Abstract
This review delves into the intricate relationship between excess folate (vitamin B9) intake, especially its synthetic form, namely, folic acid, and its implications on health and disease. While folate plays a pivotal role in the one-carbon cycle, which is essential for DNA synthesis, repair, and methylation, concerns arise about its excessive intake. The literature underscores potential deleterious effects, such as an increased risk of carcinogenesis; disruption in DNA methylation; and impacts on embryogenesis, pregnancy outcomes, neurodevelopment, and disease risk. Notably, these consequences stretch beyond the immediate effects, potentially influencing future generations through epigenetic reprogramming. The molecular mechanisms underlying these effects were examined, including altered one-carbon metabolism, the accumulation of unmetabolized folic acid, vitamin-B12-dependent mechanisms, altered methylation patterns, and interactions with critical receptors and signaling pathways. Furthermore, differences in the effects and mechanisms mediated by folic acid compared with natural folate are highlighted. Given the widespread folic acid supplementation, it is imperative to further research its optimal intake levels and the molecular pathways impacted by its excessive intake, ensuring the health and well-being of the global population.
1. Introduction
It is estimated that over a third of the population in North America is chronically exposed to high levels of dietary folate from a combination of voluntary supplementation and food fortification with folic acid (FA) [1,2,3]. Eukaryotic cells lack the capability to synthesize folate de novo, establishing folate as an essential nutrient for higher organisms and humans. While the benefits of adequate folate intake are well documented, there is mounting evidence linking its excess with deleterious effects on human health by impacting cancer, immunity, birth outcomes, cardiovascular disease, and overall mortality. The association between excess folate and these negative outcomes is well-studied, but the causative mechanisms are not. Understanding and unraveling the mechanisms by which excess folate from natural sources, supplementation, enrichment, and fortification of the food supply could negatively impact health and disease is of critical importance. Recent reports focused on the areas of nutrition, folate metabolism, cancer, and aging, with each concluding that there are substantial knowledge gaps relating to the effects of excess folate on human health [4,5,6].
A comprehensive narrative on the potential impacts of excessive folate intake on health and disease is currently lacking. Additionally, there is no consensus regarding the potentially harmful role of excess folate supplementation, and there is a need to reconcile conflicting reports to identify gaps and areas requiring further research. This review aimed to provide a synthesis of the existing literature describing the potential effects of excess folate on health and discuss the possible molecular mechanisms underlying these effects, thus contributing to a better understanding of the complex relationship between folate intake and human health.
2. Methods
PubMed and Web of science databases were searched using permutations of the terms “excess folate”, “high folate”, “folic acid”, “UMFA”, in combination with “pregnancy”, “carcinogenesis”, “methylation”, “embryogenesis”, “neurodevelopment”, “autism”, and “mortality”. The reviewers screened the results using the following inclusion criteria: availability as full text in English; published between January 2000 and June 2023; and categorized as original research, a review, a systematic review, a meta-analysis, or a letter to the editor. Titles and abstracts were screened for the above criteria and relevance to the search topic. Articles that did not meet the specified criteria, as well as articles that did not report folate intake, supplement dosage, or physiological levels, were excluded. Additional literature was obtained from the references of relevant manuscripts (snowball method).
3. Results
The field of study encompassing folate research is large and rapidly evolving. Careful analysis of the existing literature highlighted key considerations, characterizations, and definitions that are addressed herein. Additionally, the literature revealed several health outcomes impacted by excess folate, which were categorized into the following seven classifications: pregnancy outcomes, disease risk in offspring, neurodevelopment, immune function and allergies, carcinogenesis, overall mortality, and other health effects. Each category is further elaborated through a detailed discussion of the relevant literature by addressing human studies conducted in that area, as well as animal cell culture studies where applicable. This was followed by elucidating the identified molecular mechanisms implicated in the effects of excess folate in the subsequent section. These mechanisms were grouped into two broad categories encompassing one-carbon-cycle-dependent and -independent mechanisms. The mechanistic findings of individual studies were examined, synthesized, and subcategorized within this provided mechanistic framework.
4. Discussion
4.1. Key Considerations Concerning Dietary Folate Intake
4.1.1. Folic Acid versus Natural Folate
The distinction between natural folate and synthetic FA is an important consideration when evaluating the potential risks of excess intake. Natural folate, mainly in the form of 5-methyl-tetrahydrofolate (5-m-THF) and, to a lesser extent, 5-formyl-tetrahydrofolate (5-f-THF), are biologically active forms that do not necessitate activation by dihydrofolate reductase (DHFR) as folic acid does. Natural folate intake is constrained by the limited amount present in food sources, as well as its lower bioavailability and stability. In contrast, large doses of synthetic FA can be easily consumed through food fortification and supplementation, making folic acid the primary contributor to elevated folate pools in the human population. Limited research has been conducted using physiologically relevant levels of natural folate in the place of FA. Natural folates are inherently less stable and bioavailable than FA, which creates technical and logistical challenges for their integration into basic research. Additionally, the widespread use of the terms “folate” and “folic acid” interchangeably has led to the misconception that these forms are equivalent. In this review, “folate” refers to all folate species, synthetic and natural, that are found physiologically and in foods, while “folic acid” denotes the fully oxidized synthetic folate used in supplementation and food fortification.
4.1.2. Folic Acid Intake
Folic acid is used almost universally in fortification and supplementation, as well as in conducting most laboratory research. Women of childbearing age taking prenatal vitamins, as well as children and adults taking vitamins high in FA, are likely to exceed the age-adjusted tolerable upper intake level (UL) of folate intake due to a combination of food fortification and supplementation [4,7]. FA supplementation is linked to elevated circulating unmetabolized folic acid (UMFA) in a dose-dependent manner [8]. A growing body of literature linking excess FA and UMFA to potential adverse health outcomes exists. However, evidence of causality between excess FA, UMFA, and various adverse effects remains inconclusive [5].
4.1.3. Prevalence of Elevated Folate Levels in the North American Population
Mandatory fortification in combination with supplementation with FA led to a more than doubling of the USA population’s serum folate concentration in the last thirty years [9,10,11]. In the USA, up to 35% of the population is taking folic-acid-containing supplements and roughly 5 percent of the population exceeds the UL of folate intake. The average folate intake is estimated to be 813 µg/day for men and 724 µg/day for women [1]. The Canadian Health Measures Survey identified that up to 40% of participants had high folate levels (defined as RBC folate > 1360 nmol/L) [2,3,12]. Unmetabolized folic acid was detected in over 95% of Americans participating in the National Health and Nutrition Examination Survey (NHANES), with significantly higher UMFA levels recorded among supplement users and older individuals [9,13]. Similarly, high levels of FA and UMFA were found in pregnant Canadian women and umbilical cord blood, with over 97% of pregnant Canadian women having detectable plasma UMFA [14,15] and up to 26% of pregnant women exceeding the UL of FA [16].
4.1.4. Defining Excess Folate
A significant gap exists in the understanding of what constitutes excess folate in humans and research models. Determining the levels at which folate intake becomes excessive is an elusive task since there is a lack of dose–response data and comprehensive documentation of adverse effects at a high folate intake has not been established [4]. The recommended daily allowance (RDA) of folic acid varies depending on age, sex, and life stage. Adult men and women are recommended to intake 400 µg of dietary folate equivalent (DFE), increasing to 500 µg and 600 µg DFE for breastfeeding and pregnant women, respectively. The tolerable upper intake level (UL) for folic acid was set at 1000 µg per day for adults. However, the determination of folic acid UL relied on limited and low-quality observational case study data [17]. Generally, folate exposure at dosages exceeding the adult UL is considered excessive. However, this criterion does not consider chronic exposure to elevated folate intake that approaches the UL and the inherent variability and mutations in folate cycle enzymes that are prevalent in humans [18,19].
In rodent studies, excess folate can be defined as exceeding the accepted standard baseline supplementation of 2 mg/kg diet. However, the optimal folate requirements for animals may be lower, and the activity of rate-limiting one-carbon metabolism enzymes is typically much higher in rodents compared with humans [20,21,22,23]. Chow diets fed to laboratory rodents can contain a variable amount of FA ranging from 2 to 15 mg/kg diet and averaging about 8 mg/kg diet [22]. The reason for supplementing rodent diets with excessive amounts of FA is not clear. However, it is likely that suppliers of research animals and their diets defaulted to higher levels of folate supplementation to ensure rapid growth and large litter sizes.
In cell culture studies, cells and tissues are subjected to elevated levels of folates that greatly exceed the physiological levels found in tissues to support growth and proliferation. In human plasma, the physiological levels of natural folate range from 150 to 450 nM, while standard cell culture media contains 2200 nM (RPMI) or 9000 nM (DMEM) of the non-physiological synthetic folic acid [24,25]. This implies that most studies utilizing standard cell culture conditions could be confounded by these supraphysiological concentrations [25,26].
4.2. Health Outcomes of Excess Folate
4.2.1. Pregnancy Outcomes
Prenatal Folate Intake and Neural Tube Defects
The primary goal of the folic acid food fortification mandates that were instituted in various countries around the world is to reduce the incidence of births with neural tube defects (NTDs). The folate fortification mandate has been notably successful in reducing the incidence of NTDs in populations where it has been implemented. Folate adequacy for preventing NTDs is critical in the first 4 weeks of gestation. Folate supplementation in the following weeks, postnatally, and during breastfeeding could potentially provide benefits to maternal and fetal health with varying and inconsistent outcomes [27]. Supplementation at the WHO recommended levels of 400 µg/day in the periconceptual period protects against NTDs, with largely positive health outcomes reported. However, FA intake at higher doses, especially when approaching or surpassing the upper intake limit and administered for extended durations, is at times associated with deleterious pregnancy and offspring outcomes. A detailed analysis of the effects of FA supplementation on gestation and long-term health was comprehensively reviewed elsewhere by Silva et al. [27]. Common prenatal vitamins typically include an FA dose of 1 mg/day with some prescribed regimens using doses of up to 5 mg/day. On average, women taking prenatal vitamins are likely to surpass the UL [28], leading to elevated levels of total folate and UMFA in maternal and fetal samples [29,30].
Prenatal Folate Intake and Non-NTD Outcomes
The impact of FA supplementation and specifically high-dose FA on non-NTD pregnancy outcomes is not clear [27]. The link between FA supplementation and oral cleft incidence is inconsistent with studies reporting a protective effect [31,32,33,34], no clear association [35,36], or an increase in oral cleft births [37]. In one study, high FA (4 mg/day) did not compromise fetal growth or provide additional protection against orofacial clefts compared with 0.4 mg/day [35]. Another study that used the same dose of 4 mg FA/day in early pregnancy saw increased maternal UMFA and serum folate, with no significant effect on RBC folate or evidence of altered one-carbon metabolism [38]. A Cochrane Review found no conclusive evidence that FA supplementation improved focused pregnancy outcomes, such as preterm birth, stillbirth, anemia, serum, and RBC folate levels [39]. In a Greek cohort, 5 mg FA/day was found to be protective against preterm birth and low birth weight [40]. FA supplementation provided benefits in reducing the incidence of congenital heart defects, irrespective of the timing and dosage during pregnancy, with beneficial association attributed to a high dose of FA (5 and 6 mg/day) [41,42]. In a Spanish cohort where pregnant women were supplemented with FA in doses exceeding 1 mg/day, an association was found with decreased birth length and an increase in the risk of low birth weight [43]. Increased incidence of position plagiocephaly was identified in a nested case–control study performed in the Netherlands in correlation with periconceptional high doses of FA [44].
