Dietary Barriers Appear to Influence the Effects of a Dyadic Web-Based Lifestyle Intervention on Caloric Intake and Adiposity: A Mediation Analysis of the DUET Trial
Highlights
- The DUET intervention improved caloric intake, weight, and waist circumference through reductions in perceived dietary barriers, while social support and self-efficacy did not mediate these effects.
- Targeting dietary barriers is critical for improving diet and adiposity outcomes within web-based interventions. Furthermore, there is a need to incorporate strategies that enhance both social support and self-efficacy within web-based interventions in order to promote behavior change.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. DUET Intervention and Mediators
2.3. Measures
2.3.1. Dependent Variables (Y)
2.3.2. Mediators (M)
2.4. Simple Mediation Model
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Hyun, N.; Leach, C.R.; Yabroff, K.R.; Jemal, A. Association of First Primary Cancer with Risk of Subsequent Primary Cancer Among Survivors of Adult-Onset Cancers in the United States. JAMA 2020, 324, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Reeve, B.B.; Kucharska-Newton, A.M.; Foraker, R.E.; Platz, E.A.; Stearns, S.C.; Han, X.; Windham, B.G.; Irwin, D.E. Functional status declines among cancer survivors: Trajectory and contributing factors. J. Geriatr. Oncol. 2014, 5, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Heo, J.; Chun, M.; Oh, Y.T.; Noh, O.K.; Kim, L. Metabolic comorbidities and medical institution utilization among breast cancer survivors: A national population-based study. Korean J. Intern. Med. 2020, 35, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef]
- Mariotto, A.B.; Enewold, L.; Zhao, J.; Zeruto, C.A.; Yabroff, K.R. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1304–1312. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 4 September 2023).
- National Cancer Institute. Cancer Survivors and Weight. Available online: https://progressreport.cancer.gov/after/weight (accessed on 4 September 2023).
- Cao, Y.; Ma, J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prev. Res. 2011, 4, 486–501. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef]
- Byrd, D.A.; Agurs-Collins, T.; Berrigan, D.; Lee, R.; Thompson, F.E. Racial and Ethnic Differences in Dietary Intake, Physical Activity, and Body Mass Index (BMI) Among Cancer Survivors: 2005 and 2010 National Health Interview Surveys (NHIS). J. Racial Ethn. Health Disparities 2017, 4, 1138–1146. [Google Scholar] [CrossRef]
- Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Adherence to multiple health behaviours in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2019, 13, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.; Jones, D.J.; Sremanakova, J.; Sowerbutts, A.M.; Lal, S.; Pilling, M.; Todd, C. Dietary interventions for adult cancer survivors. Cochrane Database Syst. Rev. 2019, 2019, CD011287. [Google Scholar] [CrossRef]
- Irwin, M.L. Physical activity interventions for cancer survivors. Br. J. Sports Med. 2009, 43, 32–38. [Google Scholar] [CrossRef]
- Djuric, Z.; DiLaura, N.M.; Jenkins, I.; Darga, L.; Jen, C.K.; Mood, D.; Bradley, E.; Hryniuk, W.M. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes. Res. 2002, 10, 657–665. [Google Scholar] [CrossRef]
