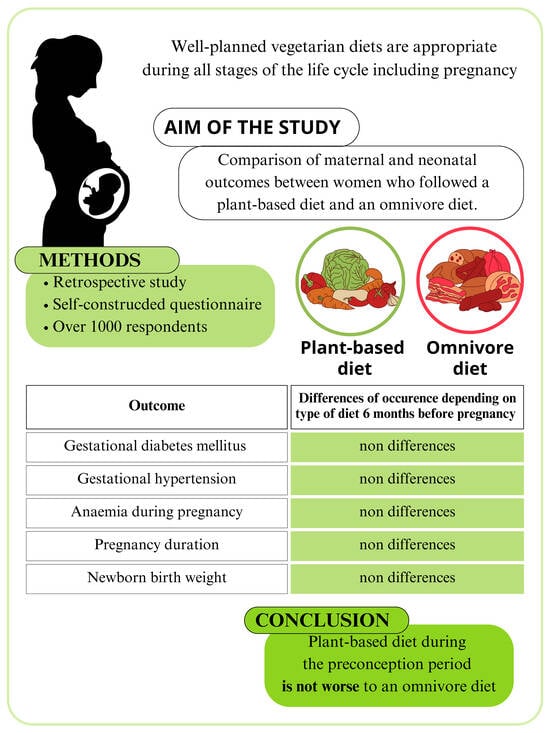
The Impact of Maternal Plant-Based Diet on Obstetric and Neonatal Outcomes—A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Cross-Sectional Survey
2.2. The Statistical Analysis and Endpoints
2.3. The Study Group
2.4. The Endpoint Definitions
3. Results
3.1. Study Population
3.2. Diet Type
3.3. Gestational Diabetes Mellitus (GDM)
3.4. Anemia during Pregnancy
3.5. Hypertension in Pregnancy
3.6. Weight Gain during Pregnancy
3.7. Duration of Pregnancy
3.8. Newborn Birth Weight
3.9. Newborn Status at Birth
3.10. Type of Delivery
3.11. Breastfeeding
3.12. Summary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Vaquero, M.P. Foods for Plant-Based Diets: Challenges and Innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Economic Weekly—Polish Economic Institute. Available online: https://pie.net.pl/wp-content/uploads/2021/11/Tygodnik-Gospodarczy-PIE_44-2021.pdf (accessed on 15 March 2023).
- Segovia-Siapco, G.; Sabaté, J. Health and sustainability outcomes of vegetarian dietary patterns: A revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur. J. Clin. Nutr. 2019, 72, 60–70. [Google Scholar] [CrossRef]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Jedut, P.; Glibowski, P.; Skrzypek, M. Comparison of the Health Status of Vegetarians and Omnivores Based on Biochemical Blood Tests, Body Composition Analysis and Quality of Nutrition. Nutrients 2023, 15, 3038. [Google Scholar] [CrossRef]
- Schiattarella, A.; Lombardo, M.; Morlando, M.; Rizzo, G. The Impact of a Plant-Based Diet on Gestational Diabetes: A Review. Antioxidants 2021, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Rizzo, G.; Goggi, S.; Giampieri, F.; Battino, M. Vegetarian diets during pregnancy: Effects on the mother’s health. A systematic review. Food Funct. 2021, 12, 466–493. [Google Scholar] [CrossRef]
- Key, T.J.; Papier, K.; Tong, T.Y.N. Plant-based diets and long-term health: Findings from the EPIC-Oxford study. Proc. Nutr. Soc. 2022, 81, 190–198. [Google Scholar] [CrossRef]
- Le, L.T.; Sabaté, J. Beyond meatless, the health effects of vegan diets: Findings from the Adventist cohorts. Nutrients 2014, 6, 2131–2147. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Papier, K.; Fensom, G.K.; Knuppel, A.; Appleby, P.N.; Tong, T.Y.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat consumption and risk of 25 common conditions: Outcome-wide analyses in 475,000 men and women in the UK Biobank study. BMC Med. 2021, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Kesary, Y.; Avital, K.; Hiersch, L. Maternal plant-based diet during gestation and pregnancy outcomes. Arch. Gynecol. Obstet. 