Gestational Diabetes Mellitus
High folate concentration was linked to an increased risk of gestational diabetes mellitus (GDM) in Chinese cohorts [45,46]. Specifically, a higher FA dose, as well as the timing and duration of the supplementation, was associated with an increased risk for the development of GDM [47,48,49,50], with a significant GDM association when high FA was combined with an imbalance in vitamin B12 levels [51].
4.2.2. Disease Risk in Offspring
Folate Intake Modulates the Epigenome
Folate intake plays a critical role in the availability of the methyl donors necessary for epigenetic programming during conception, gestation, and early development. The paradigm that maternal exposure to various stressors, dietary factors, and nutrient excess or deficiency during the prenatal period and early development can modulate the risk for developing diseases later in life is well accepted [52]. As scientific knowledge advances, much of the etiology behind these effects has been attributed to epigenetic modifications and imprinting and is influenced by one-carbon metabolism [52,53,54]. Folate and its reduced intermediates play a crucial role in the one-carbon cycle and in regulating the processes involved in methylation and epigenetic modifications. Excess FA supplementation may potentially alter gene expression and lead to aberrant epigenetic programming in progeny [55,56]. Understanding the link between folate status in the parents, offspring, and epigenetic modifications can further our understanding of the origins of adult diseases [57,58,59].
Maternal Folate Intake and Transgenerational Disease Risk in Humans
Numerous human and animal studies identified associations between excess folate intake in mothers and disease risk in offspring, though the outcomes have been inconsistent. In the Indian subcontinent, where expecting mothers are often prescribed folic acid doses as high as 5 mg/day, high folate concentrations during pregnancy were linked to insulin resistance in children [60]. Results from a UK cohort of pregnant women found that FA supplementation after the 12th week of pregnancy impacts DNA methylation in the offspring, with significant changes in the methylation of regions that regulate insulin-like growth factor 2 in infants, potentially altering susceptibility to chronic diseases [57]. A systematic review by Xie et al. aimed at exploring the association between maternal folate status and the risk of obesity and insulin resistance in offspring could not reach a definitive conclusion due to inconsistencies in data collected from animal and human studies [61].
Paternal Folate Intake and Disease Risk in Progeny
The developmental origins of health and disease hypothesis suggest that fetal programming is influenced by maternal nutrition, among other factors. Moreover, growing evidence indicates that the paternal environment and diet significantly impact progeny’s health and risk of developing disease through epigenetic changes in sperm [62]. High doses of paternal folic acid supplementation were associated with increased gestational duration in births conceived through assisted reproduction [63]. Male offspring of mice fed a high-folate diet had a decreased sperm count, altered methylation in key genes, increased postnatal mortality in offspring, and reduced litter size [64]. Similarly, high-dose folic acid resulted in sperm DNA hypomethylation in both men and mice [65]. While moderate folic acid supplementation was beneficial in reducing some assisted reproduction-induced DNA methylation variance, high-dose supplementation produced deleterious effects with sex-specific outcomes [66].
Excess Folate Induces Significant Transgenerational Effects in Animal Studies
While the clinical significance of altered methylation in response to excess folate in humans is not clear, animal studies provided a more robust causative link between maternal folate intake, epigenetic changes, and disease risk. In rodents, maternal and post-weaning supplementation with FA significantly impacted global and gene-specific methylation in offspring [67], altered embryonic development [68] and cardiac gene expression [69], and modulated the expression of genes involved in growth [70]. Alterations of DNA methylation and imprinting patterns were partially responsible for deleterious transgenerational effects observed when either folate-deficient or excess-folate diets were fed to female mice [63]. Excessive FA during pregnancy induced obesity and altered the epigenetic profile of the offspring in rats [71,72], and led to metabolic dysfunction in late adulthood [73]. Similarly, other studies found that excess perinatal folic acid supplementation caused insulin resistance, dyslipidemia, and disrupted glucose metabolism and hepatic fat metabolism in both mice [66] and rat offspring [74,75,76].
4.2.3. Neurodevelopment
Impact of Excess Prenatal Folate on Neurocognitive and Psychomotor Development
Excess folic acid supplementation during pregnancy is linked to both beneficial and detrimental effects on neurodevelopmental outcomes in children. Although FA supplementation is beneficial during the pre-conceptual period and early weeks of gestation by preventing neural tube defects, continued supplementation may significantly impact children’s neurodevelopment through epigenetic alterations [77,78]. FA supplementation at the WHO-recommended level of 400 µg/d beyond the first trimester was associated with benefits on neurocognitive development in children, with positive effects on cognitive development observed at ages 7 and 11 [79,80]. In a study where high-dose FA supplementation was implemented, cord blood analysis revealed significant changes in DNA methylation, exhibiting sex-specific and loci-specific alterations in genes related to neurodevelopment [81]. Doses exceeding the UL during the periconceptual phase were linked to impaired neurocognitive development in children [82,83]. High-dose FA (>5 mg/day) supplementation in pregnant mothers led to poor psychomotor development in their children compared with those receiving 0.4–1 mg/day [84]. In a small Greek cohort (n = 58), high-dose FA supplementation (5 mg/day) in early pregnancy improved children’s vocabulary development, verbal comprehension, and communication skills, while doses exceeding 5 mg/day showed no benefit [40].
Impact of Excess Prenatal Excess Folate on Autism Spectrum Disorders Risk
The relationship between FA supplementation and autism spectrum disorders (ASDs) is complex and marked by contradictory findings. Recent systematic reviews found inconclusive evidence associating FA supplementation and ASD [83]. However, some studies did find a significantly increased risk of ASD with elevated maternal plasma FA [83] and linked synthetic folic acid supplementation during pregnancy to an increased autism risk [85]. Some suggested that excess FA leading to unmetabolized folic acid (UMFA) can potentially heighten the ASD risk [86]. Moderate prenatal FA supplementation of at least 400 µg from the diet and supplements was associated with a decreased ASD risk in children [79,80], while excess FA corresponded with lower cognitive development and increased ASD risk [81,83,87]. This suggests a U-shaped relationship, where both inadequate and high FA exposure could increase the ASD risk [87].
Excess Prenatal FA Causes Significant Neurodevelopmental Defects in Rodents
In animal models, excess FA during the prenatal period is associated with an adverse impact on behavior, memory, embryonic growth, methyl metabolism, and neurodevelopment [88]. In rodent studies, excess maternal FA supplementation resulted in deleterious behavioral abnormalities in offspring, including anxiety-like behavior, hyperactivity, impaired reversal learning, and seizure susceptibility [83,87,89]. The collected evidence suggests that the influence of excess prenatal FA on the type of behavioral abnormalities is sex specific [70,90,91,92]. Both deficient and excess FA during pregnancy impaired cortical neurodevelopment in mice offspring [93]. In another study, moderate FA supplementation led to behavioral changes and sex-dependent alterations in one-carbon metabolism [94]. High-dose FA supplementation in mice caused neural tube defects and disrupted embryonic development [70,95,96]. Neurodevelopmental toxicity in male mouse offspring brains was observed when a 2.5-fold FA requirement was provided one week before mating and continued through pregnancy and lactation [97]. In rats, high FA doses administered periconceptually decreased the offspring seizure threshold [96], while high FA intake (20 mg/kg) impaired rat dam’s memory and Na+, K+—ATPase activity in the pup’s hippocampus [98].
4.2.4. Immune Function and Allergies
Role of Folate in Immune Function
Folate plays a crucial role in maintaining a healthy immune system, influencing both innate and adaptive immune responses. Adequate folate status is essential for optimal immune function, while deficiency or excess of folate can lead to immune dysregulation. Folate is required for the production and maintenance of fast-proliferating white blood cells and is necessary for the maturation of lymphocytes. Folate plays a role in regulating inflammation and the production of pro-inflammatory cytokines [99]. Folate deficiency has been associated with impaired immune cell function and increased levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which can contribute to impaired immunity, chronic inflammation, and the development of inflammatory diseases [100]. Considerable evidence exists associating folate intake with inflammation and autoimmune diseases [101,102,103].
Excess Folate and Dysregulation of the Immune System
Excessive folate intake can negatively impact innate immunity. In postmenopausal women, high levels of folic acid intake through supplements and a folate-rich diet were found to reduce NK cell cytotoxicity [104]. Unmetabolized folic acid (UMFA) was inversely associated with NK cell cytotoxicity in the same study [104].
The immune system plays a key role in regulating the onset and severity of airway inflammation and asthma, which is a disease characterized by an overactive immune response. Prenatal and periconceptual FA supplementation, and particularly excess FA, was associated with an increased risk of childhood asthma in several studies [55,105,106,107,108,109]. However, others could not find a clear association [110,111,112,113]. Late pregnancy FA supplementation, as well as the extended duration of the supplementation (over 6 months), was associated with the highest risk of childhood asthma [55,109]. Similarly, FA supplementation in the first trimester of pregnancy correlated with a higher probability and severity of bronchiolitis compared with non-supplemented mothers in the USA [114]. FA supplementation in pregnant women was associated with an increased risk of wheezing and lower respiratory tract infection in their offspring up to 18 months of age in a Norwegian cohort [115]. Others reported that high plasma folate in mid-pregnancy was linked to decreased odds of wheezing at three years of age [116]. In a recent systematic review and meta-analysis, Chen et al. concluded that FA intake can be a risk factor for respiratory tract allergies in infants and children; however, a significant association was found only for doses not exceeding 400 uμg/day, while the risk effect decreased with increasing FA dose. Moreover, the association between FA supplements and children’s risk of allergic diseases was significantly increased only in countries without FA fortification. This increase in risk was more pronounced in cases where mothers used folic acid supplements [117].
Atopic dermatitis is caused by the inflammatory response of an overactive immune system. A study reported that high folate and B12 levels during pregnancy were associated with a higher prevalence of atopic dermatitis in children [118], while third-trimester FA supplementation was associated with eczema in children but no other allergic outcomes [119].
A dysregulated immune system can trigger an inflammatory response that impacts multiple tissues and organs. In preclinical models, high-dose FA (50 mg/kg diet) administered intraperitoneally to a murine model of colitis improved associated inflammation [120]; however, others found that high-methyl-donor diets, including 5 mg/kg diet FA, increased susceptibility to colitis in offspring [121,122]. Similarly, excess FA supplementation in rat dams (20 mg/kg diet) exacerbated weight gain and inflammation [123]. High folic acid exposure negatively impacted human lymphocyte stress response, genomic stability, and DNA methylation in both in vitro and in vivo conditions [21,124]. Others found that folic acid supplementation could promote a proinflammatory transcriptomic milieu in the colon [125,126] and that excess FA increased inflammation in response to a high-fat diet in rats [127].
