The IRONy in Athletic Performance
Abstract
1. Introduction
2. Methodology
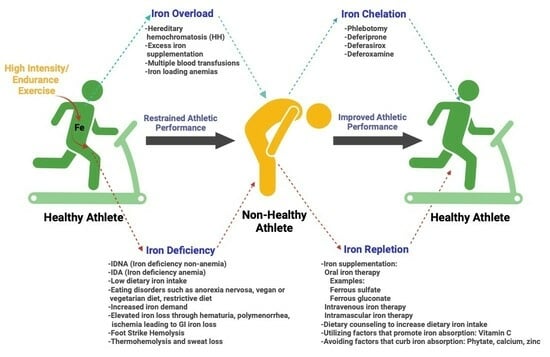
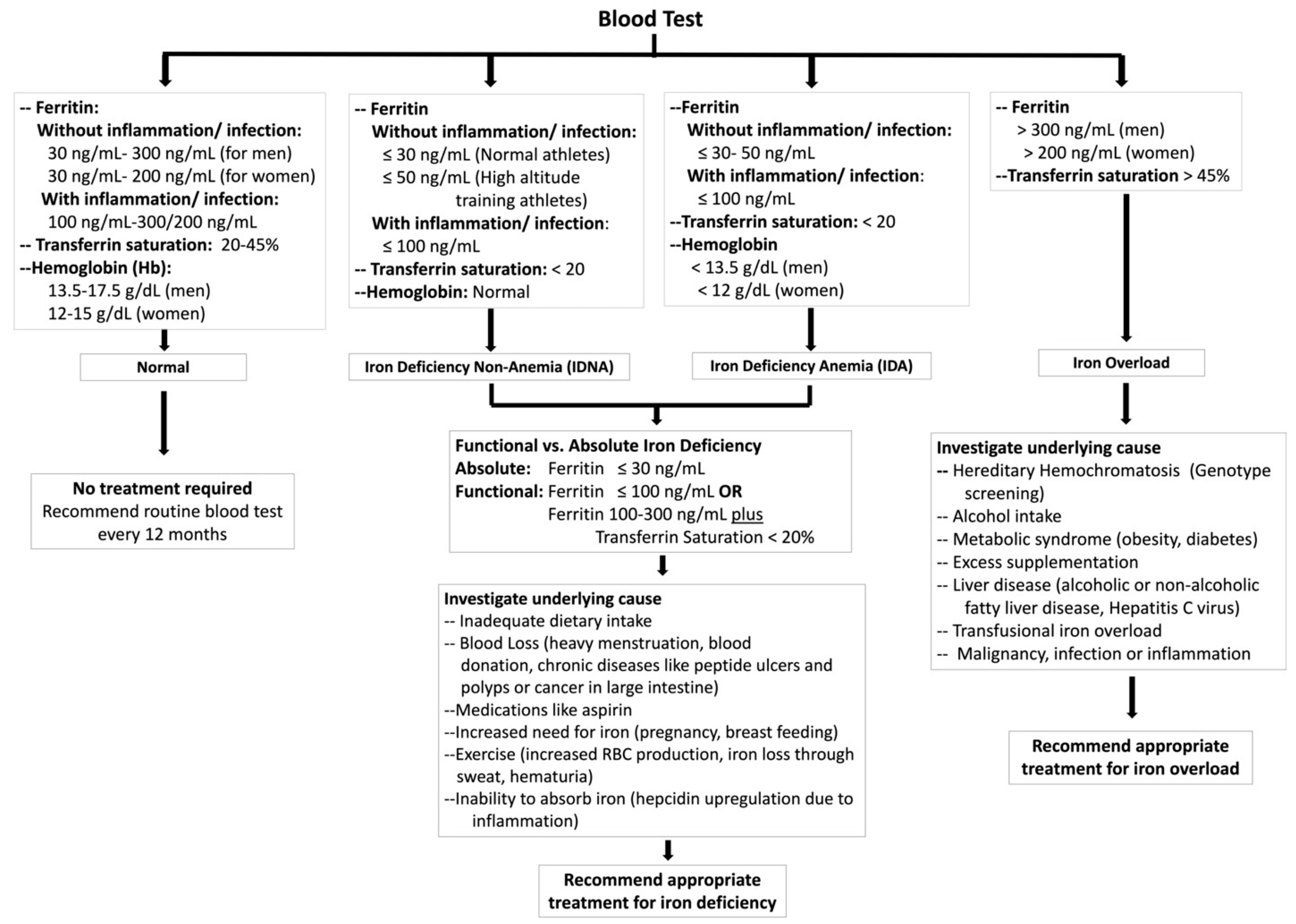
3. Exercise Leads to Iron Deficiency
4. Iron Deficiency Impairs Athletic Performance
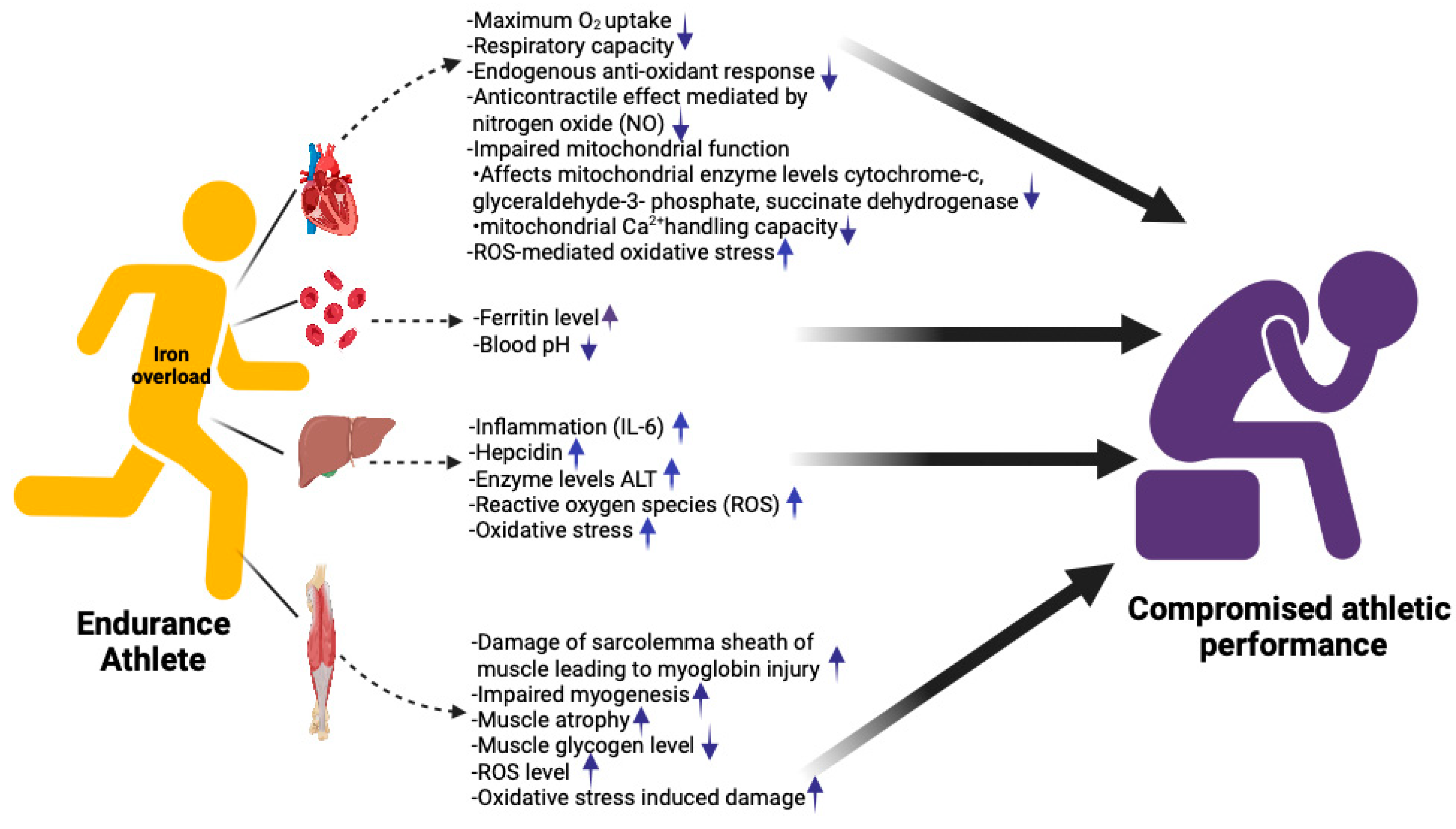
5. Iron Overload and Athletic Performance
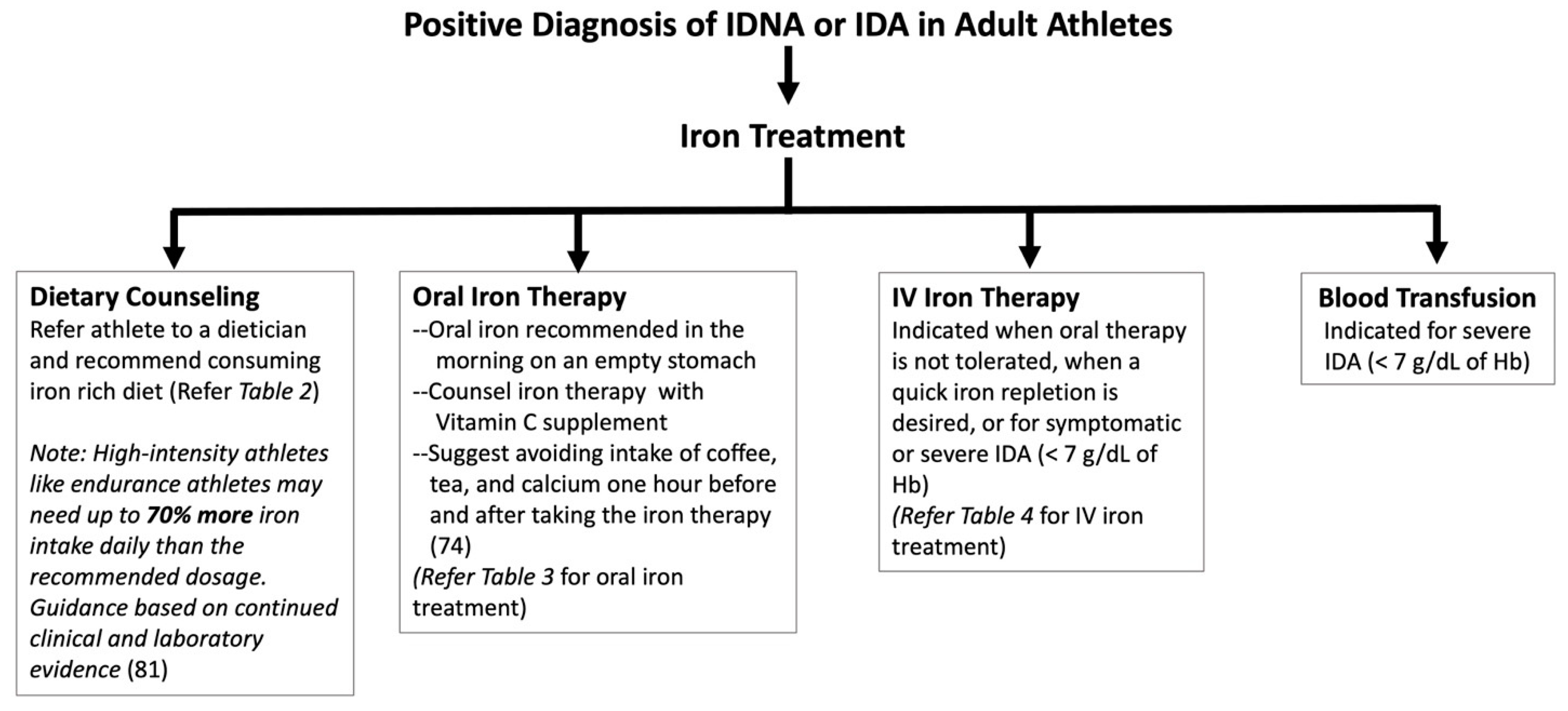
6. Treatments and Solutions
6.1. Dietary Counseling
6.2. Oral Iron Treatment
6.3. Intramuscular Iron Treatment
6.4. Intravenous Iron Treatment
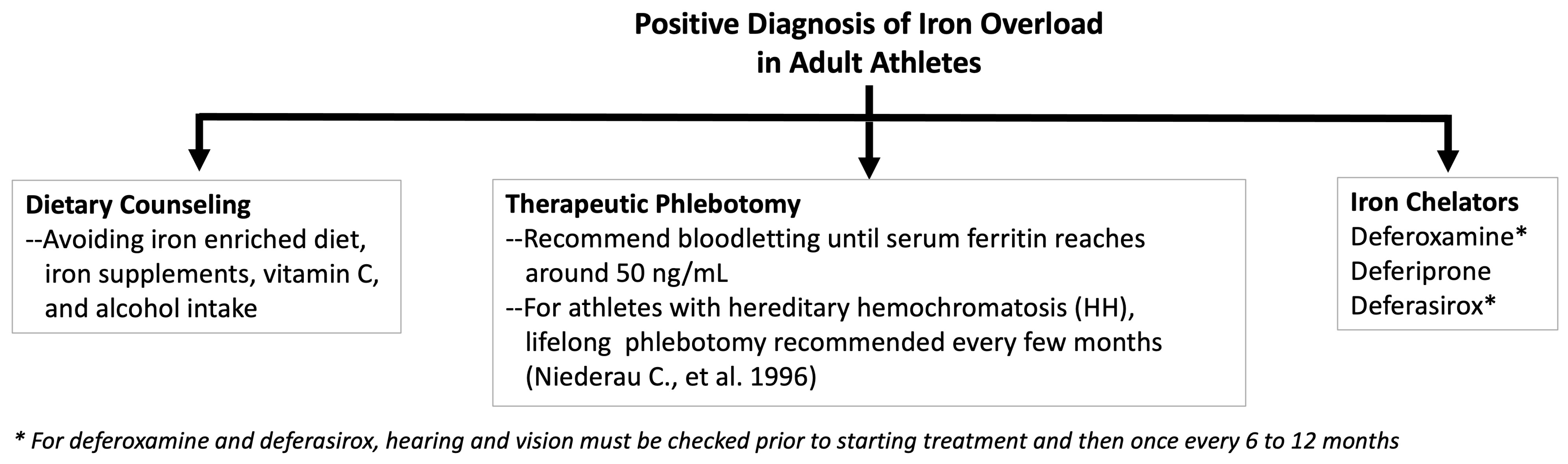
6.5. Managing Iron Overload
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mcdonald, R.; Keen, C.L. Iron, Zinc and Magnesium Nutrition and Athletic Performance. Sports Med. 1988, 5, 171–184. [Google Scholar] [CrossRef]
- Beck, K.L.; von Hurst, P.R.; O’Brien, W.J.; Badenhorst, C.E. Micronutrients and Athletic Performance: A Review. Food Chem. Toxicol. 2021, 158, 112618. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Tzilianos, M.; Christakis, J.I.; Bogdanos, D.; Tsimirika, K.; MacFarlane, J.; Goldberg, Y.P.; Sakellaropoulos, N.; Ganz, T.; Nemeth, E. Hepcidin in Iron Overload Disorders. Blood 2005, 105, 4103–4105. [Google Scholar] [CrossRef]
- Collins, J.F.; Wessling-Resnick, M.; Knutson, M.D. Hepcidin Regulation of Iron Transport. Proc. J. Nutr. 2005, 138, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Clénin, G.E.; Cordes, M.; Huber, A.; Schumacher, Y.; Noack, P.; Scales, J.; Kriemler, S. Iron Deficiency in Sports—Definition, Influence on Performance and Therapy. Swiss Med. Wkly. 2016, 64, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Solberg, A.; Reikvam, H. Iron Status and Physical Performance in Athletes. Life 2023, 13, 2007. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S. Iron and the Endurance Athlete. Appl. Physiol. Nutr. Metab. 2014, 39, 1012–1018. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br. J. Haematol. 2016, 17, 512–523. [Google Scholar] [CrossRef]
- Davidsen, E.S.; Liseth, K.; Gerdts, E. Reduced Exercise Capacity in Genetic Haemochromatosis. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 470–475. [Google Scholar] [CrossRef]
- Clark, P.; Britton, L.J.; Powell, L.W. The diagnosis and management of hereditary haemochromatosis. Clin. Biochem. Rev. 2010, 31, 3. [Google Scholar]
- Girelli, D.; Busti, F.; Brissot, P.; Cabantchik, I.; Muckenthaler, M.U.; Porto, G. Hemochromatosis classification: Update and recommendations by the BIOIRON Society. Blood Am. J. Hematol. 2022, 139, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, D.; Sicova, M.; Guest, N.S.; Garcia-Bailo, B.; El-Sohemy, A. HFE Genotype and Endurance Performance in Competitive Male Athletes. Med. Sci. Sports Exerc. 2021, 53, 1385–1390. [Google Scholar] [CrossRef]
