The Remaining Challenge to Diagnose and Manage Cow’s Milk Allergy: An Opinion Paper to Daily Clinical Practice
Abstract
:1. Introduction
2. Prevalence of Cow’s Milk Allergy
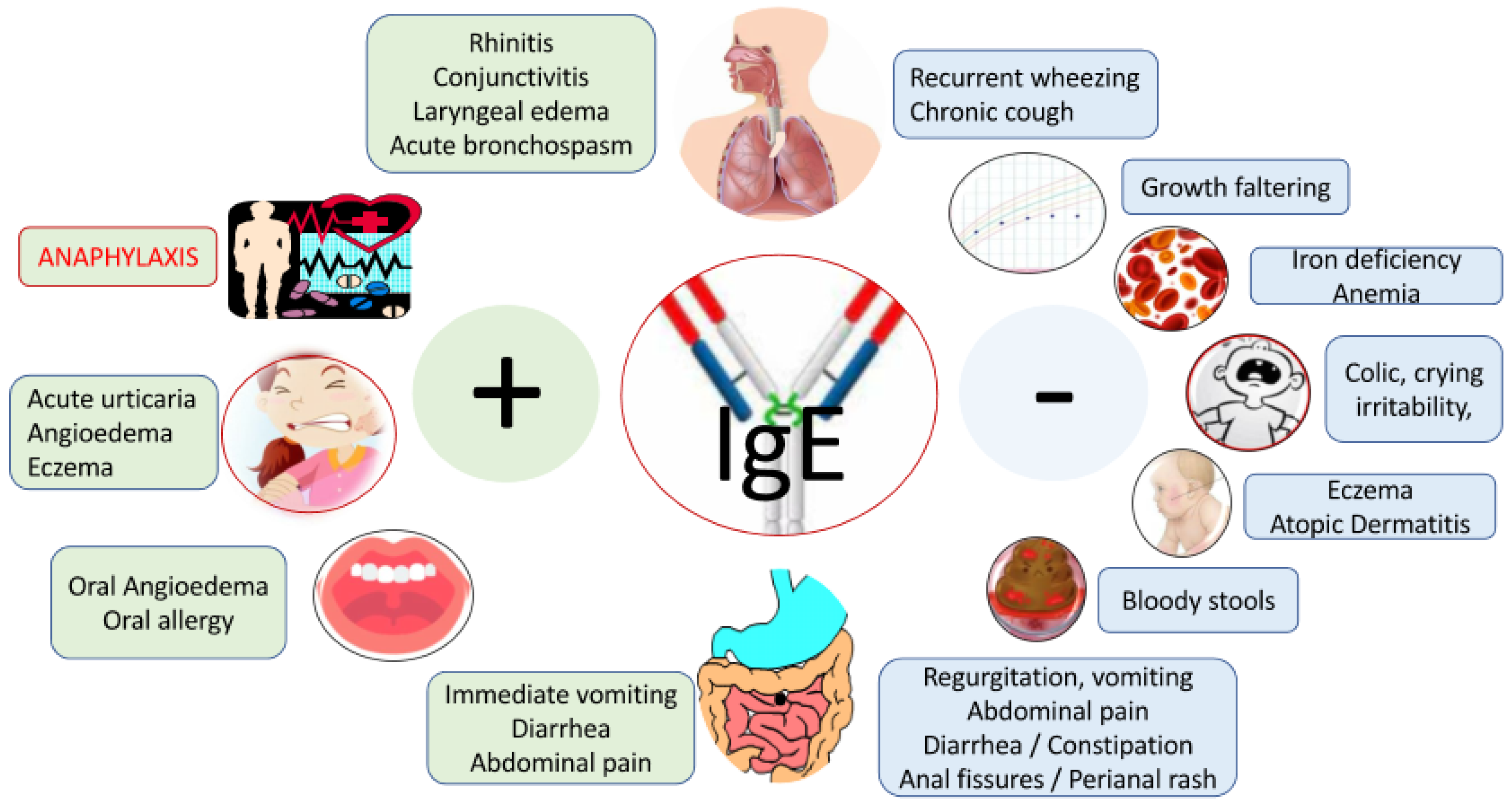
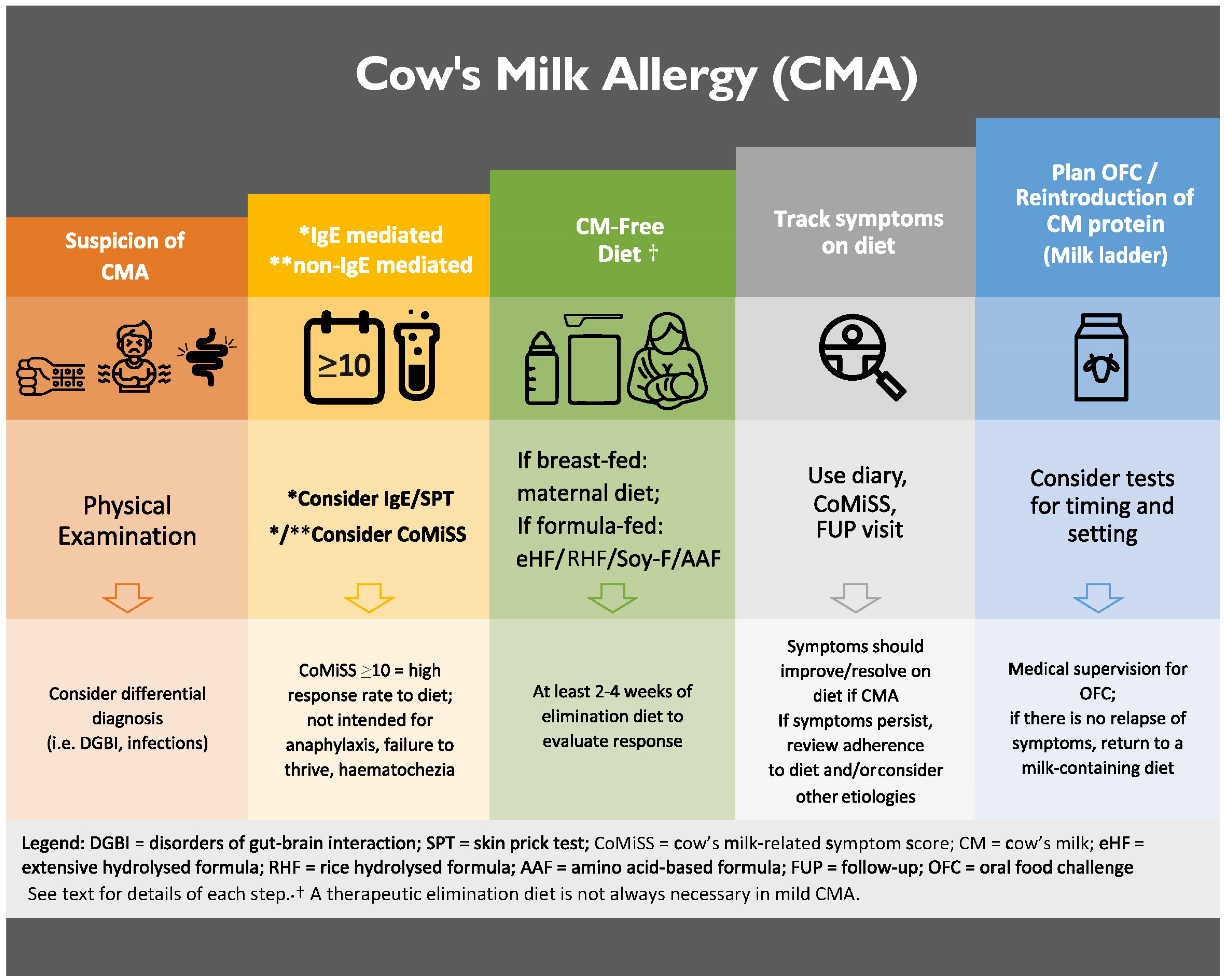
3. Symptoms and Diagnosis
3.1. IgE-Mediated CMA
3.2. Non-IgE-mediated CMA
3.3. Disorders of Gut–Brain Interaction (DGBI) and CMA
4. Awareness Tools
5. Therapeutic Elimination Diet
5.1. Hypoallergenic Formula Options for Non-Breastfed Infants
5.2. Therapeutic Elimination Diet Is Not Always Necessary in Mild CMA
5.3. “Biotics” in Infant Formula
6. Natural History of CMA and Strategies for Reintroduction of Cow’s Milk
- (A)
- Infants and young children younger than 3 years
- (B)
- Children older than 3 years
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Vandenplas, Y.; Broekaert, I.; Domellöf, M.; Indrio, F.; Lapillonne, A.; Pienar, C.; Ribes-Koninckx, C.; Shamir, R.; Szajewska, H.; Thapar, N.; et al. An ESPGHAN position paper on the diagnosis, management and prevention of cow’s milk allergy. J. Pediatr. Gastroenterol. Nutr. 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; de Silva, D.; Halken, S.; Worm, M.; Khaleva, E.; Arasi, S.; Dunn-Galvin, A.; Nwaru, B.I.; De Jong, N.W.; Del Río, P.R.; et al. Managing food allergy: GA2LEN guideline 2022. World Allergy Organ. J. 2022, 15, 100687. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Venter, C.; Fox, A.T.; Shah, N. Practical dietary management of protein energy malnutrition in young children with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2012, 23, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Winberg, A.; West, C.E.; Strinnholm, Å.; Nordström, L.; Hedman, L.; Rönmark, E. Milk allergy is a minor cause of milk avoidance due to perceived hypersensitivity among schoolchildren in Northern Sweden. Acta Paediatr. 2016, 105, 206–214. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Spolidoro, G.C.I.; Ali, M.M.; Amera, Y.T.; Nyassi, S.; Lisik, D.; Ioannidou, A.; Rovner, G.; Khaleva, E.; Venter, C.; van Ree, R.; et al. Prevalence estimates of eight big food allergies in Europe: Updated systematic review and meta-analysis. Allergy 2023, 78, 2361–2417. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Warren, C.M.; Brown-Whitehorn, T.; Cianferoni, A.; Schultz-Matney, F.; Gupta, R.S. Food protein–induced enterocolitis syndrome in the US population–based study. J. Allergy Clin. Immunol. 2019, 144, 1128–1130. [Google Scholar] [CrossRef]
- Martin, V.M.; Virkud, Y.V.; Seay, H.; Hickey, A.; Ndahayo, R.; Rosow, R.; Southwick, C.; Elkort, M.; Gupta, B.; Kramer, E.; et al. Prospective Assessment of Pediatrician-Diagnosed Food Protein–Induced Allergic Proctocolitis by Gross or Occult Blood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1692–1699.e1. [Google Scholar] [CrossRef]
