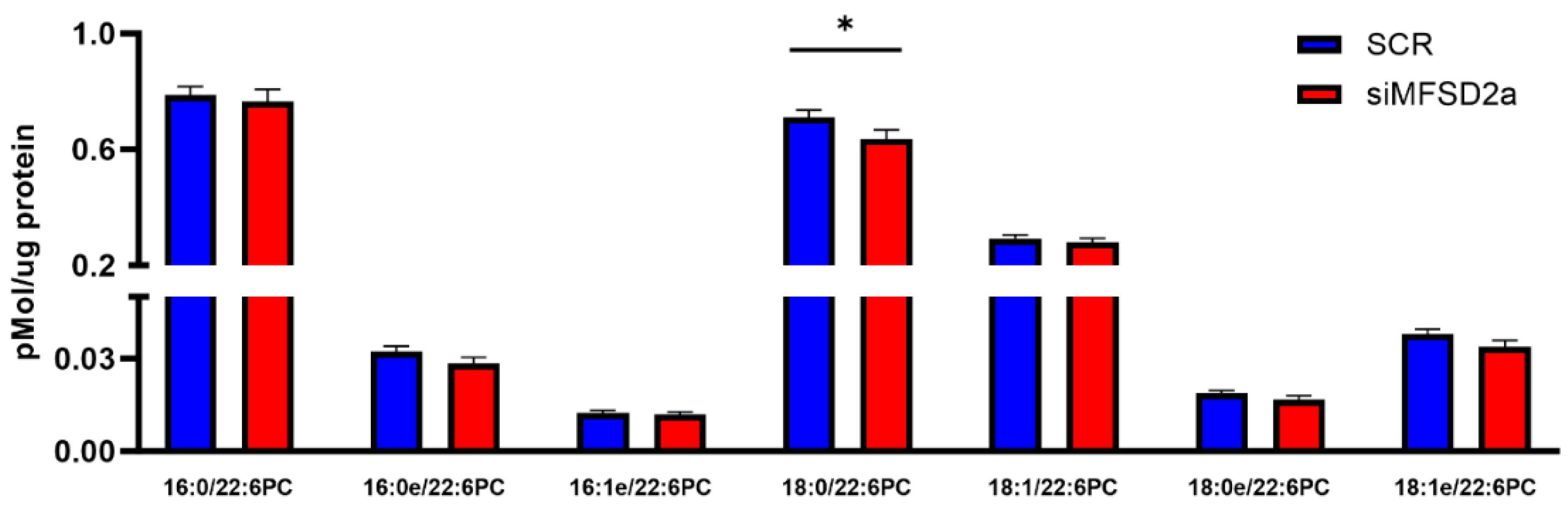
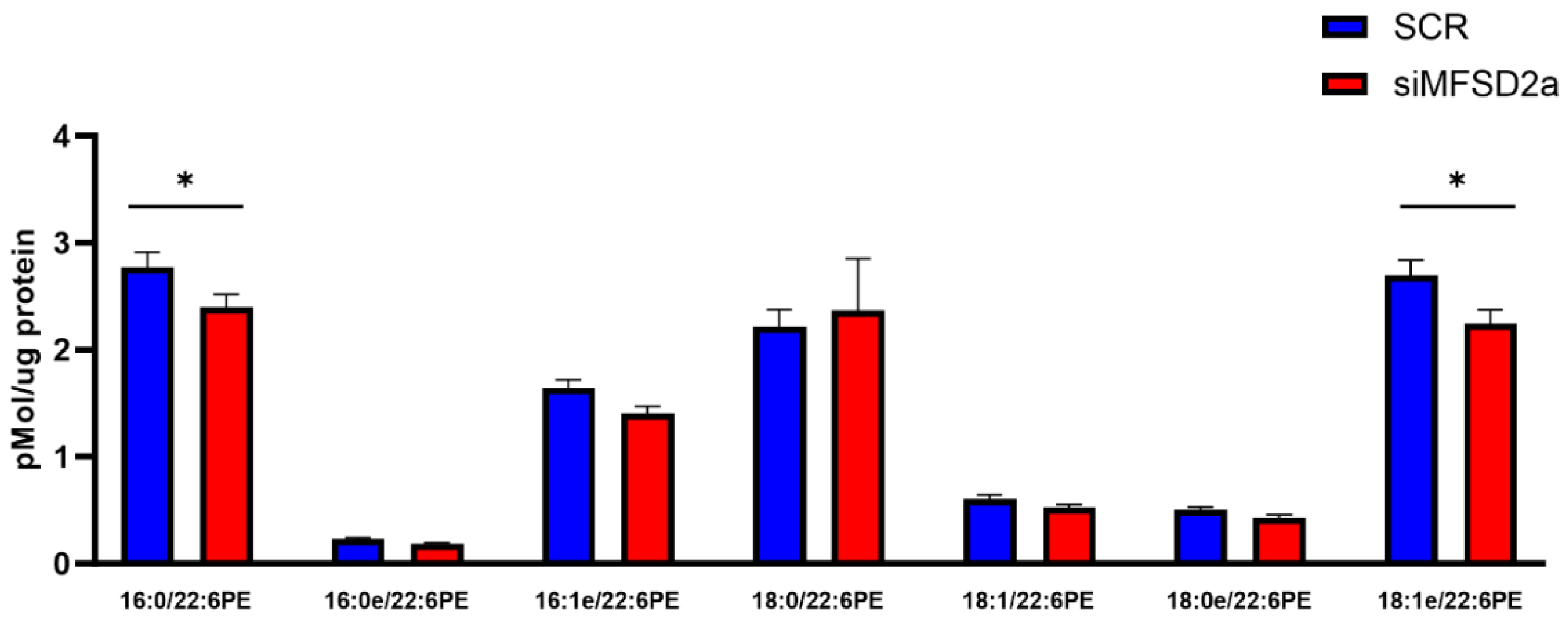
| Brain Phospholipid | Correlation Coefficient | p Value |
|---|---|---|
| PE (16:0_22:6) | 0.28 | 0.0317 |
| PE (16:0e_22:6) | 0.30 | 0.0219 |
| PE (16:1e_22:6) | 0.26 | 0.0429 |
| PE (18:0_22:6) | 0.27 | 0.0409 |
| PE (18:1_22:6) | 0.22 | 0.0943 |
| PE (18:0e_22:6) | 0.28 | 0.0324 |
| PE (18:1e_22:6) | 0.28 | 0.0288 |
| LPE (22:6) | 0.21 | 0.109 |
| Total PE containing DHA | 0.32 | 0.0122 |
| PC (16:0_22:6) | 0.17 | 0.211 |
| PC (16:0e_22:6) | 0.22 | 0.0919 |
| PC (16:1e_22:6) | 0.13 | 0.3236 |
| PC (18:0_22:6) | 0.26 | 0.0474 |
| PC (18:1_22:6) | 0.16 | 0.2302 |
| PC (18:0e_22:6) | 0.25 | 0.0527 |
| PC (18:1e_22:6) | 0.25 | 0.0546 |
| LPC (22:6) | 0.19 | 0.1533 |
| Total PC containing DHA | 0.21 | 0.1114 |
| Total Brain PE + PC DHA | 0.31 | 0.0166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, T.L.; Barentsen, K.; Vaughan, O.; Uhlson, C.; Zemski Berry, K.; Erickson, K.; Faer, K.; Chassen, S.S.; Jansson, T. Knockdown of Placental Major Facilitator Superfamily Domain Containing 2a in Pregnant Mice Reduces Fetal Brain Growth and Phospholipid Docosahexaenoic Acid Content. Nutrients 2023, 15, 4956. https://doi.org/10.3390/nu15234956
