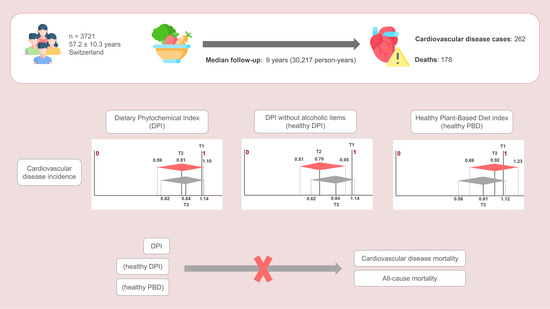
Association between Total Dietary Phytochemical Intake and Cardiometabolic Health Outcomes—Results from a 10-Year Follow-Up on a Middle-Aged Cohort Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiovascular Outcomes Ascertainment
2.3. Dietary Assessment
2.4. Dietary Indices Construction
2.5. Covariates Assessment
2.6. Ethical Considerations
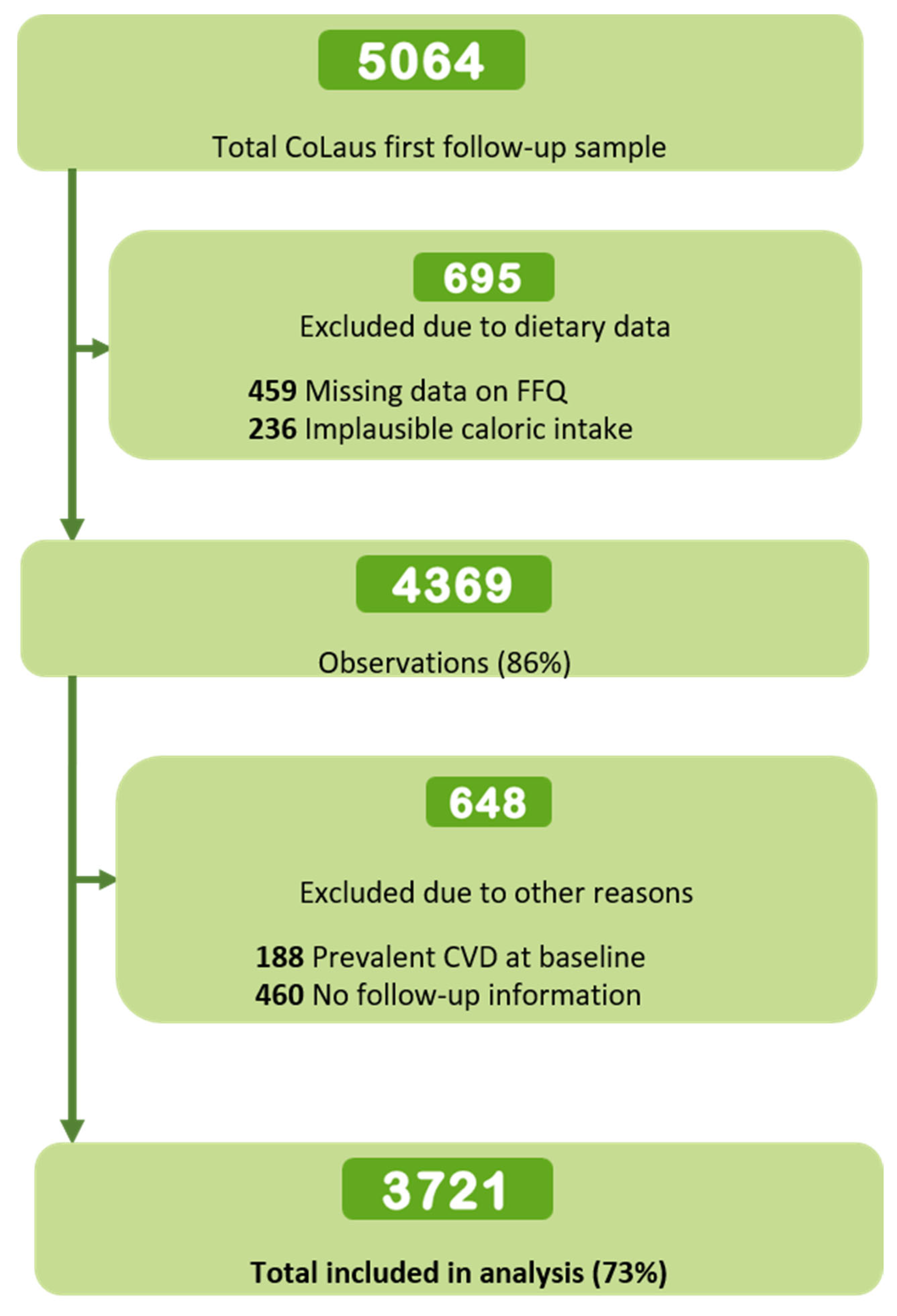
2.7. Inclusion and Exclusion Criteria
2.8. Statistical Analysis
3. Results
3.1. Sample Characteristics across Tertiles of Dietary Indexes
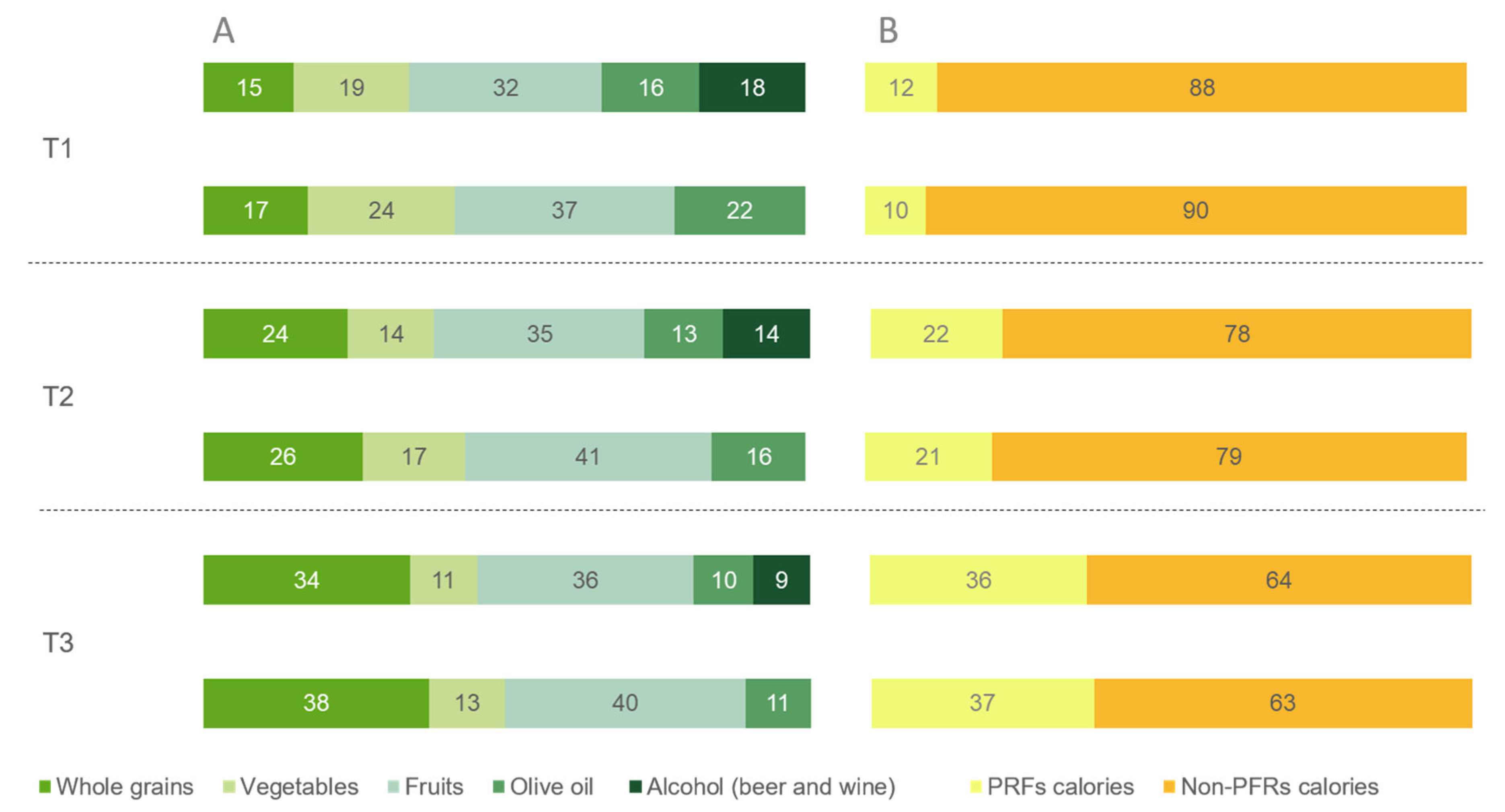
3.2. Dietary Intake across Tertiles of Dietary Indexes
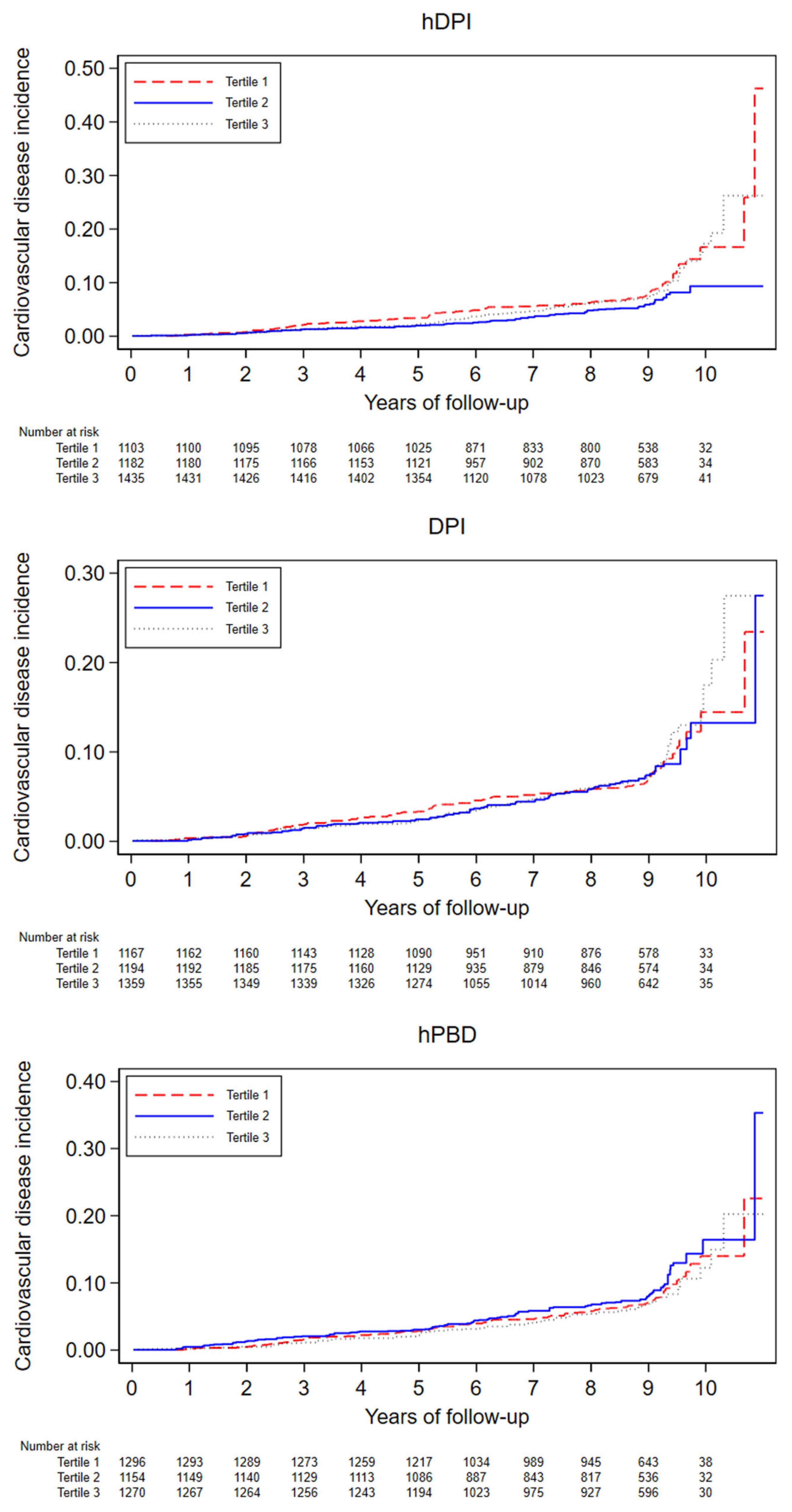
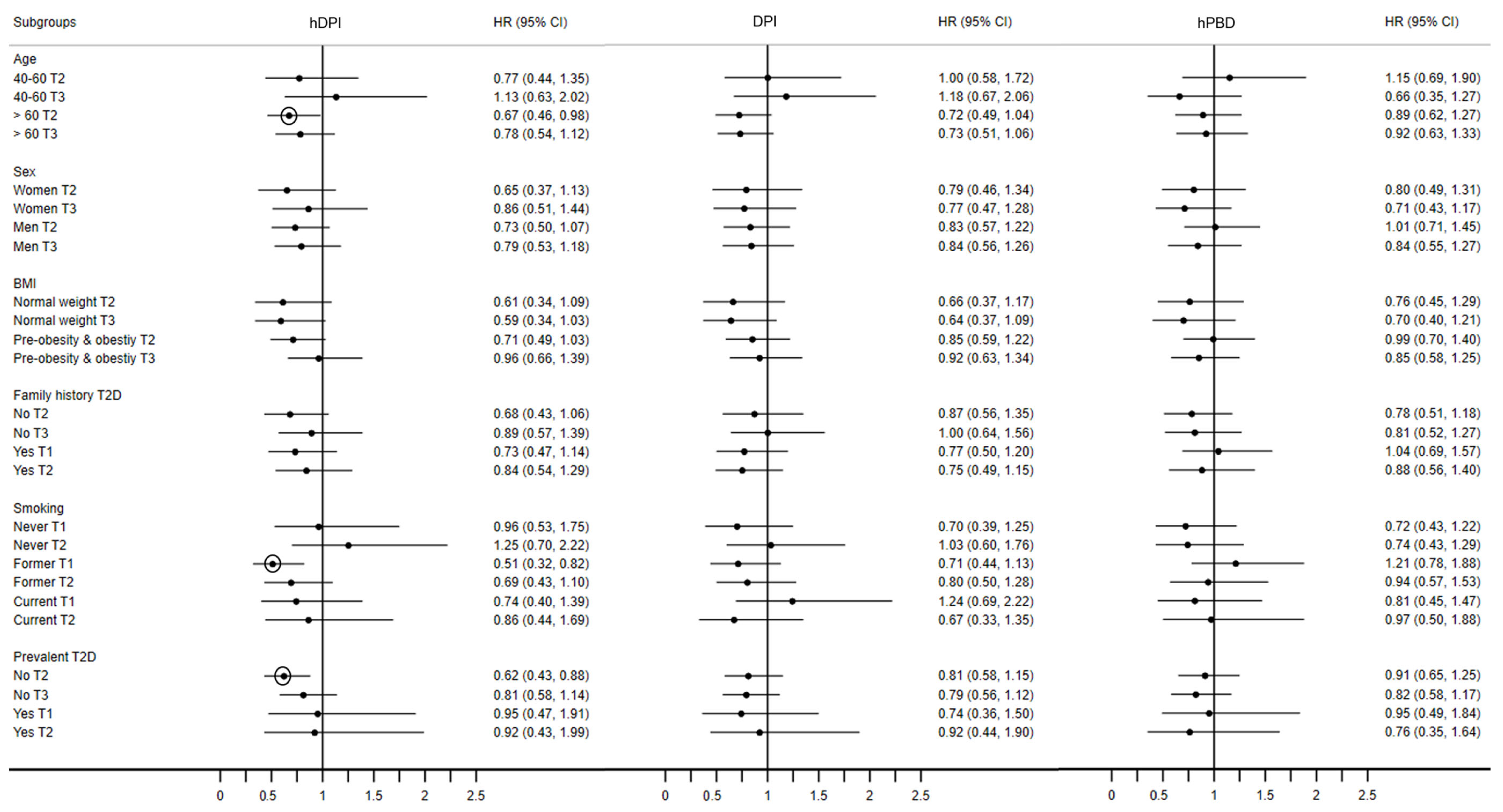
3.3. Dietary Indices Associations with CVD Incidence
3.4. Dietary Indices Associations with CVD Mortality and All-Cause Mortality
3.5. Sensitivity Analyses
4. Discussion
4.1. Dietary Indices Associations with CVD Incidence
4.2. Dietary Indices Associations with CVD Mortality and All-Cause Mortality
4.3. Strengths and Limitations
4.4. Public Health Impact and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef] [PubMed]
- European Society of Cardiology. ESC Cardiovascular Realities 2022. An illustrated Atlas of Key European Statistics; European Society of Cardiology: Sophia Antipolis, France, 2022. [Google Scholar]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The global burden of cardiovascular diseases and risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Bu, X.; Liu, J.; Wei, L.; Ma, A.; Wang, T. Cardiovascular disease burden attributable to dietary risk factors from 1990 to 2019: A systematic analysis of the Global Burden of Disease Study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 897–907. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence: Who European Office for the Prevention and Control of Noncommunicable Diseases; WHO European Office, Ed.; World Health Organization: Geneva, Switzerland, 2021; Volume WHO/EURO:2021-4007-43766-61591. [Google Scholar]
- Quek, J.; Lim, G.; Lim, W.H.; Ng, C.H.; So, W.Z.; Toh, J.; Pan, X.H.; Chin, Y.H.; Muthiah, M.D.; Chan, S.P.; et al. The association of plant-based diet with cardiovascular disease and mortality: A meta-analysis and systematic review of prospect cohort studies. Front. Cardiovasc. Med. 2021, 8, 756810. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.H.; Cheong, H.C.; Tu, Y.-K.; Kuo, P.-H. Association between plant-based dietary patterns and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Nutrients 2021, 13, 3952. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European association of preventive cardiology (eapc). Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J.; et al. 2021 Dietary guidance to improve cardiovascular health: A scientific statement from the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Oomah, B.D. Chemistry and classification of phytochemicals. In Handbook of Plant Food Phytochemicals; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 5–48. [Google Scholar]
