Abstract
Bioelectrical Impedance Analysis (BIA) is a reliable, non-invasive, objective, and cost-effective body composition assessment method, with high reproducibility. This scoping review aims to evaluate the current scientific and clinical evidence on BIA for body composition assessment in oncology patients, under active treatment. Literature search was conducted through MEDLINE, CINAHL, Scopus and Web of Science databases, following PRISMA-ScR Guidelines. Inclusion criteria comprised studies reporting the use of BIA for body composition evaluation in adults with cancer diagnosis. Studies including non-cancer pathology or only assessing nutritional status were excluded. This scoping review comprised a total of 36 studies: 25 were original studies including 18 prospective studies, six cross-sectional studies and one retrospective study and 11 were systematic reviews. Population size for the included original articles ranged from 18 to 1217 participants, comprising a total of 3015 patients with cancer with a mean baseline Body Mass Index (BMI) ranging from 20.3 to 30.0 kg/m2 and mean age ranging between 47 and 70 years. Review articles included a total of 273 studies, with a total of 78,350 participants. The current review considered studies reporting patients with head and neck cancer (HNC) (n = 8), breast cancer (BC) (n = 4), esophageal cancer (EC) (n = 2), liver cancer (n = 2), pancreatic cancer (PC) (n = 3), gastric cancer (GC) (n = 3), colorectal cancer (CRC) (n = 8), lung cancer (LC) (n = 1), skin cancer (SK) (n = 1) and multiple cancer types (n = 6). BIA is a suitable and valid method for the assessment of body composition in oncology. BIA-derived measures have shown good potential and relevant clinical value in preoperative risk evaluation, in the reduction of postoperative complications and hospital stay and as an important prognostic indicator in persons with cancer. Future research on the diagnostic value and clinical applications of BIA and BIA-derived phase angle (PhA) should be conducted in order to predict its impact on patient survival and other clinical outcomes.
1. Introduction
Nutritional deterioration and progressive unintentional weight loss are prevalent conditions in patients diagnosed with cancer, and are widely associated with poor outcomes and complications through the course of the disease and treatments [1,2].
Cancer leads to metabolic alterations that contribute to depletion in nutritional status resulting in changes in body composition, which in turn are negative predictors of therapy toxicity, clinical outcomes, quality of life and survival. Therefore, it is critical to timely identify and treat malnutrition in order to enhance clinical outcomes. Thus, body composition should be part of nutritional assessment in these patients. Nevertheless, it remains a challenge due to a variety of methods and tools to assess nutritional status and body composition in patients with cancer [1].
Methods for body composition assessment include anthropometry, bioelectrical impedance analysis (BIA), air displacement plethysmography (ADP), dual-energy X-ray absorptiometry (DXA), computed tomography (CT) and magnetic resonance imaging (MRI) [1,2]. While DXA has traditionally been regarded as the “gold standard” for assessing body composition, research has also emphasized the validity and reliability of body composition evaluation through CT and MRI [3,4]. Nonetheless, these methods are costly, may involve radiation (CT), and are not commonly performed, rendering them often inaccessible [5]. In contrast, Bioelectrical Impedance Analysis (BIA) emerges as a dependable, non-invasive, objective, and cost-effective alternative with high reproducibility and minimal training requirements [1]. Recent studies have even indicated potential advantages of utilizing BIA for body composition assessment in patients with cancer [6,7].
BIA estimates total body water (TBW) by measuring impedance (Z), which results from resistance (R) and reactance (Xc) components [1,8]. This relationship can be expressed using the equation Z2 = R2 + Xc2 [9]. R represents the body’s opposition to the flow of an alternating current, while reactance gauges the electrical charge stored in cell membranes. Impedance measurements can be obtained using single or multiple current frequencies [1,10]. BIA calculates an individual’s resistance to a weak electric current, enabling the estimation of a two-compartment model of body composition, which includes fat mass (FM) and fat-free mass (FFM), using empirically derived equations [5,11]. Lower BIA-derived FFM has been associated with malnutrition in hospitalized patients and linked to unfavorable clinical outcomes, such as prolonged hospital stays and increased 28-day mortality in intensive care settings [12,13,14].
In the context of medical oncology, BIA-derived body composition measures can be clinically relevant. Two crucial BIA-derived parameters are Bioelectrical Impedance Vector Analysis (BIVA) and Phase Angle (PhA), both of which provide insights into nutritional and hydration status [15]. BIVA assesses hydration and cell mass independently of body size and has been used to explore the connections between hydration status, symptoms, and survival in persons with advanced cancer [16,17]. By converting BIVA measurements to z-scores, researchers can compare body composition across different study populations, considering variables like cancer type, stage, gender, and ethnicity [18,19].
PhA serves as an indicator of cell membrane integrity and water distribution inside and outside the cell membrane [20]. It typically ranges from 5 to 7 in healthy populations but tends to decrease with age due to muscle mass loss and declining body fluid proportions [21]. PhA has been linked to increased mortality and morbidity in various patient groups and has demonstrated prognostic value in malignancies and chronic diseases, including CRC and PC [22,23,24].
However, in persons with advanced cancer, factors like dehydration or ascites can lead to imprecise BIA-derived FFM measurements. Discrepancies have been observed in advanced LC and CRC patients [3]. Additionally, BIA may inaccurately gauge FFM or FM in patients with breast and gynecological malignancies due to lymphedema [1].
To ensure the most accurate assessment, it is essential to determine whether BIA is being used as an indicator of nutritional and metabolic health or to assess risk based on derived body composition measurements [10]. Several studies have underscored BIA’s role in predicting preoperative risk and postoperative complications, identifying patients with sarcopenia in routine clinical practice, and providing valuable information on body composition changes in patients with cancer [5,16,25,26,27,28].
Therefore, BIA has demonstrated itself as a precise and valid tool with substantial clinical relevance, offering valuable insights that could have a significant impact on clinical outcomes [29,30,31].
This scoping review aims to evaluate the current scientific and clinical evidence concerning the use of BIA for assessing body composition in cancer patients undergoing active treatment.
2. Materials and Methods
2.1. Search Strategy
A literature search was conducted through MEDLINE (via PubMed), CINAHL (via EBSCO), Scopus (via Elsevier) and Web of Science databases, for all published articles until 3 July 2021.
Full search strategies are represented in Appendix A. This scoping review was conducted in accordance with the following research question “What is the clinical relevance of BIA as a valid tool to assess body composition in persons with malignancies for the adult population?” and followed the PCC mnemonic (The Joanna Briggs Institute Reviewer’s Manual), considering population: patients aged 18 years or older with cancer; concept: use of BIA to assess body composition; context: antineoplastic treatment (chemotherapy, radiotherapy or other).
Search followed PRISMA extension for scoping reviews (PRISMA-ScR). Search terms included Neoplasm OR Malignant Neoplasm OR Tumor OR Cancer OR Malignancy OR Malignancies AND Electric Impedance OR Electrical Impedance OR Bioelectrical Impedance OR Bioelectrical impedance analysis OR BIA OR Bioelectric Impedance OR Electrical Resistance AND Body Composition. All studies extracted from databases were imported into a systematic review tool—Rayyan QCRI 0.1.0 (Qatar Computing Research Institute, Doha, Qatar) software, and duplicates were removed. Subsequently, two reviewers independently screened the titles and abstracts of included studies using Rayyan and both inclusion and exclusion criteria. Conflicts were discussed and resolved between the reviewers. After full-text screening, study selection was conducted by one reviewer. All study designs written in English, regarding all cancer types and published within the last 10 years from 2011 to 2021 were considered.
2.2. Inclusion and Exclusion Criteria
The following inclusion criteria were used: (1) studies reporting body composition evaluation using Bioelectrical Impedance Analysis (BIA), (2) studies considering patients with cancer and (3) studies including participants ≥ 18 years. Studies including non-cancer pathology or only assessing nutritional status were excluded. After selection process according to titles and abstracts, full texts were evaluated.
2.3. Data Extraction
One reviewer extracted relevant data from the included studies and systematically organized them according to the following parameters: cancer location, author(s)/year of publication, country, study design, study objective, sample size, gender, BMI, mean age, BIA device, BIA frequencies, BIA equation, study limitations and main conclusions (Appendix B).
3. Results
A flow diagram of the included studies is represented in Figure 1. Out of 2070 total search results, 975 were duplicates. After screening all titles and abstracts, 1037 studies were excluded. A total of 58 articles were sought for retrieval, 7 of which were not retrieved because the full articles were unavailable (n = 1), or only available as conference abstracts (n = 4) or written in a foreign language (n = 2), resulting in 51 articles assessed for eligibility. Following full-text review, 15 articles were excluded (Appendix C) due to only focusing on PhA (n = 8), only assessing nutritional status (n = 3), including not only oncological patients, but also surgical patients (n = 2), focusing on fluid administration (n = 1) or including multiple diseases (n = 1). This scoping review included a total of 36 studies: 18 prospective studies [5,9,16,25,26,27,28,30,31,32,33,34,35,36,37,38,39,40], one retrospective study [41], six cross-sectional studies [29,42,43,44,45,46] and 11 systematic reviews [1,2,7,10,15,47,48,49,50,51,52].
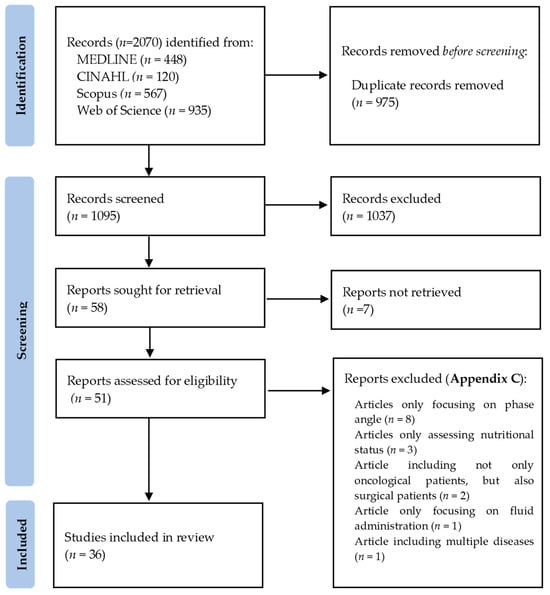
Figure 1.
Flow diagram of included studies, according to PRISMA 2020 Guidelines [53].
3.1. Study Characteristics
The studies included in this review were published from 2012 to 2021 and had been conducted in different countries: China (3) [25,32,43], USA (3) [5,7,42], Netherlands (1) [9], Finland (3) [2,16,47], Portugal (2) [2,47], Sweden (2) [16,36], Canada (3) [1,42,49], Republic of Korea (4) [30,31,33,41], Poland (5) [27,37,38,46], United Kingdom (5) [10,26,34,50,51], Japan (1) [28], Australia (1) [34], Denmark (3) [35,44,48], Norway (2) [40,48], Brazil (1) [36], Germany (3) [29,39,45], Greece (1) [15], Italy (1) [39] and United Arab Emirates (1) [15].
3.2. Population
The population size for the included original articles (n = 25) ranged from 18 [35] to 1217 participants [39], comprising a total of 3015 patients with cancer (58.6% male and 41.4% female), with a mean baseline BMI that ranged from 20.3 to 30.0 kg/m2 and mean age that ranged between 47 and 70 years. Moreover, three studies included only female participants [33,42,46], and five studies included patients with HNC, i.e., nasopharyngeal cancer [5,32], oropharyngeal cancer, sinonasal cancer, salivary gland cancer and glottic cancer [5], squamous cell carcinoma in the oral cavity, oropharynx, hypopharynx or larynx [9,16,25]. Furthermore, three studies included patients with BC [33,42,46], one with EC [49], two with hepatocellular carcinoma [27,30], one with PC (ampulla of Vater carcinoma, pancreatic ductal carcinoma [28]), one with GC (adenocarcinoma, signet ring cell carcinoma [43]), seven with CRC [31,34,35,36,37,40,41], one with LC (non-small cell lung carcinoma [44]), one with SK (malignant melanoma [29]), one which included PC, GC and CRC [38] and two which included numerous cancer types (gastrointestinal, gynecological, head and neck, lung and pleura, genito-urinary, hematological, neuroendocrine, adrenal, thyroid, skin soft tissues, central nervous system and others) [39,45].