- Hahn, J.W.; Lee, K.; Shin, J.I.; Cho, S.H.; Turner, S.; Shin, J.U.; Yeniova, A.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Global Incidence and Prevalence of Eosinophilic Esophagitis, 1976–2022: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Martin, V.M.; Virkud, Y.V.; Phadke, N.A.; Su, K.-W.; Seay, H.; Atkins, M.R.; Keet, C.; Shreffler, W.G.; Yuan, Q. Increased IgE-Mediated Food Allergy With Food Protein-Induced Allergic Proctocolitis. Pediatrics 2020, 146, e20200202. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Pereira, B.; Voigt, K.; Grundy, J.; Clayton, C.B.; Gant, C.; Higgins, B.; Dean, T. Comparison of open and double-blind placebo-controlled food challenges in diagnosis of food hypersensitivity amongst children. J. Hum. Nutr. Diet. 2007, 20, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; van Wijk, R.G.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.J.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology–European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.A.; Leonard, S.; Groetch, M.; Assa’Ad, A.; Cianferoni, A.; Clark, A.; Crain, M.; Fausnight, T.; Fleischer, D.; Green, T.; et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J. Allergy Clin. Immunol. Pract. 2020, 8, 75–90.e17. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Brueton, M.; Dupont, C.; Hill, D.; Isolauri, E.; Koletzko, S.; Oranje, A.P.; Staiano, A. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch. Dis. Child. 2007, 92, 902–908. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D.; Althera Study Group. Treating cow’s milk protein allergy: A double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr. 2013, 102, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203. [Google Scholar] [CrossRef]
- Dodi, G.; Di Filippo, P.; Di Pillo, S.; Chiarelli, F.; Attanasi, M. Total serum IgE levels as predictor of the acquisition of tolerance in children with food allergy: Findings from a pilot study. Front. Pediatr. 2022, 10, 1013807. [Google Scholar] [CrossRef]
- Venter, C.; Pereira, B.; Voigt, K.; Grundy, J.; Clayton, C.B.; Higgins, B.; Arshad, S.H.; Dean, T. Original article: Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 2008, 63, 354–359. [Google Scholar] [CrossRef]
- Meyer, R.; De Koker, C.; Dziubak, R.; Godwin, H.; Reeve, K.; Chebar-Lozinsky, A.; Foong, R.-X.; Skrapac, A.-K.; Ellmer, M.; Shah, N. The Challenge of Home Allergen Re-introductions Using the Ladder Approach in Children With Non-IgE Mediated Gastrointestinal Food Allergy. Front. Allergy 2021, 2, 721686. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; Çokura, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants From Birth to 12 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Agosti, M.; Baldassarre, M.E.; D’auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s Milk Allergy or Gastroesophageal Reflux Disease—Can We Solve the Dilemma in Infants? Nutrients 2021, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, D.K.; Talley, N.J.; Weaver, A.L.; Katusic, S.K.; De Schepper, H.; Rucker, M.J.; Locke, G.R. Incidence of Presentation of Common Functional Gastrointestinal Disorders in Children From Birth to 5 Years: A Cohort Study. Clin. Gastroenterol. Hepatol. 2007, 5, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Safe, M.; Chan, W.H.; Leach, S.T.; Sutton, L.; Lui, K.; Krishnan, U. Widespread use of gastric acid inhibitors in infants: Are they needed? Are they safe? World J. Gastrointest. Pharmacol. Ther. 2016, 7, 531–539. [Google Scholar] [CrossRef]
- Salvatore, S.; Hauser, B.; Salvatoni, A.; Vandenplas, Y. Oral ranitidine and duration of gastric pH > 4.0 in infants with persisting reflux symptoms. Acta Paediatr. 2006, 95, 176–181. [Google Scholar] [CrossRef]
- Meyer, R.; Lozinsky, A.C.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants—An EAACI Position Paper. Allergy 2019, 75, 14–32. [Google Scholar] [CrossRef]
- Ohlsson, B. Extraintestinal manifestations in irritable bowel syndrome: A systematic review. Ther. Adv. Gastroenterol. 2022, 15, 17562848221114558. [Google Scholar] [CrossRef]
- Muller, I.; Ghio, D.; Mobey, J.; Jones, H.; Hornsey, S.; Dobson, A.; Maund, E.; Santer, M. Parental perceptions and experiences of infant crying: A systematic review and synthesis of qualitative research. J. Adv. Nurs. 2023, 79, 403–417. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Salvatore, S.; Ribes-Koninckx, C.; Carvajal, E.; Szajewska, H.; Huysentruyt, K. The Cow Milk Symptom Score (CoMiSSTM) in presumed healthy infants. PLoS ONE 2018, 13, e0200603. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Ludwig, T.; Li, X.; Chen, S.; Sun, N.; Zheng, Y.; Huysentruyt, K.; Vandenplas, Y.; Zhang, T. Generation and application of a convolutional neural networks algorithm in evaluating stool consistency in diapers. Acta Paediatr. 2023, 112, 1333–1340. [Google Scholar] [CrossRef]
- Hegar, B.; Dewanti, N.R.; Kadim, M.; Alatas, S.; Firmansyah, A.; Vandenplas, Y. Natural evolution of regurgitation in healthy infants. Acta Paediatr. 2009, 98, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Hauser, B.; Salvatore, S. Functional Gastrointestinal Disorders in Infancy: Impact on the Health of the Infant and Family. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Leluyer, B.; Cazaubiel, M.; Housez, B.; Bocquet, A. Double-Blind Comparative Trial With 2 Antiregurgitation Formulae. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Abkari, A.; Cai, W.; Catto-Smith, A.; Cruchet, S.; Gottrand, F.; Hegar, B.; Lifschitz, C.; Ludwig, T.; Shah, N.; et al. Review shows that parental reassurance and nutritional advice help to optimise the management of functional gastrointestinal disorders in infants. Acta Paediatr. 2018, 107, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; A Brough, H.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256. [Google Scholar] [CrossRef]