- Yang, J.; Zhang, Y.; Na, X.; Zhao, A. Β-carotene supplementation and risk of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2022, 14, 1284. [Google Scholar] [CrossRef]
- Kiyimba, T.; Yiga, P.; Bamuwamye, M.; Ogwok, P.; Van der Schueren, B.; Matthys, C. Efficacy of dietary polyphenols from whole foods and purified food polyphenol extracts in optimizing cardiometabolic health: A meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 270–282. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, lifestyle and cardiovascular diseases: Linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Morand, C.; Tomás-Barberán, F.A. Contribution of plant food bioactives in promoting health effects of plant foods: Why look at interindividual variability? Eur. J. Nutr. 2019, 58, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, T.D.; Ramasamy, J.R.; Palanisamy, K. Nutraceutical potentials of synergic foods: A systematic review. J. Ethn. Foods 2019, 6, 27. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Mehranfar, S.; Jalilpiran, Y.; Ejtahed, H.S.; Seif, E.; Shahrestanaki, E.; Mahdavi-Gorabi, A.; Esmaeili-Abdar, M.; Larijani, B.; Qorbani, M. Association of Dietary Phytochemical Index with cardiometabolic risk factors. Int. J. Vitam. Nutr. Res. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Proposal for a Dietary “Phytochemical Index”. Med. Hypotheses 2004, 63, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Qorbani, M.; Angoorani, P.; Ejtahed, H.