Review articles included in this scoping review (n = 11) comprised a total of 273 studies, ranging from 12 [10,47] to 42 articles [50], with a total sample of 78,350 participants. Of these, three studies considered patients with HNC [2,15,47], one with BC [48], one with EC [49], one with PC [50], one with GC [51] and four which included multiple cancer types (head and neck, lung, breast, gastric, esophageal, hepatocellular, pancreatic, colorectal, gynecological (including ovarian and endometrial), prostate and hematological malignancies) [1,7,10,52].
3.3. Body Composition Assessment: BIA
Appendix D and Appendix E resume the information collected from original research articles (n = 25) and review articles (n = 11) included in this review. From the 25 original articles comprised, 13 had exclusively used BIA as a method to assess body composition [16,25,26,27,28,30,32,33,37,38,39,41,46]. Eight studies had chosen both BIA and CT [5,29,31,34,36,43,44,45]; three selected BIA and DXA [9,40,42] and only one included BIA, MRI and skinfold thickness (ST) [35].
Moreover, 11 studies did not report the frequencies applied [5,25,27,28,29,32,35,39,41,43,46]; 10 used a frequency of 50 kHz [16,33,34,36,37,38,40,42,44,45]; one used frequencies of 50 and 1000 kHz [31]; one used frequencies of 5, 50 and 200 kHz [9]; one used frequencies of 0.5, 50 and 100 kHz [9] and one used frequencies of 1, 5, 50, 260, 500 and 1000 kHz [30]. Only five studies referred BIA equations used: two mentioned Geneva equation [9,40]; one mentioned Janssen equation [36]; two mentioned Sun et al. equation [39]. and one had used the equation in the BIA software [28].
Appendix F comprises information on BIA methodologies regarding BIA measurements, position of participants during assessment, position of participants during assessment, specific body composition measures assessed and raw BIA measures.
4. Discussion
This scoping review examines the current evidence regarding the use of BIA for body composition assessment in cancer and its implications for patient outcomes and prognosis of long-term survival.
4.1. Head and Neck Cancer
Persons diagnosed with HNC often face a heightened risk of malnutrition due to the location of the tumor and the impact of oncological treatments on their ability to consume food. This challenge is particularly pronounced among those with oral, oropharyngeal, and hypopharyngeal cancers. Consequently, there is a need for tools to identify malnutrition in this specific patient group.
Almada-Correia et al. [2] conducted a comprehensive review of the existing literature on body composition assessment in patients with HNC to determine the most appropriate method for this population. Their review considered various methods for assessing body composition in clinical settings, including anthropometry, BIA, CT, and DXA. The findings indicated that CT and DXA were the established standards for evaluating body composition in patients with cancer, although they are not routinely used in the management of HNC cases [2].
Another review [47] investigated changes in body composition among patients with HNC to identify the most effective methods for assessing body composition. This study underscored the significance of body composition evaluation and concluded that both skinfold thickness (ST), BIA, DXA, and CT demonstrated substantial reductions in lean body mass (LBM), FFM, and skeletal muscle mass (SMM), along with increases in body FM, among HNC patients undergoing chemoradiotherapy [47]. Importantly, it highlighted the regular availability of BIA, which holds promise for assessing body composition in patients with HNC.
Mantzorou et al. [15] summarized and discussed clinical data on the effectiveness of assessment tools, such as BIA, in evaluating malnutrition in persons diagnosed with HNC. The authors recommended additional studies to explore the role of BIA-derived measures in assessing the nutritional status of these patients [15]. Notably, there are only a limited number of studies that have examined the use of BIA in HNC patients.
Malecka-Massalska et al. [54] also emphasized the relevance of BIA, particularly BIVA, as a method that could provide objective measures to enhance clinical decision-making and predict outcomes in HNC patients. Similarly, Axelsson et al. [55] highlighted three different factors derived from BIA variables: Fat-Free Mass Index (FFMI), PhA and Standardized Phase Angle (SPA), adjusted for age and sex. A PhA cutoff value at 5.95° was identified as the most accurate predictor of 5-year survival. Both PhA and SPA were considered valuable prognostic tools for patients with advanced HNC [55]. In a prospective study conducted by Ding et al. [32], the study aimed to assess the changes in body composition among patients with nasopharyngeal carcinoma undergoing chemoradiotherapy. The findings of this study highlighted the significance of the BIA-derived FFMI in diagnosing malnutrition. The decrease in FFMI was found to be associated with a decline in the patients’ quality of life (QoL). As a result, the authors emphasized the importance of incorporating BIA into nutritional assessments [32].
In another prospective study [5], which sought to determine whether BIA could effectively identify sarcopenia in HNC patients, the primary outcomes revealed a robust correlation between BIA measurements and CT-based estimates. BIA demonstrated the ability to accurately identify sarcopenia, particularly among male patients, with a high level of sensitivity and specificity (>90%). This reinforced the practical utility of BIA in clinical settings for identifying patients with sarcopenia [5].
Additionally, Jager-Wittenaar et al. [9] conducted a study to evaluate the validity of BIA in patients with HNC and concluded that it serves as an acceptable tool for assessing FFM in clinical practice.
Two other studies [16,25] focused on describing BIA and assessing the correlation of BIA parameters with complication rates and other relevant indicators in patients with HNC. The first study [16] emphasized the utility of BIA parameters such as PhA and BIVA as valuable screening tools that provide essential information about body composition. The second study [25] highlighted BIA’s role as a clinically valuable tool in preoperative risk assessment, contributing to the reduction in complications and hospital stays.
Collectively, it is evident that BIA represents a cost-effective, non-invasive, rapid, and user-friendly approach for estimating the nutritional status of patients with HNC. It can play a crucial role in identifying patients who require nutritional care before the initiation of treatment, potentially reducing complications and hospital stays.
4.2. Breast Cancer
BC has emerged as the predominant cause of cancer incidence globally. The development of BC has been associated with the accumulation of adipose tissue in adulthood over the years. Recommendations for patients with BC include weight loss for those who are obese and a reduction in adiposity reserves.
In these patients, undetected or unaddressed malnutrition can lead to severe adverse outcomes. The existing body of literature demonstrates that malnutrition is linked to elevated morbidity and mortality, and the nutritional status plays a pivotal role in the prognosis of BC, potentially influencing the progression of the disease [56].
Nutrition-related symptoms, such as nausea, vomiting, loss of appetite, constipation, diarrhea, stomach pain, altered taste perception, sore mouth, and difficulty swallowing, can arise from the tumor itself or as side effects of treatment. These symptoms adversely affect dietary intake and elevate the risk of malnutrition. Malnourished patients with BC can tolerate fewer treatment cycles, rendering them more vulnerable to treatment-related toxicities and increased hospitalizations [56,57].
The impact of body composition on BC risk and outcomes in BC survivors is well-documented. Therefore, the integration of body composition assessment into the comprehensive care of these patients is essential. A scoping review involving persons with BC [48] aimed to investigate changes in weight and body composition, suggesting the need for further investigations with long-term prospective designs and consistent assessment of weight and body composition using the same measurement tools. BIA was mentioned as a cost-effective and user-friendly tool that could contribute to standardizing measurements [48].
In a cross-sectional study, Bell et al. [42] compared the accuracy of previously published Single Frequency-BIA equations predicting FFM with DXA measurements of FFM in a group of patients diagnosed with BC undergoing treatment. The study found that BIA consistently overestimated FFM. However, the authors acknowledged the need for future studies to develop and validate BIA prediction equations specifically tailored to BC patients [42].
Terada et al. [58] focused, as previously mentioned [1], on the limitations of single-use BIA in detecting lymphedema and measuring patient-reported outcomes. Similarly, in a study by Blaney et al. [59], BIA was capable of identifying 80% of true lymphedema cases but produced false negatives for 20%. Consequently, the authors recognized that BIA might not be a suitable tool for assessing established lymphedema [59].
Jung et al. [33] explored changes in weight, body composition, and physical activity in BC patients undergoing adjuvant chemotherapy, concluding that BIA could provide more concrete and objective results. Wilczyński et al. [46] reported that BIA does not cause ionization and is considered a gold standard in the field of body composition analysis.
4.3. Oesophageal Cancer
Somewhat similarly to HNC, patients with EC are at nutritional risk due to the location of the disease and side effects of anticancer therapies and/or surgery. Consequently, most patients with EC become malnourished. Thus, these patients represent a group of persons with cancer who are nutritionally compromised, due to dysphagia and oncological treatments. In addition, patients undergoing surgery for cancer are at particular risk of post-operative complications [60]. BIA may offer an additional method of identifying patients at risk of post-operative morbidity.
A recent review [49] on current literature regarding the assessment of body composition in EC patients could not agree on the best tool, due to inconsistencies in methods of assessing and reporting body composition, although authors recognized its usefulness regarding decision-making support in patients with EC.
Powell et al. [26] conducted a study with the objective of establishing a connection between low muscle volume (LMV) defined by Bioelectrical Impedance Analysis (BIA) in patients undergoing EC surgery and clinical outcomes. The findings of this study revealed that BIA-derived LMV served as a crucial prognostic indicator in patients undergoing potentially curative esophagectomy for cancer [26].
In a study by Ida et al. [61], the aim was to determine the impact of neoadjuvant chemotherapy (NAC) on the body composition of patients with EC and to assess the relationship between changes in body composition and the occurrence of postoperative complications. BIA-derived measurements such as BCM, FFM, and SMM exhibited significant reductions in patients who experienced postoperative complications. The study underscored the value of nutritional assessment through body composition in predicting postoperative complications following NAC in EC patients [61].
Similarly, after employing BIA to evaluate body composition, Motoori et al. [62] reported similar findings, concluding that the loss of SMM during NAC represented a significant risk factor for postoperative infectious complications in patients with EC undergoing esophagectomy.
Conversely, a different outcome emerged in the study by Miyata et al. [63], which aimed to assess changes in body composition using BIA during NAC for EC. According to their study [63], pre-NAC sarcopenia was not linked to the occurrence of postoperative complications, and a significant reduction in SMM was not observed in all persons diagnosed with EC [26,61,62,63].
4.4. Hepatocellular Cancer
Although common, malnutrition is frequently an underdiagnosed condition in patients with hepatocellular carcinoma (HCC). These patients are at a special increased risk for malnutrition as the liver is the central organ involved in nutrients metabolism [64].
In a prospective study conducted by Lee et al. [30], the impact of various factors, including Bioelectrical Impedance Analysis (BIA)-derived PhA, the presence of sarcopenia, and edema index, was evaluated in relation to postoperative complications in patients with hepatocellular carcinoma (HCC). The study found that BIA offered valuable additional clinical insights into the occurrence of postoperative complications in patients with HCC scheduled for surgery. However, it should be noted that due to a relatively short follow-up duration, the precise role of BIA in predicting short-term survival remained unclear [30].
In another study by Pagano et al. [65], the potential of BIA as a valuable tool for chronic liver diseases was highlighted. The study concluded that BIA-derived PhA had the capacity to predict the severity of liver disease and reflect the nutritional risk in HCC patients.
A cross-sectional study by Peres et al. [66] also recognized the relevance of BIA-derived PhA as an essential tool for nutritional assessment in the context of chronic hepatitis, liver cirrhosis, and HCC [30,65,66].