The impact of FA supplementation on inflammatory markers and associated diseases in humans remains inconclusive [128]. Further research is needed to elucidate the potential mechanisms underlying these associations and to determine the optimal FA supplementation strategies for minimizing immune-related risks.
4.2.5. Carcinogenesis
Due to the importance of the folate cycle in DNA replication and repair, inadequate consumption of folate is considered an independent risk factor for cancer. A folate-deficient diet results in uracil misincorporation into DNA, leading to single- and double-stranded breaks [129]. On the other hand, excess folate may increase the cancer risk in the presence of preneoplastic lesions [17]. In rapidly proliferating pre-neoplastic or transformed cells, excess folate could help sustain high rates of growth by providing thymidylate and purines for DNA synthesis [130].
Folate and Colorectal Cancer: An Unsettled Debate
Colorectal cancer (CRC) is the second most prevalent cancer in Western societies, and it is most closely linked to folate intake. Following the fortification mandate, a small increase in the incidence of colorectal cancer in the USA and Canada was observed [131]. This association is considered controversial, as data from human and animal studies yielded contradictory results. Higher folate intake in natural and synthetic forms was associated with a decreased risk of CRC in women participating in the nurses’ health study [132]. Others found that vitamin B12 and folic acid supplementation significantly increased CRC incidence [131]. Long-term supplementation of FA at 1 mg/day was associated with increased multiplicity of colorectal adenomas, advanced lesions, and increased risk of prostate cancer in some studies [133,134,135]. However, in a recent systematic review, high folate intake was associated with reduced risk of colorectal cancer in the USA and Europe, particularly among people with moderate or high alcohol consumption [136].
Folate Intake and Breast Cancer Risk
Conflicting associations were found between folate intake and breast cancer risk [137,138,139]. In a meta-analysis by Chen et al., a U-shaped relationship was observed in prospective studies, where moderate folate intake (153–400 µg/day) decreased breast cancer risk compared with <153 µg/day, while an intake > 400 µg/day had no effect [138]. In another analysis by Zhang et al., moderate folate intake between 200–320 µg/day lowered the risk of breast cancer, while a significantly increased risk was observed with a daily folate intake exceeding 400 µg [137]. Recent studies concluded that a high intake of one-carbon-related vitamins, including folic acid, may be protective against estrogen-receptor-negative/progesterone-receptor-negative tumors and in individuals with moderate-to-high alcohol consumption [139,140].
Folate Intake Association with Other Cancers and Overall Cancer Risk
The association of folate intake with the incidence of non-CRC cancers is inconsistent and was reviewed in detail elsewhere [141]. Higher intake of folate was associated with a reduced risk of squamous cell carcinoma of the head and neck [142] and esophageal [143,144,145], oral [146], pancreatic [147,148], and bladder cancers [149,150]. We should note that the higher intake in these studies was unlikely to represent excess folate intake and the analysis was not stratified to isolate the effects of intake that approach or exceed the UL. No clear association between high folate intake and lung cancer was observed in a meta-analysis [151]. However, a study from Poland found that high systemic folate levels (RBC folate concentrations above 506.5 nmol/L) increased lung cancer risk in heavy smokers [152]. High intake of folate was not protective against ovarian cancer [153]. Despite an overall marginally negative association between endometrial cancer and folate intake, at higher intake levels ranging between 205 and 987 µg/day, cancer risk increased in a dose-dependent manner, suggesting a threshold effect [154]. In two randomized controlled trials, treatment with a moderate dose of FA and vitamin B12 caused an increased overall cancer incidence and mortality [142]. Similarly, a meta-analysis found a borderline significant increase in overall cancer risk with FA supplementation [155]. However, a more recent independent meta-analysis of RCTs did not find any significant effect of FA supplementation on overall cancer or colorectal cancer risk [156].
Folate in Preclinical Cancer Studies
Studies in rodents similarly revealed a complex and sometimes contradictory relationship between folic acid intake and tumorigenesis. The difference in models, timing, duration, dose, and experimental design led to inconsistent findings in rodents. The impact of folic acid supplementation on colorectal cancer in rodents was reviewed in detail elsewhere [157]. Notably, in some rodent studies that used high doses of folic acid (8 mg/kg), supplementation led to an increased occurrence of preneoplastic lesions and CRC [143,144,145]. Increased proliferation of colorectal epithelia and intake of folate after the development of neoplastic lesions was hypothesized to be responsible for the observed increased risk.
In an inducible breast cancer mouse model, a high-FA diet (10 mg/kg) significantly increased the tumor volume compared with baseline diets (2 mg/kg) [158]. Moderately high FA supplementation (5 mg FA/kg) prior to and during pregnancy and lactation in rats increased the risk and multiplicity of mammary adenocarcinomas in the offspring and reduced global DNA methylation [159]. In another rat model, FA supplementation (ranging from 5 to 10 mg/kg) promoted the progression of established mammary tumors [160].
In a chemically inducible prostatitis model, maternal folic acid supplementation at a dose of 2 mg/kg in rats led to a heightened incidence and severity of inflammation in their offspring [135]. This is especially relevant to the observation that folic acid supplementation was associated with an increased risk of prostate cancer in humans [161]. However, further research is needed to validate the effects and explore the underlying mechanisms.
In cell culture studies, FA depletion and supplementation enhanced the transformation of keratinocytes infected by the human papilloma virus [162,163]. Furthermore, depletion and over-supplementation of FA led to increased progression, migration, and invasion of the hepatocarcinoma HepG2 cells [164]. Similarly, in human lymphoblastoid cell lines, excess FA mimicked folate restriction by similarly impacting the oxidative stress response, DNA damage repair, and DNA methylation [124].
4.2.6. Overall Mortality
Several studies sought to determine the relationship between high folate intake, chronic disease risk, and overall mortality. A systematic review by Colapinto et al. observed a negative association between high folate levels and adverse health outcomes. However, the variability in study methods, designs, and high folate cutoffs prevented definitive conclusions [12]. A large UK population cohort study (n = 115,664) found that higher dietary folate intake correlated with lower CVD risk and overall mortality [165], and a similar trend was noted in a recent NHANES survey data analysis [166]. Other studies, however, could not find a significant association between high folic acid intake and overall mortality [167,168]. A prospective analysis of 2011–2014 NHANES data found a higher mortality risk associated with increased serum folate levels, such as 5-m-THF, UMFA, non-methyl folate, and MeFox (a biologically inactive degradation product of 5-m-THF), as well as with 5-m-THF insufficiency [169]. Another cohort study based on 1999–2010 NHANES data reported modestly elevated all-cause and cardiovascular mortality in participants with high folate levels (>40 nmol/L) [170].
4.2.7. Other Health Effects
Multiple studies offered insights into the relationship between folate intake and non-cancer health outcomes, particularly metabolic and cardiovascular diseases. A recent systematic review and meta-analysis found that folate supplementation improved fasting glucose, insulin resistance, and insulin levels, but had no effect on diabetes or HbA1c [171]. A large meta-analysis of randomized controlled trials could not establish a connection between folic acid supplementation and reduced cardiovascular risk, although a modest benefit was observed in stroke prevention [172]. However, high folate intake was associated with an increased risk of thyroid and endocrine disorders [5,172]. Studies in rats that compared the effects of 5-m-THF and FA supplementation showed that high doses of either form led to distinct adverse and harmful effects on metabolism [173]. Research that compared the effects of high intakes of natural and synthetic folate on health and disease is of particular significance, given that very few comparative studies have been conducted in human and animal models.
Folic acid supplementation is commonly used to treat hyperhomocysteinemia, which is a condition characterized by elevated homocysteine levels [174]. Homocysteine is a risk factor for stroke, cardiovascular disease, cognitive function, and adverse pregnancy outcomes [175,176,177,178]. In two separate studies, folic acid supplementations at 0.5 and 5 mg per day were similarly effective at reducing homocysteine levels [179,180]. A dose-finding interventional trial concluded that long-term doses as low as 0.2 mg of folic acid or lower effectively lowered homocysteine levels, with no further significant reduction observed at higher doses [181]. However, a high dose of folic acid (5 mg/day) for three months failed to normalize homocysteine levels in over 40% of participants in a recent study [182].
5. Molecular Mechanisms Underlying the Adverse Effects of Excess Folate Intake
While the previous sections outlined what is known about the association between excess folate intake and potentially deleterious health effects, the underlying mechanisms are not well defined. Isolating the responsible mechanisms is necessary for establishing causation. A diverse array of potential mechanisms from human and animal studies were identified in the literature. This section elaborates and discusses these potential molecular and genetic mechanisms that are hypothesized to mediate some of the potential deleterious effects of excess folate (Figure 1).
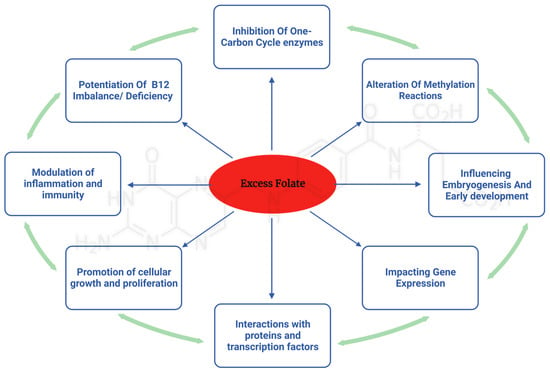
Figure 1.
Implications of excess folic acid: impact on multifaceted pathways and their biological consequences.
5.1. One-Carbon-Metabolism-Dependent Mechanisms
The one-carbon metabolism plays a crucial role in numerous cellular processes, including DNA synthesis, repair, and methylation. Excess folic acid can disrupt the balance of this metabolic cycle, leading to various adverse health effects. The mechanisms related to the one-carbon cycle can be further divided into the following categories:
5.1.1. Vitamin-B12-Dependent Mechanisms
The aging population is particularly at risk for vitamin B12 deficiency, and there is concern that folic acid (FA) supplementation might mask and exacerbate the severe, potentially irreversible neurological damage caused by B12 deficiency [178,179,183,184]. Excess FA can conceal hematological and neurological symptoms of B12 deficiency, worsening the condition and delaying diagnosis, leading to irreversible morbidities [6,185]. Reynolds et al.’s comprehensive review highlighted evidence that long-term exposure to excess FA, even at doses below the upper limit (0.5–1 mg), combined with B12 deficiency, can lead to neurological harm and cognitive decline [183]. FA supplementation may increase the demand for vitamin B12, exacerbating its depletion, pernicious anemia, and associated neurological and hematological decline [186,187,188]. Following fortification mandates in countries like the United States and Australia, epidemiological studies found a link between excess folic acid and cognitive decline in older adults at risk of B12 deficiency [189,190,191,192]. However, there is still no consensus regarding the role of excess FA in potentiating neurological and cognitive impairment in individuals with a low or deficient B12 status [193,194].