- von Gruenigen, V.; Frasure, H.; Kavanagh, M.B.; Janata, J.; Waggoner, S.; Rose, P.; Lerner, E.; Courneya, K.S. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): A randomized controlled trial. Gynecol. Oncol. 2012, 125, 699–704. [Google Scholar] [CrossRef]
- Bluethmann, S.M.; Bartholomew, L.K.; Murphy, C.C.; Vernon, S.W. Use of Theory in Behavior Change Interventions. Health Educ. Behav. 2017, 44, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Stacey, F.G.; James, E.L.; Chapman, K.; Courneya, K.S.; Lubans, D.R. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J. Cancer Surviv. 2015, 9, 305–338. [Google Scholar] [CrossRef] [PubMed]
- Glanz, K.; Lewis, F.M.; Rimer, B.K. How Individuals, Environments, and Health Behavior Interact: Social Cognitive Theory. Health Behavior and Health Education: Theory, Research, and Practice, 2nd ed.; American Psychological Association (APA): San Francisco, CA, USA, 1997; pp. 153–174. [Google Scholar]
- Mosher, C.E.; Lipkus, I.; Sloane, R.; Snyder, D.C.; Lobach, D.F.; Demark-Wahnefried, W. Long-term outcomes of the FRESH START trial: Exploring the role of self-efficacy in cancer survivors’ maintenance of dietary practices and physical activity. Psychooncology 2013, 22, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Markwell, S.; Hopkins-Price, P.; Vicari, S.; Courneya, K.S.; Hoelzer, K.; Verhulst, S. Reduced barriers mediated physical activity maintenance among breast cancer survivors. J. Sport. Exerc. Psychol. 2011, 33, 235–254. [Google Scholar] [CrossRef]
- Ellis, K.R.; Raji, D.; Olaniran, M.; Alick, C.; Nichols, D.; Allicock, M. A systematic scoping review of post-treatment lifestyle interventions for adult cancer survivors and family members. J. Cancer Surviv. 2022, 16, 233–256. [Google Scholar] [CrossRef]
- Usta, Y.Y. Importance of social support in cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 3569–3572. [Google Scholar] [CrossRef]
- Martin, E.C.; Basen-Engquist, K.; Cox, M.G.; Lyons, E.J.; Carmack, C.L.; A Blalock, J.; Demark-Wahnefried, W. Interest in Health Behavior Intervention Delivery Modalities Among Cancer Survivors: A Cross-Sectional Study. JMIR Cancer 2016, 2, e1. [Google Scholar] [CrossRef]
- Wijesooriya, N.R.; Mishra, V.; Brand, P.L.P.; Rubin, B.K. COVID-19 and telehealth, education, and research adaptations. Paediatr. Respir. Rev. 2020, 35, 38–42. [Google Scholar] [CrossRef]
- Roberts, A.L.; Fisher, A.; Smith, L.; Heinrich, M.; Potts, H.W.W. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2017, 11, 704–719. [Google Scholar] [CrossRef]
- Furness, K.; Sarkies, M.N.; Huggins, C.E.; Croagh, D.; Haines, T.P. Impact of the Method of Delivering Electronic Health Behavior Change Interventions in Survivors of Cancer on Engagement, Health Behaviors, and Health Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e16112. [Google Scholar] [CrossRef]
- Haberlin, C.; O’Dwyer, T.; Mockler, D.; Moran, J.; O’Donnell, D.M.; Broderick, J. The use of eHealth to promote physical activity in cancer survivors: A systematic review. Support. Care Cancer 2018, 26, 3323–3336. [Google Scholar] [CrossRef]
- Lazar, D.E.; Postolica, R.; Hanganu, B.; Mocanu, V.; Ioan, B.G. Web-based nutrition: A useful resource for cancer patients? Front. Nutr. 2023, 10, 1134793. [Google Scholar] [CrossRef] [PubMed]