- Ruffner, M.A.; Wang, K.Y.; Dudley, J.W.; Cianferoni, A.; Grundmeier, R.W.; Spergel, J.M.; Brown-Whitehorn, T.F.; Hill, D.A. Elevated Atopic Comorbidity in Patients with Food Protein–Induced Enterocolitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1039–1046. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Bajerova, K.; Dupont, C.; Eigenmann, P.; Kuitunen, M.; Meyer, R.; Ribes-Koninckx, C.; Salvatore, S.; Shamir, R.; Szajewska, H. The Cow’s Milk Related Symptom Score: The 2022 Update. Nutrients 2022, 14, 2682. [Google Scholar] [CrossRef]
- Meyer, R.; Meyer, R.; Vandenplas, Y.; Vandenplas, Y.; Reese, I.; Reese, I.; Vieira, M.C.; Vieira, M.C.; Ortiz-Piedrahita, C.; Ortiz-Piedrahita, C.; et al. The role of online symptom questionnaires to support the diagnosis of cow’s milk allergy in children for healthcare professionals—A Delphi consensus study. Pediatr. Allergy Immunol. 2023, 34, e13975. [Google Scholar] [CrossRef]
- Sladkevicius, E.; Nagy, E.; Lack, G.; Guest, J.F. Resource implications and budget impact of managing cow milk allergy in the UK. J. Med. Econ. 2010, 13, 119–128. [Google Scholar] [CrossRef]
- Petrus, N.C.M.; Schoemaker, A.-F.A.; Van Hoek, M.W.; Jansen, L.; Der Weide, M.C.J.-V.; Van Aalderen, W.M.C.; Sprikkelman, A.B. Remaining symptoms in half the children treated for milk allergy. Eur. J. Pediatr. 2015, 174, 759–765. [Google Scholar] [CrossRef]
- Latcham, F.; Merino, F.; Lang, A.; Garvey, J.; Thomson, M.A.; Walker-Smith, J.A.; Davies, S.E.; Phillips, A.D.; Murch, S.H. A consistent pattern of minor immunodeficiency and subtle enteropathy in children with multiple food allergy. J. Pediatr. 2003, 143, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Maslin, K.; Dean, T.; Arshad, S.H.; Venter, C. Fussy eating and feeding difficulties in infants and toddlers consuming a cows’ milk exclusion diet. Pediatr. Allergy Immunol. 2015, 26, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Nagy, E. Modelling the resource implications and budget impact of managing cow milk allergy in Australia. Curr. Med. Res. Opin. 2009, 25, 339–349. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.B.; Spolidoro, J.V.; Vieira, M.C.; Cardoso, A.L.; Clark, O.; Nishikawa, A.; Castro, A.P.M. Amino acid formula as a new strategy for diagnosing cow’s milk allergy in infants: Is it cost-effective? J. Med. Econ. 2016, 19, 1207–1214. [Google Scholar] [CrossRef]
- Guler, N.; Cokugras, F.; Sapan, N.; Selimoglu, A.; Turktas, I.; Cokugras, H.; Aydogan, M.; Beser, O. Diagnosis and management of cow’s milk protein allergy in Turkey: Region-specific recommendations by an expert-panel. Allergol. Immunopathol. 2020, 48, 202–210. [Google Scholar] [CrossRef]