A B vitamin imbalance resulting from high FA combined with inadequate B12 may lead to a functional folate deficiency, contributing to insulin resistance and gestational diabetes mellitus [195]. In pregnant rats, high FA supplementation combined with a B12-deficient diet led to oxidative stress in both the mothers and pups compared with the same supplementation with adequate B12 status [196]. Excess FA supplementation alongside B12 deficiency during pregnancy and lactation resulted in diet-dependent metabolic impairment in female rat offspring [197]. Similar studies in rats demonstrated that adequate B12 levels are required to mitigate the potential harmful effects of high FA on DNA methylation and neurodevelopment in offspring [198,199].
Mechanistically, the paradoxical combination of excess folate with B12 deficiency leads to a unique metabolic situation known as the methyl trap. The primary enzymatic reaction implicated in the methyl trap is catalyzed by methionine synthase (MTR), which is a vitamin B12 (cobalamin)-dependent enzyme. MTR catalyzes the conversion of homocysteine to methionine, with 5-m-THF acting as the methyl donor. During this process, 5-m-THF transfers a methyl group to cobalamin, generating methylcobalamin, which subsequently transfers the methyl group to homocysteine, yielding methionine and tetrahydrofolate (THF). THF is essential for purine and pyrimidine synthesis, which are required for DNA and RNA production. In situations of vitamin B12 deficiency, MTR activity is compromised, leading to an accumulation of 5-m-THF, as the enzyme is unable to utilize it as a methyl donor, leading to the methyl trap (Figure 2). This sequestration of folate in the form of 5-m-THF results in a functional folate deficiency, as the intracellular pool of THF is depleted. The reduced availability of THF impairs the synthesis of purines and pyrimidines, ultimately affecting DNA and RNA synthesis. Consequently, this disruption in nucleotide synthesis manifests as megaloblastic anemia, which is characterized by large, immature erythrocytes. Additionally, vitamin B12 deficiency and the subsequent impairment of MTR activity leads to an accumulation of homocysteine, as it cannot be converted to methionine. Elevated homocysteine levels are associated with an increased risk of cardiovascular disease, neurodegenerative disorders, and other adverse health outcomes.
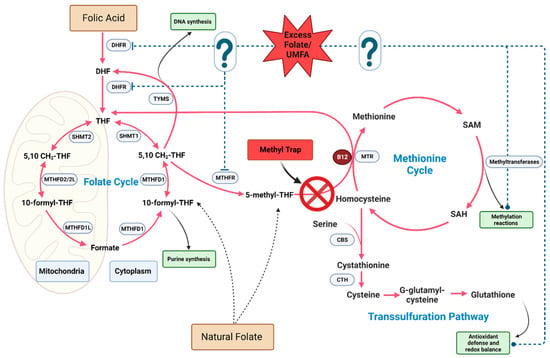
Figure 2.
Potential biochemical impact of excess folate on one-carbon metabolism pathways. The question marks and dotted blue line represent the potential molecular targets of excess folate and/or UMFA on one-carbon meatbolic enzymes and biosynthetic outputs.
5.1.2. Accumulation of Unmetabolized Folic Acid
When folic acid intake is high, UMFA can accumulate in the bloodstream, as the body’s ability to convert folic acid to its active reduced form, 5-m-THF, in the intestine and liver becomes saturated [4,200,201]. Specifically, UMFA accumulation is associated with the saturation of the rate-limiting one-carbon enzyme DHFR. The enzymatic activation of FA by DHFR is slow in humans, leading to a persistent elevation of UMFA after limited exposure to dietary FA [201,202]. Higher levels of circulating UMFA generally correlate with increased folic acid intake [203,204]. Intakes of FA that surpass the UL are associated with an increased likelihood of having elevated UMFA levels [205,206,207,208,209]. Furthermore, a daily intake of FA in doses as low as 200 µg was found to positively correlate with circulating UMFA levels [9,200,210,211]. UMFA can act as either a competitive or non-competitive inhibitor of DHFR depending on the intracellular concentration of dihydrofolate (DHF), which is a folate cycle intermediate generated during thymidylate synthesis (Figure 2) [201]. This inhibition of DHFR can lead to the accumulation of DHF, which is a potent inhibitor of methylenetetrahydrofolate reductase (MTHFR), ultimately disrupting the one-carbon cycle [212,213,214]. As a result, essential reactions in the cycle can be dysregulated, impacting DNA synthesis and repair, as well as methylation processes. Evidence exists to support the use of UMFA as a marker for excess folate in humans, as UMFA levels generally increase in response to supplemental folic acid intake [38,215]; however, newer sensitive methods were able to detect UMFA in the majority of samples, irrespective of folic acid intake [9,216].
5.1.3. Pseudo-MTHFR Deficiency
MTHFR is an enzyme that catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-m-THF, which is the biologically active form of folate. This conversion is a critical step in the folate metabolic pathway, as 5-m-THF participates in the re-methylation of homocysteine to methionine. As outlined previously, the presence of excess folic acid could competitively inhibit the enzymes involved in the conversion of other forms of folate, such as DHFR, which converts dietary folic acid to DHF and subsequently to THF. This competitive inhibition can exacerbate an existing MTHFR deficiency by further disrupting the folate metabolic pathway, leading to a functional folate deficiency despite adequate folate intake. Polymorphisms in the MTHFR gene are associated with reduced enzyme activity and increased health risks. In humans, C677T and A1298C polymorphisms are common, with an estimated 20–40% of the US population harboring one or both mutations [19].
MTHFR inhibition by excess folate is not exclusively linked to cases where genetic polymorphisms are present. In the absence of known MTHFR polymorphisms, elevated levels of folic acid resulted in a pseudo-MTHFR deficiency [68,97,217,218,219]. This leads to the disruption of the one-carbon cycle, negatively impacting methylation reactions, and potentially precipitating various health issues, including abnormal embryonic growth and neurodevelopment [218].
5.1.4. Disruption of Methylation and Epigenetics
A significant portion of the negative effects linked to excess folate appeared to be associated with epigenetic alterations. The methionine cycle, which supplies one-carbon units to methylation reactions, is intricately linked to the folate cycle, with both sharing common regulatory signals. Folate is vital for the generation of S-adenosylmethionine (SAM), which is the primary methyl donor for DNA methylation.
Disturbances in folate levels arising from either low or high folate status can lead to hypo- or hypermethylation, resulting in epigenetic instability, dysregulation of gene expression, and ultimately contributing to the initiation and progression of cancer [220,221,222]. DNA hypomethylation is considered an early event in colon carcinogenesis [223]. Excess FA is capable of inducing hypomethylation in cultured human cells [224] and an alteration in methyl metabolism in mice [94,217]. Work with multiple models suggests that both folate deficiency and excess FA can disrupt the folate cycle and the connected methionine cycle, leading to dysregulated methylation [93,218,225,226]. In human lymphocytes, high folic acid intake relative to natural folate can influence DNA methylation, disrupt genomic stability, and diminish the capacity to withstand oxidative stress [21,124]. Excess folic acid supplemented to cancer cell organoids can counteract methionine dependency, thus promoting cancer stem cell formation [227]. In mice, a high-FA diet promoted hepatocellular carcinoma development through the stabilization of the methionine cycle enzyme MATIIα.
The in vivo and in vitro mechanisms through which excess maternal FA supplementation can influence neurological development were reviewed elsewhere [228], detailing behavioral, morphological, and molecular changes in the offspring’s brain exposed to FA over-supplementation. A recent investigation into some of the underlying mechanisms discovered significant changes in DNA methylation of neurodevelopmental genes, which play a crucial role in transcriptional regulation [229]. Notably, these modified epigenetic patterns persisted throughout an individual’s life, as demonstrated in the Aberdeen Folic Acid Supplementation Trial [230]. Although both animal and human studies have identified a correlation between excessive maternal FA intake and metabolic abnormalities in offspring, a clear causative link with the modified epigenetic landscape has not yet been established [60,74,75,76,231,232].
While the precise mechanisms by which excess folate impacts methylation are not clearly understood, disruption of one-carbon metabolism is the primary suspect. The prevailing theory is that excess folic acid can disrupt the balance of SAM and its demethylated product, namely, S-adenosylhomocysteine (SAH). This disruption could lead to global DNA hypomethylation, resulting in genomic instability, altered gene expression, and an increased risk of diseases. Supporting evidence is available from studies reporting that high FA levels were linked to the dysregulation of intracellular one-carbon metabolism [233], imbalance in the distribution of folate coenzymes and derivatives [218], and perturbation of methylation reactions [70,91,234,235,236,237]. Studies in mice found that excess maternal FA intake disrupted the offspring’s folate metabolism, creating an altered metabolic flux within one-carbon metabolism that mimicked folate deficiency, favoring DNA synthesis over methylation reactions [93]. Other reports confirmed that both folate deficiency and excess supplementation similarly compromised folate-dependent pathways [238]. In a zebrafish model, excessive FA led to abnormal cardiac development and defects in folate metabolism similar to folate depletion using the antifolate drug methotrexate [126]. Both excess paternal FA supplementation and FA depletion in mice resulted in similar deleterious transgenerational outcomes that potentially stem from altered methylation of imprinted sperm genes [64].
The literature presents compelling evidence that excess folate disrupts one-carbon metabolism, significantly impacting methylation reactions. It remains unclear whether the resulting epigenetic changes are caused solely by the direct disruption of one-carbon metabolism by excess FA or if other unidentified pathways are involved.
5.2. One-Carbon-Metabolism-Independent Mechanisms
A significant limitation to previously discussed reports hypothesizing that UMFA could be the driver behind the effects of excess folate is that UMFA is not known to accumulate in biological tissues and inside cellular compartments. Unlike reduced folate, UMFA has a low affinity for the reduced folate carrier transporter (RFC) and is not a substrate for the enzyme folate polyglutamate synthase (FPGS) [239,240]. UMFA cannot directly participate in one-carbon cycle metabolism and binds weekly to folate enzymes. However, UMFA can bind to and be transported into tissues by the folate receptor (FOLR) and the proton-coupled folate transporters (PCFTs). This prompts an inquiry into whether the effects of excess folate on one-carbon metabolism, as observed across multiple studies, arise from UMFA’s direct interactions with one-carbon enzymes, indirectly by increasing total systemic folate, or through alternate pathways independently of one-carbon metabolism. The following studies detail the effects of excess FA and natural folate that are mediated via non-canonical pathways.
Folic acid could operate through mechanisms different from natural folate. One notable mechanism concerns the folate receptor (FOLR) binding to FA, facilitating its transport, and potentially activating downstream effectors. Folate receptors have a much higher affinity to FA compared with natural folates. FOLRα facilitates the intake of FA through receptor-meditated endocytosis. FOLRα is ubiquitously expressed in the epithelia of normal tissues (kidney, placenta, blood–brain barrier), as well as being overexpressed in many cancers, such as ovarian, breast, and colon cancers [241]. The role of FOLRα in mediating tumorigenesis and the associated physiological function in cancer are not clear [158,242]. In the presence of FA, FOLRα could translocate into the nucleus and function as a transcription factor, impacting the genes responsible for cellular pluripotency [203,204]. FA-bound FOLR acts as a signaling molecule that activates downstream targets, such as the pro-oncogene STAT3, extracellular signal-regulated kinase (ERK) kinases, and cellular Sarcoma (SRC) pathways [205,206,207,208,209].