4.5. Pancreatic Cancer
PC has a poor overall prognosis, with a low 5-year survival rate. However, patients with early tumor resection have a higher chance of more successful treatments. Patients with PC often are malnourished and suffer from cancer cachexia. PC has been characterized as a highly catabolism inducer, with rapid depletion of the host’s body compartments. In fact, a large proportion of these patients have already lost 10% of body weight at the diagnosis. Early detection of wasting is central in the clinical approach of these patients [67]. Therefore, it is crucial to early assess nutritional status to identify and treat malnutrition and also to prevent or counteract cachexia. In clinical practice, anthropometric methods have been used but are not ideal: they are time-consuming and difficult to perform, especially in bed-ridden patients. Additionally, objective indicators such as serum albumin and transferrin are difficult to interpreter due to non-nutritional factors [50].
Regarding specifically PC, the only available systematic review on this topic was conducted by Bundred et al. [50], who summarized the existing literature on body composition assessment in patients with PC and assessed its impact on perioperative outcomes and long-term survival. Despite the lack of consensus regarding optimal methodology, this review highlighted the need for standardized assessment of body composition, reinforcing its importance to support future decision-making in PC patients [50].
Mikamori et al. [28] conducted a study with the goal of utilizing BIA for postoperative nutritional assessment in patients who had undergone pancreaticoduodenectomy. Their findings indicated that BIA proved to be a valuable tool for assessing body composition and nutritional status in patients who had undergone surgery for PC and other related conditions.
Gupta et al. [24] investigated the correlation between BIA-derived PhA and overall survival in patients with stage IV PC. Their study suggested that BIA-derived PhA served as a more robust predictor of survival compared to nutritional indices such as albumin, pre-albumin, and transferrin in patients with advanced PC.
In a similar way, Yasui-Yamada et al. [68] reported parallel findings concerning the significance of PhA. They highlighted PhA as a valuable prognostic marker for both short-term and long-term postoperative outcomes in patients with hepatobiliary-pancreatic cancer.
4.6. Gastric Cancer
GC leads to important depletion of muscle and fat tissue due to surgical interventions, chemo- and radiotherapy. In line with this, GC patients are at high nutritional risk and malnutrition-related to malignant disease, leading to lower compliance with treatment and complications during surgery. Complete surgical resection remains the only curative modality for early-stage GC. These patients in the perioperative period first consume LBM, which might not be evident from BMI nor other nutritional scores. BIA can overcome these difficulties [69].
In a cross-sectional study conducted by Gao et al. [43], the accuracy of BIA in estimating visceral fat area (VFA) in individuals with GC was explored. The study’s findings revealed a significant correlation and satisfactory reliability between VFA measurements obtained by CT and BIA [43].
In a retrospective study by Yuet al. [70], it was demonstrated that preoperative low BIA-derived PhA was a predictor of the risk of overall and severe complications in patients with GC. This suggests the potential use of BIA in assessing the risk of postoperative complications, especially in elderly patients with GC.
A review conducted by Kamarajah et al. [51] regarding the current literature on body composition assessment in patients with GC highlighted the absence of a consensus on the optimal methodology. It also emphasized the need for establishing guidelines for body composition assessment in patients with GC. Dzierżek et al. [38] examined body composition and its changes in patients who had undergone surgeries for GC, PC, and CRC. The authors concluded that BIA was a straightforward and effective method for assessing body composition and its alterations in patients undergoing major surgery [38,43,51,70].
4.7. Colorectal Cancer
Excess body weight and metabolic alterations have been identified as risk factors for cancer of the colon-rectum. Patients with advanced disease stage can develop a wasting-like phenotype of nutritional status, and gastrointestinal cancers globally show high prevalence of malnutrition.
In a prospective study led by Jones et al. [34], the primary objective was to assess the agreement between BIA and MAMC in comparison to CT scans for the measurement of muscle mass and the identification of sarcopenia in patients with CRC. The study’s conclusion revealed that both BIA and MAMC were found to be inadequate methods for detecting reduced muscle mass in patients with CRC when compared to the measurements of CT-derived muscle mass at L3 [34]. Another study [31] explored the relationships between a single cross-sectional area of skeletal muscle at the lumbar region (L3) measured by CT and total body SMM assessed by BIA in primary CRC patients. Kim et al. [31] identified BIA as an alternative method to CT scans, offering a non-invasive and cost-effective tool for assessing body composition status, including SMM, in patients with CRC.
Palle et al. [35] examined the correlation between a single cross-sectional thigh MRI, SMM as a reference, and multi-frequency BIA-derived FFM in CRC patients who had undergone chemotherapy. This study considered BIA and thigh MRI as suitable alternatives to the more complex and expensive MRI due to their consistent and correctable errors [35]. Ræder et al. [40] aimed to validate two different BIA devices against DXA in persons with CRC. Their study concluded that FFM estimated from both BIA devices exhibited good agreement with DXA, as long as the appropriate equation was used [40]. However, Bärebring et al. [71] reached contrasting conclusions in their study, which aimed to validate the ability of BIA, compared to DXA, to assess changes in FFM in non-metastatic CRC patients. BIA yielded imprecise data on changes in FFM, regardless of the equation used, and was therefore not deemed a valid option for quantifying changes in FFM in patients with CRC.
In a retrospective study, Song et al. [41] delved into the relationship between body composition and the platelet-to-lymphocyte ratio (PLR) in patients diagnosed with CRC. They discovered that body composition indices, including fat and muscle indices measured by BIA, were associated with PLR in these patients and could be related to a poorer prognosis in CRC cases [41]. A study by Souza et al. [36] aimed to evaluate the agreement between CT and surrogate methods commonly applied in clinical practice. The study revealed that while physical examination demonstrated the best agreement for assessing low muscle mass, Patient-Generated Subjective Global Assessment (PG-SGA), BIA, and CT exhibited similar prognostic values for survival [36]. In another study, conducted by Szefel et al. [37], the purpose was to determine the usefulness of BIA in detecting and monitoring cancer cachexia (CC) in patients with CRC. The study successfully associated BIA with the identification of differences in body composition depending on the cancer stage and the advancement of CC [37].
Gupta et al. conducted two separate studies investigating the prognostic role of PhA in advanced CRC [23] and the association between BIA-derived PhA and Subjective Global Assessment (SGA) [72]. The findings from both studies recognized BIA-derived PhA as a prognostic and potential nutritional indicator in advanced CRC patients [23,31,34,35,36,37,40,41,71,72].
4.8. Lung Cancer
Hansen et al. [44] conducted a study to explore the agreement between BIA assessment of body composition and software analysis of CT scans in patients with non-small cell LC. The study’s conclusion indicated that both methods were not directly comparable for body composition measurements. Furthermore, BIA was found to overestimate SMM and underestimate FM [44]. In another study, Kovarik et al. [73] provided a summary of recent evidence concerning various methods, including BIA, for assessing changes in body composition in LC patients. The study underscored the importance of BIA and BIA-derived PhA in predicting outcomes for LC patients. Similarly, Gupta et al. [74] observed that BIA-derived PhA served as an independent prognostic indicator in patients with stage IIIB and IV non-small cell LC [44,73,74].
4.9. Skin Cancer
A prospective study conducted by Zopfs et al. [29] evaluated the correlation between simple, planimetric measurements in CT slices and measurements of patient body composition and anthropometric data, performed with BIA and metric clinical assessments. This study concluded that simple measurements in a single axial CT slice could determine body composition parameters, with high clinical relevance [29].
4.10. All Cancer Types
Adequate body composition has been proved essential for neoplastic disease outcome. BIA has been found to be a prognostic indicator in several chronic conditions, including cancer [24].
Cereda et al. [39] conducted a study with the aim of investigating the potential independent prognostic roles of FFMI, BMI, and weight loss (WL), and their associations with quality of life (QoL) in a substantial cohort of patients with cancer. While acknowledging the versatility and non-invasive nature of BIA for bedside assessments, the study emphasized the necessity for additional confirmatory studies to validate the usefulness and prognostic value of BIA-derived FFMI in people diagnosed with cancer [39].
In a cross-sectional study by Mueller et al. [45], the validity of BIA as a diagnostic tool in patients with malignancies, both with and without malnutrition, was investigated. BIA was recognized as a valid diagnostic tool for assessing muscle and FM, and its use was recommended for the early detection and short-term follow-up of malnutrition and cachexia [45].
A review conducted by Di Sebastiano et al. [1] aimed to identify key considerations for body composition analysis in diverse populations with cancer and to discuss various methods of body composition assessment, such as anthropometric measures, BIA, ADP, DXA, CT, and MRI. The review deemed DXA as the optimal method for whole-body composition analysis in prospective use with cancer populations due to its precision, accuracy, and fewer limitations compared to other methods. The authors highlighted the limited applicability of BIA in advanced and BC patients, as it relies on water volume and cannot distinguish tumor or lymphedema within lean and fat tissue depots [1]. Aleixo et al. [75] summarized the existing literature on BIA’s role in assessing sarcopenia in adults with cancer. BIA was considered an accurate method for detecting sarcopenia and a viable alternative to CT, DXA, and MRI in the field of oncology.
Grundmann et al. [7] conducted a review of the current scientific and clinical evidence regarding the utility of BIA in patients with malignant neoplasms. The study noted that BIA and PhA provided valuable information for assessing nutritional and overall health status in patients diagnosed with cancer. While acknowledging the need for further research, the authors encouraged the use of BIA in clinical practice [7]. A recent study by Di Vincenzo et al. [76] found that BIA-derived PhA was decreased in patients with sarcopenia, suggesting PhA as a valuable parameter for detecting low muscle quality and identifying sarcopenia. Małecka-Massalska et al. [52] described the existing literature on the utility of BIA in assessing cancer-related malnutrition. The study considered BIA to be an objective, reliable, and non-invasive method for assessing malnutrition [52].
A systematic review conducted by Matthews et al. [10] aimed to assess whether BIA measures and estimates of body composition could identify adults with oncological diseases at risk of complications. The use of BIA in the peri-operative period was found to be useful in predicting the risk of complications following elective cancer surgery [10].
4.11. General Considerations for the Use of BIA
Appendix G focused on BIA’s advantages and disadvantages, as a method for estimating body composition in patients with cancer.
Bioelectric impedance analysis (BIA) is a safe [49], non-invasive [1,2,7,15,47,50,51,52], easy-to-use [1,2,15,50,51], reproducible [1,2], indirect method [2] for measuring body composition. It appears to show good correlations, when compared to other methods [47], despite being considered less accurate than radiological assessment methods [49].
Although it has good application consistency and is considered a useful tool for assessing the nutritional status of persons with cancer [2,52], BIA can underestimate FFM in patients with advanced cancer, compared with DXA [1]. Moreover, considering BIA depends on water volume, it has limited use in people with advanced-staged cancer and patients with BC and is not capable of distinguishing tumor or lymphedema in the lean and fat tissue depots [1]. While BIA is recognized as a validated tool to assess body composition in patients with cancer [2,47,52], some studies reported some inconsistent findings, with poorer accuracy and precision in obese and edematous individuals [50,51]. As a practical and objective assessment method [1,50,51,52], BIA is also considered to be relatively inexpensive, when compared to more sophisticated methods like DXA, CT or MRI [1,2,7,47,49], although it is usually more expensive than anthropometric measures [1]. Often reliable [52], one of BIA’s disadvantages resides in the fact that it is unable to measure the entire body, which results in incomplete information [2].
BIA is a portable [1,7,15,47], time and cost-effective technique [15], that requires little training [1]. However, this tool is not routinely available outside the research setting [49]. Many prediction equations using linear regression rely on BIA to estimate body composition, based on variables that may differ between different populations and were derived from healthy individuals [1,2,15,47]. Nonetheless, BIA-derived PhA, a prognostic factor of patient survival [15], which, along with BIVA, is considered to reflect both nutritional and hydration status [7,15], does not depend on regression equations to be calculated [47]. BIA-derived measures (FFM, FM, body weight, BMI) are correlated with an increased risk of developing colon cancer and potentially other cancers [7] and may serve as early indicators for improvement in nutritional and health status [7,52], but can also be useful to evaluate and predict outcomes, such as post-operative complications [10].