2020, 302, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Clari, R.; Vigotti, F.N.; Leone, F.; Attini, R.; Cabiddu, G.; Mauro, G.; Castelluccia, N.; Colombi, N.; Capizzi, I.; et al. Vegan-vegetarian diets in pregnancy: Danger or panacea? A systematic narrative review. BJOG 2015, 122, 623–633. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Iron Deficiency Anemia Assessment Prevention and Control: A Guide for Programme Managers; 132 (WHO/NHD/01.3); World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Gestational Hypertension and Preeclampsia, ACOG Practice Bulletin, Clinical Management Guidelines for Obstetrician-Gynecologists, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Kruszewski, A.; Przybysz, P.; Kacperczyk-Bartnik, J.; Dobrowolska-Redo, A.; Romejko-Wolniewicz, E. Physical Activity during Preconception Impacts Some Maternal Outcomes—A Cross-Sectional Study on a Population of Polish Women. Int. J. Environ. Res. Public Health 2023, 20, 3581. [Google Scholar] [CrossRef]
- IOM. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Wielgoś, M.; Bomba-Opoń, D.; Bręborowicz, G.H.; Czajkowski, K.; Dębski, R.; Leszczyńska-Gorzelak, B.; Oszukowski, P.; Radowicki, S.; Zimmer, M. Recommendations of the Polish Society of Gynecologists and Obstetricians regarding caesarean sections. Ginekol. Pol. 2018, 89, 644–657. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat-The Relationship between Diet, Microbiota, and Metabolic Disorders-A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Jaworsky, K.; DeVillez, P.; Basu, A. The Role of Phytochemicals and Plant-Based Diets in Gestational Diabetes: Evidence from Clinical Trials. Int. J. Environ. Res. Public Health 2023, 20, 4188. [Google Scholar] [CrossRef]
- Yisahak, S.F.; Hinkle, S.N.; Mumford, S.L.; Li, M.; Andriessen, V.C.; Grantz, K.L.; Zhang, C.; Grewal, J. Vegetarian diets during pregnancy, and maternal and neonatal outcomes. Int. J. Epidemiol. 2021, 50, 165–178. [Google Scholar] [CrossRef]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.D.; Zhang, C. Prepregnancy dietary protein intake, major dietary protein sources, and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef]
- The InterAct Consortium. Association between dietary meat consumption and incident type 2 diabetes: The EPICInterAct study. Diabetologia 2013, 56, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G.; Knu, S. Food groups and risk of type 2 diabetes mellitus: A systematic review and metaanalysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.P.; Furman, T.; Hutcheson, H.R. Preeclampsia and reproductive performance in a community of vegans. South Med. J. 1987, 80, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Sumalla Cano, S.; Elio, I.; Masias Vergara, M.; Giampieri, F.; Battino, M. Plant-Plant-Based and Plant-Rich Diet Patterns during Gestation: Beneficial Effects and Possible Shortcomings. Adv. Nutr. 2015, 6, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal Diet and Nutrient Requirements in Pregnancy and Breastfeeding. An Italian Consensus Document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef]