- Pekmezi, D.W.; Crane, T.E.; Oster, R.A.; Rogers, L.Q.; Hoenemeyer, T.; Farrell, D.; Cole, W.W.; Wolin, K.; Badr, H.; Demark-Wahnefried, W. Rationale and Methods for a Randomized Controlled Trial of a Dyadic, Web-Based, Weight Loss Intervention among Cancer Survivors and Partners: The DUET Study. Nutrients 2021, 13, 3472. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Oster, R.A.; Crane, T.E.; Rogers, L.Q.; Cole, W.W.; Kaur, H.; Farrell, D.; Parrish, K.B.; Badr, H.J.; Wolin, K.Y.; et al. Results of DUET: A Web-Based Weight Loss Randomized Controlled Feasibility Trial among Cancer Survivors and Their Chosen Partners. Cancers 2023, 15, 1577. [Google Scholar] [CrossRef] [PubMed]
- Demark-Wahnefried, W.; Jones, L.W.; Snyder, D.C.; Sloane, R.J.; Kimmick, G.G.; Hughes, D.C.; Badr, H.J.; Miller, P.E.; Burke, L.E.; Lipkus, I.M. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer 2014, 120, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Ashford, S.; Sniehotta, F.F.; Dombrowski, S.U.; Bishop, A.; French, D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: The CALO-RE taxonomy. Psychol. Health 2011, 26, 1479–1498. [Google Scholar] [CrossRef] [PubMed]
- Glanz, K.; Lewis, F.M.; Rimer, B.K. Social Networks and Social Support. Health Behavior and Health Education: Theory, Research, and Practice, 2nd ed.; American Psychological Association (APA): San Francisco, CA, USA, 1997; pp. 179–205. [Google Scholar]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; McAuley, E.; Anton, P.M.; Courneya, K.S.; Vicari, S.; Hopkins-Price, P.; Verhulst, S.; Mocharnuk, R.; Hoelzer, K. Better exercise adherence after treatment for cancer (BEAT Cancer) study: Rationale, design, and methods. Contemp. Clin. Trials 2012, 33, 124–137. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J. Cancer Surviv. 2015, 9, 532–540. [Google Scholar] [CrossRef]
- Freudenheim, J.L.; Darrow, S.L. Accuracy of self-measurement of body fat distribution by waist, hip, and thigh circumferences. Nutr. Cancer. 1991, 15, 179–186. [Google Scholar] [CrossRef]
- Hoenemeyer, T.W.; Cole, W.W.; Oster, R.A.; Pekmezi, D.W.; Pye, A.; Demark-Wahnefried, W. Test/Retest Reliability and Validity of Remote vs. In-Person Anthropometric and Physical Performance Assessments in Cancer Survivors and Supportive Partners. Cancers 2022, 14, 21. [Google Scholar] [CrossRef]
- Clark, M.M.; Abrams, D.B.; Niaura, R.S.; Eaton, C.A.; Rossi, J.S. Self-efficacy in weight management. J. Consult. Clin. Psychol. 1991, 59, 739–744. [Google Scholar] [CrossRef]
- McAuley, E.; Blissmer, B. Self-efficacy determinants and consequences of physical activity. Exerc. Sport Sci. Rev. 2000, 28, 85–88. [Google Scholar]
- Sallis, J.F.; Grossman, R.M.; Pinski, R.B.; Patterson, T.L.; Nader, P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987, 16, 825–836. [Google Scholar] [CrossRef]
- Arroyave, W.D.; Clipp, E.C.; Miller, P.E.; Jones, L.W.; Ward, D.S.; Bonner, M.J.; Rosoff, P.M.; Snyder, D.C.; Demark-Wahnefried, W. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol. Nurs. Forum 2008, 35, 121–130. [Google Scholar] [CrossRef]