Despite the many advantages associated with BIA use in general and specifically in patients with cancer, the literature presents a multiplicity of equipment used to measure BIA, methods and participant preparation protocols, which in turn can lead to different readings obtained by BIA. Therefore, evaluations should consider an adequate fluid balance and food intake and be performed under the same circumstances, taking into consideration possible sources of error. Moreover, its efficiency may be affected by nutrition status, physical activity, phase of the menstrual cycle, placement of electrodes, limb length, blood chemistry, altered fluid balance, oedema, endocrine diseases, treatment with growth hormone, acute illness, intensive care treatment, organ transplantation, position of the body and movements during the measure, type of electrodes, use of oral contraceptives [1,2]. This method can also be compromised by loss of accuracy when patients are in the extremes of BMI ranges (≤16 kg/m2 or ≥35 kg/m2) [2,47].
4.12. Limitations
The overall strength of conclusions from this review is limited by the heterogeneity of the studies, different objectives, BIA devices and methodologies, and findings regarding BIA’s assessment of body composition in patients with oncological diseases. Furthermore, the initial literature search was only conducted in four different databases and research articles were only considered when written in English, which may have excluded other potentially relevant studies. Different cancer locations were taken into consideration in this review, making it challenging to condense information. Also, the limited number of existing studies by cancer location, included in this review, highlights the need for further studies.
Additionally, this scoping review did not perform a quality evaluation on included articles, which may also be a limitation of this review. Overall findings drawn from this scoping review must be interpreted carefully, considering different types of study designs included.
5. Conclusions
The identified studies have considered BIA to be a suitable and valid method for the assessment of body composition in oncology. BIA-derived measures have shown good potential and relevant clinical value in preoperative risk evaluation, in the reduction of postoperative complications and hospital stay and as an important prognostic indicator in patients with cancer. Hence, research encourages the implementation of this method in the nutritional assessment at a larger extent.
Future research focusing on the diagnostic value and clinical applications of BIA and BIA-derived PhA should be conducted in order to increase knowledge and strengthen evidence on its impact on patient survival and other clinical outcomes, including postoperative complications and treatment-related toxicity, that justify the increase of its use in clinical practice.
Author Contributions
Conceptualization and study design, M.G.B.; data interpretation and manuscript revision, M.G.B. and C.M.; writing—original draft preparation, M.G.B.; research supervision, review and editing, M.L.C., P.R., N.P., A.M., C.T., S.G.-A. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Search Strategy
| Database | Search | Results | |
| MEDLINE (via PubMed) Date searched: 3 July 2021 | (((((((((Neoplasm*[MeSH Terms]) OR (Neoplasm*[Title/Abstract])) OR (Malignant Neoplasm*[Title/Abstract])) OR (Neoplasia*[Title/Abstract])) OR (Tumor*[Title/Abstract])) OR (Cancer*[Title/Abstract])) OR (Malignancy[Title/Abstract])) OR (Malignancies[Title/Abstract])) AND ((((((((Electric Impedance[MeSH Terms]) OR (Electric Impedance[Title/Abstract])) OR (Electrical Impedance[Title/Abstract])) OR (Bioelectrical Impedance[Title/Abstract])) OR (Bioelectrical impedance analysis[Title/Abstract])) OR (BIA[Title/Abstract])) OR (Bioelectric Impedance[Title/Abstract])) OR (Electrical Resistance[Title/Abstract]))) AND ((Body Composition*[MeSH Terms]) OR (Body Composition*[Title/Abstract])) | 448 | |
| CINAHL (via EBSCO) Date searched: 3 July 2021 | ((MM Neoplasm* OR TI Neoplasm* OR AB Neoplasm* OR TI Malignant Neoplasm* OR AB Malignant Neoplasm* OR TI Neoplasia* OR AB Neoplasia* OR TI Tumor* OR AB Tumor* OR TI Cancer* OR AB Cancer* OR TI Malignancy OR AB Malignancy OR TI Malignancies OR AB Malignancies)) AND ((MM Electric Impedance OR TI Electric Impedance OR AB Electric Impedance OR TI Electrical Impedance OR AB Electrical Impedance OR TI Bioelectrical Impedance OR AB Bioelectrical Impedance OR TI Bioelectrical impedance analysis OR AB Bioelectrical impedance analysis OR TI BIA OR AB BIA OR TI Bioelectric Impedance OR AB Bioelectric Impedance OR TI Electrical Resistance OR AB Electrical Resistance)) AND ((MM Body Composition* OR TI Body Composition* OR AB Body Composition*)) | 120 | |
| Scopus (via Elsevier) Date searched: 2 July 2021 | ((TITLE-ABS-KEY (neoplasm*)) OR (TITLE-ABS-KEY (malignant AND neoplasm*)) OR (TITLE-ABS-KEY (neoplasia*)) OR (TITLE-ABS-KEY (tumor*)) OR (TITLE-ABS-KEY (cancer*)) OR (TITLE-ABS-KEY (malignancy)) OR (TITLE-ABS-KEY (malignancies))) AND ((TITLE-ABS-KEY (electric AND impedance)) OR (TITLE-ABS-KEY (electrical AND impedance)) OR (TITLE-ABS-KEY (bioelectrical AND impedance)) OR (TITLE-ABS-KEY (bioelectrical AND impedance AND analysis)) OR (TITLE-ABS-KEY (bia)) OR (TITLE-ABS-KEY (bioelectric AND impedance)) OR (TITLE-ABS-KEY (electrical AND resistance))) AND (TITLE-ABS-KEY (body AND composition*)) | 567 | |
| Web of Science | #1 | Neoplasm* | 935 |
| #2 | Malignant Neoplasm* | ||
| Date searched: | #3 | Neoplasia* | |
| 3 July 2021 | #4 | Tumor* | |
| #5 | Cancer* | ||
| #6 | Malignancy | ||
| #7 | Malignancies | ||
| #8 | #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 | ||
| #9 | Electric Impedance | ||
| #10 | Electrical Impedance | ||
| #11 | Bioelectrical Impedance | ||
| #12 | Bioelectrical impedance analysis | ||
| #13 | BIA | ||
| #14 | Bioelectric Impedance | ||
| #15 | Electrical Resistance | ||
| #16 | #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 | ||
| #17 | Body Composition* | ||
| #18 | #17 AND #16 AND #8 | ||
Appendix B. Data Extraction Instrument
| Review Title: Bioelectrical Impedance Analysis (BIA) for the Assessment of Body Composition in Oncology: a scoping review |
| Review Objective: To evaluate the current scientific and clinical evidence on Bioelectrical Impedance Analysis (BIA) for body composition assessment in patients with cancer, under active treatment. |
| Review Question: 1—What is the clinical relevance of BIA as a valid tool to assess body composition in cancer patients for the adult population? |
| Inclusion/Exclusion Criteria Population: Cancer patients aged 18 years or older. Concept: Use of BIA to assess body composition. Context: Antineoplastic treatment (chemotherapy, radiotherapy or other). |
| Types of Study: Only published studies, both quantitative and qualitative data, and systematic reviews, with abstract available. |
| Study Details and Characteristics Cancer location________________________________________________________________________________________ Author(s)/Year of publication____________________________________________________________________________ Country______________________________________________________________________________________________ Study design__________________________________________________________________________________________ Study objective________________________________________________________________________________________ Sample size___________________________________________________________________________________________ Gender_______________________________________________________________________________________________ Body Mass Index_______________________________________________________________________________________ Mean age _____________________________________________________________________________________________ BIA device____________________________________________________________________________________________ BIA Frequencies_______________________________________________________________________________________ BIA Equation__________________________________________________________________________________________ Study limitations ______________________________________________________________________________________ Main conclusions ______________________________________________________________________________________ |
Appendix C. Studies Ineligible Following Full Text Review
|
Appendix D. Original Research Articles Included in the Review (n = 25)
| Cancer Location | Authors (Year) | Country | Study Design | Objective | Sample Size (n) | Gender (n/%) | BMI (kg/m2) | Mean Age ± SD | BIA Device | Frequencies (kHz)/ Equation | Sensitivity/ Specificity | Main Conclusions |
| Head and neck cancer | Ding et al., 2018 [32] | China | Prospective study | To investigate body composition changes in patients with nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy and assess the use of the PG-SGA 1 and the ESPEN diagnostic criteria as evaluation methods. | 48 | M: 36 (75%) F: 12 (25%) | 23.34 ± 0.6 | 47 | InBody S10 Biospace Model JMW140, Seoul, Republic of Korea | NM/NM | NM 2 | Body composition parameters, specifically FFMI 3, are important in the diagnosis of malnutrition. BIA 4 should be implemented for nutritional assessment. |
| Grossberg et al., 2021 [5] | USA | Prospective study | To explore if BIA is useful to identify sarcopenia associated with decreased survival in HNC 5 patients treated with RT 6. | 48 | M: 40 (83%) F: 8 (17%) | M: 30 ± 5 F: 24 ± 5 | 60 ± 12 | SECA mBCA 515 scale, Hamburg, Germany | NM/NM | SMMI 7 (M) 92%/ 93% SMM 8 (F) 100%/ 0% | BIA showed high sensitivity and specificity to identify patients with sarcopenia, a negative prognostic factor in HNC. BIA seems a practical solution to identify patients with sarcopenia in routine clinical practice. | |
| Jager-Wittenaar et al., 2014 [9] | Netherlands | Prospective study | To assess the validity of BIA with Geneva equation, for the assessment of FFM 9 in patients with HNC in pretreatment and posttreatment. | 24 | M: 20 (83%) F: 4 (17%) | 23.7 ± 4.7 | 60.4 ± 8.3 | BodyStat QuadScan 4000, BodyStat, Douglas, Isle of Man, UK | 5, 50, 200/ Geneva equation | NM | BIA seems to be valuable to assess FFM in HNC patients in the clinic, with good concordance in group mean-level comparisons. | |
| Lundberg et al., 2017 [16] | Finland | Prospective study | To describe BIA measures in Finnish patients with HNC at diagnosis. | 41 | M: 32 (78%) F: 9 (22%) | M: 25.2 F: 27.0 | 62.5 | SECA mBCA 515 scale, Hamburg, Germany | 50/NM | NM | BIA was fast, non-invasive, inexpensive tool and both PhA 10 and BIVA 11 are easily analyzed by an inexperienced clinician. PhA and BIVA seemed useful and also provided information on body composition. | |
| Lundberg et al., 2019 [25] | Finland | Prospective study | To evaluate correlation of BIA with complication rate and other related indicators after major HNC surgery. | 61 | M: 47 (77%) F: 14 (23%) | PhA low: 23.2 PhA normal range: 27.3 | 61 | SECA mBCA 515 scale, Hamburg, Germany | NM/NM | NM | BIA is cheap, quick, easy, non-invasive and feasible to analyze body composition in patients with cancer. BIA can be of clinical value in preoperative risk evaluation and might reduce complications and hospital stay. | |
