Impact of Caloric Restriction in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Prospective Case Control Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Patients
2.2. Nutritional Assessment and Blood Sampling
2.3. Statistical Analysis
3. Results
3.1. Impact of CR in Response to NACT
3.2. Nutritional Efficacy and Safety of CR in NACT Patients
3.2.1. IC and BIA
3.2.2. Anthropometric Parameters
3.2.3. Biochemical Analysis
3.2.4. Compliance with CR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| BCM | Body Cell Mass |
| BIA | Bioimpedentiometry |
| BMI | Body Mass Index |
| CHO | Carbohydrates |
| CI | Confidence Intervals |
| CR | Caloric Restriction |
| CT | Chemotherapy |
| ER | Estrogen Receptor |
| FFM | Free Fat Mass |
| FM | Fat Mass |
| HDL | High-density Lipoprotein |
| HOMA | Homeostatic Model Assessment |
| IC | Indirect Calorimetry |
| LDL | Low-density Lipoprotein |
| NACT | Neoadjuvant Chemotherapy |
| OR | Odd Ratio |
| PA | Phase Angle |
| pCR | Pathological Complete Response |
| PR | Progesterone Receptor |
| REE | Resting Energy Expenditure |
| RQ | Respiratory Quotient |
| RR | Relative Risk |
| TEE | Total Energy Expenditure |
| TBW | Total Body Water |
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Brown, K.A. Obesity and breast cancer—Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell. Endocrinol. 2017, 466, 15–30. [Google Scholar] [CrossRef]
- Reggiani, F.; Bertolini, F. Roles of obesity in the development and progression of breast cancer. Discov. Med. 2017, 24, 183–190. [Google Scholar]
- Bhandari, R.; Kelley, G.A.; Hartley, T.A.; Rockett, I.R.H. Metabolic syndrome is associated with increased breast cancer risk: A systematic review with meta-analysis. Int. J. Breast Cancer 2014, 2014, 189384. [Google Scholar] [CrossRef] [PubMed]
- Lashinger, L.M.; O’flanagan, C.H.; Dunlap, S.M.; Rasmussen, A.J.; Sweeney, S.; Guo, J.Y.; Lodi, A.; Tiziani, S.; White, E.; Hursting, S.D. Starving cancer from the outside and inside: Separate and combined effects of calorie restriction and autophagy inhibition on Ras-driven tumors. Cancer Metab. 2016, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Walford, R.L. The Retardation of Aging and Disease by Dietary Restriction; Charles C Thomas Publisher: Springfield, IL, USA, 1988. [Google Scholar]
- Albanes, D. Total calories, body weight, and tumor incidence in mice. Cancer Res. 1987, 47, 1987–1992. [Google Scholar]
- Kritchevsky, D. Caloric restriction and experimental mammary carcinogenesis. Breast Cancer Res. Treat. 1997, 46, 161–167. [Google Scholar] [CrossRef]