-S. Dietary Phytochemical Index as a biomarker in nutritional studies: Features and applications. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 307–328. [Google Scholar]
- Gamba, M.; Roa-Diaz, Z.M.; Raguindin, P.F.; Glisic, M.; Bano, A.; Muka, T.; Franco, O.H.; Marques-Vidal, P. Association between Dietary Phytochemical Index, cardiometabolic risk factors and metabolic syndrome in Switzerland. The CoLaus study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Firmann, M.; Mayor, V.; Vidal, P.M.; Bochud, M.; Pécoud, A.; Hayoz, D.; Paccaud, F.; Preisig, M.; Song, K.S.; Yuan, X.; et al. The colaus study: A population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc. Disord. 2008, 8, 6. [Google Scholar] [CrossRef]
- Beuret, H.; Hausler, N.; Nanchen, D.; Méan, M.; Marques-Vidal, P.; Vaucher, J. Comparison of swiss and european risk algorithms for cardiovascular prevention in Switzerland. Eur. J. Prev. Cardiol. 2021, 28, 204–210. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Bernstein, L.H.I.; Morabia, A. Amélioration des performances d’un questionnaire alimentaire semi-quantitatif comparé à un rappel des 24 heures (improvement in the performance of a semi-quantitative food questionnaire compared to a 24-hour recall). Sante Publique 1995, 7, 407–413. [Google Scholar]
- ANSES-CIQUAL French Food Composition Table. Available online: https://ciqual.anses.fr/ (accessed on 15 October 2023).