| Breast cancer | Bell et al., 2020 [42] | Canada & USA | Cross-sectional study | To compare the ability of previously published SF-BIA 12 equations that predict FFM with a reference method (DXA 13) in a group of patients with BC 14 undergoing treatment. | 48 | F: 48 (100%) | 27.5 ± 5.5 | 52 ± 10 | BIA Quantum IV, Clinton Township, MI, USA | 50/NM | NM | BIA overestimated FFM, and underestimated FM 15 in patients with BC. Future studies are needed to develop and validate BIA prediction equations specific to BC throughout the disease trajectory. |
| Jung et al., 2020 [33] | Republic of Korea | Prospective study | To analyze changes in weight, body composition, and physical activity in patients with BC under adjuvant chemotherapy. | 37 | F: 37 (100%) | 23.42 ± 3.06 | 50.9 ± 9.4 | Inbody S10, Seoul, Republic of Korea | 50/NM | NM | No significant change in weight, body composition, and physical activity during adjuvant chemotherapy in patients with BC. Using BIA could provide more concrete and objective results. | |
| Wilczyński et al., 2020 [46] | Poland | Cross-sectional study | To investigate body composition of women following radical mastectomy. | 60 | F: 60 (100%) SG 16: 30 CG 17: 30 | SG: 27.56 CG: 24.96 | SG: 55.07 ± 4.71 CG: 50.27 ± 5.13 | TANITA MC-780, Tokyo, Japan | NM/NM | NM | The use of BIA does not cause ionisation and is a gold standard in the field of body composition analysis. | |
| Esophageal cancer | Powell et al., 2020 [26] | United Kingdom | Prospective study | To assess the association between BIA defined low FFM, in patients undergoing surgery for OC 18 and clinical outcomes, related to post-operative morbidity graded by Clavien- Dindo MSS 19, and both Disease-Free and OS 20. | 122 | M: 104 (85.2%) F: 18 (14.8%) | NMV 21: 28.1 LMV: 20.3 | 65 | Maltron Bioscan 920, Essex, UK | 0.5, 50, 100/ NM | NM | BIA derived LMV 22 was a prognostic indicator in patients undergoing potentially curative oesophagectomy for cancer. |
| Hepatocellular cancer | Lee et al., 2021 [30] | Republic of Korea | Prospective Study | To explore if PhA, presence of sarcopenia, and EI 23, measured through BIA, affect postoperative complications and prognosis after liver resection in patients with HCC 24. | 79 | M: 66 (83.5%) F: 13 (16.5%) | 23.7 ± 3.3 | 56.1 ± 10.9 | InBody 770 scanner, Seoul, Republic of Korea | 1, 5, 50, 260, 500, 1000/ NM | EI (ECW 25/TBW 26) 68.6%/ 70.5% | BIA can provide additional clinical information regarding postoperative complications in patients with HCC scheduled for surgery. |
| Skroński et al., 2018 [27] | Poland | Prospective Study | To evaluate changes in body composition before and after resection of liver tumors and radiofrequency ablation of lesions. | 50 | M: 23 (46%) F: 27 (54%) | NM | 60 | BIA 101 Anniversary analyzer, Akern, Florence, Italy | NM/NM | NM | BIA is a suitable method to assess changes in body composition of patients undergoing liver resection. | |
| Pancreatic cancer | Mikamori et al., 2016 [28] | Japan | Prospective Study | To explore postoperative changes in body composition of patients submitted to PG 27 Vs GT 28, and assess nutrition with BIA postoperatively. | 60 | M: 43 (71.7%) F: 17 (28.3%) | PD:21.4 ± 2.7 TG 29:21.6 ± 3.0 DG 30:21.9 ± 3.7 | 65.8 ± 7.4 | InBody 720, Tokyo, Japan | NM/NM | NM | BIA can be used to assess body composition in patients who have undergone surgery. |
| Gastric cancer | Gao et al., 2020 [43] | China | Cross-sectional Study | To investigate the accuracy of BIA in estimating VFA 31 in individuals with GC 32 in the Chinese population, as well as to determine the threshold for diagnosing visceral obesity using BIA. | 157 | M: 109 (69.4%) F: 48 (30.6%) | 23.28 ± 2.93 | 60.61 ± 11.95 | InBody 720, Seoul, Republic of Korea | NM/NM | VFA 65.6/88.2% | VFA given by CT 33 and BIA had significant correlation and satisfactory reliability. Nevertheless, the absolute values of the two methods were not interchangeable directly. |
| Colorectal cancer | Jones et al., 2020 [34] | United Kingdom & Australia | Prospective study | To determine the agreement between BIA and MAMC 34 against CT scans for the measurement of muscle mass and identification of sarcopenia in patients with CRC 35. | 100 | M: 67 (67%) F: 33 (33%) | 25.8 ± 4.7 | 69.6 ± 11.5 | Bodystat 1500 machine, Douglas, Isle of Man, UK | 50/NM | BIA low MM 36 80%/52% MAMC low ‘MM 38% 88% | BIA and MAMC were inadequate to measure muscle mass in CRC patients Vs CT measurements at L3. Neither method can match the high precision of CT scans. |
| Kim et al., 2020 [31] | Republic of Korea | Prospective Study | To explore relationships between CT scans at the L3 level for muscle assessment and total SMM assessed by BIA in CRC patients. | 50 | M: 28 (56%) F: 22 (44%) | 24.3 ± 3.4 | 63.4 | InBody 770, Seoul, Republic of Korea | 50, 1000/ NM | NM | BIA could be an alternative method to CT scan, and it could be a non-invasive and cost-effective tool for the assessment of body composition, including SMM in a CRC patient that could be associated with clinical results. | |
| Palle et al., 2016 [35] | Denmark | Prospective Study | To assess associations between single cross-sectional thighs given by MRI 37, SMM as reference and multi-frequency BIA FFM in CRC patients undergoing chemotherapy. | 18 | M: 10 (56%) F: 8 (44%) | M: 25.3 ± 2.6 F: 23.1 ± 3.9 | 67 ± 6 | Tanita MC780MA, Tokyo, Japan | NM/NM | NM | BIA and ST 38 were the best alternatives to MRI since they showed constant and subsequently corrected errors. | |
| Ræder et al., 2018 [40] | Norway | Prospective study | To evaluate two different BIA devices, a whole-body BIA and a segmental BIA device, against DXA in CRC patients, and to investigate the ability of 14 empiric equations to predict DXA FFM. | 43 | M: 17 (39.5%) F: 26 (60.5%) | 25.8 | 67.0 | BIA, BIA-101, Würzburg, Germany Seca mBCA515, Birmingham, UK | 50/ Geneva equation | Whole-body BIA 78.6%/100% Segmental BIA 85.7%/77.8% | Both BIA-devices showed good ability to detect low FFM with an optimal equation. We recommend using one of these combinations of device and equation to determine FFM in this population. | |
| Song et al., 2019 [41] | Republic of Korea | Retrospective study | To determine the relationship between body composition and PLR 39 in patients with CRC. | 110 | M: 77 (70%) F: 33 (30%) | NM | 68.3 ± 9.6 | InBody 770, Biospace, Seoul, Republic of Korea | NM/NM | NM | Fat and muscle indices measured by InBody 770 were related to PLR in CRC. These results suggest that low muscle and fat may be related to poor prognosis of CRC. | |
| Souza et al., 2020 [36] | Brazil & Sweden | Prospective Study | To assess the agreement between computed tomography (CT) and surrogate methods employed in clinical practice for the assessment of low muscle mass. | 188 | M: 108 (57%) F: 80 (43%) | 27.1 ± 5.4 | 61.0 ± 11.4 | Quantum II, RJL Systems, Detroit, MI, USA | 50/ Janssen equation | SMI-BIA 93.9%/54.2% | Physical examination Vs CT had the best agreement to assess low muscle mass. Low muscle mass given by PG-SGA, BIA, and CT showed similar prognostic values for survival. | |
| Szefel et al., 2020 [37] | Poland | Prospective Study | To determine the effectiveness of BIA to detect and monitor cancer cachexia CC 40 in patients with CRC. | 158 | M: 72 (46%) F: 86 (54%) | NM | NM | Seca mBCA525, seca GmbH and Co., Hamburg, Germany | 50/NM | FFMI-BIA (M) 100%/39% FFMI-BIA (F) 88%/50% | BIA identified differences in body composition according to cancer stage and advancement of CC. After CRC diagnosis, periodic assessment by BIA seems useful. | |
| Pancreatic, gastric and colorectal cancer | Dzierżek et al., 2020 [38] | Poland | Prospective Study | To assess body composition and its impact on patients undergoing surgery due to GC, PC 41, and CRC. | 56 | M: 31 (55.4%) F: 25 (44.6%) | 25.8 | 66.0 | BIA-101 Akern, Italy | 50/NM | NM | BIA can be easy and effective to assess body composition and its change in patients undergoing major surgery. |
| Lung cancer | Hansen et al., 2021 [44] | Denmark | Cross-sectional Study | To investigate the agreement between body composition recorded with BIA and software analysis of CT scans of patients with cancer with a particular emphasis on MM. | 60 | M: 35 (58.3%) F: 25 (41.7%) | 23.96 ± 3.78 | 67.07 ± 7.54 | Tanita Segmental Body Composition Analyzer (BC-418), Tokyo, JapanC | 50/NM | NM | BIA and CT image analysis were not comparable to assess body composition. BIA overestimated MM and underestimated FM with LoA 42 outside that of the clinically acceptable difference. Bias was lower in the subgroup analysis, but not to acceptable levels. |
| Skin cancer | Zopfs et al., 2020 [29] | Germany | Cross-sectional study | To analyze if anthropometric measures, and body composition derived from BIA, as well as clinical anthropometric data, can be estimated from simple and reliable 2D measurements in routine CT scans. | 62 | M: 31 (50%) F: 31 (50%) | NM | 63.32 ± 15.92 | Seca mBCA 515, Hamburg, Germany | NM/NM | NM | Using simple measurements in a single axial CT slice, body composition can accurately be determined in clinical examinations by using simple measurements. |
| All cancer types | Cereda et al., 2021 [39] | Italy & Germany | Prospective study | To assess the potential prognostic role of FFMI in addition to BMI 43 and WL 44). The association with QoL 45 was also explored. | 1217 | M: 713 (58.6%) F: 504 (41.4%) | 23.6 ± 4.3 | 63.0 ± 12.6 | NUTRILAB Akern srl, Florence, Italy Nutriguard-M Data Input GmbH, Darmstadt, Germany | NM/ Equation of Sun et al. [77] | NM | In all patients with cancer, altered body composition should always be considered as an additional phenotypic criterion of poor prognosis and BIA provides the possibility of multiple, non-invasive bedside assessments. |
| Mueller et al., 2020 [45] | Germany | Cross-sectional Study | To determine if BIA is a reliable diagnostic tool even in patients with cancer with and without malnutrition, and could thus be safely used for short-term follow-up or in non-specialized/out-patient settings. | 118 NMG: 64 MG: 54 | NMG 46 M: 29 (45.3%) F: 35 (54.7%) MG 47 M: 28 (51.9%) F: 26 (48.1%) | NMG: 25.0 MG: 22.5 | NMG: 56 MG: 63 | BIA 101 anniversary SE, Akern Bioresearch, Italy | 50/NM | NM | BIA is a reliable diagnostic tool for the assessment of muscle and FM, even in patients with malnutrition, and could be utilized for the early detection and short-term follow-up of malnutrition and cachexia. | |
| 1 Patient Generated Subjective Global Assessment. 2 Not mentioned. 3 Fat-free mass index. 4 Bioelectrical impedance analysis. 5 Head and neck cancer. 6 Radiotherapy. 7 Skeletal muscle mass index. 8 Skeletal muscle mass. 9 Fat-free mass. 10 Phase angle. 11 Bioelectrical impedance vector analysis. 12 Single-frequency BIA. 13 Dual-energy X-ray absorptiometry. 14 Breast cancer. 15 Fat mass. 16 Study group. 17 Control group. 18 Oesophageal Cancer. 19 Morbidity Severity Score. 20 Overall Survival. 21 Normal muscle volume. 22 Low muscle volume. 23 Edema index. 24 Hepatocellular carcinoma. 25 Extracellular water. 26 Total body water. 27 Pancreaticoduodenectomy. 28 Gastrectomy. 29 Total gastrectomy. 30 Distal gastrectomy. 31 Visceral fat area. 32 Gastric cancer. 33 Computed tomography. 34 Mid arm muscle circumference. 35 Colorectal cancer. 36 Muscle mass. 37 Magnetic resonance imaging. 38 Skin-fold thickness. 39 Platelet-to-lymphocyte ratio. 40 Cancer cachexia. 41 Pancreatic cancer. 42 Limits of agreement. 43 Body mass index. 44 Weight loss. 45 Quality of life. 46 No Malnutrition Group. 47 Malnutrition Group. | ||||||||||||
Appendix E. Review Articles Included in the Review (n = 11)
| Cancer Location | Authors (Year) | Country | Objective | Articles | Sample (n) | Gender (n/%) | Main Conclusions |