- Mierzejewska, E. Comprehensive Assessment of pro-Health Behaviors and Their Determinants among Pregnant Women in the Context of Current National and International Recommendations on the Management of Healthy Pregnancy. Unpublished. Doctoral Dissertation, Institute of Mother and Child, Warsaw, Poland, 2022. [Google Scholar]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lattey, A.; Black, R.E.; Lancet Nutrition Interventions Review Group; et al. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Avnon, T.; Anbar, R.; Lavie, I.; Ben-Mayor Bashi, T.; Paz Dubinsky, E.; Shaham, S.; Yogev, Y. Does vegan diet influence umbilical cord vitamin B12, folate, and ferritin levels? Arch. Gynecol. Obstet. 2020, 301, 1417–1422. [Google Scholar] [CrossRef]
- Dyson, L.; McCormick, F.M.; Renfrew, M.J. Interventions for promoting the initiation of breastfeeding. Cochrane Database Syst. Rev. 2005, 2, CD001688. [Google Scholar]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet Assoc. 2009, 109, 1266–1282. [Google Scholar]

| Total Study Group N = 1015 | Plant-Based Diet Subgroup N = 83 | Omnivore Diet Subgroup N = 932 | p | |
|---|---|---|---|---|
| % (N) | % (N) | % (N) | ||
| Age (years) | ||||
| 16–20 | 1.9 (19) | 1.2 (1) | 1.9 (18) | 1.00 |
| 21–25 | 12.3 (125) | 6.0 (5) | 12.9 (120) | 0.08 |
| 26–30 | 36.9 (375) | 44.6 (37) | 36.3 (338) | 0.15 |
| 31–35 | 33.2 (337) | 28.9 (24) | 33.6 (313) | 0.47 |
| ≥36 | 15.7 (159) | 19.3 (16) | 15.3 (143) | 0.36 |
| Habitation | ||||
| Countryside | 19.4 (197) | 16.9 (14) | 19.7 (183) | 0.66 |
| Town < 50,000 | 15.1 (153) | 6.0 (5) | 15.9 (148) | 0.0153 |
| City 50,000–100,000 | 10.4 (106) | 14.5 (12) | 10.1 (94) | 0.26 |
| City 100,000–500,000 | 14.0 (142) | 16.9 (14) | 13.7 (128) | 0.41 |
| City > 500,000 | 41.1 (417) | 45.8 (38) | 40.7 (379) | 0.42 |
| Education | ||||
| Primary | 0.6 (6) | 0.0 (0) | 0.6 (6) | 1.00 |
| Vocational | 2.2 (22) | 2.4 (2) | 2.1 (20) | 0.70 |
| Secondary | 22.2 (225) | 19.3 (16) | 22.4 (209) | 0.58 |
| Higher | 75.1 (762) | 78.3 (65) | 74.8 (697) | 0.51 |
| Prepregnancy BMI (kg/m2) | ||||
| Underweight (<18.5) | 7.7 (78) | 12.0 (10) | 7.3 (68) | 0.13 |
| Normal (18.5–24.9) | 66.0 (670) | 75.9 (63) | 65.1 (607) | 0.05 |
| Overweight (25–29.9) | 18.3 (186) | 8.4 (7) | 19.2 (179) | 0.0119 |
| Obese (≥30) | 8.0 (81) | 3.6 (3) | 8.4 (78) | 0.14 |
| Prepregnancy BMI | Plant-Based Diet Subgroup | Omnivore Diet Subgroup | ||
|---|---|---|---|---|
| % (N) | % (N) | p a | p b | |
| During the 6 months before pregnancy | ||||
| Gestational Diabetes Mellitus (GDM) | ||||
| Underweight | 11.1 (1) | 5.7 (8) | 0.26 | 0.0002 |