- Hursting, S.D.; Lavigne, J.A.; Berrigan, D.; Perkins, S.N.; Barrett, J.C. Barrett. Calorie restriction, aging, and cancer prevention: Mechanisms of action and applicability to humans. Annu. Rev. Med. 2003, 54, 131–152. [Google Scholar] [CrossRef]
- Pariza, M.W. Dietary fat, calorie restriction, ad libitum feeding, and cancer risk. Nutr. Rev. 1987, 45, 1–7. [Google Scholar] [CrossRef]
- De Groot, S.; Vreeswijk, M.P.G.; Welters, M.J.P.; Gravesteijn, G.; Boei, J.J.W.A.; Jochems, A.; Houtsma, D.; Putter, H.; Van Der Hoeven, J.J.M.; Nortier, J.W.R.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Fusco, N.; Rizzo, A.; Costarelli, L.; Santinelli, A.; Cerbelli, B.; Scatena, C.; Macrì, E.; Pietribiasi, F.; D’amati, G.; Sapino, A.; et al. Pathological examination of breast cancer samples before and after neoadjuvant therapy: Recommendations from the Italian Group for the Study of Breast Pathology—Italian Society of Pathology (GIPaM-SIAPeC). Pathologica 2002, 114, 104–110. [Google Scholar] [CrossRef]
- Lee, K.; Sami, N.; Sweeney, F.C.; Dieli-Conwright, C.M. Body Composition with Dual-Energy X-Ray Absorptiometry and Bioelectrical Impedance Analysis in Breast Cancer Survivors. Nutr. Clin. Pract. 2019, 34, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part I and part II: Review of principles and methods and utilization in clinical practice. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Armitage, P.; Berry, G. Statistical Methods in Medical Research, 3rd ed.; Blackwell Scientific Publication: Oxford, UK, 1994. [Google Scholar]
- Altmann, D.G. Practical Statistics for Medical Research; Chapman & Hall: London, UK, 1991. [Google Scholar]
- Parman, M.K.B.; Machin, D. Survival Analysis: A Practical Approach; John Wiley: Chichester, UK, 1995. [Google Scholar]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2016, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Katzmarzyk, P.T.; Ross, R. Waist circumference and not body mass index explains obesity-related health risk. Am. J. Clin. Nutr. 2004, 79, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Kim, D.-S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A. Metabolic pathways in obesity-related breast cancer. Nat. Rev. Endocrinol. 2012, 17, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Lam, M.F.; DeMichael, K.M. Calorie restriction and breast cancer treatment: A mini-review. J. Mol. Med. 2022, 100, 1095–1109. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef]
- Longo, V.D.; Di Tano, M.; Mattson, M.P.; Guidi, N. Intermittent and periodic fasting, longevity and disease. Nat. Aging 2021, 1, 47–59. [Google Scholar] [CrossRef]