| Head and neck cancer | Almada-Correia et al., 2019 [2] | Portugal & Finland | To examine the existing literature regarding body composition evaluation in patients with HNC to determine, which is the most suitable approach for this population. | 41 | 2708 | M: 2193 (81%) F: 515 (19%) | The reference methods for body composition assessment in patients with cancer are DXA and CT at L3, but these examinations are not frequently performed in the management of HNC. |
| Ferrão et al., 2020 [47] | Portugal & Finland | To examine and map the body composition changes in HNC, under active treatment, and to determine which methods are suitable to evaluate body composition in these patients. | 12 | 891 | M: 671 (75.3%) F: 220 (24.7%) | During chemoradiotherapy, persons with HNC experience significant depletion of LBM, FFM, and SMM, accompanied by body FM demonstrated either by the TSF, BIA, DXA, or CT. Body composition assessment should become an integral component of the care of HNC, beyond weight and BMI, and should be carried out at different times throughout treatment. | |
| Mantzorou et al., 2020 [15] | Greece & United Arab Emirates | To summarize and discuss the current clinical data on the effectiveness of easily accessible nutritional status assessment tools such as weight loss and BIA measures in the evaluation of malnutrition in patients with HNC. | 27 | 7215 | NM | Further studies are recommended to clarify the role of BIA-derived measures for nutritional status. | |
| Breast cancer | Pedersen et al., 2019 [48] | Denmark & Norway | To investigate changes in weight and body composition associated with anticancer medication and to examine factors that may influence the assessment and diversity of the findings. | 19 | 24,575 | NM | Based on this review, further investigation is recommend applying long-term prospective designs, measurements at certain time points, and assessing weight and body composition changes via the same kind of device. For example, bioelectrical impedance analysis that is cheap and easy to use could help to standardize measurement. |
| Esophageal cancer | Boshier et al., 2018 [49] | Canada | To present current literature on the assessment of body composition in patients with EC and to assess its potential implication for survival and perioperative morbidity. | 29 | 3193 | NM | The strength of the overall conclusions that can be drawn from this review is however limited by the lack of consensus in regard to optimal methodology and reporting standards. Priority should be given to established consensus guidelines for body composition assessment in EC. |
| Pancreatic cancer | Bundred et al., 2019 [50] | United Kingdom | To analyze current literature regarding body composition assessment in patients with PC and assess its impact on perioperative outcomes and long-term survival. | 42 | 7619 | NM | This review highlights the need for standardized assessment of body composition as it has the potential to contribute to future decisions in patients with PC. |
| Gastric cancer | Kamarajah et al., 2019 [51] | United Kingdom | To examine current literature regarding body composition assessment in patients with GC and assess its impact on perioperative outcomes and long-term survival. | 39 | 8402 | NM | This review highlights the need for standardized assessment of body composition as it has the potential to support future decision-making in patients with GC. With lack of consensus in regard to optimal methodology and reporting standards, future efforts should be focused at establishing consensus guidelines for body composition assessment in GC. |
| All cancer types | Di Sebastiano et al., 2012 [1] | Canada | To identify potential considerations for body composition analysis among different cancer populations and to discuss several methods of body composition analysis (anthropometric measures, BIA, ADP, DXA, CT, MRI) as they may provide viable options for use in people diagnosed with cancer. | NM | NM | NM | DXA is the ideal whole-body composition analysis method for prospective use in cancer populations as it is more precise, accurate, and provides fewer limitations than other methods. Since BIA relies on water volume, it has limited use in persons with advanced cancer as well as in persons with BC and cannot distinguish tumor or lymphedema in the lean and fat tissue depots. |
| Grundmann et al., 2015 [7] | USA | To summarize the current scientific and clinical evidence of BIA utility in persons with cancer and the implementation of BIA for evaluating outcomes of symptom management and providing supportive care in patients with cancers. | 27 | 20,239 | NM | BIA and PhA provide practitioners for the evaluation of nutritional and overall health status in patients with cancer with a convenient and non-invasive technique and should be encouraged. Further research on the diagnostic value and clinical applications of the BIA and the PhA should be conducted to strengthen and increase its use in clinical practice. | |
| Małecka-Massalska et al., 2017 [52] | Poland | To provide a literature review of bioelectrical impedance analysis in cancer malnutrition assessment. | 25 | 2000 | NM | BIA is an objective, reliable, and non-invasive method of malnutrition assessment. | |
| Matthews et al., 2021 [10] | United Kingdom | To assess whether BIA measures and estimates of body composition determined by BIA can identify adult patients at risk of complications after elective surgery for cancer. | 12 | 1508 | NM | BIA in the perioperative period may be advantageous in predicting the risk of complications following elective cancer surgery. | |
| NM: not mentioned. | |||||||
Appendix F. BIA Methodologies (Original Research Articles)
| Article | BIA Measurement (Whole-Body or Segmental Body Composition) | Position of Participants During Assessment (Supine or Standing) | Participant Preparation Protocols (e.g., Fasting Requirements, Exercise Restrictions) | Specific Body Composition Measures Assessed and Raw BIA Measures |
| Ding et al., 2018 [32] | Segmental | Supine—Lying position and the electrodes were attached in both ankles for legs and thumbs and middle fingers for arms | Free intake period before fasting | BCM 1, FM 2, FFM 3, and SMM 4 were obtained weekly using the InBody software from baseline until the end of treatment. FFMI 5 and FMI 6 values were calculated by dividing a patient’s FFM and FM values by the height squared (m2). |
| Grossberg et al., 2021 [5] | Segmental | Standing—One of three possible hand positions was selected such that the angle between the body and arms was as close to 30 degrees as possible. | To avoid additional barriers to adequate nutrition and exercise, participants were instructed to not alter their food intake or activity prior to measurement. | SMM, FFM, and FM. |
| Jager-Wittenaar et al., 2014 [9] | Segmental | Supine—Patients were put in a supine position 15 min before and during the measurement. | Patients were not allowed to eat or drink during the 4 h preceding the measurements. Patients were measured in their underwear, without shoes, and after voiding their bladders. | (ECW 7/ICW 8) ratio was calculated. R 9 and Xc 10 measured at 50 kHz were used to estimate FFM with the Geneva equation. |
| Lundberg et al., 2017 [16] | Segmental | Standing | Not mentioned. | BMI 11, R, Xc, PhA 12, FFMI, FMI, PhA, as well as a BIVA 13 |
| Lundberg et al., 2019 [25] | Segmental | Standing | Not mentioned. | The BIA parameters registered included PhA, FFMI and SMMI 14. |
| Bell et al., 2020 [42] | Segmental | Supine—Four electrodes were placed on the right side of the body in the following locations: (1) hand, (2) wrist, (3) foot and (4) ankle. | After voiding their bladder and removing all jewelry | R and Xc measurements. Measurements were performed twice, and the average R and Xc values were used to calculate FFM. |
| Jung et al., 2020 [33] | Segmental | Supine—Before the measurement, the patients rested in a lying down state for 15 min. In a stable position, patients’ arms were abducted to about 15 min. | Not mentioned. | TBW 15, total body skeleton, body FM, SMM, FFM and body fat percentage |
| Wilczyński et al., 2020 [46] | Segmental | Supine | In order to obtain the most accurate measurements, they were always carried out at the same time by the same trained person (between 18:00 and 20:00 p.m.) and under the same conditions, e.g., always before a meal. | Body mass (kg), BMI, FM, (%), FM (kg), FFM, MM (kg), TBW (kg) and TBW (%), MMLUL 16 (kg), MMRUL 17 (kg), LULFM 18 (kg) and RULFM 19 (kg). |
| Powell et al., 2020 [26] | Whole-body | Supine—The patient was supine and motionless throughout the test and the right arm was held equidistant from the torso during each assessment. | Not mentioned. | SMM (kg), FFM (kg), body fat (kg), ICW %, ECW %, TBW and PhA |
| Lee et al., 2021 [30] | Segmental | Supine—BIA was performed with patients in a standing position according to the manufacturer’s instructions after shoes, coats, and sweaters had been removed. | BIA was performed on the day of admission before intravenous hydration. | PhA, extracellular fluid and total body fluid, BCM, and appendicular skeletal muscle |
| Skroński et al., 2018 [27] | Segmental | Supine | The patients undergoing surgery were subjected to a two-fold body composition analysis procedure. The first measurement was carried out 1 day before the scheduled operation, the second measurement was made between the 5th and 6th day after surgery, before the patient was discharged from hospital. The measurement was carried out at 25 °C after at least 2.5 h from the last meal. | TBW, ICW, ECW, FFM, SMM, FM, BCM |
| Mikamori et al., 2016 [28] | Segmental | Standing | Not mentioned. | SMM, FM, VFA 20, and the ratio ECW/TBW |
| Gao et al., 2020 [43] | Segmental | Standing | Patients with fasting condition and empty bladder stand with both arms 45° apart from the body trunk and with both feet bared on the spots of the platform. | TBW, total FM, fat percentage, LBM, SBM 21 and FFM. |
| Jones et al., 2020 [34] | Whole-body | Supine—The patient lay in a supine position, with shoes and socks removed. | Not mentioned. | FFM, FFMI. |
| Kim et al., 2020 [31] | Segmental | Standing | Body composition of all participants was measured after a minimum of 3 h of fasting and voiding before measurements. | TBW, ICW, ECW, the ECW ratio (ECW/TBW), SLM 22, FFMFM, percent body fat, SMM, BCM, and bone mineral content. |
| Palle et al., 2016 [35] | Segmental | Standing | The patients were instructed not to eat, drink or exercise one hour before the determination of FFM | FM Fat at torso BIA |
| Ræder et al., 2018 [40] | Two different BIA devices were used—one whole-body single-frequency BIA and a multifrequency segmental BIA | Supine (whole-body) vs. standing (segmental) | The patients were instructed to fast overnight and until all measurements were completed. They were also encouraged to void their bladders before measurements. | FFM (whole-body BIA) vs. FFM (segmental BIA) |
| Song et al., 2019 [41] | Segmental | Standing | Not mentioned. | Body FM, percent body fat, body fat mass of trunk, VFA, FMI, and measured fat thickness of the abdomen SLM, FFM, SMM, SLM of trunk (kg) |
| Souza et al., 2020 [36] | Segmental | Supine | Participants under 6 h of fast. | SMM and PhA |
| Szefel et al., 2020 [37] | Segmental | Supine | Not mentioned. | FFMI, SMMI, ECW/TBW, and PhA |
| Dzierżek et al., 2020 [38] | Segmental | Supine | Not mentioned. | FM, FFM, TBW, ICW, ECW and BCM |