- Luszczyk, M.; Kaczorowska-Hac, B.; Milosz, E.; Adamkiewicz-Drozynska, E.; Ziemann, E.; Laskowski, R.; Flis, D.; Rokicka-Hebel, M.; Antosiewicz, J. Reduction of Skeletal Muscle Power in Adolescent Males Carrying H63D Mutation in the HFE Gene. Biomed. Res. Int. 2017, 2017, 5313914. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Mickey, M.C.; Receno, C.N.; Atalay, M.; DeRuisseau, K.C. Functional and Biochemical Responses of Skeletal Muscle Following a Moderate Degree of Systemic Iron Loading in Mice. J. Appl. Physiol. 2019, 126, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.; Ferrari, N.; Kuriakose, P. Iron-Overload Myopathy. Int. J. Hematol. 2011, 94, 503–504. [Google Scholar] [CrossRef][Green Version]
- Muiesan, P.; Rela, M.; Kane, P.; Dawan, A.; Baker, A.; Ball, C.; Mowat, A.P.; Williams, R.; Heaton, N.D. Liver Transplant. Neonatal Haemochromatosis 1995, 73, 178–180. [Google Scholar]
- Damian MTVulturar, R.; Login, C.C.; Damian, L. Anemia in sports: A narrative review. Life 2021, 11, 987. [Google Scholar] [CrossRef]
- Brune, M.; Magnusson, B.; Persson, H.; Hallberg, L. Iron losses in sweat. Am. J. Clin. Nutr. 1986, 43, 438–443. [Google Scholar] [CrossRef]
- Urakami, S.; Ogawa, K.; Oka, S.; Hayashida, M.; Hagiwara, K.; Nagamoto, S.; Okaneya, T. Macroscopic hematuria caused by running-induced traumatic bladder mucosal contusions. IJU Case Rep. 2019, 2, 27–29. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron Considerations for the Athlete: A Narrative Review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Suedekum, N.A.; Dimeff, R.J. Iron and the athlete. Curr. Sports Med. Rep. 2005, 4, 199–202. [Google Scholar] [CrossRef]
- Ter Steege, R.W.; Kolkman, J.J. Review article: The pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment. Pharmacol. Ther. 2012, 35, 516–528. [Google Scholar] [CrossRef]
- Olaf Schumacher, Y.; Schmid, A.; Grathwohl, D.; Berg, A.; Schumacher, A.; Schmid, A.; Grathwohl, D. Hematological Indices and Iron Status in Athletes of Various Sports and Performances. Med. Sci. Sports Exerc. 2002, 34, 869–875. [Google Scholar] [CrossRef] [PubMed]
- McLane, J.A.; Fell, R.D.; Mckay, R.H.; Winder, W.W.; Brown, E.B.; Fell, R.; Hmckay, R.; Winder, W. Physiological and Biochemical Effects of Iron Deficiency on Rat Skeletal Muscle. Am. J. Physiol. 1981, 241, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ohira, Y.; Gill, S.L. Effects of Dietary Iron Deficiency on Muscle Fiber Characteristics and Whole-Body Distribution of Hemoglobin in Mice. J. Nutr. 1983, 113, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med.Sci. 2014, 19, 164–174. [Google Scholar]
- Whiting, S.J.; Barabash, W.A. Dietary Reference Intakes for the Micronutrients: Considerations for Physical Activity. Appl. Physiol. Nutri Metab. 2006, 31, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pedlar, C.R.; Brugnara, C.; Bruinvels, G.; Burden, R. Iron Balance and Iron Supplementation for the Female Athlete: A Practical Approach. Eur. J. Sport. Sci. 2018, 18, 295–305. [Google Scholar] [CrossRef]
- Al-Naseem, A.; Sallam, A.; Choudhury, S.; Thachil, J. Iron Deficiency without Anaemia: A Diagnosis That Matters. Clinl. Med. 2021, 21, 107–113. [Google Scholar] [CrossRef]
- Miller, J.L. Iron Deficiency Anemia: A Common and Curable Disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef]