- Vijan, S.; Stuart, N.S.; Fitzgerald, J.T.; Ronis, D.L.; Hayward, R.A.; Slater, S.; Hofer, T.P. Barriers to following dietary recommendations in Type 2 diabetes. Diabet. Med. 2005, 22, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.Q.; Courneya, K.S.; Shah, P.; Dunnington, G.; Hopkins-Price, P. Exercise stage of change, barriers, expectations, values and preferences among breast cancer patients during treatment: A pilot study. Eur. J. Cancer Care 2007, 16, 55–66. [Google Scholar] [CrossRef] [PubMed]
- MPLUS Software, Version 8; Muthén & Muthén (1998–2011). Available online: https://www.statmodel.com/ (accessed on 2 June 2023).
- Fritz, M.S.; Mackinnon, D.P. Required sample size to detect the mediated effect. Psychol. Sci. 2007, 18, 233–239. [Google Scholar] [CrossRef]
- Burbidge, J.B.; Magee, L.; Robb, A.L. Alternative Transformations to Handle Extreme Values of the Dependent Variable. J. Am. Stat. Assoc. 1988, 83, 123–127. [Google Scholar] [CrossRef]
- Allison, P.D. Handling Missing Data by Maximum Likelihood. 2012. Available online: https://statisticalhorizons.com/wp-content/uploads/MissingDataByML.pdf (accessed on 4 September 2023).
- Ikram, M.A. The disjunctive cause criterion by VanderWeele: An. easy solution to a complex problem? Eur. J. Epidemiol. 2019, 34, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Kindred, M.M.; Pinto, B.M.; Dunsiger, S.I. Mediators of physical activity adoption and maintenance among breast cancer survivors. J. Behav. Med. 2020, 43, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Stacey, F.G.; James, E.L.; Chapman, K.; Lubans, D.R. Social cognitive theory mediators of physical activity in a lifestyle program for cancer survivors and carers: Findings from the ENRICH randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 49. [Google Scholar] [CrossRef]
- Ko, L.K.; Turner-McGrievy, G.M.; Campbell, M.K. Information processing versus social cognitive mediators of weight loss in a podcast-delivered health intervention. Health Educ. Behav. 2014, 41, 197–206. [Google Scholar] [CrossRef]
- Leslie, E.; Marshall, A.L.; Owen, N.; Bauman, A. Engagement and retention of participants in a physical activity website. Prev. Med. 2005, 40, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, K.; Bao, X.; Liu, X.; Yao, J. Patterns of eHealth Website User Engagement Based on Cross-site Clickstream Data: Correlational Study. J. Med. Internet Res. 2021, 23, e29299. [Google Scholar] [CrossRef]
- Rentscher, K.E.; Zhou, X.; Small, B.J.; Cohen, H.J.; Dilawari, A.A.; Patel, S.K.; Bethea, T.N.; Van Dyk, K.M.; Nakamura, Z.M.; Ahn, J.; et al. Loneliness and mental health during the COVID-19 pandemic in older breast cancer survivors and noncancer controls. Cancer 2021, 127, 3671–3679. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.E.; Eirich, R.; Racine, N.; Madigan, S. Prevalence of posttraumatic and general psychological stress during COVID-19: A rapid review and meta-analysis. Psychiatry Res. 2020, 292, 113347. [Google Scholar] [CrossRef]
- Anderson, E.S.; Winett, R.A.; Wojcik, J.R.; Williams, D.M. Social cognitive mediators of change in a group randomized nutrition and physical activity intervention: Social support, self-efficacy, outcome expectations and self-regulation in the guide-to-health trial. J. Health Psychol. 2010, 15, 21–32. [Google Scholar] [CrossRef]