| Hansen et al., 2021 [44] | Segmental | Standing | BIA took place on the same day as the first cycle of treatment, but prior to commencing treatment. Patients were instructed to fast at least 4 h (consumption of water was allowed until 2 h), refrain from exercise 8 h, and to urinate within 30 min prior to testing. | MM and FM |
| Zopfs et al., 2020 [29] | Segmental | Standing | Overnight-fasted patients within the morning hours | MM, FM, FFM, and VFA |
| Cereda et al., 2021 [39] | Segmental | Standing | Not mentioned. | FFM |
| 1 Body cell mass. 2 Fat mass. 3 Fat-free mass. 4 Skeletal muscle mass. 5 Fat-free mass index. 6 Fat mass index. 7 Extracellular water. 8 Intracellular water. 9 Resistance. 10 Reactance. 11 Body mass index. 12 Phase angle. 13 Bioelectrical Impedance Vector Analysis. 14 Skeletal muscle mass index. 15 Total body water. 16 Muscle mass of the left upper limb. 17 Muscle mass of the right upper limb. 18 Left upper limb fat mass. 19 Right upper limb fat mass. 20 Visceral fat area. 21 Skeletal body mass. 22 Soft lean mass. | ||||
Appendix G. General Considerations of BIA as an Assessment Tool in Patients with Cancer
| Advantages |
| Indirect method Safe Practical and objective tool Reliable Portable Easy-to-use Non-invasive Reproducible Time and cost effective technique Relatively inexpensive, when compared to more sophisticated methods like DXA, CT or MRI Requires little training to use the equipment BIA appears to show good correlations when compared with gold standard methods Validated method to assess body composition in patients with cancer Good application consistency in cancer patients Usefulness as a tool for assessing the nutritional status of patients with cancer BIA measures may serve as early indicators for improvement in nutritional and health status BIA measures can useful to evaluate and predict outcomes, such as post-operative complications BIA-derived PhA does not depend on regression equations to be calculated and as prognostic factor of patient survival BIA-derived measures (FFM, FM, body weight, BMI) are correlated with the risk of developing colon cancer and potentially other cancers BIA-derived PhA and BIVA are considered to reflect both nutritional and hydration status |
| Disadvantages |
| Does not measure the entire body, gives incomplete information Not routinely available outside the research setting More expensive than using anthropometric measures Generally considered less accurate than radiological assessment methods Evaluations should be done under the same circumstances and taking into consideration an adequate fluid balance and food intake Possible sources of error: nutrition status, physical activity, phase of the menstrual cycle, placement of electrodes, limb length, blood chemistry, altered fluid balance, edema, endocrine diseases, treatment with growth hormone, acute illness, intensive care treatment, organ transplantation, position of the body and movements during the measure, type of electrodes, use of oral contraceptives Loses accuracy when patients are in the extremes of BMI ranges (≤16 kg/m2 or ≥35 kg/m2) Regarding the hydration status, dehydration or over hydration may underestimate or overestimate LBM or FBM As a consequence of the fluid accumulation, BIA may imprecisely measure FFM or FM in persons diagnosed with BC and gynaecological cancer BIA has provided inconsistent findings, with poorer accuracy and precision in obese/oedematous individuals It has limited use in advanced cancer and BC because of the large fluid shifts that occur in these cancer cohorts and cannot distinguish tumor or lymphedema in the lean and fat tissue depots Underestimates FFM in patients with advanced cancer, compared with DXA Relies on a large number of prediction equations using linear regression to estimate body composition based on a variety of predetermined variables that may differ between different populations and were derived from healthy individuals |
References
- Di Sebastiano, K.M.; Mourtzakis, M. A critical evaluation of body composition modalities used to assess adipose and skeletal muscle tissue in cancer. Appl. Physiol. Nutr. Metab. 2012, 37, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Almada-Correia, I.; Neves, P.M.; Mäkitie, A.; Ravasco, P. Body Composition Evaluation in Head and Neck Cancer Patients: A Review. Front. Oncol. 2019, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.; Goh, V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Rock, C.D.; Edwards, J.; Mohamed, A.S.; Ruzensky, D.; Currie, A.; Rosemond, P.; Phan, J.; Gunn, G.B.; Frank, S.J.; et al. Bioelectrical impedance analysis as a quantitative measure of sarcopenia in head and neck cancer patients treated with radiotherapy. Radiother. Oncol. 2021, 159, 21–27. [Google Scholar] [CrossRef]
- Mulasi, U.; Kuchnia, A.J.; Cole, A.J.; Earthman, C.P. Bioimpedance at the Bedside: Current Applications, Limitations, and Opportunities. Nutr. Clin. Pract. 2015, 30, 180–193. [Google Scholar] [CrossRef]
- Grundmann, O.; Yoon, S.L.; Williams, J.J. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—A comprehensive review. Eur. J. Clin. Nutr. 2015, 69, 1290–1297. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis? Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Earthman, C.P.; Krijnen, W.P.; Langendijk, J.A.; van der Laan, B.F.; Pruim, J.; Roodenburg, J.L. Validity of bioelectrical impedance analysis to assess fat-free mass in patients with head and neck cancer: An exploratory study. Head Neck 2013, 36, 585–591. [Google Scholar] [CrossRef]
- Matthews, L.; Bates, A.; Wootton, S.; Levett, D. The use of bioelectrical impedance analysis to predict post-operative complications in adult patients having surgery for cancer: A systematic review. Clin. Nutr. 2021, 40, 2914–2922. [Google Scholar] [CrossRef]
- Ellis, K.J. Innovative Non-or Minimally-Invasive Technologies for Monitoring Health and Nutritional Status in Mothers and Young Children Selected Body Composition Methods Can Be Used in Field Studies 1. J. Nutr. 2001, 131, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Morabia, A.; Slosman, D.O.; Mensi, N.; Unger, P.; Pichard, C. Contribution of body composition to nutritional assessment at hospital admission in 995 patients: A controlled population study. Br. J. Nutr. 2001, 86, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Pichard, C.; Kyle, U.G.; Morabia, A.; Perrier, A.; Vermeulen, B.; Unger, P. Nutritional assessment: Lean body mass depletion at hospital admission is associated with an increased length of stay. Am. J. Clin. Nutr. 2004, 79, 613–618. [Google Scholar] [CrossRef]
- Thibault, R.; Makhlouf, A.-M.; Mulliez, A.; Gonzalez, M.C.; Kekstas, G.; Kozjek, N.R.; Preiser, J.-C.; Rozalen, I.C.; Dadet, S.; Krznaric, Z.; et al. Fat-free mass at admission predicts 28-day mortality in intensive care unit patients: The international prospective observational study Phase Angle Project. Intensive Care Med. 2016, 42, 1445–1453. [Google Scholar] [CrossRef]
- Mantzorou, M.; Tolia, M.; Poultsidi, A.; Pavlidou, E.; Papadopoulou, S.K.; Papandreou, D.; Giaginis, C. Can Bioelectrical Impedance Analysis and BMI Be a Prognostic Tool in Head and Neck Cancer Patients? A Review of the Evidence. Cancers 2020, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, M.; Nikander, P.; Tuomainen, K.; Orell-Kotikangas, H.; Mäkitie, A. Bioelectrical impedance analysis of head and neck cancer patients at presentation. Acta Oto-Laryngol. 2017, 137, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, A.C.; Mayland, C.R.; Mason, S.; Cox, T.F.; Varro, A.; Ellershaw, J. The Association of Hydration Status with Physical Signs, Symptoms and Survival in Advanced Cancer—The Use of Bioelectrical Impedance Vector Analysis (BIVA) Technology to Evaluate Fluid Volume in Palliative Care: An Observational Study. PLoS ONE 2016, 11, e0163114. [Google Scholar] [CrossRef]
- Piccoli, A.; Pillon, L.; Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: Standard reference intervals as bivariate Z scores. Nutrition 2002, 18, 153–167. [Google Scholar] [CrossRef]
- Nwosu, A.C.; Mayland, C.R.; Mason, S.; Cox, T.F.; Varro, A.; Stanley, S.; Ellershaw, J. Bioelectrical impedance vector analysis (BIVA) as a method to compare body composition differences according to cancer stage and type. Clin. Nutr. ESPEN 2019, 30, 59–66. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.G.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Zocher, D.; Bosy-Westphal, A.; Szramek, A.; Scheufele, R.; Smoliner, C.; Pirlich, M. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am. J. Clin. Nutr. 2010, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lammersfeld, C.A.; Burrows, J.L.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Hoffman, S.; Lis, C.G. Bioelectrical Impedance Phase Angle in Clinical Practice: Implications for Prognosis in Advanced Colorectal Cancer. Am. J. Clin. Nutr. 2004, 80, 1634–1638. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Lammersfeld, C.A. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br. J. Nutr. 2004, 92, 957–962. [Google Scholar] [CrossRef]
- Lundberg, M.; Dickinson, A.; Nikander, P.; Orell, H.; Mäkitie, A. Low-phase angle in body composition measurements correlates with prolonged hospital stay in head and neck cancer patients. Acta Oto-Laryngol. 2019, 139, 383–387. [Google Scholar] [CrossRef]
- Powell, A.; Mulla, M.; Eley, C.; Patel, N.; Abdelrahman, T.; Blake, P.; Barlow, R.; Bailey, D.; Lewis, W. Prognostic significance of low muscle volume in patients undergoing surgery for oesophageal cancer. Clin. Nutr. ESPEN 2020, 40, 220–225. [Google Scholar] [CrossRef]
- Skroński, M.; Andrzejewska, M.; Fedosiejew, M.; Ławiński, M.; Włodarek, D.; Ukleja, A.; Nyckowski, P.; Słodkowski, M. Assessment of changes in the body composition in patients qualified for the operational treatment of the primary and metastatic liver tumors with the use of bioelectric impedance. Ann. Surg. 2018, 90, 1–5. [Google Scholar] [CrossRef]
- Mikamori, M.; Miyamoto, A.; Asaoka, T.; Maeda, S.; Hama, N.; Yamamoto, K.; Hirao, M.; Ikeda, M.; Sekimoto, M.; Doki, Y.; et al. Postoperative Changes in Body Composition After Pancreaticoduodenectomy Using Multifrequency Bioelectrical Impedance Analysis. J. Gastrointest. Surg. 2015, 20, 611–618. [Google Scholar] [CrossRef]
- Zopfs, D.; Theurich, S.; Hokamp, N.G.; Knuever, J.; Gerecht, L.; Borggrefe, J.; Schlaak, M.; dos Santos, D.P. Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur. Radiol. 2019, 30, 1701–1708. [Google Scholar] [CrossRef]
- Lee, G.H.; Cho, H.J.; Lee, G.; Kim, H.G.; Wang, H.J.; Kim, B.-W.; Lee, M.Y.; Yoon, S.Y.; Noh, C.-K.; Seo, C.W.; et al. Bioelectrical impedance analysis for predicting postoperative complications and survival after liver resection for hepatocellular carcinoma. Ann. Transl. Med. 2021, 9, 190. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, S.R.; Won, D.D.; Choi, M.H.; Lee, I.K. Multifrequency Bioelectrical Impedance Analysis Compared With Computed Tomography for Assessment of Skeletal Muscle Mass in Primary Colorectal Malignancy: A Predictor of Short-Term Outcome After Surgery. Nutr. Clin. Pract. 2019, 35, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Dou, S.; Ling, Y.; Zhu, G.; Wang, Q.; Wu, Y.; Qian, Y. Longitudinal Body Composition Changes and the Importance of Fat-Free Mass Index in Locally Advanced Nasopharyngeal Carcinoma Patients Undergoing Concurrent Chemoradiotherapy. Integr. Cancer Ther. 2018, 17, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.H.; Kim, J.H.; Chung, M.S. Changes in weight, body composition, and physical activity among patients with breast cancer under adjuvant chemotherapy. Eur. J. Oncol. Nurs. 2019, 44, 101680. [Google Scholar] [CrossRef]