Unlike natural folate, folic acid is linked to the regulation of neurogenesis in the ventral hippocampus in rats [243]. A study in piglets found that a high-FA diet altered the expression of liver proteins involved in oxidative responses, metabolic regulation, and cancer pathways compared with a control diet (30 mg FA/kg vs 1.3 mg FA/kg diet) [244]. Excess periconceptual FA led to upregulated Fos expression in mice [91] and activation of β-catenin (Wnt pathway) in the brain of male mouse offspring [98]. In studies that compared the effects of supplementing excess synthetic and natural folate in rats, the authors observed contrasting metabolic shifts leading to weight gain, leptin dysregulation, and increased food intake in the female offspring when dams were fed excess 5-m-THF compared with excess FA, with FA significantly impacting neurotransmitter signaling genes [173,245]. In a prostate cancer model, folate was found to be directly involved in oncogenic signaling pathways, specifically the PI3K-AKT-mTOR cascade [246]. Similarly, in a colon carcinogenesis mouse model, a significant increase in mTOR signaling was recorded in mice supplemented with folic acid compared with those without supplementation [20]. In addition to the observed effects on gene expression, FA was found to impact posttranslational modifications and protein–protein interaction [241]. These findings highlight an extensive interplay between folate intake and regulatory processes that mediate gene expression and protein interactions that require further research and analysis.
6. Folate: A Regulator of Organismal Aging?
Recently, the one-carbon cycle has been identified as a central regulator of organismal aging [247,248]. Metabolomic analysis of long-lived C. elegans and mouse models revealed common signatures and regulation of the one-carbon folate cycle. In a robust analysis, Annibal et al. demonstrated that supplementing long-lived C. elegans mutants with physiological levels of folate partially abrogated the lifespan extension, while RNAi knockdown of DHFR extended it in wild-type worms [247]. Our preliminary work confirmed these reports; we observed increased longevity in C57/Bl6 mice on a folic-acid-restricted diet compared with those receiving a folic-acid-supplemented diet. Furthermore, exposing C. elegans to the DHFR inhibitor methotrexate significantly increased the worm’s lifespan, while supplementing the media with folate shortened it in a dose-dependent manner. These findings highlight the folate cycle as a shared causal mechanism for longevity and add to the urgency for further research.
7. Limitations
The expansive domain of nutrition research focusing on folate’s impact on health has witnessed significant diversity and evolution over the last three decades. Historically, a vast majority of studies emphasized the dangers of folate deficiency and the benefits of maintaining adequate folate intake. This predominant focus led to a relative sidelining of the potential ramifications of excessive folate consumption, which is an issue gaining relevance due to escalating folic acid intake in contemporary diets. However, a surge in recent literature shed light on the potential deleterious effects associated with high folate intake. The increasing volume and frequency of these publications prompted a re-evaluation of longstanding paradigms related to folate intake and health.
Given the breadth and depth of this field, constructing a comprehensive review encompassing every nuance, controversy, and equivocal finding is inherently daunting. Hence, this review did not intend to serve as an exhaustive or entirely impartial examination of the folate research spectrum. Instead, its primary objective lay in accentuating the emergent findings and mechanisms that robustly correlate excessive folate consumption with health effects and the etiology of diseases. Nevertheless, efforts were made to ensure a balanced discourse, underscore prevailing controversies, and highlight areas necessitating further investigation.
Caution must be exercised when interpreting the results from folate studies conducted in preclinical models. Rodents display significant and measurable differences in folate requirements, metabolism, and the activity of rate limiting one-carbon enzymes [201]. Rats and mice blood folate levels are on average 6-8 fold higher than humans [157]. This observation should lead to careful consideration when extrapolating rodent data to human disease. However, the phenotypes induced by excess folate in rodents can undoubtedly provide valuable insights into the risks and mechanisms involved.
8. Conclusions
As researchers, including ourselves, strive to unravel the conserved and intricate dynamics governing the relationship between folate, health, disease, and longevity, a crucial question arises: could exposing the human population to excessive levels of folate potentially accelerate aging and increase the risk of disease?
The key to answering this question lies in identifying causal underlying mechanisms that can robustly link excess folate intake to the observed detrimental effects. Achieving this goal requires substantial research efforts and collaboration across multiple scientific disciplines to identify and address the existing knowledge gaps. The importance of pursuing such research cannot be overstated, as these gaps in knowledge may be unknowingly and insidiously compromising the health of hundreds of millions of people worldwide who are consuming excess folate.
Author Contributions
Writing—original draft preparation, A.M.F.; writing—review and editing, A.M.F. and A.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
National Institutes of Health to A.R.H. (CA121298).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figures were created with BioRender.com (accessed on 28 September 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; McDowell, M.A.; Yetley, E.A.; Sempos, C.A.; Burt, V.L.; Radimer, K.L.; Picciano, M.F. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 2010, 91, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Colapinto, C.K.; O’Connor, D.L.; Tremblay, M.S. Folate status of the population in the Canadian Health Measures Survey. Can. Med. Assoc. J. 2011, 183, E100–E106. [Google Scholar] [CrossRef] [PubMed]
- Colapinto, C.K.; O’Connor, D.L.; Dubois, L.; Tremblay, M.S. Prevalence and correlates of high red blood cell folate concentrations in the Canadian population using 3 proposed cut-offs. Appl. Physiol. Nutr. Metab. 2015, 40, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Maruvada, P.; Stover, P.J.; Mason, J.B.; Bailey, R.L.; Davis, C.D.; Field, M.S.; Finnell, R.H.; Garza, C.; Green, R.; Gueant, J.-L.; et al. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: A summary, and perspectives, from an NIH workshop. Am. J. Clin. Nutr. 2020, 112, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Field, M.S.; Stover, P.J. Safety of folic acid. Ann. N. Y. Acad. Sci. 2018, 1414, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Rosenberg, I.H. Excessive folic acid intake and relation to adverse health outcome. Biochimie 2016, 126, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.D.; O’Connor, D.L. Maternal folic acid and multivitamin supplementation: International clinical evidence with considerations for the prevention of folate-sensitive birth defects. Prev. Med. Rep. 2021, 24, 101617. [Google Scholar] [CrossRef]
- Obeid, R.; Kirsch, S.H.; Dilmann, S.; Klein, C.; Eckert, R.; Geisel, J.; Herrmann, W. Folic acid causes higher prevalence of detectable unmetabolized folic acid in serum than B-complex: A randomized trial. Eur. J. Nutr. 2016, 55, 1021–1028. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Fazili, Z.; Yetley, E.A.; Lacher, D.A.; Bailey, R.L.; Johnson, C.L. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J. Nutr. 2015, 145, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Johnson, C.L.; Jain, R.B.; Yetley, E.A.; Picciano, M.F.; Rader, J.I.; Fisher, K.D.; Mulinare, J.; Osterloh, J.D. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am. J. Clin. Nutr. 2007, 86, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos, C.T.; Johnson, C.L. Estimation of Trends in Serum and RBC Folate in the U.S. Population from Pre- to Postfortification Using Assay-Adjusted Data from the NHANES 1988–2010. J. Nutr. 2012, 142, 886–893. [Google Scholar] [CrossRef]
- Colapinto, C.K.; O’Connor, D.L.; Sampson, M.; Williams, B.; Tremblay, M.S. Systematic review of adverse health outcomes associated with high serum or red blood cell folate concentrations. J. Public Health 2016, 38, e84–e97. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Z.; Sternberg, M.R.; Potischman, N.; Wang, C.-Y.; Storandt, R.J.; Yeung, L.; Yamini, S.; Gahche, J.J.; Juan, W.; Qi, Y.P.; et al. Demographic, Physiologic, and Lifestyle Characteristics Observed with Serum Total Folate Differ among Folate Forms: Cross-Sectional Data from Fasting Samples in the NHANES 2011–2016. J. Nutr. 2019, 150, 851–860. [Google Scholar] [CrossRef]
- Plumptre, L.; Tammen, S.A.; Sohn, K.J.; Masih, S.P.; Visentin, C.E.; Aufreiter, S.; Malysheva, O.; Schroder, T.H.; Ly, A.; Berger, H.; et al. Maternal and Cord Blood Folate Concentrations Are Inversely Associated with Fetal DNA Hydroxymethylation, but Not DNA Methylation, in a Cohort of Pregnant Canadian Women. J. Nutr. 2020, 150, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.-J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.L.; Kim, Y.-I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Gómez, M.F.; Field, C.J.; Olstad, D.L.; Loehr, S.; Ramage, S.; McCargar, L.J.; APrON Study Team. Use of micronutrient supplements among pregnant women in Alberta: Results from the Alberta Pregnancy Outcomes and Nutrition (APrON) cohort. Matern. Child Nutr. 2015, 11, 497–510. [Google Scholar] [CrossRef]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Fardous, A.M.; Beydoun, S.; James, A.A.; Ma, H.; Cabelof, D.C.; Unnikrishnan, A.; Heydari, A.R. The Timing and Duration of Folate Restriction Differentially Impacts Colon Carcinogenesis. Nutrients 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Alnabbat, K.I.; Fardous, A.M.; Shahab, A.; James, A.A.; Bahry, M.R.; Heydari, A.R. High Dietary Folic Acid Intake Is Associated with Genomic Instability in Peripheral Lymphocytes of Healthy Adults. Nutrients 2022, 14, 3944. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Laboratory Animals, 4th Revised ed.; The National Academies Press: Washington, DC, USA, 1995; p. 192. [Google Scholar] [CrossRef]