- Lambert, J.; Taylor, A.; Streeter, A.; Greaves, C.; Ingram, W.M.; Dean, S.; Jolly, K.; Mutrie, N.; Taylor, R.S.; Yardley, L.; et al. A process evaluation, with mediation analysis, of a web-based intervention to augment primary care exercise referral schemes: The e-coachER randomised controlled trial. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 128. [Google Scholar] [CrossRef]
- Beasley, J.M.; Newcomb, P.A.; Trentham-Dietz, A.; Hampton, J.M.; Ceballos, R.M.; Titus-Ernstoff, L.; Egan, K.M.; Holmes, M.D. Social networks and survival after breast cancer diagnosis. J. Cancer Surviv. 2010, 4, 372–380. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Smith, M.L.; Lee, S.; Towne, S.D.; Ory, M.G. The role of improved social support for healthy eating in a lifestyle intervention: Texercise Select. Public Health Nutr. 2021, 24, 146–156. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Carraça, E.V.; Marques, M.M.; Rutter, H.; Oppert, J.-M.; De Bourdeaudhuij, I.; Lakerveld, J.; Brug, J. Successful behavior change in obesity interventions in adults: A systematic review of self-regulation mediators. BMC Med. 2015, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, J.; Jenkins, S.; A Patten, C.; Hayes, S.N.; Jones, C.; A Cooper, L.; Brewer, L.C. Improvements in Diet and Physical Activity-Related Psychosocial Factors Among African Americans Using a Mobile Health Lifestyle Intervention to Promote Cardiovascular Health: The FAITH! (Fostering African American Improvement in Total Health) App Pilot Study. JMIR Mhealth Uhealth 2021, 9, e28024. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.K.; Carr, C.; DeVellis, B.; Switzer, B.; Biddle, A.; Amamoo, M.A.; Walsh, J.; Zhou, B.; Sandler, R. A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control. Ann. Behav. Med. 2009, 38, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Horton, L.A.; Ayala, G.X.; Slymen, D.J.; Ibarra, L.; Hernandez, E.; Parada, H.; Rock, C.L.; Arredondo, E.M.; Elder, J.P. A Mediation Analysis of Mothers’ Dietary Intake: The Entre Familia: Reflejos de Salud Randomized Controlled Trial. Health Educ. Behav. 2018, 45, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.V.; Edney, S.M.; Plotnikoff, R.C.; Olds, T.; Vandelanotte, C.; Ryan, J.; Curtis, R.; Maher, C.A. Examining social-cognitive theory constructs as mediators of behaviour change in the active team smartphone physical activity program: A mediation analysis. BMC Public. Health 2021, 21, 88. [Google Scholar] [CrossRef]
- Fjeldsoe, B.S.; Miller, Y.D.; Marshall, A.L. Social cognitive mediators of the effect of the MobileMums intervention on physical activity. Health Psychol. 2013, 32, 729–738. [Google Scholar] [CrossRef]
- Suminski, R.R.; Petosa, R. Web-assisted instruction for changing social cognitive variables related to physical activity. J. Am. Coll. Health 2006, 54, 219–225. [Google Scholar] [CrossRef]
- Carmack, C.L.; Parker, N.H.; Demark-Wahnefried, W.; Shely, L.; Baum, G.; Yuan, Y.; Giordano, S.H.; Rodriguez-Bigas, M.; Pettaway, C.; Basen-Engquist, K. Healthy Moves to Improve Lifestyle Behaviors of Cancer Survivors and Their Spouses: Feasibility and Preliminary Results of Intervention Efficacy. Nutrients 2021, 13, 4460. [Google Scholar] [CrossRef]



| Consequent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antecedent | Dietary Barriers (M) | Diet Quality (Y) | Indirect and Total Effects (SE, Standard Errors, [95% CI]) | ||||||||