- Testa, I.; Salvatori, C.; Di Cara, G.; Latini, A.; Frati, F.; Troiani, S.; Principi, N.; Esposito, S. Soy-Based Infant Formula: Are Phyto-Oestrogens Still in Doubt? Front. Nutr. 2018, 5, 110. [Google Scholar] [CrossRef]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A Comprehensive Review of Sensitization and Allergy to Soy-Based Products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281. [Google Scholar] [CrossRef]
- Lozinsky, A.C.; Meyer, R.; De Koker, C.; Dziubak, R.; Godwin, H.; Reeve, K.; Ortega, G.D.; Shah, N. Time to symptom improvement using elimination diets in non-IgE-mediated gastrointestinal food allergies. Pediatr. Allergy Immunol. 2015, 26, 403–408. [Google Scholar] [CrossRef]
- Meyer, R.; Fleming, C.; Dominguez-Ortega, G.; Lindley, K.; Michaelis, L.; Thapar, N.; Elawad, M.; Chakravarti, V.; Fox, A.T.; Shah, N. Manifestations of food protein induced gastrointestinal allergies presenting to a single tertiary paediatric gastroenterology unit. World Allergy Organ. J. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Agostoni, C.; Axelsson, I.; Goulet, O.; Koletzko, B.; Michaelsen, K.F.; Puntis, J.; Rieu, D.; Rigo, J.; Shamir, R.; Szajewska, H.; et al. Soy Protein Infant Formulae and Follow-On Formulae: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 352–361. [Google Scholar] [CrossRef]
- Meyer, R.; Venter, C.; Bognanni, A.; Szajewska, H.; Shamir, R.; Nowak-Wegrzyn, A.; Fiocchi, A.; Vandenplas, Y. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guideline update—VII—Milk elimination and reintroduction in the diagnostic process of cow’s milk allergy. World Allergy Organ. J. 2023, 16, 100785. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Barrio-Torres, J.; Dupont, C.; Howells, H.E.; Shamir, R.; Venter, C.; Meyer, R. Hydrolyzed rice formula for dietary management of infants with cow’s milk allergy. World Allergy Organ. J. 2022, 15, 100717. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Travaini, M.; D’Auria, E.; Banderali, G.; Bernardo, L.; Riva, E. Tolerance to a rice hydrolysate formula in children allergic to cow’s milk and soy. Clin. Exp. Allergy 2003, 33, 1576–1580. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Restani, P.; Bernardini, R.; Lucarelli, S.; Lombardi, G.; Magazzù, G.; Marseglia, G.L.; Pittschieler, K.; Tripodi, S.; Troncone, R.; et al. A hydrolysed rice-based formula is tolerated by children with cow’s milk allergy: A multi-centre study. Clin. Exp. Allergy 2006, 36, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Reche, M.; Pascual, C.; Fiandor, A.; Polanco, I.; Rivero-Urgell, M.; Chifre, R.; Johnston, S.; Martín-Esteban, M. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2010, 21, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; De Greef, E.; Hauser, B.; Halut, C.; Robberecht, M.; Balduck, N.; L’Homme, A.; Mohring, M.; Carvelli, T.; Defontaine, E.; et al. An extensively hydrolysed rice protein-based formula in the management of infants with cow’s milk protein allergy: Preliminary results after 1 month. Arch. Dis. Child. 2014, 99, 933–936. [Google Scholar] [CrossRef]