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-based dietary patterns and incidence of Type 2 Diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- Hayer, A. Swiss Food Pyramid. Available online: https://www.sge-ssn.ch/ich-und-du/essen-und-trinken/ausgewogen/schweizer-lebensmittelpyramide/ (accessed on 7 March 2023).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Bernstein, M.; Sloutskis, D.; Kumanyika, S.; Sparti, A.; Schutz, Y.; Morabia, A. Data-based approach for developing a physical activity frequency questionnaire. Am. J. Epidemiol. 1998, 147, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: An updated systematic review and meta-analysis of cohort studies. J. Acad. Nutr. Diet. 2018, 118, 74–100.e111. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Kaptoge, S.; Butterworth, A.S.; Willeit, P.; Warnakula, S.; Bolton, T.; Paige, E.; Paul, D.S.; Sweeting, M.; Burgess, S.J.T.L. Risk thresholds for alcohol consumption: Combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018, 391, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; ElSayed, A.; Beger, B.; Naidoo, P.; Shilton, T.; Jain, N.; Armstrong-Walenczak, K.; Mwangi, J.; Wang, Y.; Eiselé, J.-L.J.G.H. The impact of alcohol consumption on cardiovascular health: Myths and measures. Glob. Heart 2022, 17, 45. [Google Scholar] [CrossRef]
- Weston, L.J.; Kim, H.; Talegawkar, S.A.; Tucker, K.L.; Correa, A.; Rebholz, C.M. Plant-based diets and incident cardiovascular disease and all-cause mortality in african americans: A cohort study. PLoS Med. 2022, 19, e1003863. [Google Scholar] [CrossRef]
- Schneid Schuh, D.; Campos Pellanda, L.; Guessous, I.; Marques-Vidal, P. Trends and determinants of change in compliance to dietary guidelines in a swiss community-dwelling sample. Prev. Med. 2018, 111, 198–203. [Google Scholar] [CrossRef]
- Chen, H.; Shen, J.; Xuan, J.; Zhu, A.; Ji, J.S.; Liu, X.; Cao, Y.; Zong, G.; Zeng, Y.; Wang, X.; et al. Plant-based dietary patterns in relation to mortality among older adults in China. Nat. Aging 2022, 2, 224–230. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Nguyen, X.-M.T.; Song, R.J.; Ho, Y.-L.; Hu, F.B.; Willett, W.C.; Wilson, P.; Cho, K.; Gaziano, J.M.; et al. Degree of adherence to plant-based diet and total and cause-specific mortality: Prospective cohort study in the Million Veteran Program. Public Health Nutr. 2023, 26, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Giovannucci, E.L. Plant-based diet quality and the risk of total and disease-specific mortality: A population-based prospective study. Clin. Nutr. 2021, 40, 5718–5725. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Mirmiran, P.; Bahadoran, Z.; Alamdari, S.; Azizi, F. Dietary Phytochemical Index and subsequent changes of lipid profile: A 3-year follow-up in Tehran Lipid and Glucose Study in Iran. ARYA Atheroscler. 2014, 10, 203–210. [Google Scholar] [PubMed]
- Golzarand, M.; Bahadoran, Z.; Mirmiran, P.; Sadeghian-Sharif, S.; Azizi, F. Dietary Phytochemical Index is inversely associated with the occurrence of hypertension in adults: A 3-year follow-up (the Tehran Lipid and Glucose Study). Eur. J. Clin. Nutr. 2015, 69, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Golzarand, M.; Shiva, N.; Azizi, F. Association between Dietary Phytochemical Index and 3-year changes in weight, waist circumference and body adiposity index in adults: Tehran Lipid and Glucose Study. Nutr. Metab. 2012, 9, 108. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Chatelan, A.; Beer-Borst, S.; Randriamiharisoa, A.; Pasquier, J.; Blanco, J.M.; Siegenthaler, S.; Paccaud, F.; Slimani, N.; Nicolas, G.; Camenzind-Frey, E.; et al. Major differences in diet across three linguistic regions of Switzerland: Results from the first national nutrition survey menuch. Nutrients 2017, 9, 1163. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef]
- Heianza, Y.; Zhou, T.; Sun, D.; Hu, F.B.; Manson, J.E.; Qi, L. Genetic susceptibility, plant-based dietary patterns, and risk of cardiovascular disease. Am. J. Clin. Nutr. 2020, 112, 220–228. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the anthropocene: The EAT-Lancet commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal definition of myocardial infarction. Eur. Heart J. 2007, 28, 2525–2538. [Google Scholar]
- Advisory Council for the National Institute of Neurological and Communicative Disorders and Stroke. National Institute of Health. A classification and outline of cerebrovascular diseases II. Stroke 1975, 6, 564–616. [Google Scholar] [CrossRef]
- World Health Organization. Cerebrovascular Disorders: A Clinical and Research Classification; WHO Offset Publication 1978, No. 43. Available online: www.who.int/iris/handle/10665/37194#sthash.I8sxekCh.dpuf (accessed on 19 August 2015).