| Coeff. | SE | 95% CI | Coeff. | SE | 95% CI | ab | Total | ||||
| DUET vs. WLC (X) | a | −6.49 | 2.60 | −12.33, −1.93 | c’ | 3.38 | 3.03 | −2.14, 9.32 | 0.62 (0.57) [−0.15, 2.15] | 4.00 (2.95) [−1.33, 9.71] | |
| Dietary Barriers (M) | - | - | b | −0.10 | 0.09 | −0.26, 0.06 | |||||
| Constant | iM 1 | 10.40 | 8.43 | −4.00, 29.53 | iY 2 | 38.48 | 10.54 | 15.99, 57.55 | |||
| R2 = 0.240, p = 0.003 | R2 = 0.111, p = 0.222 | ||||||||||
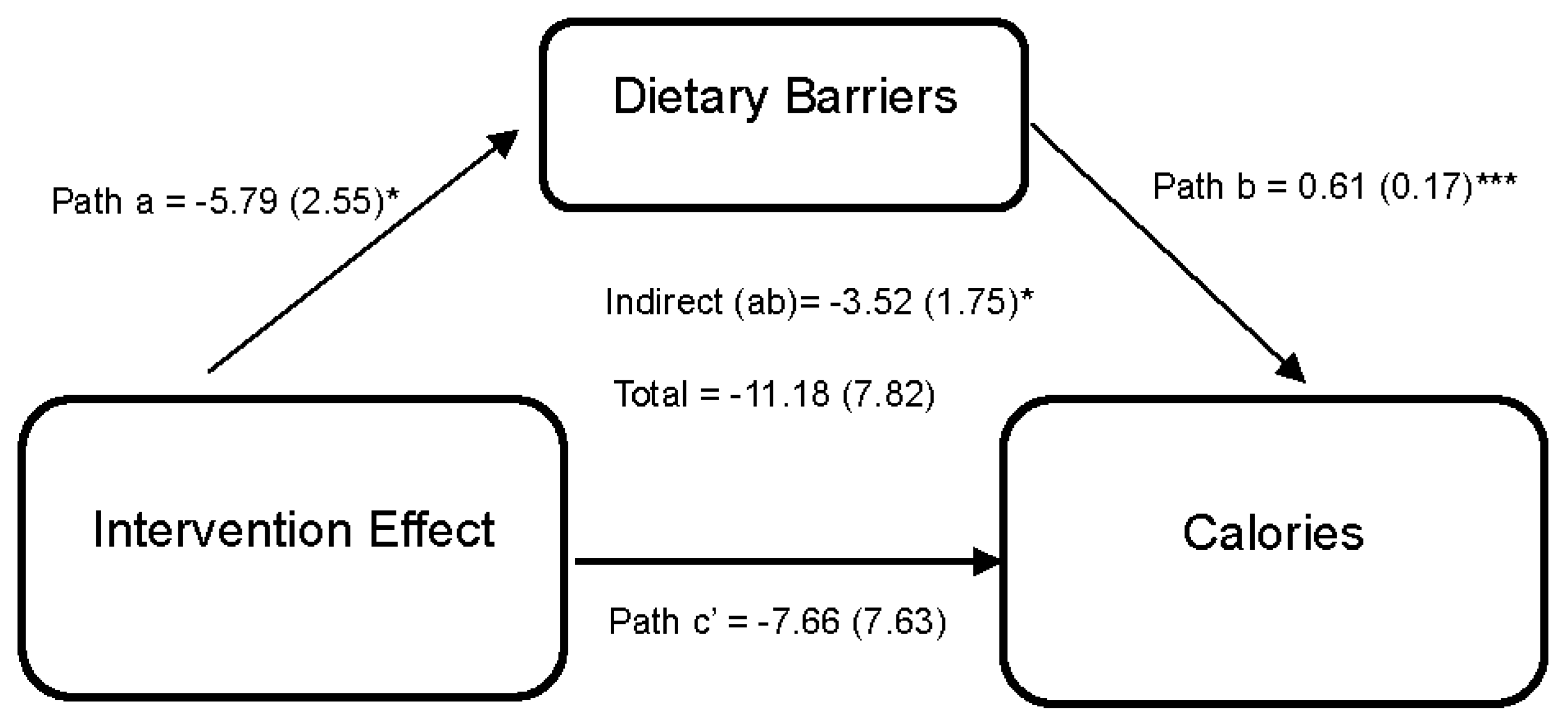
| Dietary Barriers (M) | Calories (Y) | −3.52 (1.75) [−8.08, −0.84] | −11.18 (7.82) [−27.10, 2.91] | ||||||||
| DUET vs. WLC (X) | a | −5.79 | 2.55 | −11.48, −1.26 | c’ | −7.66 | 7.63 | −23.38, 5.51 | |||
| Dietary Barriers (M) | - | - | - | b | 0.61 | 0.17 | 0.28, 0.94 | ||||
| Constant | iM | 3.63 | 8.51 | −13.66, 20.77 | iY | 68.94 | 22.71 | 17.53, 112.05 | |||
| R2 = 0.251, p = 0.001 | R2 = 0.497, p =< 0.001 | ||||||||||
| Exercise Barriers (M) | Self-report MVPA (Y) | 0.16 (0.12) [−0.01, 0.51] | 0.86 (0.42) [0.15, 1.75] | ||||||||
| DUET vs. WLC (X) | a | −6.13 | 3.48 | −12.47, 1.11 | c’ | 0.70 | 0.43 | −0.06, 1.55 | |||
| Exercise Barriers (M) | - | - | - | b | −0.03 | 0.01 | −0.06, −0.00 | ||||
| Constant | iM | 15.11 | 8.86 | −3.66, 32.96 | iY | 2.56 | 1.34 | −0.16, 4.97 | |||
| R2 = 0.271, p = 0.007 | R2 = 0.312, p = 0.001 | ||||||||||
| Exercise Barriers (M) | Accelerometer MVPA (Y) | ||||||||||
| DUET vs. WLC (X) | a | −5.92 | 3.89 | −13.42, 2.90 | c’ | 0.32 | 0.44 | −0.61, 1.08 | 0.03 (0.08) [−0.10, 0.24] | 0.35 (0.42) [−0.58, 1.10] | |
| Exercise Barriers (M) | - | - | - | b | −0.00 | 0.01 | −0.03, 0.02 | ||||
| Constant | iM | 18.78 | 12.18 | −1.12, 45.54 | iY | 2.31 | 1.35 | −0.71, 4.87 | |||
| R2 = 0.270, p = 0.011 | R2 = 0.329, p = 0.002 | ||||||||||
| Dietary Barriers (M) | Weight (Y) | ||||||||||
| DUET vs. WLC (X) | a | −7.59 | 2.84 | −13.68, −2.18 | c’ | −1.52 | 2.06 | −5.66, 2.24 | −1.60 (0.78) [−3.84, −0.47] | −3.12 (1.90) [−7.08, 0.38] | |
| Dietary Barriers (M) | - | - | - | b | 0.21 | 0.06 | 0.08, 0.32 | ||||
| Constant | iM | 33.18 | 14.53 | 4.78, 62.23 | iY | 6.35 | 8.27 | −10.79, 21.12 | |||
| R2 = 0.276, p = 0.001 | R2 = 0.951, p =< 0.001 | ||||||||||
| Dietary Barriers (M) | Waist Circumference (Y) | ||||||||||
| DUET vs. WLC (X) | a | −7.55 | 2.73 | −13.68, −2.72 | c’ | −0.46 | 1.16 | −2.67, 1.82 | −0.83 (0.41) [−1.77, −0.18] | −1.29 (1.10) [−3.51, 0.87] | |
| Dietary Barriers (M) | - | - | - | b | 0.11 | 0.04 | 0.01, 0.16 | ||||
| Constant | iM | 43.76 | 15.41 | 15.25, 74.48 | iY | 6.18 | 7.21 | −8.53, 19.21 | |||
| R2 = 0.289, p =< 0.001 | R2 = 0.862, p =< 0.001 | ||||||||||
| Consequent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antecedent | Dietary Social Support (M) | Diet Quality (Y) | Indirect and Total Effects (SE, Standard Errors, [95% CI]) | ||||||||
| Coeff. | SE | 95% CI | Coeff. | SE | 95% CI | ab | Total | ||||
| DUET vs. WLC (X) | a | 0.18 | 0.12 | −0.08, 0.40 | c’ | 3.60 | 2.79 | −1.51, 9.39 | 0.49 (0.54) [−0.21, 2.02] | 4.09 (2.77) [−0.89, 9.81] | |
| Dietary Social Support (M) | - | - | - | b | 2.82 | 1.87 | −1.07, 6.08 | ||||
| Constant | iM 1 | 1.88 | 0.42 | 1.09, 2.7 | iY 2 | 26.30 | 12.01 | 3.10, 48.73 | |||