- Meyer, R.; Carey, M.P.; Turner, P.J.; Meharg, A.A. Low inorganic arsenic in hydrolysed rice formula used for cow’s milk protein allergy. Pediatr. Allergy Immunol. 2018, 29, 561–563. [Google Scholar] [CrossRef]
- Wopereis, H.; the ASSIGN study group; van Ampting, M.T.J.; Cetinyurek-Yavuz, A.; Slump, R.; Candy, D.C.A.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Fox, A.T.; et al. A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow’s milk allergic infants: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 27. [Google Scholar] [CrossRef]
- Fox, A.T.; ASSIGN study group; Wopereis, H.; Van Ampting, M.T.J.; Oude Nijhuis, M.M.; Butt, A.M.; Peroni, D.G.; Vandenplas, Y.; Candy, D.C.A.; Shah, N.; et al. A specific synbiotic-containing amino acid-based formula in dietary management of cow’s milk allergy: A randomized controlled trial. Clin. Transl. Allergy 2019, 9, 5. [Google Scholar] [CrossRef]
- Hendrickx, D.M.; An, R.; Boeren, S.; Mutte, S.K.; Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; et al. Assessment of infant outgrowth of cow’s milk allergy in relation to the faecal microbiome and metaproteome. Sci. Rep. 2023, 13, 12029. [Google Scholar] [CrossRef]
- Fiocchi, A.; Knol, J.; Koletzko, S.; O’mahony, L.; Papadopoulos, N.G.; Salminen, S.; Szajewska, H.; Nowak-Węgrzyn, A. Early-Life Respiratory Infections in Infants with Cow’s Milk Allergy: An Expert Opinion on the Available Evidence and Recommendations for Future Research. Nutrients 2021, 13, 3795. [Google Scholar] [CrossRef] [PubMed]
- Cool, R.; Vandenplas, Y. The Link between Different Types of Prebiotics in Infant Formula and Infection Rates: A Review. Nutrients 2023, 15, 1942. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhou, Z.; Li, W.; Lu, H.; Qiu, Z. Lactobacillus rhamnosus GG for Cow’s Milk Allergy in Children: A Systematic Review and Meta-Analysis. Front. Pediatr. 2021, 9, 727127. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, L.; Bouygue, G.R.; Sarratud, T.; Veglia, F.; Martelli, A.; Fiocchi, A. Impact of dietary regimen on the duration of cow’s milk allergy: A random allocation study. Clin. Exp. Allergy 2010, 40, 637–642. [Google Scholar] [CrossRef]
- Nowak-Węgrzyn, A.; Chehade, M.; Groetch, M.E.; Spergel, J.M.; Wood, R.A.; Allen, K.; Atkins, D.; Bahna, S.; Barad, A.V.; Berin, C.; et al. International consensus guidelines for the diagnosis and management of food protein–induced enterocolitis syndrome: Executive summary—Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2017, 139, 1111–1126.e4. [Google Scholar] [CrossRef]