| Normal | 66.7 (6) | 52.5 (74) | ||
| Overweight | 22.2 (2) | 25.5 (36) | ||
| Obese | 0.0 (0) | 16.3 (23) | ||
| Nongestational Diabetes Mellitus (Non-GDM) | ||||
| Underweight | 12.2 (9) | 7.6 (60) | ||
| Normal | 77.0 (57) | 67.8 (532) | ||
| Overweight | 6.8 (5) | 17.7 (139) | ||
| Obese | 4.0 (3) | 6.9 (54) | ||
| During pregnancy | ||||
| Gestational Diabetes Mellitus (GDM) | ||||
| Underweight | 14.3 (1) | 5.6 (8) | 0.0495 | 0.0001 |
| Normal | 71.4 (5) | 52.4 (75) | ||
| Overweight | 14.3 (1) | 25.9 (37) | ||
| Obese | 0.0 (0) | 16.1 (23) | ||
| Nongestational Diabetes Mellitus (Non-GDM) | ||||
| Underweight | 11.4 (9) | 7.7 (60) | ||
| Normal | 75.9 (60) | 67.8 (529) | ||
| Overweight | 8.9 (7) | 17.6 (137) | ||
| Obese | 3.8 (3) | 6.9 (54) | ||
| Subgroup | Less Physically Active | Adequately Physically Active | p | |
|---|---|---|---|---|
| % (N) | % (N) | % (N) | ||
| Plant-based diet | ||||
| Non-GDM | 89.2 (74) | 91.7 (44) | 85.7 (30) | 0.48 |
| GDM | 10.8 (9) | 8.3 (4) | 14.3 (5) | |
| Omnivore diet | ||||
| Non-GDM | 84.8 (785) | 83.4 (616) | 90.4 (169) | 0.0166 |
| GDM | 15.2 (141) | 16.6 (123) | 9.6 (18) | |
| Prepregnancy BMI | Plant-Based Diet Subgroup | Omnivore Diet Subgroup | ||
|---|---|---|---|---|
| % (N) | % (N) | p a | p b | |
| During 6 months before pregnancy | ||||
| Anemia | ||||
| Underweight | 16.7 (4) | 8.4 (19) | 0.24 | 0.05 |
| Normal | 70.8 (17) | 69.9 (158) | ||
| Overweight | 8.3 (2) | 15.5 (35) | ||
| Obese | 4.2 (1) | 6.2 (14) | ||
| Nonanemia | ||||
| Underweight | 10.9 (6) | 7.0 (49) | ||
| Normal | 76.4 (42) | 63.5 (446) | ||
| Overweight | 9.1 (5) | 20.4 (143) | ||
| Obese | 3.6 (2) | 9.1 (64) | ||
| During pregnancy | ||||
| Anemia | ||||
| Underweight | 17.4 (4) | 8.4 (19) | 0.53 | 0.08 |
| Normal | 65.2 (15) | 70.5 (160) | ||
| Overweight | 13.0 (3) | 15.0 (34) | ||
| Obese | 4.3 (1) | 6.2 (14) | ||
| Nonanemia | ||||
| Underweight | 10.0 (6) | 7.0 (49) | ||
| Normal | 76.7 (46) | 63.4 (442) | ||
| Overweight | 10.0 (6) | 20.4 (142) | ||
| Obese | 3.3 (2) | 9.2 (64) | ||
| Prepregnancy BMI | Plant-Based Diet Subgroup | Omnivore Diet Subgroup | ||
|---|---|---|---|---|
| % (N) | % (N) | p a | p b | |
| During 6 months before pregnancy | ||||
| Gestational Hypertension | ||||
| Underweight | 0.0 (0) | 3.4 (2) | 0.26 | <0.0001 |
| Normal | 33.3 (1) | 45.8 (27) | ||
| Overweight | 33.3 (1) | 32.2 (19) | ||
| Obese | 33.3 (1) | 18.6 (11) | ||
| Nonhypertension | ||||
| Underweight | 12.5 (10) | 7.7 (65) | ||
| Normal | 77.5 (62) | 67.3 (568) | ||
| Overweight | 7.5 (6) | 18.4 (155) | ||
| Obese | 2.5 (2) | 6.6 (56) | ||
| During pregnancy | ||||
| Gestational Hypertension | ||||
| Underweight | 0.0 (0) | 3.3 (2) | 0.07 | 0.0001 |
| Normal | 50.0 (1) | 45.0 (27) | ||