- Duregon, E.; Pomatto-Watson, L.C.D.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent fasting: From calories to time restriction. GeroScience 2021, 43, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.; Ehsan, I.; Vreeswijk, M.P.; Smit, V.T.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.; et al. Fasting mimicking dide as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Methods Mol. Biol. 2016, 207, 241–266. [Google Scholar]
- Cramer, T.; Schmitt, C.A. Metabolism in Cancer; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Simone, B.A.; Palagani, A.; Strickland, K.; Ko, K.; Jin, L.; Lim, M.K.; Dan, T.D.; Sarich, M.; Monti, D.A.; Cristofanilli, M.; et al. Caloric restriction counteracts chemotherapy-induced inflammation and increases response to therapy in a triple negative breast cancer model. Cell Cycle 2018, 17, 1536–1544. [Google Scholar] [CrossRef]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef] [PubMed]
- Huisman, S.A.; Bijman-Lagcher, W.; IJzermans, J.N.; Smits, R.; de Bruin, R.W. Fasting protects against the side effects of irinotecan but preserves its anti-tumor effect in Apc15lox mutant mice. Cell Cycle 2015, 14, 2333–2339. [Google Scholar] [CrossRef]
- Bozzetti, F. Calorie restriction in cancer patients undergoing chemotherapy: Facts, phantasy or misunderstanding. Clin. Nutr. 2022, 41, 1316–1319. [Google Scholar] [CrossRef]
- Komatsu, T.; Park, S.; Hayashi, H.; Mori, R.; Yamaza, H.; Shimokawa, I. Mechanisms of Calorie Restriction: A Review of Genes Required for the Life-Extending and Tumor-Inhibiting Effects of Calorie Restriction. Nutrients 2019, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Cha, M.-J.; Lee, S.-K.; Song, B.-W.; Jin, X.; Lee, J.M.; Park, J.H.; Lee, J.D. Curcumin Treatment in Combination with Glucose Restriction Inhibits Intracellular Alkalinization and Tumor Growth in Hepatoma Cells. Int. J. Mol. Sci. 2019, 20, 2375. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Harvie, M.; Pegington, M.; Howell, S.J.; Bundred, N.; Foden, P.; Adams, J.; Graves, L.; Greystoke, A.; Mattson, M.P.; Cutler, R.G.; et al. Randomised controlled trial of intermittent vs continuous energy restriction during chemotherapy for early breast cancer. Br. J. Cancer 2022, 126, 1157–1167. [Google Scholar] [CrossRef]
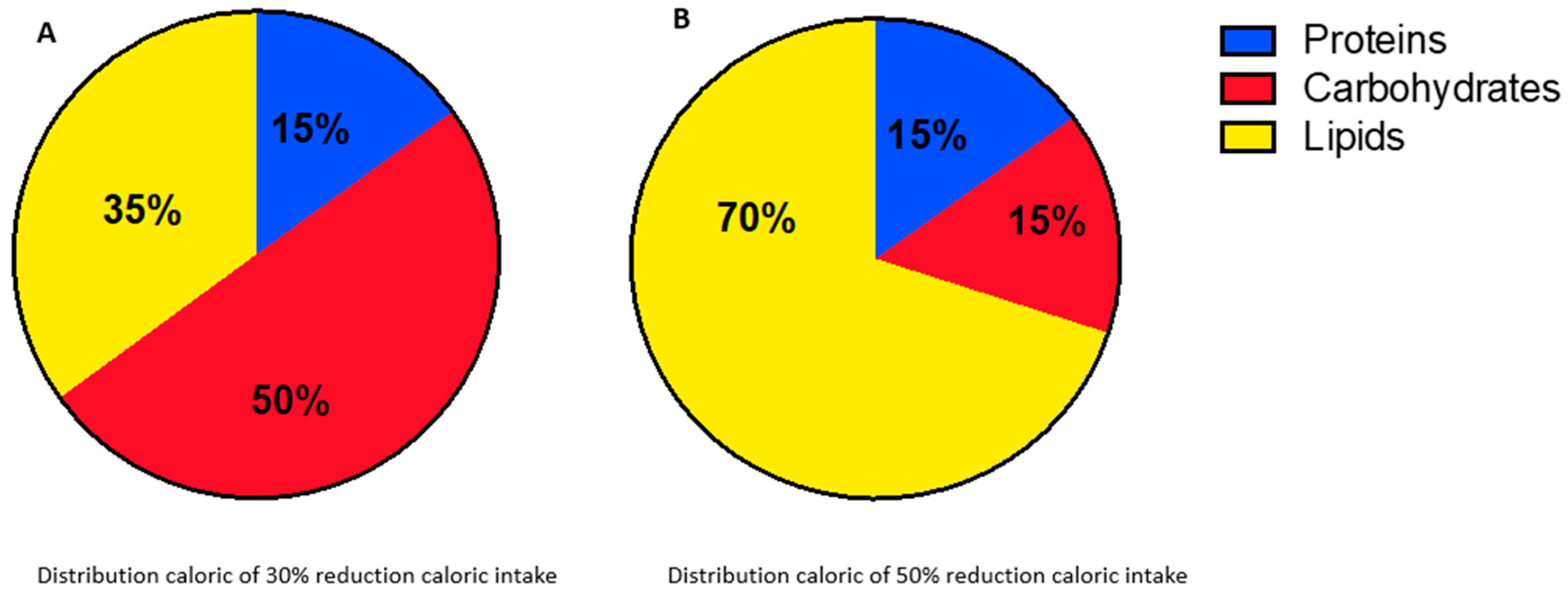

| Diet Group (39) | Control Group (60) | Chi-Square | p-Value | |
|---|---|---|---|---|
| Age years (median) | 46 | 52 | ||
| Size (mm) | ||||
| ≤20 | 11 | 3 | 11.586 | 0.003 |
| 20–50 | 21 | 36 | ||
| >50 | 7 | 21 | ||
| Lymph node tatus | ||||
| Positive | 23 | 45 | 2.822 | ns |
| Negative | 16 | 15 | ||
| Histotype | ||||
| Non-special type | 39 | 55 | 3.423 | ns |
| Others | 0 | 5 | ||
| Histological grade | ||||
| 1 | 2 | 1 | 1.22 | ns |
| 2 | 17 | 24 | ||
| 3 | 20 | 35 | ||