- Sampson, H.A.; Aceves, S.; Bock, S.A.; James, J.; Jones, S.; Lang, D.; Nadeau, K.; Nowak-Wegrzyn, A.; Oppenheimer, J.; Perry, T.T.; et al. Food allergy: A practice parameter update—2014. J. Allergy Clin. Immunol. 2014, 134, 1016–1025.e43. [Google Scholar] [CrossRef]
- Upton, J.; Nowak-Wegrzyn, A. The Impact of Baked Egg and Baked Milk Diets on IgE- and Non-IgE-Mediated Allergy. Clin. Rev. Allergy Immunol. 2018, 55, 118–138. [Google Scholar] [CrossRef]
- Ball, H.B.; Luyt, D. Home-based cow’s milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin. Exp. Allergy 2019, 49, 911–920. [Google Scholar] [CrossRef]
- D’art, Y.M.; Forristal, L.; Byrne, A.M.; Fitzsimons, J.; van Ree, R.; DunnGalvin, A.; Hourihane, J.O. Single low-dose exposure to cow’s milk at diagnosis accelerates cow’s milk allergic infants’ progress on a milk ladder programme. Allergy 2022, 77, 2760–2769. [Google Scholar] [CrossRef]
- Venter, C.; Meyer, R.; Ebisawa, M.; Athanasopoulou, P.; Mack, D.P. Food allergen ladders: A need for standardization. Pediatr. Allergy Immunol. 2022, 33, e13714. [Google Scholar] [CrossRef]
- Berghi, O.; Dumitru, M.; Caragheorgheopol, R.; Tucureanu, C.; Simioniuc-Petrescu, A.; Sfrent-Cornateanu, R.; Giurcaneanu, C. The Relationship between Chemokine Ligand 3 and Allergic Rhinitis. Cureus 2020, 12, e7783. [Google Scholar] [CrossRef] [PubMed]
- Shek, L.P.; Soderstrom, L.; Ahlstedt, S.; Beyer, K.; Sampson, H.A. Determination of food specific IgE levels over time can predict the development of tolerance in cow’s milk and hen’s egg allergy. J. Allergy Clin. Immunol. 2004, 114, 387–391. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandenplas, Y.; Meyer, R.; Nowak-Wegrzyn, A.; Salvatore, S.; Venter, C.; Vieira, M.C. The Remaining Challenge to Diagnose and Manage Cow’s Milk Allergy: An Opinion Paper to Daily Clinical Practice. Nutrients 2023, 15, 4762. https://doi.org/10.3390/nu15224762
Vandenplas Y, Meyer R, Nowak-Wegrzyn A, Salvatore S, Venter C, Vieira MC. The Remaining Challenge to Diagnose and Manage Cow’s Milk Allergy: An Opinion Paper to Daily Clinical Practice. Nutrients. 2023; 15(22):4762. https://doi.org/10.3390/nu15224762
Chicago/Turabian StyleVandenplas, Yvan, Rosan Meyer, Anna Nowak-Wegrzyn, Silvia Salvatore, Carina Venter, and Mario C. Vieira. 2023. "The Remaining Challenge to Diagnose and Manage Cow’s Milk Allergy: An Opinion Paper to Daily Clinical Practice" Nutrients 15, no. 22: 4762. https://doi.org/10.3390/nu15224762
APA StyleVandenplas, Y., Meyer, R., Nowak-Wegrzyn, A., Salvatore, S., Venter, C., & Vieira, M. C. (2023). The Remaining Challenge to Diagnose and Manage Cow’s Milk Allergy: An Opinion Paper to Daily Clinical Practice. Nutrients, 15(22), 4762. https://doi.org/10.3390/nu15224762