- Fazili, Z.; Pfeiffer, C.M.; Zhang, M. Comparison of Serum Folate Species Analyzed by LC-MS/MS with Total Folate Measured by Microbiologic Assay and Bio-Rad Radioassay. Clin. Chem. 2007, 53, 781–784. [Google Scholar] [CrossRef]
- Lee, W.D.; Pirona, A.C.; Sarvin, B.; Stern, A.; Nevo-Dinur, K.; Besser, E.; Sarvin, N.; Lagziel, S.; Mukha, D.; Raz, S.; et al. Tumor Reliance on Cytosolic versus Mitochondrial One-Carbon Flux Depends on Folate Availability. Cell Metab. 2021, 33, 190–198. [Google Scholar] [CrossRef] [PubMed]
- López, J.M.; Outtrim, E.L.; Fu, R.; Sutcliffe, D.J.; Torres, R.J.; Jinnah, H.A. Physiological levels of folic acid reveal purine alterations in Lesch-Nyhan disease. Proc. Natl. Acad. Sci. USA 2020, 117, 12071–12079. [Google Scholar] [CrossRef]
- Silva, C.; Keating, E.; Pinto, E. The impact of folic acid supplementation on gestational and long term health: Critical temporal windows, benefits and risks. Porto Biomed. J. 2017, 2, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Ledowsky, C.; Mahimbo, A.; Scarf, V.; Steel, A. Women Taking a Folic Acid Supplement in Countries with Mandatory Food Fortification Programs May Be Exceeding the Upper Tolerable Limit of Folic Acid: A Systematic Review. Nutrients 2022, 14, 2715. [Google Scholar] [CrossRef]
- Cheng, T.L.; Mistry, K.B.; Wang, G.; Zuckerman, B.; Wang, X. Folate Nutrition Status in Mothers of the Boston Birth Cohort, Sample of a US Urban Low-Income Population. Am. J. Public Health 2018, 108, 799–807. [Google Scholar] [CrossRef]
- Page, R.; Robichaud, A.; Arbuckle, T.E.; Fraser, W.D.; Macfarlane, A.J. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am. J. Clin. Nutr. 2017, 105, 1101–1109. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Q.; Huang, Y.-Q.; Wang, Y.; Li, S.; Lu, D.-W.; Shi, B.; Chen, H.-Q. Significant Evidence of Association Between Polymorphisms in ZNF533, Environmental Factors, and Nonsyndromic Orofacial Clefts in the Western Han Chinese Population. DNA Cell Biol. 2010, 30, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Lie, R.T.; Solvoll, K.; Taylor, J.; McConnaughey, D.R.; Abyholm, F.; Vindenes, H.; Vollset, S.E.; Drevon, C.A. Folic acid supplements and risk of facial clefts: National population based case-control study. BMJ 2007, 334, 464. [Google Scholar] [CrossRef]
- Bille, C.; Olsen, J.; Vach, W.; Knudsen, V.K.; Olsen, S.F.; Rasmussen, K.; Murray, J.C.; Andersen, A.M.N.; Christensen, K. Oral clefts and life style factors—A case-cohort study based on prospective Danish data. Eur. J. Epidemiol. 2007, 22, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.-L.; Shi, B.; Chen, C.-H.; Shi, J.-Y.; Wu, J.; Xu, X. Maternal malnutrition, environmental exposure during pregnancy and the risk of non-syndromic orofacial clefts. Oral. Dis. 2011, 17, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Wehby, G.; Félix, T.; Goco, N.; Richieri-Costa, A.; Chakraborty, H.; Souza, J.; Pereira, R.; Padovani, C.; Moretti-Ferreira, D.; Murray, J. High Dosage Folic Acid Supplementation, Oral Cleft Recurrence and Fetal Growth. Int. J. Environ. Res. Public Health 2013, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
- Little, J.; Gilmour, M.; Mossey, P.A.; Fitzpatrick, D.; Cardy, A.; Clayton-Smith, J.; Fryer, A.E. Folate and Clefts of the Lip and Palate—A U.K.-Based Case-Control Study: Part I: Dietary and Supplemental Folate. Cleft Palate-Craniofac. J. 2008, 45, 420–427. [Google Scholar] [CrossRef]
- Rozendaal, A.M.; Van Essen, A.J.; Te Meerman, G.J.; Bakker, M.K.; Van Der Biezen, J.J.; Goorhuis-Brouwer, S.M.; Vermeij-Keers, C.; De Walle, H.E.K. Periconceptional folic acid associated with an increased risk of oral clefts relative to non-folate related malformations in the Northern Netherlands: A population based case-control study. Eur. J. Epidemiol. 2013, 28, 875–887. [Google Scholar] [CrossRef]
- Murphy, M.S.Q.; Muldoon, K.A.; Sheyholislami, H.; Behan, N.; Lamers, Y.; Rybak, N.; White, R.R.; Harvey, A.L.J.; Gaudet, L.M.; Smith, G.N.; et al. Impact of high-dose folic acid supplementation in pregnancy on biomarkers of folate status and 1-carbon metabolism: An ancillary study of the Folic Acid Clinical Trial (FACT). Am. J. Clin. Nutr. 2021, 113, 1361–1371. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Salam, R.A.; Haider, B.A.; Bhutta, Z.A. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst. Rev. 2013, 3, CD006896. [Google Scholar] [CrossRef]
- Chatzi, L.; Papadopoulou, E.; Koutra, K.; Roumeliotaki, T.; Georgiou, V.; Stratakis, N.; Lebentakou, V.; Karachaliou, M.; Vassilaki, M.; Kogevinas, M. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: The mother-child cohort ‘Rhea’ study in Crete, Greece. Public Health Nutr. 2012, 15, 1728–1736. [Google Scholar] [CrossRef]
- Csáky-Szunyogh, M.; Vereczkey, A.; Kósa, Z.; Gerencsér, B.; Czeizel, A.E. Risk Factors in the Origin of Congenital Left-Ventricular Outflow-Tract Obstruction Defects of the Heart: A Population-Based Case–Control Study. Pediatr. Cardiol. 2014, 35, 108–120. [Google Scholar] [CrossRef]
- Vereczkey, A.; Kósa, Z.; Csáky-Szunyogh, M.; Czeizel, A.E. Isolated atrioventricular canal defects: Birth outcomes and risk factors: A population-based hungarian case–control study, 1980–1996. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Valero, M.; Navarrete-Muoz, E.M.; Rebagliato, M.; Iñiguez, C.; Murcia, M.; Marco, A.; Ballester, F.; Vioque, J. Periconceptional folic acid supplementation and anthropometric measures at birth in a cohort of pregnant women in Valencia, Spain. Br. J. Nutr. 2011, 105, 1352–1360. [Google Scholar] [CrossRef]
- Michels, A.; Bakkali, N.E.; Bastiaenen, C.H.; De Bie, R.A.; Colla, C.G.; Van der Hulst, R.R. Periconceptional Folic Acid Use and the Prevalence of Positional Plagiocephaly. J. Craniofac. Surg. 2008, 19, 37–39. [Google Scholar] [CrossRef]
- Xie, K.; Xu, P.; Fu, Z.; Gu, X.; Li, H.; Cui, X.; You, L.; Zhu, L.; Ji, C.; Guo, X. Association of maternal folate status in the second trimester of pregnancy with the risk of gestational diabetes mellitus. Food Sci. Nutr. 2019, 7, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Ge, X.; Huang, K.; Mao, L.; Yan, S.; Xu, Y.; Huang, S.; Hao, J.; Zhu, P.; Niu, Y.; et al. Folic Acid Supplement Intake in Early Pregnancy Increases Risk of Gestational Diabetes Mellitus: Evidence from a Prospective Cohort Study. Diabetes Care 2016, 39, e36–e37. [Google Scholar] [CrossRef]
- Cheng, G.; Sha, T.; Gao, X.; He, Q.; Wu, X.; Tian, Q.; Yang, F.; Tang, C.; Wu, X.; Xie, Q.; et al. The Associations between the Duration of Folic Acid Supplementation, Gestational Diabetes Mellitus, and Adverse Birth Outcomes based on a Birth Cohort. Int. J. Environ. Res. Public Health 2019, 16, 4511. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Li, L.; Chen, Y.; Yang, Y.; Yang, Y.; Hu, Y.; Zhao, Y.; Tang, H.; Xu, D.; et al. Duration of periconceptional folic acid supplementation and risk of gestational diabetes mellitus. Asia Pac. J. Clin. Nutr. 2019, 28, 321–329. [Google Scholar] [CrossRef]
- Petersen, J.M.; Parker, S.E.; Benedum, C.M.; Mitchell, A.A.; Tinker, S.C.; Werler, M.M. Periconceptional folic acid and risk for neural tube defects among higher risk pregnancies. Birth Defects Res. 2019, 111, 1501–1512. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chen, H.; Jiang, Y.; Wang, Y.; Wang, D.; Li, M.; Dou, Y.; Sun, X.; Huang, G.; et al. Association of Maternal Folate and Vitamin B12 in Early Pregnancy With Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2021, 44, 217–223. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Yan, X.; Wang, Y.; Shi, C.; Wu, X.; Liu, H.; Zhang, L.; Zhang, X.; Liu, J.; et al. Joint effects of folate and vitamin B12 imbalance with maternal characteristics on gestational diabetes mellitus. J. Diabetes 2019, 11, 744–751. [Google Scholar] [CrossRef]
- James, P.; Sajjadi, S.; Tomar, A.S.; Saffari, A.; Fall, C.H.D.; Prentice, A.M.; Shrestha, S.; Issarapu, P.; Yadav, D.K.; Kaur, L.; et al. Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: A review of existing evidence in humans with specific focus on one-carbon metabolism. Int. J. Epidemiol. 2018, 47, 1910–1937. [Google Scholar] [CrossRef]
- Callinan, P.A.; Feinberg, A.P. The emerging science of epigenomics. Hum. Mol. Genet. 2006, 15, R95–R101. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Whitrow, M.J.; Moore, V.M.; Rumbold, A.R.; Davies, M.J. Effect of Supplemental Folic Acid in Pregnancy on Childhood Asthma: A Prospective Birth Cohort Study. Am. J. Epidemiol. 2009, 170, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Lillycrop, K.A. Folic acid supplementation in pregnancy: Are there devils in the detail? Br. J. Nutr. 2012, 108, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P.; Hoad, G.; Campbell, D.M.; Horgan, G.W.; Piyathilake, C.; McNeill, G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr. 2012, 97, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Forman, M.R.; Calingaert, B.; Demark-Wahnefried, W.; Kurtzberg, J.; Jirtle, R.L.; Murphy, S.K. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 2011, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, C.; Murtha, A.P.; Schildkraut, J.M.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Iversen, E.S.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011, 6, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Veena, S.R.; Karat, S.C.; Yajnik, C.S.; Fall, C.H.D. Association between maternal folate concentrations during pregnancy and insulin resistance in Indian children. Diabetologia 2014, 57, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.H.; Liu, Y.J.; Retnakaran, R.; Macfarlane, A.J.; Hamilton, J.; Smith, G.; Walker, M.C.; Wen, S.W. Maternal folate status and obesity/insulin resistance in the offspring: A systematic review. Int. J. Obes. 2016, 40, 1–9. [Google Scholar] [CrossRef]