- Sinclair, L.M.; Hinton, P.S. Prevalence of Iron Deficiency with and without Anemia in Recreationally Active Men and Women. J. Am. Diet. Assoc. 2005, 105, 975–978. [Google Scholar] [CrossRef]
- Auersperger, I.; Škof, B.; Leskošek, B.; Knap, B.; Jerin, A.; Lainscak, M. Exercise-Induced Changes in Iron Status and Hepcidin Response in Female Runners. PLoS ONE 2013, 8, E58090. [Google Scholar] [CrossRef]
- Soppi, E.T. Iron Deficiency without Anemia—A Clinical Challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Tansarli, G.S.; Karageorgopoulos, D.E.; Kapaskelis, A.; Gkegkes, I.; Falagas, M.E. Iron deficiency and susceptibility to infections. J. Infect. 2014, 69, S23–S27. [Google Scholar]
- Lands, R.; Isang, E. Secondary hemochromatosis due to chronic oral iron supplementation. Case Rep. Hematol. 2017, 2017, 2494167. [Google Scholar] [CrossRef] [PubMed]
- Makker, J.; Hanif, A.; Bajantri, B.; Chilimuri, S. Dysmetabolic hyperferritinemia: All iron overload is not hemochromatosis. Case Rep. Gastroenterol. 2015, 9, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Farrag, K.; Stein, J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. I.J. Chronic. Dis. 2018, 18, 9394060. [Google Scholar] [CrossRef]
- Kölmel, S.; Nowak, A.; Krayenbuehl, P.A. Iron Overload Associated Symptoms and Laboratory Changes in the Swiss Haemochromatosis Cohort—When a Clinician Should Become Attentive. Swiss Med. Wkly. 2020, 150, w20294. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Satoh, A.; Horinouchi, Y.; Hamano, H.; Watanabe, H.; Imao, M.; Imanishi, M.; Zamami, Y.; Takechi, K.; Izawa-Ishizawa, Y.; et al. Iron Accumulation Causes Impaired Myogenesis Correlated with MAPK Signaling Pathway Inhibition by Oxidative Stress. FASEB J. 2019, 33, 9551–9564. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Halon-Golabek, M.; Borkowska, A.; Herman-Antosiewicz, A.; Antosiewicz, J. Iron Metabolism of the Skeletal Muscle and Neurodegeneration. Front. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef]
- Mancardi, D.; Mezzanotte, M.; Arrigo, E.; Barinotti, A.; Roetto, A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants 2021, 10, 1864. [Google Scholar] [CrossRef]
- Rossi, E.M.; Andrade Ávila, R.; Tereza, M.; Carneiro, W.D.; Almenara, C.C.P.; Santos, L. Dos Chronic Iron Overload Restrains the Benefits of Aerobic Exercise to the Vasculature. Biol. Trace Elem. Res. 2011, 198, 521–534. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Cytochrome c Oxidase Dysfunction in Oxidative Stress. Free Radic. Biol. Med. 2012, 53, 1252–1263. [Google Scholar] [CrossRef]
- Guerra-Castellano, A.; Díaz-Quintana, A.; Pérez-Mejías, G.; Elena-Real, C.A.; González-Arzola, K.; García-Mauriño, S.M.; De la Rosa, M.A.; Díaz-Moreno, I. Oxidative Stress Is Tightly Regulated by Cytochrome c Phosphorylation and Respirasome Factors in Mitochondria. Proc. Natl. Acad. Sci. USA 2018, 115, 7955–7960. [Google Scholar] [CrossRef] [PubMed]