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009, 40, 2276–2293. [Google Scholar]
| Healthy Dietary Phytochemical Index (hDPI) | Dietary Phytochemical Index (DPI) | Healthy Plant-Based Diet Index (hPBD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | |
| Number of participants | 1127 | 1200 | 1394 | 1184 | 1212 | 1325 | 1371 | 1209 | 1141 |
| Index median (IQR) | 11 (8–14) | 21 (19–24) | 36 (31–43) | 15 (12–18) | 26 (23–28) | 39 (35–46) | 42 (39–44) | 48 (47–50) | 56 (53–59) |
| Age, mean (SD) | 55.2 (10.2) | 56.5 (10.2) | 59.2 (10.2) | 55.2 (10.4) | 57.1 (10.2) | 59.3 (10.0) | 55.7 (10.4) | 57.6 (10.4) | 58.6 (9.9) |
| Sex, n (% women) | 454 (37.4) | 694 (55.5) | 892 (70.7) | 531 (42.8) | 687 (55.2) | 822 (66.3) | 611 (43.7) | 704 (57.7) | 725 (65.7) |
| Education attainment, n (%) | |||||||||
| University education | 246 (21.8) | 231 (19.2) | 319 (22.9) | 258 (21.8) | 232 (19.1) | 321 (24.2) | 329 (24.0) | 233 (19.3) | 281 (24.6) |
| High school | 293 (26.0) | 327 (27.2) | 399 (28.6) | 317 (26.8) | 330 (27.2) | 375 (28.3) | 361 (26.3) | 333 (27.5) | 336 (29.4) |
| Apprenticeship | 420 (37.3) | 449 (37.4) | 482 (34.6) | 433 (36.6) | 461 (38.1) | 446 (33.7) | 509 (37.1) | 443 (36.7) | 360 (31.6) |
| Mandatory education | 168 (14.9) | 194 (16.1) | 194 (13.9) | 176 (14.9) | 189 (15.6) | 184 (13.9) | 172 (12.6) | 200 (16.5) | 164 (14.3) |
| Smoking status, n (%) | |||||||||
| Never | 430 (38.1) | 521 (43.4) | 675 (48.4) | 502 (42.4) | 523 (43.2) | 595 (44.9) | 569 (41.5) | 526 (43.5) | 502 (44.0) |
| Former | 403 (35.7) | 475 (39.6) | 513 (36.8) | 396 (33.4) | 474 (39.1) | 528 (39.9) | 486 (35.5) | 455 (37.7) | 465 (40.7) |
| Smoker | 295 (26.1) | 204 (17.0) | 206 (14.8) | 286 (24.2) | 214 (17.7) | 202 (15.3) | 315 (23.0) | 227 (18.8) | 174 (15.3) |
| Alcohol intake (units/week), median (IQR) | 6 (2–13) | 3 (1–8) | 2 (0–6) | 4 (1–8) | 4 (1–9) | 3 (0–7) | 5 (1–10) | 3 (0–8) | 3 (0–7) |
| Alcohol intake (categories), n (%) | |||||||||
| Non-drinker | 194 (17.2) | 283 (23.6) | 408 (29.2) | 295 (24.9) | 247 (20.4) | 332 (25.1) | 253 (18.4) | 307 (25.4) | 308 (26.9) |
| Low | 389 (34.5) | 523 (43.5) | 686 (49.2) | 493 (41.7) | 519 (42.8) | 568 (42.9) | 559 (40.8) | 515 (42.6) | 495 (43.4) |
| Moderate | 271 (24.0) | 245 (20.4) | 231 (16.5) | 257 (21.7) | 251 (20.7) | 255 (19.2) | 327 (23.9) | 230 (19.0) | 210 (18.4) |
| High | 274 (24.3) | 149 (12.5) | 69 (5.0) | 139 (11.8) | 196 (16.1) | 170 (12.8) | 232 (16.9) | 157 (13.0) | 128 (11.3) |
| Physical activity (total minutes/day), mean (SD) | 440.5 (175.2) | 439.2 (155.4) | 455.3 (145.0) | 444.7 (174.8) | 439.6 (154.5) | 447.2 (145.1) | 433.6 (165.7) | 447.4 (155.8) | 453.2 (153.0) |
| BMI, kg/m2, mean (SD) | 26.5 (4.3) | 26.0 (4.7) | 25.2 (4.5) | 26.5 (4.5) | 26.0 (4.5) | 25.2 (4.4) | 26.3 (4.3) | 25.8 (4.7) | 25.4 (4.5) |
| Family history of CVD, n (%) | 349 (31.0) | 476 (39.7) | 650 (46.6) | 384 (32.4) | 456 (37.7) | 621 (46.8) | 447 (32.6) | 493 (40.8) | 491 (43.0) |
| Family history of diabetes, n (%) | 389 (34.5) | 405 (33.7) | 460 (33.0) | 403 (34.0) | 420 (34.6) | 429 (32.4) | 458 (33.4) | 423 (35.0) | 363 (31.8) |
| Type 2 diabetes, n (%) | 109 (9.7) | 106 (8.8) | 111 (8.0) | 103 (8.7) | 120 (9.9) | 117 (8.8) | 113 (8.2) | 121 (10.0) | 99 (8.7) |