- Soubry, A. POHaD: Why we should study future fathers. Environ. Epigenet. 2018, 4, dvy007. [Google Scholar] [CrossRef]
- Ly, L.; Chan, D.; Landry, M.; Angle, C.; Martel, J.; Trasler, J. Impact of mothers’ early life exposure to low or high folate on progeny outcome and DNA methylation patterns. Environ. Epigenet. 2020, 6, dvaa018. [Google Scholar] [CrossRef] [PubMed]
- Ly, L.; Chan, D.; Aarabi, M.; Landry, M.; Behan, N.A.; MacFarlane, A.J.; Trasler, J. Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation. Mol. Hum. Reprod. 2017, 23, 461–477. [Google Scholar] [CrossRef]
- Aarabi, M.; Christensen, K.E.; Chan, D.; Leclerc, D.; Landry, M.; Ly, L.; Rozen, R.; Trasler, J. Testicular MTHFR deficiency may explain sperm DNA hypomethylation associated with high dose folic acid supplementation. Hum. Mol. Genet. 2018, 27, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Kintaka, Y.; Wada, N.; Shioda, S.; Nakamura, S.; Yamazaki, Y.; Mochizuki, K. Excessive folic acid supplementation in pregnant mice impairs insulin secretion and induces the expression of genes associated with fatty liver in their offspring. Heliyon 2020, 6, e03597. [Google Scholar] [CrossRef]
- Sie, K.K.Y.; Li, J.; Ly, A.; Sohn, K.-J.; Croxford, R.; Kim, Y.-I. Effect of maternal and postweaning folic acid supplementation on global and gene-specific DNA methylation in the liver of the rat offspring. Mol. Nutr. Food Res. 2013, 57, 677–685. [Google Scholar] [CrossRef]
- Mikael, L.G.; Deng, L.; Paul, L.; Selhub, J.; Rozen, R. Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 97, 47–52. [Google Scholar] [CrossRef]
- Caldwell, P.T.; Manziello, A.; Howard, J.; Palbykin, B.; Runyan, R.B.; Selmin, O. Gene expression profiling in the fetal cardiac tissue after folate and low-dose trichloroethylene exposure. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 111–127. [Google Scholar] [CrossRef]
- Barua, S.; Chadman, K.K.; Kuizon, S.; Buenaventura, D.; Stapley, N.W.; Ruocco, F.; Begum, U.; Guariglia, S.R.; Brown, W.T.; Junaid, M.A. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS ONE 2014, 9, e101674. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, R.; Pannia, E.; Kubant, R.; Wasek, B.; Bottiglieri, T.; Malysheva, O.V.; Caudill, M.A.; Anderson, G.H. Choline and Folic Acid in Diets Consumed during Pregnancy Interact to Program Food Intake and Metabolic Regulation of Male Wistar Rat Offspring. J. Nutr. 2021, 151, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Sánchez-Hernández, D.; Reza-López, S.A.; Huot, P.S.; Kim, Y.I.; Anderson, G.H. High folate gestational and post-weaning diets alter hypothalamic feeding pathways by DNA methylation in Wistar rat offspring. Epigenetics 2013, 8, 710–719. [Google Scholar] [CrossRef]
- Tojal, A.; Neves, C.; Veiga, H.; Ferreira, S.; Rodrigues, I.; Martel, F.; Calhau, C.; Negrão, R.; Keating, E. Perigestational high folic acid: Impact on offspring’s peripheral metabolic response. Food Funct. 2019, 10, 7216–7226. [Google Scholar] [CrossRef]
- Keating, E.; Correia-Branco, A.; Araújo, J.R.; Meireles, M.; Fernandes, R.; Guardão, L.; Guimarães, J.T.; Martel, F.; Calhau, C. Excess perigestational folic acid exposure induces metabolic dysfunction in post-natal life. J. Endocrinol. 2015, 224, 245–259. [Google Scholar] [CrossRef]
- Huang, Y.; He, Y.; Sun, X.; He, Y.; Li, Y.; Sun, C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int. J. Mol. Sci. 2014, 15, 6298–6313. [Google Scholar] [CrossRef]
- Morakinyo, A.O.; Samuel, T.A.; Awobajo, F.O.; Oludare, G.O.; Mofolorunso, A. High-Dose Perinatal Folic-Acid Supplementation Alters Insulin Sensitivity in Sprague-Dawley Rats and Diminishes the Expression of Adiponectin. J. Diet. Suppl. 2019, 16, 14–26. [Google Scholar] [CrossRef]
- Irwin, R.E.; Pentieva, K.; Cassidy, T.; Lees-Murdock, D.J.; McLaughlin, M.; Prasad, G.; McNulty, H.; Walsh, C.P. The interplay between DNA methylation, folate and neurocognitive development. Epigenomics 2016, 8, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Liu, S.M.; Zhang, Y.Z. Maternal Folic Acid Supplementation Mediates Offspring Health via DNA Methylation. Reprod. Sci. 2020, 27, 963–976. [Google Scholar] [CrossRef]
- McNulty, H.; Rollins, M.; Cassidy, T.; Caffrey, A.; Marshall, B.; Dornan, J.; McLaughlin, M.; McNulty, B.A.; Ward, M.; Strain, J.J.; et al. Effect of continued folic acid supplementation beyond the first trimester of pregnancy on cognitive performance in the child: A follow-up study from a randomized controlled trial (FASSTT Offspring Trial). BMC Med. 2019, 17, 196. [Google Scholar] [CrossRef]
- Caffrey, A.; McNulty, H.; Rollins, M.; Prasad, G.; Gaur, P.; Talcott, J.B.; Witton, C.; Cassidy, T.; Marshall, B.; Dornan, J.; et al. Effects of maternal folic acid supplementation during the second and third trimesters of pregnancy on neurocognitive development in the child: An 11-year follow-up from a randomised controlled trial. BMC Med. 2021, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Valera-Gran, D.; Navarrete-Muñoz, E.M.; Garcia de la Hera, M.; Fernández-Somoano, A.; Tardón, A.; Ibarluzea, J.; Balluerka, N.; Murcia, M.; González-Safont, L.; Romaguera, D.; et al. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4–5 y of age: The prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am. J. Clin. Nutr. 2017, 106, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Compañ Gabucio, L.M.; García de la Hera, M.; Torres Collado, L.; Fernández-Somoano, A.; Tardón, A.; Guxens, M.; Vrijheid, M.; Rebagliato, M.; Murcia, M.; Ibarluzea, J.; et al. The Use of Lower or Higher Than Recommended Doses of Folic Acid Supplements during Pregnancy Is Associated with Child Attentional Dysfunction at 4–5 Years of Age in the INMA Project. Nutrients 2021, 13, 327. [Google Scholar] [CrossRef]
- Egorova, O.; Myte, R.; Schneede, J.; Hägglöf, B.; Bölte, S.; Domellöf, E.; Ivars A’roch, B.; Elgh, F.; Ueland, P.M.; Silfverdal, S.A. Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: A study of 62 serum biomarkers. Mol. Autism 2020, 11, 7. [Google Scholar] [CrossRef]
- Valera-Gran, D.; García de la Hera, M.; Navarrete-Muñoz, E.M.; Fernandez-Somoano, A.; Tardón, A.; Julvez, J.; Forns, J.; Lertxundi, N.; Ibarluzea, J.M.; Murcia, M.; et al. Folic Acid Supplements During Pregnancy and Child Psychomotor Development After the First Year of Life. JAMA Pediatr. 2014, 168, e142611. [Google Scholar] [CrossRef]
- Wiens, D.; Desoto, M. Is High Folic Acid Intake a Risk Factor for Autism?—A Review. Brain Sci. 2017, 7, 149. [Google Scholar] [CrossRef]
- Caffrey, A.; Irwin, R.E.; McNulty, H.; Strain, J.J.; Lees-Murdock, D.J.; McNulty, B.A.; Ward, M.; Walsh, C.P.; Pentieva, K. Gene-specific DNA methylation in newborns in response to folic acid supplementation during the second and third trimesters of pregnancy: Epigenetic analysis from a randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 566–575. [Google Scholar] [CrossRef]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef]
- Liu, X.; Zou, M.; Sun, C.; Wu, L.; Chen, W.-X. Prenatal Folic Acid Supplements and Offspring’s Autism Spectrum Disorder: A Meta-analysis and Meta-regression. J. Autism Dev. Disord. 2022, 52, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Selhub, J.; Paul, L.; Ji, Y.; Wang, G.; Hong, X.; Zuckerman, B.; Fallin, M.D.; Wang, X. A prospective birth cohort study on cord blood folate subtypes and risk of autism spectrum disorder. Am. J. Clin. Nutr. 2020, 112, 1304–1317. [Google Scholar] [CrossRef]
- Yang, X.; Sun, W.; Wu, Q.; Lin, H.; Lu, Z.; Shen, X.; Chen, Y.; Zhou, Y.; Huang, L.; Wu, F.; et al. Excess Folic Acid Supplementation before and during Pregnancy and Lactation Alters Behaviors and Brain Gene Expression in Female Mouse Offspring. Nutrients 2021, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Li, L.; Jiang, Y.; Tan, J.; Ji, J.; Zhang, Y.; Jin, N.; Liu, F. Excess Folic Acid Supplementation Before and During Pregnancy and Lactation Activates Fos Gene Expression and Alters Behaviors in Male Mouse Offspring. Front. Neurosci. 2019, 13, 313. [Google Scholar] [CrossRef]
- Huot, P.S.; Ly, A.; Szeto, I.M.; Reza-López, S.A.; Cho, D.; Kim, Y.I.; Anderson, G.H. Maternal and postweaning folic acid supplementation interact to influence body weight, insulin resistance, and food intake regulatory gene expression in rat offspring in a sex-specific manner. Appl. Physiol. Nutr. Metab. 2016, 41, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Harlan De Crescenzo, A.; Panoutsopoulos, A.A.; Tat, L.; Schaaf, Z.; Racherla, S.; Henderson, L.; Leung, K.-Y.; Greene, N.D.E.; Green, R.; Zarbalis, K.S. Deficient or Excess Folic Acid Supply during Pregnancy Alter Cortical Neurodevelopment in Mouse Offspring. Cereb. Cortex 2020, 31, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomás, M.; Luan, Y.; Leclerc, D.; Malysheva, O.V.; Lauzon, N.; Bahous, R.H.; Christensen, K.E.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Behavioral Alterations in Offspring with Sex-Specific Changes in Methyl Metabolism. Nutrients 2020, 12, 1716. [Google Scholar] [CrossRef]
- Henzel, K.S.; Ryan, D.P.; Schröder, S.; Weiergräber, M.; Ehninger, D. High-dose maternal folic acid supplementation before conception impairs reversal learning in offspring mice. Sci. Rep. 2017, 7, 3098. [Google Scholar] [CrossRef] [PubMed]
- Girotto, F.; Scott, L.; Avchalumov, Y.; Harris, J.; Iannattone, S.; Drummond-Main, C.; Tobias, R.; Bello-Espinosa, L.; Rho, J.M.; Davidsen, J.; et al. High dose folic acid supplementation of rats alters synaptic transmission and seizure susceptibility in offspring. Sci. Rep. 2013, 3, 1465. [Google Scholar] [CrossRef]
- Pickell, L.; Brown, K.; Li, D.; Wang, X.L.; Deng, L.; Wu, Q.; Selhub, J.; Luo, L.; Jerome-Majewska, L.; Rozen, R. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, D.; Wu, R.; Shi, R.; Shen, X.; Jin, N.; Gu, J.; Gu, J.H.; Liu, F.; Chu, D. Excess folic acid supplementation before and during pregnancy and lactation activates β-catenin in the brain of male mouse offspring. Brain Res. Bull. 2022, 178, 133–143. [Google Scholar] [CrossRef]