- Sheokand, N.; Malhotra, H.; Kumar, S.; Ajit Tillu, V.; Singh, A.; Iyengar Raje, C.; Raje, M. Moonlighting Cell Surface GAPDH Recruits Apo Transferrin to Effect Iron Egress from 1 Mammalian Cells. J. Cell Sci. 2014, 127, 4279–4291. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Mao, C.; Kondiparthi, L.; Poyurovsky, M.V.; Olszewski, K.; Gan, B. A Ferroptosis Defense Mechanism Mediated by Glycerol-3-Phosphate Dehydrogenase 2 in Mitochondria. Proc. Natl. Acad. Sci. USA 2022, 119, e21219871. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Bardou-Jacquet, E. Revisiting Hemochromatosis: Genetic vs. Phenotypic Manifestations. Ann. Transl. Med. 2021, 9, 731. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial Dysfunction: Roles in Skeletal Muscle Atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and Iron: Current Questions. Expert. Rev. Hematol. 2017, 10, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, R.N.; Boeno, F.P.; Dowllah, I.M.; Moritz, C.E.J.; Nguyen, B.L.; Doerr, V.; Bomkamp, M.P.; Smuder, A.J. Exercise and Doxorubicin Modify Markers of Iron Overload and Cardiolipin Deficiency in Cardiac Mitochondria. Int. J. Mol. Sci. 2023, 24, 7689. [Google Scholar] [CrossRef]
- Duan, G.; Li, J.; Duan, Y.; Zheng, C.; Guo, Q.; Li, F.; Zheng, J.; Yu, J.; Zhang, P.; Wan, M.; et al. Mitochondrial Iron Metabolism: The Crucial Actors in Diseases. Molecules 2023, 28, 29. [Google Scholar] [CrossRef]
- Seo, A.Y.; Xu, J.; Servais, S.; Hofer, T.; Marzetti, E.; Wohlgemuth, S.E.; Knutson, M.D.; Chung, H.Y.; Leeuwenburgh, C. Mitochondrial Iron Accumulation with Age and Functional Consequences. Aging Cell 2008, 7, 706–716. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum Ferritin Is an Important Inflammatory Disease Marker, as It Is Mainly a Leakage Product from Damaged Cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Zotter, H.; Robinson, N.; Zorzoli, M.; Schattenberg, L.; Saugy, M.; Mangin, P. Abnormally High Serum Ferritin Levels among Professional Road Cyclists. Br. J. Sports Med. 2004, 38, 704–708. [Google Scholar] [CrossRef]
- Bari, M.A.; MahmoodAlobaidi, M.A.; Ansari, H.A.; Parrey, J.A.; Ajhar, A.; Nuhmani, S.; Alghadir, A.H.; Khan, M. Effects of an Aerobic Training Program on Liver Functions in Male Athletes: A Randomized Controlled Trial. Sci. Rep. 2023, 13, 9427. [Google Scholar] [CrossRef]
- Mettler, S.; Zimmermann, M.B. Iron Excess in Recreational Marathon Runners. Eur. J. Clin. Nutr. 2010, 64, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Zeller, M.P. Management of iron deficiency. Hematol. Am. J. Hematol. 2019, 2019, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Schrage, B.; Rübsamen, N.; Schulz, A.; Münzel, T.; Pfeiffer, N.; Wild, P.S.; Beutel, M.; Schmidtmann, I.; Lott, R.; Blankenberg, S.; et al. Iron deficiency is a common disorder in general population and independently predicts all-cause mortality: Results from the Gutenberg Health Study. Clin. Res. Cardiol. 2020, 109, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Lopez Amaro, M.A.; Martoz, C. Iron availability: An updated review. Int. J. Food Sci. Nutri 2004, 55, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of Black Bean (Phaseolus Vulgaris L.) Polyphenols That Inhibit and Promote Iron Uptake by Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting Phytate-Element Interactions: Implications for Iron, Zinc and Calcium Bioavailability, with Emphasis on Legumes. Crit. Rev. Food Sci. Nutr. 2022, 62, 1696–1712. [Google Scholar] [CrossRef]