| Hypertension, n (%) | 434 (38.5) | 464 (38.7) | 550 (39.5) | 416 (35.1) | 497 (41.0) | 550 (41.5) | 502 (36.6) | 491 (40.7) | 431 (37.7) |
| Hypercholesterolemia, n (%) | 805 (71.5) | 788 (65.6) | 943 (67.7) | 850 (71.8) | 790 (65.2) | 899 (67.8) | 969 (70.7) | 802 (66.4) | 763 (66.9) |
| Dieting, n (%) | 253 (22.4) | 389 (32.4) | 537 (38.5) | 278 (23.5) | 385 (31.8) | 523 (39.4) | 329 (24.0) | 392 (32.5) | 439 (38.4) |
| Dietary supplements use, n (%) | 24 (2.2) | 96 (8.0) | 97 (6.9) | 31 (2.6) | 86 (7.1) | 96 (7.2) | 46 (3.4) | 78 (6.5) | 81 (7.1) |
| Tertile 1 | Tertile 2 | Tertile 3 | ||
|---|---|---|---|---|
| H.R. (95% CI) | H.R. (95% CI) | p-Trend | ||
| Healthy Dietary Phytochemical Index (hDPI) | ||||
| Incident CVD (N cases) | 101 | 72 | 90 | |
| Person-years | 9779 | 10,186 | 10,251 | |
| Incidence rate * (CI) | 10.2 (8.4, 12.4) | 7.0 (5.6, 8.9) | 8.7 (7.1, 10.7) | |
| Crude model | 1.00 (reference) | 0.64 * (0.47, 0.86) | 0.64 * (0.48, 0.85) | 0.005 |
| Model 1 | 1.00 (reference) | 0.71 * (0.52, 0.96) | 0.82 (0.61, 1.11) | 0.275 |
| Model 2 | 1.00 (reference) | 0.72 * (0.53, 0.99) | 0.89 (0.66, 1.21) | 0.570 |
| Model 3 | 1.00 (reference) | 0.70 * (0.51, 0.95) | 0.84 (0.62, 1.14) | 0.362 |
| Dietary Phytochemical Index (DPI) | ||||
| Incident CVD (N cases) | 96 | 80 | 87 | |
| Person-years | 10,113 | 10,053 | 10,050 | |
| Incidence rate * (CI) | 9.4 (7.7, 11.5) | 7.8 (6.3, 9.7) | 8.6 (7, 10.6) | |
| Crude model | 1.00 (reference) | 0.73 * (0.54, 0.98) | 0.67 * (0.50, 0.90) | 0.011 |
| Model 1 | 1.00 (reference) | 0.77 (0.57, 1.05) | 0.79 (0.59, 1.07) | 0.153 |
| Model 2 | 1.00 (reference) | 0.85 (0.62, 1.15) | 0.89 (0.66, 1.21) | 0.514 |
| Model 3 | 1.00 (reference) | 0.81 (0.59, 1.10) | 0.84 (0.62, 1.14) | 0.303 |
| Healthy Plant-Based Diet Index (hPBD) | ||||
| Incident CVD (N cases) | 103 | 90 | 70 | |
| Person-years | 11,372 | 9850 | 8994 | |
| Incidence rate * (CI) | 8.9 (7.3, 10.8) | 9.1 (7.4, 11.2) | 7.7 (6.1, 9.8) | |
| Crude model | 1.00 (reference) | 0.87 (0.66, 1.16) | 0.68 * (0.50, 0.93) | 0.015 |
| Model 1 | 1.00 (reference) | 0.95 (0.71, 1.26) | 0.80 (0.58, 1.09) | 0.155 |
| Model 2 | 1.00 (reference) | 0.97 (0.72, 1.29) | 0.83 (0.60, 1.15) | 0.271 |
| Model 3 | 1.00 (reference) | 0.92 (0.69, 1.23) | 0.81 (0.58, 1.12) | 0.206 |
| All-Cause Mortality | Cardiovascular Disease Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | Tertile 1 | Tertile 2 | Tertile 3 | |||
| H.R. (95% CI) | H.R. (95% CI) | p-Trend | H.R. (95% CI) | H.R. (95% CI) | p-Trend | |||
| Healthy Dietary Phytochemical Index (hDPI) | ||||||||
| Cases | 60 | 62 | 56 | 14 | 16 | 19 | ||
| Person-years | 10,046 | 10,443 | 10,468 | 10,088 | 10,459 | 10,477 | ||
| Incidence rate * (CI) | 5.9 (4.6, 7.6) | 5.9 (4.6, 7.6) | 5.3 (4.1, 6.9) | 1.4 (0.8, 2.3) | 1.5 (0.9, 2.4) | 1.8 (1.1, 2.8) | ||
| Crude model | 1.00 (reference) | 0.87 (0.61, 1.25) | 0.68 * (0.47, 0.98) | 0.034 | 1.00 (reference) | 0.94 (0.45, 1.96) | 0.89 (0.44, 1.78) | 0.738 |
| Model 1 | 1.00 (reference) | 0.95 (0.66, 1.36) | 0.79 (0.54, 1.15) | 0.206 | 1.00 (reference) | 1.09 (0.52, 2.27) | 1.15 (0.57, 2.36) | 0.699 |
| Model 2 | 1.00 (reference) | 0.96 (0.66, 1.39) | 0.88 (0.60, 1.30) | 0.509 | 1.00 (reference) | 1.05 (0.49, 2.21) | 1.31 (0.61, 2.78) | 0.465 |