| ER (%) | ||||
| 0 | 17 | 12 | 6.35 | 0.01 |
| >1 | 22 | 48 | ||
| PR (%) | ||||
| 0 | 26 | 26 | 5.161 | 0.02 |
| >1 | 13 | 34 | ||
| HER2 | ||||
| Negative | 21 | 33 | 0.013 | ns |
| Positive | 18 | 27 | ||
| Ki67 (%) | ||||
| ≤20 | 2 | 8 | 1.752 | ns |
| >20 | 37 | 52 |
| Diet Group (39) | Control Group (60) | Chi-Square | p-Value | |
|---|---|---|---|---|
| Surgery | ||||
| Conservative | 32 | 24 | 6.915 | 0.008 |
| Mastectomy | 7 | 36 | ||
| Size (mm) | ||||
| Not determined | 11 | 7 | 11.505 | 0.009 |
| ≤20 | 19 | 23 | ||
| 20–50 | 9 | 20 | ||
| >50 | 0 | 10 | ||
| Residual tumor | ||||
| No | 20 | 17 | 0.015 | |
| Yes | 19 | 43 | ||
| Lymph node status | ||||
| Positive | 9 | 29 | 6.375 | 0.01 |
| Negative | 30 | 31 | ||
| ypT | ||||
| Is | 1 | 7 | 22.324 | <0.001 |
| 0 | 19 | 10 | ||
| 1 | 17 | 24 | ||
| 2 | 1 | 13 | ||
| 3 | 0 | 6 | ||
| 4 | 1 | 0 | ||
| ypN | ||||
| 0 | 30 | 29 | 9.431 | 0.008 |
| 1 | 7 | 29 | ||
| 2 | 2 | 2 | ||
| Pinder tumor stage | ||||
| 1 | 20 | 17 | 10.767 | 0.004 |
| 2 | 18 | 32 | ||
| 3 | 0 | 11 | ||
| Pinder lymph node stage | ||||
| 1 | 24 | 17 | 15.081 | 0.001 |
| 2 | 6 | 14 | ||
| 3 | 9 | 17 | ||
| 4 | 0 | 12 | ||
| ER (%) | ||||
| 0 | 9 | 8 | 5.478 | 0.01 |
| >1 | 10 | 35 | ||
| PR (%) | ||||
| 0 | 14 | 22 | 4.938 | 0.02 |
| >1 | 3 | 21 | ||
| Not performed | 2 | 0 | ||
| HER2 | ||||
| Negative | 13 | 33 | 0.477 | ns |
| Positive | 6 | 10 | ||
| Ki67 (%) | ||||
| ≤20 | 9 | 25 | 0.166 | ns |
| >20 | 5 | 18 | ||
| Not performed | 5 | 0 |
| Lymph Nodes Status Post Nact | Total | ||||
|---|---|---|---|---|---|
| yN0 | yN1–2 | ||||
| Control Group | Lymph Nodes Status Pre Nact | Negative | 13 | 2 | 15 |
| Positive | 16 | 29 | 45 | ||
| Total | 29 | 31 | 60 | ||
| Diet Group | Negative | 16 | 0 | 16 | |
| Positive | 14 | 9 | 23 | ||
| Total | 30 | 9 | 39 | ||
| pCR vs. Residual Cancer | ||||||
|---|---|---|---|---|---|---|
| Breast Cancer | Lymph Nodes | |||||
| OR (Crude) | 95% Confidence Interval | OR (Crude) | 95% Confidence Interval | |||
| Inf | Sup | Inf | Sup | |||
| Diet group vs. control group | 2.66 | 1.15 | 6.18 | 4.05 | 1.72 | 9.52 |
| OR (adj) | Inf | Sup | OR (adj) | Inf | Sup | |
| Diet group vs. control group | 2.94 | 1.07 | 8.10 | 3.22 | 1.22 | 8.56 |
| Size in mm (T0) | 0.94 | 0.44 | 2.01 | 0.77 | 0.36 | 1.63 |
| ER (T0) | 1.09 | 0.32 | 3.69 | 0.54 | 0.16 | 1.83 |
| PGR (T0) | 0.45 | 0.14 | 1.42 | 0.38 | 0.12 | 1.19 |
| Ki67 (T0) | 1.02 | 0.84 | 15.34 | 5.17 | 0.47 | 56.30 |
| HER2 (T0) | 1.66 | 1.05 | 2.63 | 1.09 | 0.69 | 1.73 |
| Parameter | Time | Median (Range) | p |
|---|---|---|---|
| Resistance (Ohm) | T0 | 598 (416–700) | |
| T2 | 565 (426–727) | ns | |
| Reactance (Ohm) | T0 | 57 (40–75) | |