- Jones, D.J.; Lal, S.; Strauss, B.J.; Todd, C.; Pilling, M.; Burden, S.T. Measurement of Muscle Mass and Sarcopenia Using Anthropometry, Bioelectrical Impedance, and Computed Tomography in Surgical Patients with Colorectal Malignancy: Comparison of Agreement Between Methods. Nutr. Cancer 2019, 72, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Palle, S.S.; Møllehave, L.T.; Taheri-Kadkhoda, Z.; Johansen, S.; Larsen, L.; Hansen, J.W.; Jensen, N.K.; Elingaard, A.O.; Møller, A.H.; Larsen, K.; et al. Multi-frequency bioelectrical impedance analysis (BIA) compared to magnetic resonance imaging (MRI) for estimation of fat-free mass in colorectal cancer patients treated with chemotherapy. Clin. Nutr. ESPEN 2016, 16, 8–15. [Google Scholar] [CrossRef]
- Souza, N.C.; Gonzalez, M.C.; Martucci, R.B.; Rodrigues, V.D.; de Pinho, N.B.; Qureshi, A.R.; Avesani, C.M. Comparative Analysis Between Computed Tomography and Surrogate Methods to Detect Low Muscle Mass Among Colorectal Cancer Patients. J. Parenter. Enter. Nutr. 2019, 44, 1328–1337. [Google Scholar] [CrossRef]
- Szefel, J.; Kruszewski, W.J.; Szajewski, M.; Ciesielski, M.; Danielak, A. Bioelectrical Impedance Analysis to Increase the Sensitivity of Screening Methods for Diagnosing Cancer Cachexia in Patients with Colorectal Cancer. J. Nutr. Metab. 2020, 2020, 3874956. [Google Scholar] [CrossRef]
- Dzierżek, P.; Kurnol, K.; Hap, W.; Frejlich, E.; Diakun, A.; Karwowski, A.; Kotulski, K.; Rudno-Rudzińska, J. Assessment of body composition measure of bioelectrical impedance in patients operated for pancreatic, gastric and colorectal cancer. Ann. Surg. 2020, 92, 1–5. [Google Scholar] [CrossRef]
- Cereda, E.; Pedrazzoli, P.; Lobascio, F.; Masi, S.; Crotti, S.; Klersy, C.; Turri, A.; Stobäus, N.; Tank, M.; Franz, K.; et al. The prognostic impact of BIA-derived fat-free mass index in patients with cancer. Clin. Nutr. 2021, 40, 3901–3907. [Google Scholar] [CrossRef]
- Ræder, H.; Kværner, A.S.; Henriksen, C.; Florholmen, G.; Henriksen, H.B.; Bøhn, S.K.; Paur, I.; Smeland, S.; Blomhoff, R. Validity of bioelectrical impedance analysis in estimation of fat-free mass in colorectal cancer patients. Clin. Nutr. 2017, 37, 292–300. [Google Scholar] [CrossRef]
- Song, W.J.; Kim, K.E.; Bae, S.U.; Jeong, W.K.; Baek, S.K. Association between body composition measured by bioelectrical impedance analysis and platelet-to-lymphocyte ratio in colorectal cancer. Korean J. Clin. Oncol. 2019, 15, 7–14. [Google Scholar] [CrossRef]
- Bell, K.E.; Schmidt, S.; Pfeiffer, A.; Bos, L.; Earthman, C.; Russell, C.; Mourtzakis, M. Bioelectrical Impedance Analysis Overestimates Fat-Free Mass in Breast Cancer Patients Undergoing Treatment. Nutr. Clin. Pract. 2019, 35, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Liu, Y.; Ding, C.; Liu, S.; Chen, X.; Bian, X. Comparison of visceral fat area measured by CT and bioelectrical impedance analysis in Chinese patients with gastric cancer: A cross-sectional study. BMJ Open 2020, 10, e036335. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Tobberup, R.; Rasmussen, H.H.; Delekta, A.M.; Holst, M. Measurement of body composition: Agreement between methods of measurement by bioimpedance and computed tomography in patients with non-small cell lung cancer. Clin. Nutr. ESPEN 2021, 44, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.C.; Reik, L.; Prokopchuk, O.; Friess, H.; Martignoni, M.E. Measurement of body mass by bioelectrical impedance analysis and computed tomography in cancer patients with malnutrition—A cross-sectional observational study. Medicine 2020, 99, e23642. [Google Scholar] [CrossRef]
- Wilczyński, J.; Sobolewski, P.; Zieliński, R.; Kabała, M. Body Composition in Women after Radical Mastectomy. Int. J. Environ. Res. Public Health 2020, 17, 8991. [Google Scholar] [CrossRef]
- Ferrão, B.; Neves, P.M.; Santos, T.; Capelas, M.L.; Mäkitie, A.; Ravasco, P. Body composition changes in patients with head and neck cancer under active treatment: A scoping review. Support. Care Cancer 2020, 28, 4613–4625. [Google Scholar] [CrossRef]
- Pedersen, B.; Delmar, C.; Lörincz, T.; Falkmer, U.; Grønkjær, M. Investigating Changes in Weight and Body Composition Among Women in Adjuvant Treatment for Breast Cancer: A Scoping Review. Cancer Nurs. 2019, 42, 91–105. [Google Scholar] [CrossRef]
- Boshier, P.R.; Heneghan, R.; Markar, S.R.; E Baracos, V.; E Low, D. Assessment of body composition and sarcopenia in patients with esophageal cancer: A systematic review and meta-analysis. Dis. Esophagus 2018, 31, doy047. [Google Scholar] [CrossRef]
- Bundred, J.; Kamarajah, S.K.; Roberts, K.J. Body composition assessment and sarcopenia in patients with pancreatic cancer: A systematic review and meta-analysis. HPB 2019, 21, 1603–1612. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J.; Tan, B.H.L. Body composition assessment and sarcopenia in patients with gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2018, 22, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Małecka-Massalska, T.; Powrózek, T.; Mlak, R. Handbook of Famine, Starvation, and Nutrient Deprivation; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2017; pp. 1–23. ISBN 9783319400075. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Malecka-Massalska, T.; Smolen, A.; Morshed, K. Body composition analysis in head and neck squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 2775–2779. [Google Scholar] [CrossRef][Green Version]
- Axelsson, L.; Silander, E.; Bosaeus, I.; Hammerlid, E. Bioelectrical phase angle at diagnosis as a prognostic factor for survival in advanced head and neck cancer. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2379–2386. [Google Scholar] [CrossRef]
- Adam, R.; Haileselassie, W.; Solomon, N.; Desalegn, Y.; Tigeneh, W.; Suga, Y.; Gebremedhin, S. Nutritional status and quality of life among breast Cancer patients undergoing treatment in Addis Ababa, Ethiopia. BMC Women’s Health 2023, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Sulaiman, S.; Koon, P.B.; Amani, R.; Hosseini, S.M. Association of Nutritional Status with Quality of Life in Breast Cancer Survivors. Asian Pac. J. Cancer Prev. 2013, 14, 7749–7755. [Google Scholar] [CrossRef]
- Terada, M.; Yoshimura, A.; Sawaki, M.; Hattori, M.; Naomi, G.; Kotani, H.; Adachi, Y.; Iwase, M.; Kataoka, A.; Sugino, K.; et al. Patient-reported outcomes and objective assessments with arm measurement and bioimpedance analysis for lymphedema among breast cancer survivors. Breast Cancer Res. Treat. 2019, 179, 91–100. [Google Scholar] [CrossRef]
- Blaney, J.M.; McCollum, G.; Lorimer, J.; Bradley, J.; Kennedy, R.; Rankin, J.P. Prospective surveillance of breast cancer-related lymphoedema in the first-year post-surgery: Feasibility and comparison of screening measures. Support. Care Cancer 2014, 23, 1549–1559. [Google Scholar] [CrossRef]
- Jordan, T.; Mastnak, D.M.; Palamar, N.; Kozjek, N.R. Nutritional Therapy for Patients with Esophageal Cancer. Nutr. Cancer 2017, 70, 23–29. [Google Scholar] [CrossRef]
- Ida, S.; Watanabe, M.; Karashima, R.; Imamura, Y.; Ishimoto, T.; Baba, Y.; Iwagami, S.; Sakamoto, Y.; Miyamoto, Y.; Yoshida, N.; et al. Changes in Body Composition Secondary to Neoadjuvant Chemotherapy for Advanced Esophageal Cancer are Related to the Occurrence of Postoperative Complications After Esophagectomy. Ann. Surg. Oncol. 2014, 21, 3675–3679. [Google Scholar] [CrossRef]
- Motoori, M.; Fujitani, K.; Sugimura, K.; Miyata, H.; Nakatsuka, R.; Nishizawa, Y.; Komatsu, H.; Miyazaki, S.; Komori, T.; Kashiwazaki, M.; et al. Skeletal Muscle Loss during Neoadjuvant Chemotherapy Is an Independent Risk Factor for Postoperative Infectious Complications in Patients with Advanced Esophageal Cancer. Oncology 2018, 95, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Sugimura, K.; Motoori, M.; Fujiwara, Y.; Omori, T.; Yanagimoto, Y.; Ohue, M.; Yasui, M.; Miyoshi, N.; Tomokuni, A.; et al. Clinical Assessment of Sarcopenia and Changes in Body Composition During Neoadjuvant Chemotherapy for Esophageal Cancer. Anticancer Res. 2017, 37, 3053–3059. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schütte, K.; Tippelt, B.; Schulz, C.; Feneberg, A.; Seidensticker, R.; Arend, J.; Malfertheiner, P. Malnutrition is a prognostic factor in patients with hepatocellular carcinoma (HCC). Clin. Nutr. 2014, 34, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.P.; Sicchieri, J.M.F.; Schiavoni, I.L.; Barbeiro, D.; Manca, C.S.; da Silva, B.R.; Bezerra, A.E.; Pinto, L.C.M.; Araújo, R.C.; Teixeira, A.C.; et al. Phase angle as a severity indicator for liver diseases. Nutrition 2020, 70, 110607. [Google Scholar] [CrossRef] [PubMed]
- Peres, W.A.F.; Lento, D.F.; Baluz, K.; Ramalho, A. Phase Angle as a Nutritional Evaluation Tool in Ail Stages of Chronic Liver Disease. Nutr. Hosp. 2012, 27, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, A.; Krampitz, J.; Rosenberger, F.; Kind, S.; Rötzer, I. Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers 2022, 14, 2212. [Google Scholar] [CrossRef]
- Yasui-Yamada, S.; Oiwa, Y.; Saito, Y.; Aotani, N.; Matsubara, A.; Matsuura, S.; Tanimura, M.; Tani-Suzuki, Y.; Kashihara, H.; Nishi, M.; et al. Impact of phase angle on postoperative prognosis in patients with gastrointestinal and hepatobiliary-pancreatic cancer. Nutrition 2020, 79–80, 110891. [Google Scholar] [CrossRef]
- Kirac, I.; Fila, J.; Misir, Z.; Čugura, J.F.; Žaja, A.; Benčić, I.; Štefančić, L. Nutritional evaluation in the perioperative period of gastric cancer patients using bioelectrical impedance analysis (BIA). Libr. Oncol. Croat. J. Oncol. 2019, 47, 13–16. [Google Scholar] [CrossRef]
- Yu, B.; Park, K.B.; Park, J.Y.; Lee, S.S.; Kwon, O.K.; Chung, H.Y. Bioelectrical Impedance Analysis for Prediction of Early Complications after Gastrectomy in Elderly Patients with Gastric Cancer: The Phase Angle Measured Using Bioelectrical Impedance Analysis. J. Gastric Cancer 2019, 19, 278–289. [Google Scholar] [CrossRef]
- Bärebring, L.; Kværner, A.S.; Skotnes, M.; Henriksen, H.B.; Skjetne, A.J.; Henriksen, C.; Ræder, H.; Paur, I.; Bøhn, S.K.; Wiedswang, G.; et al. Use of bioelectrical impedance analysis to monitor changes in fat-free mass during recovery from colorectal cancer– a validation study. Clin. Nutr. ESPEN 2020, 40, 201–207. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; King, J.; Vashi, P.G.; Grutsch, J.F.; A Lammersfeld, C. The relationship between bioelectrical impedance phase angle and subjective global assessment in advanced colorectal cancer. Nutr. J. 2008, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Kovarik, M.; Hronek, M.; Zadak, Z. Clinically relevant determinants of body composition, function and nutritional status as mortality predictors in lung cancer patients. Lung Cancer 2014, 84, 1–6. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical Impedance Phase Angle in Clinical Practice: Implications for Prognosis in Stage IIIB and IV Non-Small Cell Lung Cancer. BMC Cancer 2009, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bioelectrical Impedance Phase Angle in Clinical Practice: Implications for Prognosis in Advanced Colorectal Cancer. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Di Gregorio, A.; Pasanisi, F.; Scalfi, L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: A systematic review. Clin. Nutr. 2021, 40, 3052–3061. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).