| Model 3 | 1.00 (reference) | 0.93 (0.64, 1.34) | 0.84 (0.56, 1.24) | 0.367 | 1.00 (reference) | 1.06 (0.50, 2.24) | 1.25 (0.59, 2.65) | 0.546 |
| Dietary Phytochemical Index (DPI) | ||||||||
| Cases | 55 | 65 | 58 | 12 | 21 | 16 | ||
| Person-years | 10,394 | 10,303 | 10,261 | 10,412 | 10,342 | 10,270 | ||
| Incidence rate * (CI) | 5.3 (4.0, 6.8) | 6.3 (4.9, 8.0) | 5.6 (4.3, 7,3) | 1.1 (0.6, 2.0) | 2.0 (1.3, 3.1) | 1.5 (0.9, 2.5) | ||
| Crude model | 1.00 (reference) | 1.17 (0.81, 1.68) | 0.90 (0.61, 1.31) | 0.461 | 1.00 (reference) | 1.71 (0.82, 3.58) | 1.04 (0.48, 2.25) | 0.832 |
| Model 1 | 1.00 (reference) | 1.17 (0.81, 1.68) | 0.96 (0.66, 1.41) | 0.755 | 1.00 (reference) | 1.79 (0.86, 3.73) | 1.19 (0.55, 2.58) | 0.858 |
| Model 2 | 1.00 (reference) | 1.29 (0.88, 1.88) | 1.11 (0.75, 1.65) | 0.712 | 1.00 (reference) | 2.12 (0.99, 4.54) | 1.55 (0.68, 3.55) | 0.415 |
| Model 3 | 1.00 (reference) | 1.27 (0.87, 1.86) | 1.07 (0.72, 1.59) | 0.862 | 1.00 (reference) | 2.21* (1.01, 4.80) | 1.49 (0.65, 3.43) | 0.518 |
| Healthy Plant-Based Diet Index (hPBD) | ||||||||
| Cases | 67 | 64 | 47 | 16 | 17 | 16 | ||
| Person-years | 11,683 | 10,120 | 9155 | 11,705 | 10,137 | 9182 | ||
| Incidence rate * (CI) | 5.7 (4.5, 7.2) | 6.3 (4.9, 8.0) | 5.1 (3.8, 6.8) | 1.3 (0.8, 2.2) | 1.6 (1.0, 2.6) | 1.7 (1.0, 2.8) | ||
| Crude model | 1.00 (reference) | 0.98 (0.69, 1.38) | 0.70 (0.48, 1.02) | 0.064 | 1.00 (reference) | 1.01 (0.51, 2.01) | 0.87 (0.42, 1.79) | 0.694 |
| Model 1 | 1.00 (reference) | 0.99 (0.70, 1.40) | 0.75 (0.51, 1.10) | 0.142 | 1.00 (reference) | 1.08 (0.54, 2.15) | 1.03 (0.50, 2.13) | 0.949 |
| Model 2 | 1.00 (reference) | 1.02 (0.72, 1.45) | 0.81 (0.54, 1.22) | 0.323 | 1.00 (reference) | 1.12 (0.56, 2.26) | 1.03 (0.47, 2.26) | 0.934 |
| Model 3 | 1.00 (reference) | 0.96 (0.67, 1.36) | 0.77 (0.51, 1.16) | 0.209 | 1.00 (reference) | 1.14 (0.56, 2.30) | 0.93 (0.42, 2.04) | 0.843 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamba, M.; Pano, O.; Raguindin, P.F.; Roa-Diaz, Z.M.; Muka, T.; Glisic, M.; Franco, O.H.; Marques-Vidal, P. Association between Total Dietary Phytochemical Intake and Cardiometabolic Health Outcomes—Results from a 10-Year Follow-Up on a Middle-Aged Cohort Population. Nutrients 2023, 15, 4793. https://doi.org/10.3390/nu15224793
Gamba M, Pano O, Raguindin PF, Roa-Diaz ZM, Muka T, Glisic M, Franco OH, Marques-Vidal P. Association between Total Dietary Phytochemical Intake and Cardiometabolic Health Outcomes—Results from a 10-Year Follow-Up on a Middle-Aged Cohort Population. Nutrients. 2023; 15(22):4793. https://doi.org/10.3390/nu15224793
Chicago/Turabian StyleGamba, Magda, Octavio Pano, Peter Francis Raguindin, Zayne M. Roa-Diaz, Taulant Muka, Marija Glisic, Oscar H. Franco, and Pedro Marques-Vidal. 2023. "Association between Total Dietary Phytochemical Intake and Cardiometabolic Health Outcomes—Results from a 10-Year Follow-Up on a Middle-Aged Cohort Population" Nutrients 15, no. 22: 4793. https://doi.org/10.3390/nu15224793
APA StyleGamba, M., Pano, O., Raguindin, P. F., Roa-Diaz, Z. M., Muka, T., Glisic, M., Franco, O. H., & Marques-Vidal, P. (2023). Association between Total Dietary Phytochemical Intake and Cardiometabolic Health Outcomes—Results from a 10-Year Follow-Up on a Middle-Aged Cohort Population. Nutrients, 15(22), 4793. https://doi.org/10.3390/nu15224793