| T2 | 50 (37–66) | 0.0009 | |
| PA (°) Reference values: 5.2–7.2° | T0 | 5.6 (4.3–6.6) | |
| T2 | 5.0 (4.2–6.5) | 0.003 | |
| FFM (kg) | T0 | 44.2 (36.2–53.5) | |
| T2 | 45 (38.6–55.2) | ns | |
| FM (kg) | T0 | 22.4 (17.1–29.6) | |
| T2 | 12.1 (2.3–32) | 0.0001 | |
| TBW (L) | T0 | 32.4 (26.5–39.2) | |
| T2 | 32.5 (25.7–38.6) | ns |
| Median | T0 | T2 | p | Δ% T0–T2 |
|---|---|---|---|---|
| Body Weight (kg) | 63 | 59 | 0.001 | −6.35 |
| BMI (kg/m2) | 24.22 | 22.49 | 0.02 | −7.14 |
| Waist Circumference (cm) | 81 | 74 | 0.0001 | −9.46 |
| Hip Circumference (cm) | 97.5 | 91.5 | 0.0001 | −6.56 |
| Time | CR Diet 50% TEE | CR Diet 70% TEE | ||
|---|---|---|---|---|
| Prescription | Value | Prescription | ||
| Energy (kcal/die) Median (range) | T0 | 900 (704–1097) | 918 (450–1160) | 1300 (1012–1660) |
| T1 | 980 (532–1400) | |||
| T2 | 945 (550–1126) | |||
| CHO (g/die) Median (range) | T0 | 47 (37–53) | 56 (20–117) | 117 (85–145) |
| T1 | 60 (20–126) | |||
| T2 | 58 (30–110) | |||
| Protein (g/die) Median (range) | T0 | 75 (45–98) | 68 (31–83) | 65 (55–80) |
| T1 | 67 (34–100) | |||
| T2 | 67 (33–82) | |||
| Lipid (g/die) Median (range) | T0 | 50 (37–59) | 49 (20–77) | 65 (44–92) |
| T1 | 52 (30–80) | |||
| T2 | 50 (25–75) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellano, I.; Gallo, F.; Durelli, P.; Monge, T.; Fadda, M.; Metovic, J.; Cassoni, P.; Borella, F.; Raucci, C.; Menischetti, M.; et al. Impact of Caloric Restriction in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Prospective Case Control Study. Nutrients 2023, 15, 4677. https://doi.org/10.3390/nu15214677
Castellano I, Gallo F, Durelli P, Monge T, Fadda M, Metovic J, Cassoni P, Borella F, Raucci C, Menischetti M, et al. Impact of Caloric Restriction in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Prospective Case Control Study. Nutrients. 2023; 15(21):4677. https://doi.org/10.3390/nu15214677
Chicago/Turabian StyleCastellano, Isabella, Francesco Gallo, Paola Durelli, Taira Monge, Maurizio Fadda, Jasna Metovic, Paola Cassoni, Fulvio Borella, Carlo Raucci, Monica Menischetti, and et al. 2023. "Impact of Caloric Restriction in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Prospective Case Control Study" Nutrients 15, no. 21: 4677. https://doi.org/10.3390/nu15214677
APA StyleCastellano, I., Gallo, F., Durelli, P., Monge, T., Fadda, M., Metovic, J., Cassoni, P., Borella, F., Raucci, C., Menischetti, M., Beano, A., Migliaretti, G., & Finocchiaro, C. (2023). Impact of Caloric Restriction in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: A Prospective Case Control Study. Nutrients, 15(21), 4677. https://doi.org/10.3390/nu15214677