- Hallberg, L.; Rossander-Hulthèn, L.; Brune, M.; Gleerup, A. Inhibition of Haem-Iron Absorption in Man by Calcium. British J. Nutri 1993, 69, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Benkhedda, K.; L’Abbé, M.R.; Cockell, K.A. Effect of Calcium on Iron Absorption in Women with Marginal Iron Status. British J. Nutri 2010, 103, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Pizarro, F.; Ruz, M.; De Romaña, D.L. Acute Inhibition of Iron Bioavailability by Zinc: Studies in Humans. BioMetals 2012, 25, 657–664. [Google Scholar] [CrossRef]
- Yamaji, S.; Tennant, J.; Tandy, S.; Williams, M.; Singh Srai, S.K.; Sharp, P. Zinc Regulates the Function and Expression of the Iron Transporters DMT1 and IREG1 in Human Intestinal Caco-2 Cells. FEBS Lett. 2001, 507, 137–141. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Ganasen, M.; Togashi, H.; Takeda, H.; Asakura, H.; Tosha, T.; Yamashita, K.; Hirata, K.; Nariai, Y.; Urano, T.; Yuan, X.; et al. Structural Basis for Promotion of Duodenal Iron Absorption by Enteric Ferric Reductase with Ascorbate. Commun. Biol. 2018, 1, 120. [Google Scholar] [CrossRef]
- Lane, D.J.R.; Chikhani, S.; Richardson, V.; Richardson, D.R. Transferrin Iron Uptake Is Stimulated by Ascorbate via an Intracellular Reductive Mechanism. Biochim. Biophys. Acta 2013, 1833, 1527–1541. [Google Scholar] [CrossRef]
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; MacPhail, P.; Schmidt, U.; Ta, A.; Mayet, F. Ascorbic Acid Prevents the Dose-Dependent Inhibitory Effects of Polyphenols and Phytates on Nonheme-Iron Absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, T.; Muthayya, S.; Wegmüller, R.; Thankachan, P.; Sierksma, A.; Frenken, L.G.J.; Thomas, T.; Kurpad, A.; Hurrell, R.F. Inhibition of Iron Absorption by Calcium Is Modest in an Iron-Fortified, Casein- and Whey-Based Drink in Indian Children AndIs Easily Compensated for by Addition of Ascorbic Acid. J. Nutr. 2014, 144, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Pacier, C.; Martirosyan, D.M. Vitamin D: Optimal dosages, supplementation and use in disease prevention. Func. Food Health Dis. 2015, 5, 89–107. [Google Scholar] [CrossRef]
- von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B.; Stoffel, N.U. Effect of Dietary Factors and Time of Day on Iron Absorption from Oral Iron Supplements in Iron Deficient Women. Am. J. Hematol. 2023, 98, 1356–1363. [Google Scholar] [CrossRef]
- Hinton, P.S.; Sinclair, L.M. Iron Supplementation Maintains Ventilatory Threshold and Improves Energetic Efficiency in Iron-Deficient Nonanemic Athletes. Eur. J. Clin. Nutr. 2007, 61, 30–39. [Google Scholar] [CrossRef]