| R2 = 0.323, p =< 0.001 | R2 =0.133, p = 0.176 | ||||||||||
| Dietary Social Support (M) | Calories (Y) | 1.09 (1.15) [−0.17, 5.58] | −11.53 (7.67) [−26.95, 2.07] | ||||||||
| DUET vs. WLC (X) | a | 0.18 | 0.12 | −0.09, 0.41 | c’ | −12.62 | 7.70 | −28.33, 0.98 | |||
| Dietary Social Support (M) | - | - | - | B | 6.20 | 4.37 | −1.55, 17.50 | ||||
| Constant | iM | 2.22 | 0.53 | 1.29, 3.25 | iY | 71.84 | 28.69 | 13.99, 125.16 | |||
| R2 = 0.310, p =< 0.001 | R2 = 0.476, p =< 0.001 | ||||||||||
| Exercise Social Support (M) | Self-report MVPA (Y) | 0.14 (0.14) [−0.04, 0.52] | 0.84 (0.43) [0.10, 1.72] | ||||||||
| DUET vs. WLC (X) | a | 0.19 | 0.15 | −0.08, 0.47 | c’ | 0.70 | 0.42 | −0.052, 1.55 | |||
| Exercise Social Support (M) | - | - | - | B | 0.69 | 0.27 | 0.19, 1.24 | ||||
| Constant | iM | 1.44 | 0.53 | 0.43, 2.46 | iY | −0.92 | 1.32 | −3.92, 1.23 | |||
| R2 = 0.267, p =< 0.001 | R2 = 0.283, p = 0.002 | ||||||||||
| Exercise Social Support (M) | Accelerometer MVPA (Y) | ||||||||||
| DUET vs. WLC (X) | a | 0.20 | 0.17 | −0.09, 0.57 | c’ | 0.27 | 0.42 | −0.60, 1.03 | 0.11 (0.11) [−0.04, 0.39] | 0.38 (0.42) [−0.56, 1.11] | |
| Exercise Social Support (M) | - | - | - | B | 0.55 | 0.22 | 0.15, 1.00 | ||||
| Constant | iM | 1.72 | 0.74 | 0.22, 3.19 | iY | 1.50 | 1.42 | −1.41, 4.80 | |||
| R2 = 0.273, p = 0.002 | R2 = 0.356, p =< 0.001 | ||||||||||
| Dietary Social Support (M) | Weight (Y) | ||||||||||
| DUET vs. WLC (X) | a | 0.18 | 0.12 | −0.09, 0.41 | c’ | −3.13 | 1.90 | −6.61, 0.51 | −0.15 (0.36) [−1.10, 0.39] | −3.28 (1.90) [−7.03, 0.44] | |
| Dietary Social Support (M) | - | - | - | B | −0.81 | 1.50 | −3.31, 2.83 | ||||
| Constant | iM | 2.04 | 0.77 | 0.49, 3.36 | iY | 15.24 | 10.24 | −6.54, 32.86 | |||
| R2 = 0.311, p =< 0.001 | R2 = 0.945, p =< 0.001 | ||||||||||
| Dietary Social Support (M) | Waist Circumference (Y) | ||||||||||
| DUET vs. WLC (X) | a | 0.19 | 0.13 | −0.09, 0.42 | c’ | −1.09 | 1.08 | −3.12, 1.05 | −0.09 (0.24) [−0.89, 0.17] | −1.19 (1.09) [−3.39, 0.93] | |
| Dietary Social Support (M) | - | - | - | B | −0.51 | 0.91 | −2.23, 1.30 | ||||
| Constant | iM | 1.85 | 0.80 | 0.43, 3.60 | iY | 9.51 | 9.26 | −8.76, 26.48 | |||
| R2 = 0.313, p =< 0.001 | R2 = 0.850, p =< 0.001 | ||||||||||
| Consequent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antecedent | Dietary Self-Efficacy (M) | Diet Quality (Y) | Indirect and Total Effects (SE, Standard Errors, [95% CI]) | ||||||||
| Coeff. | SE | 95% CI | Coeff. | SE | 95% CI | ab | Total | ||||
| DUET vs. WLC (X) | a | 0.05 | 0.03 | −0.02, 0.11 | c’ | 3.42 | 2.92 | −1.99, 9.06 | 0.53 (0.60) [−0.19, 2.27] | 3.95 (2.89) [−1.03, 9.57] | |
| Dietary Self-Efficacy (M) | - | - | - | b | 10.87 | 8.09 | −6.72, 27.38 | ||||
| Constant | iM 1 | 0.34 | 0.13 | 0.06, 0.58 | iY 2 | 33.28 | 10.17 | 11.73, 52.96 | |||
| R2 = 0.344, p =< 0.001 | R2 = 0.117, p = 0.181 | ||||||||||
| Dietary Self-Efficacy (M) | Calories (Y) | −1.30 (1.61) [−6.38, 0.40] | −12.88 (8.42) [−30.01, 1.80] | ||||||||
| DUET vs. WLC (X) | A | 0.04 | 0.03 | −0.03, 0.10 | c’ | −11.58 | 8.13 | −27.84, 2.44 | |||
| Dietary Self-Efficacy (M) | - | - | - | b | −36.45 | 20.42 | −75.04, 3.06 | ||||
| Constant | iM | 0.43 | 0.14 | 0.15, 0.69 | iY | 110.88 | 38.23 | 34.46, 179.19 | |||
| R2 = 0.360, p =< 0.001 | R2 = 0.485, p =< 0.001 | ||||||||||