| Overweight | 50.0 (1) | 31.7 (19) | ||
| Obese | 0.0 (0) | 20.0 (12) | ||
| Nonhypertension | ||||
| Underweight | 11.7 (10) | 7.7 (65) | ||
| Normal | 75.3 (64) | 67.5 (566) | ||
| Overweight | 9.4 (8) | 18.2 (153) | ||
| Obese | 3.5 (3) | 6.6 (55) | ||
| Subgroup | Less Physically Active | Adequately Physically Active | p | |
|---|---|---|---|---|
| % (N) | % (N) | % (N) | ||
| Plant-based diet subgroup | ||||
| Gestational hypertension | 3.6 (3) | 4.2 (2) | 2.9 (1) | 1.00 |
| Nonhypertension | 96.4 (80) | 95.8 (46) | 97.1 (34) | |
| Omnivore diet subgroup | ||||
| Gestational hypertension | 6.5 (59) | 5.8 (42) | 9.3 (17) | 0.09 |
| Nonhypertension | 93.5 (844) | 94.2 (679) | 90.7 (165) | |
| Prepregnancy BMI | Plant-Based Diet Subgroup | Omnivore Diet Subgroup | ||
|---|---|---|---|---|
| % (N) | % (N) | p a | p b | |
| Weight gain during pregnancy | ||||
| Insufficient weight gain | ||||
| Underweight | 10.3 (3) | 8.7 (26) | 0.463 | <0.001 |
| Normal | 86.2 (25) | 75.6 (226) | ||
| Overweight | 3.4 (1) | 15.7 (47) | ||
| Obese | 0.0 (0) | 0.0 (0) | ||
| Adequate weight gain | ||||
| Underweight | 19.4 (6) | 7.0 (22) | ||
| Normal | 67.7 (21) | 69.0 (216) | ||
| Overweight | 6.5 (2) | 14.4 (45) | ||
| Obese | 6.5 (2) | 9.6 (30) | ||
| Excessive weight gain | ||||
| Underweight | 4.3 (1) | 6.3 (20) | ||
| Normal | 73.9 (17) | 51.6 (165) | ||
| Overweight | 17.4 (4) | 27.2 (87) | ||
| Obese | 4.3 (1) | 15.0 (48) | ||
| Pregnancy duration | ||||
| Premature birth | ||||
| Underweight | 12.5 (1) | 8.8 (9) | 0.23 | 0.04 |
| Normal | 75.0 (6) | 60.8 (62) | ||
| Overweight | 12.5 (1) | 17.6 (18) | ||
| Obese | 0.0 (0) | 12.7 (13) | ||
| Term delivery | ||||
| Underweight | 14.3 (9) | 7.0 (44) | ||
| Normal | 71.4 (45) | 65.3 (411) | ||
| Overweight | 9.5 (6) | 19.1 (120) | ||
| Obese | 4.8 (3) | 8.6 (54) | ||
| Post-term delivery | ||||
| Underweight | 0.0 (0) | 7.5 (15) | ||
| Normal | 100.0 (12) | 66.7 (134) | ||
| Overweight | 0.0 (0) | 20.4 (41) | ||
| Obese | 0.0 (0) | 5.5 (11) | ||
| Newborn birth weight | ||||
| Birth weight < 2500 g | ||||
| Underweight | 20.0 (1) | 14.8 (8) | 0.16 | 0.06 |
| Normal | 60.0 (3) | 63.0 (34) | ||
| Overweight | 20.0 (1) | 16.7 (9) | ||
| Obese | 0.0 (0) | 5.6 (3) | ||
| Birth weight 2500–4000 g | ||||
| Underweight | 12.2 (9) | 6.9 (55) | ||
| Normal | 77.0 (57) | 66.0 (523) | ||
| Overweight | 8.1 (6) | 18.7 (148) | ||
| Obese | 2.7 (2) | 8.4 (67) | ||
| Birth weight > 4000 g | ||||
| Underweight | 0.0 (0) | 6.7 (7) | ||
| Normal | 75.0 (3) | 60.6 (63) | ||
| Overweight | 0.0 (0) | 24.0 (25) | ||
| Obese | 25.0 (1) | 8.7 (9) | ||
| Apgar score at 5 min after birth | ||||
| ≥ 8 | ||||
| Underweight | 12.2 (10) | 7.6 (68) | 0.21 | 0.001 |
| Normal | 75.6 (62) | 65.6 (590) | ||
| Overweight | 8.5 (7) | 19.2 (173) | ||