- Lamanca, J.J.; Haymes, E.M. Effects of iron repletion on VO2 max, endurance and blood lactate in women. Med. Sci. Sports Exerc. 1993, 25, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of Iron Supplementation on Fatigue and Physical Capacity in Non-Anaemic Iron-Deficient Adults: A Systematic Review of Randomised Controlled Trials. BMJ Open 2018, 8, e019240. [Google Scholar] [CrossRef]
- Klingshirn, L.A. Effect on iron supplementation on endurance capacity in iron depleted female rumors. Med. Sci. Sports Exerc. 1991, 24, 819–824. [Google Scholar]
- Rubeor, A.; Goojha, C.; Manning, J.; White, J. Does Iron Supplementation Improve Performance in Iron-Deficient Nonanemic Athletes? Sports Health 2018, 10, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Peeling, P.; Nemeth, E.; Bergland, D.; Mccluskey, W.T.P.; Stellingwerff, T. Single versus Split. Dose of Iron Optimizes Hemoglobin Mass Gains at 2106 m Altitude. Med. Sci. Sports Exerc. 2019, 51, 751–759. [Google Scholar] [CrossRef]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- McCormick, R.; Moretti, D.; McKay, A.K.A.; Laarakkers, C.M.; Vanswelm, R.; Trinder, D.; Cox, G.R.; Zimmerman, M.B.; Sim, M.; Goodman, C.; et al. The Impact of Morning versus Afternoon Exercise on Iron Absorption in Athletes. Med. Sci. Sports Exerc. 2019, 51, 2147–2155. [Google Scholar] [CrossRef]
- Santiago, P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: A clinical overview. Sci. World J. 2012, 2012, 846824. [Google Scholar] [CrossRef]
- Balendran, S.; Forsyth, C. Non-Anaemic Iron Deficiency. Aust. Prescr. 2021, 44, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.A.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef] [PubMed]
- Hussain, U.; Zia, K.; Iqbal, R.; Saeed, M.; Ashraf, N. Efficacy of a Novel Food Supplement (Ferfer®) Containing Microencapsulated Iron in Liposomal Form in Female Iron Deficiency Anemia. Cureus 2019, 11, e4063. [Google Scholar] [CrossRef]
- Li, S.; Guo, T.; Guo, W.; Cui, X.; Zeng, M.; Wu, H. Polyphosphates as an Effective Vehicle for Delivery of Bioavailable Nanoparticulate Iron(III). Food Chem. 2022, 373, 131477. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, K.; Ratkowski, W.; Wołyniec, W.; Kaczmarczyk, M.; Witek, K.; Żmijewski, P.; Renke, M.; Jastrzębski, Z.; Rosemann, T.; Nikolaidis, P.T.; et al. The effect of vitamin D3 supplementation on hepcidin, iron, and IL-6 responses after a 100 km ultra-marathon. Int. J. Environ. Res. Public. Health 2020, 17, 2962. [Google Scholar] [CrossRef]
- Dawson, B.; Goodman, C.; Blee, T.; Claydon, G.; Peeling, P.; Beilby, J.; Prins, A. Iron Supplementation: Oral Tablets Versus Intramuscular Injection. Int. J. Sport. Nutri Exerc. Metab. 2006, 16, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Blee, T.; Goodman, C.; Dawson, B.; Stapff, A. The Effect of Intramuscular Iron Injections on Serum Ferr|tin Levels and Physical Performance in E|ite Netba|lers. J. Sci. Med. Sport. 1999, 2, 311–321. [Google Scholar] [CrossRef]