| Exercise Self-Efficacy (M) | Self-report MVPA (Y) | 0.06 (0.11) [−0.04, 0.55] | 0.89 (0.39) [0.15, 1.71] | ||||||||
| DUET vs. WLC (X) | A | 0.04 | 0.05 | −0.07, 0.14 | c’ | 0.83 | 0.39 | 0.11, 1.67 | |||
| Exercise Self-Efficacy (M) | - | - | - | b | 1.32 | 1.00 | −0.58, 3.30 | ||||
| Constant | iM | 0.16 | 0.13 | −0.10, 0.41 | iY | −0.46 | 1.29 | −3.24, 1.87 | |||
| R2 = 0.199, p = 0.032 | R2 = 0.336, p =< 0.001 | ||||||||||
| Exercise Self-Efficacy (M) | Accelerometer MVPA (Y) | ||||||||||
| DUET vs. WLC (X) | A | 0.04 | 0.06 | −0.07, 0.15 | c’ | 0.32 | 0.40 | −0.60, 0.99 | 0.05 (0.08) [−0.04, 0.32] | 0.37 (0.40) [−0.59, 1.07] | |
| Exercise Self-Efficacy (M) | - | - | - | b | 1.10 | 0.77 | −0.43, 2.49 | ||||
| Constant | iM | 0.18 | 0.19 | −0.20, 0.56 | iY | 1.12 | 1.40 | −1.49, 4.14 | |||
| R2 = 0.230, p = 0.022 | R2 = 0.356, p =< 0.001 | ||||||||||
| Dietary Self-Efficacy (M) | Weight (Y) | ||||||||||
| DUET vs. WLC (X) | A | 0.06 | 0.04 | −0.01, 0.13 | c’ | −2.19 | 1.98 | −6.18, 1.43 | −0.74 (0.76) [−3.26, 0.11] | −2.93 (1.89) [−6.54, 0.36] | |
| Dietary Self-Efficacy (M) | - | - | - | b | −13.37 | 7.95 | −27.42, 4.67 | ||||
| Constant | iM | 0.12 | 0.19 | −0.23, 0.49 | iY | 10.66 | 8.23 | −6.18, 27.18 | |||
| R2 = 0.345, p =< 0.001 | R2 = 0.949, p =< 0.001 | ||||||||||
| Dietary Self-Efficacy (M) | Waist Circumference (Y) | ||||||||||
| DUET vs. WLC (X) | A | 0.05 | 0.03 | −0.01, 0.13 | c’ | −0.92 | 1.18 | −3.51, 1.16 | −0.24 (0.31) [−1.26, 0.05] | −1.16 (1.11) [−3.50, 0.95] | |
| Dietary Self-Efficacy (M) | - | - | - | b | −4.62 | 3.66 | −11.83, 1.79 | ||||
| Constant | iM | 0.13 | 0.22 | −0.25, 0.58 | iY | 11.67 | 8.10 | −5.08, 26.37 | |||
| R2 = 0.340, p =< 0.001 | R2 = 0.849, p =< 0.001 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Pavela, G.; Pekmezi, D.W.; Rogers, L.Q.; Cole, W.W.; Parrish, K.B.; Sayer, R.D.; Wyatt, H.R.; Demark-Wahnefried, W. Dietary Barriers Appear to Influence the Effects of a Dyadic Web-Based Lifestyle Intervention on Caloric Intake and Adiposity: A Mediation Analysis of the DUET Trial. Nutrients 2023, 15, 4918. https://doi.org/10.3390/nu15234918
Kaur H, Pavela G, Pekmezi DW, Rogers LQ, Cole WW, Parrish KB, Sayer RD, Wyatt HR, Demark-Wahnefried W. Dietary Barriers Appear to Influence the Effects of a Dyadic Web-Based Lifestyle Intervention on Caloric Intake and Adiposity: A Mediation Analysis of the DUET Trial. Nutrients. 2023; 15(23):4918. https://doi.org/10.3390/nu15234918
Chicago/Turabian StyleKaur, Harleen, Gregory Pavela, Dori W. Pekmezi, Laura Q. Rogers, William W. Cole, Kelsey B. Parrish, R. Drew Sayer, Holly R. Wyatt, and Wendy Demark-Wahnefried. 2023. "Dietary Barriers Appear to Influence the Effects of a Dyadic Web-Based Lifestyle Intervention on Caloric Intake and Adiposity: A Mediation Analysis of the DUET Trial" Nutrients 15, no. 23: 4918. https://doi.org/10.3390/nu15234918
APA StyleKaur, H., Pavela, G., Pekmezi, D. W., Rogers, L. Q., Cole, W. W., Parrish, K. B., Sayer, R. D., Wyatt, H. R., & Demark-Wahnefried, W. (2023). Dietary Barriers Appear to Influence the Effects of a Dyadic Web-Based Lifestyle Intervention on Caloric Intake and Adiposity: A Mediation Analysis of the DUET Trial. Nutrients, 15(23), 4918. https://doi.org/10.3390/nu15234918