| Obese | 3.7 (3) | 7.7 (69) | ||
| <8 | ||||
| Underweight | 0.0 (0) | 0.0 (0) | ||
| Normal | 100.0 (1) | 53.1 (17) | ||
| Overweight | 0.0 (0) | 18.8 (6) | ||
| Obese | 0.0 (0) | 28.1 (9) | ||
| Newborn Birth Weight | Plant-Based Diet Subgroup | Omnivore Diet Subgroup | ||
|---|---|---|---|---|
| % (N) | % (N) | p a | p b | |
| Type of delivery | ||||
| Vaginal delivery | ||||
| <2500 g | 1.9 (1) | 3.3 (17) | 0.16 | 0.036 |
| 2500 g–4000 g | 92.5 (49) | 86.0 (443) | ||
| >4000 g | 5.7 (3) | 10.7 (55) | ||
| Cesarean section (medical indications) | ||||
| <2500 g | 14.8 (4) | 5.8 (23) | ||
| 2500 g–4000 g | 81.5 (22) | 81.9 (326) | ||
| >4000 g | 3.7 (1) | 12.3 (49) | ||
| Cesarean section (undefined indications) | ||||
| <2500 g | 0.0 (0) | 5.3 (1) | ||
| 2500 g–4000 g | 100.0 (3) | 94.7 (18) | ||
| >4000 g | 0.0 (0) | 0.0 (0) | ||
| Plant-Based Diet Subgroup | Omnivore Diet Subgroup | p 1 | |
|---|---|---|---|
| % (N) | % (N) | ||
| Nonbreastfeeding difficulties | 75.3 (58) | 66.3 (544) | 0.13 |
| Breastfeeding difficulties | 24.7 (19) | 33.7 (276) |
| Outcome | Plant-Based or Omnivorous Diet 6 Months before Pregnancy | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | p | AUC | |
| Gestational diabetes mellitus | 1.134 | 0.790–1.628 | 0.494 | 0.596 ± 0.0262 |
| Anemia during pregnancy | 0.877 | 0.681–1.129 | 0.308 | 0.544 ± 0.0209 |
| Gestational hypertension | 1.217 | 0.669–2.213 | 0.521 | 0.663 ± 0.0368 |
| Plant-based or Omnivorous Diet during Pregnancy | ||||
| Gestational diabetes mellitus | 1.350 | 0.905–2.014 | 0.141 | 0.602 ± 0.0260 |
| Anemia during pregnancy | 0.939 | 0.729–1.210 | 0.625 | 0.539 ± 0.0208 |
| Gestational hypertension | 1.580 | 0.770–3.240 | 0.212 | 0.666 ± 0.0366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybysz, P.; Kruszewski, A.; Kacperczyk-Bartnik, J.; Romejko-Wolniewicz, E. The Impact of Maternal Plant-Based Diet on Obstetric and Neonatal Outcomes—A Cross-Sectional Study. Nutrients 2023, 15, 4717. https://doi.org/10.3390/nu15224717
Przybysz P, Kruszewski A, Kacperczyk-Bartnik J, Romejko-Wolniewicz E. The Impact of Maternal Plant-Based Diet on Obstetric and Neonatal Outcomes—A Cross-Sectional Study. Nutrients. 2023; 15(22):4717. https://doi.org/10.3390/nu15224717
Chicago/Turabian StylePrzybysz, Paulina, Adrian Kruszewski, Joanna Kacperczyk-Bartnik, and Ewa Romejko-Wolniewicz. 2023. "The Impact of Maternal Plant-Based Diet on Obstetric and Neonatal Outcomes—A Cross-Sectional Study" Nutrients 15, no. 22: 4717. https://doi.org/10.3390/nu15224717
APA StylePrzybysz, P., Kruszewski, A., Kacperczyk-Bartnik, J., & Romejko-Wolniewicz, E. (2023). The Impact of Maternal Plant-Based Diet on Obstetric and Neonatal Outcomes—A Cross-Sectional Study. Nutrients, 15(22), 4717. https://doi.org/10.3390/nu15224717