- Burden, R.J.; Pollock, N.; Whyte, G.P.; Richards, T.; Moore, B.; Busbridge, M.; Srai, S.K.; Otto, J.; Pedlar, C.R. Effect of Intravenous Iron on Aerobic Capacity and Iron Metabolism in Elite Athletes. Med. Sci. Sports Exerc. 2015, 47, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron Deficiency Anaemia Revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef]
- Available online: https://www.allergan.com/assets/pdf/infed_pi (accessed on 30 October 2023).
- Available online: http://products.sanofi.us/ferrlecit/ferrlecit.html (accessed on 30 October 2023).
- Available online: https://www.venofer.com/pdfs/19-VFR-1222_VenoferDosingAdmin_04-05-2019.pdf (accessed on 30 October 2023).
- Available online: https://injectaferhcp.com/iron-deficiency-anemia-dosing (accessed on 30 October 2023).
- Golubov, J.; Flanagan, P.; Adams, P. Inhibition of Iron Absorption by Omeprazole in Rat Model. Dig. Dis. Sci. 1991, 36, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Deloughery, T. Single-dose intravenous iron for iron deficiency: A new paradigm. Hematology Am. Soc. Hematol. Educ. Program. 2016, 2016, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Schena, F.; Franchini, M.; Salvagno, G.L.; Guidi, G.C. Serum Ferritin as a Marker of Potential Biochemical Iron Overload in Athletes. Clin. J. Sport. Med. 2005, 15, 356–358. [Google Scholar] [CrossRef]
- Dellavalle, D.M. Iron Supplementation for Female Athletes: Effects on Iron Status and Performance Outcomes. Curr. Sports Med. Rep. 2013, 12, 234–239. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Erce, J.A.; Remacha, Á.F. Disorders of Iron Metabolism. Part II: Iron Deficiency and Iron Overload. J. Clin. Pathol. 2011, 64, 287–296. [Google Scholar] [CrossRef]
- Kaye, P.; Abdulla, K.; Wood, J.; James, P.; Foley, S.; Ragunath, K.; Atherton, J. Iron-Induced Mucosal Pathology of the Upper Gastrointestinal Tract: A Common Finding in Patients on Oral Iron Therapy. Histopathology 2008, 53, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Proksell, S.; Kuan, S.-F.; Behari, J. Iron Pill-Induced Gastritis. ACG Case Rep. J. 2013, 1, 13–15. [Google Scholar] [CrossRef][Green Version]
- Haig, A.; Driman, D.K. Iron-Induced Mucosal Injury to the Upper Gastrointestinal Tract. Histopathology 2006, 48, 808–812. [Google Scholar] [CrossRef]
- Sunkara, T.; Caughey, M.E.; Nigar, S.; Olivo, R.; Gaduputi, V. Iron Pill Gastritis: An Under Diagnosed Condition with Potentially Serious Outcomes. Gastroenterol. Res. 2017, 10, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Sussman, H.H. Iron in Cancer. Pathobiology 1992, 60, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Niederau, C.; Fischer, R.; Pürschel, A.; Purschel, P.; Stremmel, W.; Haüssinger, D.; Haüssinger, H.; Strohmeyer, G. Liver, Pancreas, and Biliary Tract Long-Term Survival in Patients With Hereditary Hemochromatosis. Gastroenterology 1996, 110, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.L.; Girelli, M.D.; Phatak, P. Patient education: Hereditary hemochromatosis (Beyond the Basics). 2023. Available online: https://www.uptodate.com/contents/hereditary-hemochromatosis-beyond-the-basics (accessed on 1 November 2023).