Powell TL, Barentsen K, Vaughan O, Uhlson C, Zemski Berry K, Erickson K, Faer K, Chassen SS, Jansson T. Knockdown of Placental Major Facilitator Superfamily Domain Containing 2a in Pregnant Mice Reduces Fetal Brain Growth and Phospholipid Docosahexaenoic Acid Content. Nutrients. 2023; 15(23):4956. https://doi.org/10.3390/nu15234956
Chicago/Turabian StylePowell, Theresa L., Kenneth Barentsen, Owen Vaughan, Charis Uhlson, Karin Zemski Berry, Kathryn Erickson, Kelsey Faer, Stephanie S. Chassen, and Thomas Jansson. 2023. "Knockdown of Placental Major Facilitator Superfamily Domain Containing 2a in Pregnant Mice Reduces Fetal Brain Growth and Phospholipid Docosahexaenoic Acid Content" Nutrients 15, no. 23: 4956. https://doi.org/10.3390/nu15234956
APA StylePowell, T. L., Barentsen, K., Vaughan, O., Uhlson, C., Zemski Berry, K., Erickson, K., Faer, K., Chassen, S. S., & Jansson, T. (2023). Knockdown of Placental Major Facilitator Superfamily Domain Containing 2a in Pregnant Mice Reduces Fetal Brain Growth and Phospholipid Docosahexaenoic Acid Content. Nutrients, 15(23), 4956. https://doi.org/10.3390/nu15234956