- Christopher, A.F. Iron Chelation therapy in myelodysplastic syndromes. Am. J. Health Syst. Pharm. 2010, 67, S10–S14. [Google Scholar]
- Adams, P.C.; Barton, J.C. How I treat hemochromatosis. Blood J. Am. Soci. Hematol. 2010, 116, 317–325. [Google Scholar] [CrossRef]
- Chen, W.J.; Kung, G.P.; Gnana-Prakasam, J.P. Role of Iron in Aging Related Diseases. Antioxidants 2022, 11, 865. [Google Scholar] [CrossRef]
- Wood, J.C. Guidelines for quantifying iron overload. Hematology. Am. J. Hematol. 2014, 2014, 210–215. [Google Scholar]
| Gene | Location | Geographical Distribution | Inheritance |
|---|---|---|---|
| HFE (p. Cys282Tyr) | Chromosome 6 | Highest prevalence in Northern Europeans | Autosomal recessive |
| HFE (non-p.Cys282Tyr) | Chromosome 6 | Highest prevalence in Northern Europeans | Autosomal recessive |
| HJV | Chromosome 1 | Highest in Southern Asia | Autosomal recessive |
| TFR2 | Chromosome 7 | Highest among Non-Finnish Europeans | Autosomal recessive |
| HAMP | Chromosome 19 | Several populations | Autosomal recessive |
| SLC40A1 | Chromosome 2 | Several populations, highest among African populations | Autosomal dominant |
| Animal-Source (Heme) | Iron (mg) per 100 g |
| Chicken, liver, simmered | 11.6 |
| Oyster, moist heat | 9.21 |
| Mussels, moist heat | 6.72 |
| Beef, liver, braised | 6.54 |
| Beef, tenderloin, roast, choice, roasted | 3.11 |
| Sardines, canned in oil | 2.92 |
| Duck, meat, roasted | 2.7 |
| Lamb, sirloin chop, broiled | 2.34 |
| Beef, ground, 90% lean meat | 2.3 |
| Turkey, ground, cooked | 1.52 |
| Turkey, dark meat, roasted | 1.43 |
| Tuna, light, canned in oil | 1.39 |
| Egg, whole, scrambled | 1.31 |
| Pork, tenderloin, roasted | 1.15 |
| Salmon, Atlantic wild, dry heat | 1.03 |
| Plant-Source (Non-heme) | Iron (mg) per 100 g |
| Tofu, raw, regular | 5.36 |
| Soybeans, boiled | 5.14 |
| Molasses | 4.72 |
| Bread, wheat, toasted | 4.09 |
| Pistachios, raw | 3.92 |
| Lentils, boiled | 3.33 |
| Red kidney beans, boiled | 2.94 |
| Walnuts, raw | 2.91 |
| Chickpeas, boiled | 2.89 |
| Spinach, raw | 2.71 |
| Pecans, raw | 2.51 |
| Lima beans, boiled | 2.39 |
| Black beans, boiled | 2.1 |
| Pinto beans, boiled | 2.09 |
| Pasta, whole-wheat, cooked | 1.72 |
| Compound | Formulation | Elemental Iron Content (mg) | Recommended Dosage |
|---|---|---|---|
| Ferrous gluconate | Tablet, 240 mg | 27 | 1–3 tablets once per day or on an alternate-day schedule [58] |
| Ferrous sulfate | Tablet, 325 mg | 65 | 1–2 tablets once per day or on an alternate-day schedule [58] |
| Ferrous fumarate | Tablet, 324 mg | 106 | 1 tablet once per day or on an alternate-day schedule [58] |
| Form | Amount per Dose Recommended (mg) | Infusion Time | Reference |
|---|---|---|---|
| Iron dextran (Low-molecular-weight) | 100 | 2 to 6 h | [93] |
| Ferrous gluconate | 125 | 12.5 mg per min | [94] |
| Iron sucrose | 200–300 | 100 mg per 5 min | [95] |
| Ferric carboxymaltose | 750 | 15 min | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kardasis, W.; Naquin, E.R.; Garg, R.; Arun, T.; Gopianand, J.S.; Karmakar, E.; Gnana-Prakasam, J.P. The IRONy in Athletic Performance. Nutrients 2023, 15, 4945. https://doi.org/10.3390/nu15234945
Kardasis W, Naquin ER, Garg R, Arun T, Gopianand JS, Karmakar E, Gnana-Prakasam JP. The IRONy in Athletic Performance. Nutrients. 2023; 15(23):4945. https://doi.org/10.3390/nu15234945
Chicago/Turabian StyleKardasis, William, Ethan R. Naquin, Richa Garg, Tejas Arun, Jyotsna S. Gopianand, Eshani Karmakar, and Jaya P. Gnana-Prakasam. 2023. "The IRONy in Athletic Performance" Nutrients 15, no. 23: 4945. https://doi.org/10.3390/nu15234945
APA StyleKardasis, W., Naquin, E. R., Garg, R., Arun, T., Gopianand, J. S., Karmakar, E., & Gnana-Prakasam, J. P. (2023). The IRONy in Athletic Performance. Nutrients, 15(23), 4945. https://doi.org/10.3390/nu15234945