- Cianciulli, A.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A. Folic Acid Is Able to Polarize the Inflammatory Response in LPS Activated Microglia by Regulating Multiple Signaling Pathways. Mediat. Inflamm. 2016, 2016, 5240127. [Google Scholar] [CrossRef]
- Lisboa, J.V.C.; Ribeiro, M.R.; Luna, R.C.P.; Lima, R.P.A.; Nascimento, R.; Monteiro, M.; Lima, K.Q.F.; Fechine, C.; Oliveira, N.F.P.; Persuhn, D.C.; et al. Food Intervention with Folate Reduces TNF-α and Interleukin Levels in Overweight and Obese Women with the MTHFR C677T Polymorphism: A Randomized Trial. Nutrients 2020, 12, 361. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Scarlett, C.J.; Veysey, M.; Beckett, E.L. Folate and Inflammation–links between folate and features of inflammatory conditions. J. Nutr. Intermed. Metab. 2019, 18, 100104. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Guo, H.; Jabir, M.S.; Liu, X.; Cui, W.; Li, D. Associations between Folate and Vitamin B12 Levels and Inflammatory Bowel Disease: A Meta-Analysis. Nutrients 2017, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Selvi, E.; Lorenzini, S.; Bisogno, S.; Galeazzi, M.; Laghi Pasini, F. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun. Rev. 2007, 6, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Cordero, A.M.; Qi, Y.P.; Mulinare, J.; Dowling, N.F.; Berry, R.J. Prenatal folic acid and risk of asthma in children: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Zetstra-van der Woude, P.A.; De Walle, H.E.; Hoek, A.; Bos, H.J.; Boezen, H.M.; Koppelman, G.H.; de Jong-van den Berg, L.T.; Scholtens, S. Maternal high-dose folic acid during pregnancy and asthma medication in the offspring. Pharmacoepidemiol. Drug Saf. 2014, 23, 1059–1065. [Google Scholar] [CrossRef]
- Veeranki, S.P.; Gebretsadik, T.; Mitchel, E.F.; Tylavsky, F.A.; Hartert, T.V.; Cooper, W.O.; Dupont, W.D.; Dorris, S.L.; Hartman, T.J.; Carroll, K.N. Maternal Folic Acid Supplementation During Pregnancy and Early Childhood Asthma. Epidemiology 2015, 26, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, L.; Bi, M.; Jia, X.; Wang, Y.; He, C.; Yao, Y.; Wang, J.; Wang, Z. High dose of maternal folic acid supplementation is associated to infant asthma. Food Chem. Toxicol. 2015, 75, 88–93. [Google Scholar] [CrossRef]
- Chu, S.; Zhang, J. Periconceptional folic acid supplementation is a risk factor for childhood asthma: A case-control study. BMC Pregnancy Childbirth 2022, 22, 220. [Google Scholar] [CrossRef]
- Magdelijns, F.J.; Mommers, M.; Penders, J.; Smits, L.; Thijs, C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics 2011, 128, e135–e144. [Google Scholar] [CrossRef]
- Martinussen, M.P.; Risnes, K.R.; Jacobsen, G.W.; Bracken, M.B. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am. J. Obstet. Gynecol. 2012, 206, 72.e1–72.e7. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Sharma, S.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Weiss, S.T.; Oken, E.; Gillman, M.W.; Gold, D.R.; DeMeo, D.L.; Litonjua, A.A. Folic Acid in Pregnancy and Childhood Asthma: A US Cohort. Clin. Pediatr. 2018, 57, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Vereen, S.; Gebretsadik, T.; Johnson, N.; Hartman, T.J.; Veeranki, S.P.; Piyathilake, C.; Mitchel, E.F.; Kocak, M.; Cooper, W.O.; Dupont, W.D.; et al. Association between Maternal 2nd Trimester Plasma Folate Levels and Infant Bronchiolitis. Matern. Child. Health J. 2019, 23, 164–172. [Google Scholar] [CrossRef]
- Veeranki, S.P.; Gebretsadik, T.; Dorris, S.L.; Mitchel, E.F.; Hartert, T.V.; Cooper, W.O.; Tylavsky, F.A.; Dupont, W.; Hartman, T.J.; Carroll, K.N. Association of Folic Acid Supplementation during Pregnancy and Infant Bronchiolitis. Am. J. Epidemiol. 2014, 179, 938–946. [Google Scholar] [CrossRef][Green Version]
- Håberg, S.E.; London, S.J.; Stigum, H.; Nafstad, P.; Nystad, W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch. Dis. Child. 2009, 94, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Kocak, M.; Hartman, T.J.; Vereen, S.; Adgent, M.; Piyathilake, C.; Tylavsky, F.A.; Carroll, K.N. Association of prenatal folate status with early childhood wheeze and atopic dermatitis. Pediatr. Allergy Immunol. 2018, 29, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xing, Y.; Yu, X.; Dou, Y.; Ma, D. Effect of Folic Acid Intake on Infant and Child Allergic Diseases: Systematic Review and Meta-Analysis. Front. Pediatr. 2021, 8, 615406. [Google Scholar] [CrossRef]
- Kiefte-de Jong, J.C.; Timmermans, S.; Jaddoe, V.W.V.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; de Jongste, J.C.; Moll, H.A. High Circulating Folate and Vitamin B-12 Concentrations in Women During Pregnancy Are Associated with Increased Prevalence of Atopic Dermatitis in Their Offspring. J. Nutr. 2012, 142, 731–738. [Google Scholar] [CrossRef]
- Dunstan, J.A.; West, C.; McCarthy, S.; Metcalfe, J.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; D’Vaz, N.; Prescott, S.L. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy 2012, 67, 50–57. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Keely, S.; MacManus, C.F.; Glover, L.E.; Scully, M.; Collins, C.B.; Bowers, B.E.; Campbell, E.L.; Colgan, S.P. An Endogenously Anti-Inflammatory Role for Methylation in Mucosal Inflammation Identified through Metabolite Profiling. J. Immunol. 2011, 186, 6505–6514. [Google Scholar] [CrossRef]
- Schaible, T.D.; Harris, R.A.; Dowd, S.E.; Smith, C.W.; Kellermayer, R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Human. Mol. Genet. 2011, 20, 1687–1696. [Google Scholar] [CrossRef]
- Mir, S.A.; Nagy-Szakal, D.; Dowd, S.E.; Szigeti, R.G.; Smith, C.W.; Kellermayer, R. Prenatal Methyl-Donor Supplementation Augments Colitis in Young Adult Mice. PLoS ONE 2013, 8, e73162. [Google Scholar] [CrossRef] [PubMed]
- Pannia, E.; Cho, C.E.; Kubant, R.; Sánchez-Hernández, D.; Huot, P.S.P.; Chatterjee, D.; Fleming, A.; Anderson, G.H. A high multivitamin diet fed to Wistar rat dams during pregnancy increases maternal weight gain later in life and alters homeostatic, hedonic and peripheral regulatory systems of energy balance. Behav. Brain Res. 2015, 278, 1–11. [Google Scholar] [CrossRef]
- Alnabbat, K.I.; Fardous, A.M.; Cabelof, D.C.; Heydari, A.R. Excessive Folic Acid Mimics Folate Deficiency in Human Lymphocytes. Curr. Issues Mol. Biol. 2022, 44, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Kadaveru, K.; Protiva, P.; Greenspan, E.J.; Kim, Y.I.; Rosenberg, D.W. Dietary methyl donor depletion protects against intestinal tumorigenesis in Apc(Min/+) mice. Cancer Prev. Res. 2012, 5, 911–920. [Google Scholar] [CrossRef]
- Protiva, P.; Mason, J.B.; Liu, Z.; Hopkins, M.E.; Nelson, C.; Marshall, J.R.; Lambrecht, R.W.; Pendyala, S.; Kopelovich, L.; Kim, M.; et al. Altered folate availability modifies the molecular environment of the human colorectum: Implications for colorectal carcinogenesis. Cancer Prev. Res. 2011, 4, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.B.; Kennelly, J.P.; Ordonez, M.; Nelson, R.; Leonard, K.; Stabler, S.; Gomez-Munoz, A.; Field, C.J.; Jacobs, R.L. Excess Folic Acid Increases Lipid Storage, Weight Gain, and Adipose Tissue Inflammation in High Fat Diet-Fed Rats. Nutrients 2016, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Moosavian, S.P.; Nazarian, B.; Afrisham, R.; Kelishadi, M.R.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Inflammatory Markers: A Grade-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2327. [Google Scholar] [CrossRef]
- Courtemanche, C.; Huang, A.C.; Elson-Schwab, I.; Kerry, N.; Ng, B.Y.; Ames, B.N. Folate deficiency and ionizing radiation cause DNA breaks in primary human lymphocytes: A comparison. FASEB J. 2004, 18, 209–211. [Google Scholar] [CrossRef]
- Palmer, A.M.; Kamynina, E.; Field, M.S.; Stover, P.J. Folate rescues vitamin B(12) depletion-induced inhibition of nuclear thymidylate biosynthesis and genome instability. Proc. Natl. Acad. Sci. USA 2017, 114, E4095–E4102. [Google Scholar] [CrossRef]
- Mason, J.B.; Dickstein, A.; Jacques, P.F.; Haggarty, P.; Selhub, J.; Dallal, G.; Rosenberg, I.H. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: A hypothesis. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, K.; Li, Y.; Song, R.; Wu, Y.; Zhang, X.; Song, M.; Liang, L.; A Smith-Warner, S.; Giovannucci, E.L.; et al. Association of folate intake and colorectal cancer risk in the postfortification era in US women. Am. J. Clin. Nutr. 2021, 114, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.F.; Baron, J.A.; Sandler, R.S.; Haile, R.W.; Ahnen, D.J.; Bresalier, R.S.; McKeown-Eyssen, G.; Summers, R.W.; Rothstein, R.I.; Burke, C.A.; et al. Folic Acid for the Prevention of Colorectal Adenomas. JAMA 2007, 297, 2351–2359. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Grau, M.V.; Haile, R.W.; Sandler, R.S.; Summers, R.W.; Bresalier, R.S.; Burke, C.A.; McKeown-Eyssen, G.E.; Baron, J.A. Folic acid and risk of prostate cancer: Results from a randomized clinical trial. J. Natl. Cancer Inst. 2009, 101, 432–435. [Google Scholar] [CrossRef]
- Liss, M.A.; Ashcraft, K.; Satsangi, A.; Bacich, D. Rise in serum folate after androgen deprivation associated with worse prostate cancer-specific survival. Urol. Oncol. 2020, 38, 682.e21–682.e27. [Google Scholar] [CrossRef]
- Fu, H.; He, J.; Li, C.; Deng, Z.; Chang, H. Folate intake and risk of colorectal cancer: A systematic review and up-to-date meta-analysis of prospective studies. Eur. J. Cancer Prev. 2023, 32, 103–112. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Shi, W.W.; Gao, H.F.; Zhou, L.; Hou, A.J.; Zhou, Y.H. Folate intake and the risk of breast cancer: A dose-response meta-analysis of prospective studies. PLoS ONE 2014, 9, e100044. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, C.; Li, X.; Li, J.; Chu, R.; Wang, H. Higher dietary folate intake reduces the breast cancer risk: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Gu, Y.; Fu, H.; Liu, C.; Zou, Y.; Chang, H. Association Between One-carbon Metabolism-related Vitamins and Risk of Breast Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Clin. Breast Cancer 2020, 20, e469–e480. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, K.; Ye, F.; Lei, L.; Zhou, Y.; Chen, J.; Zhao, G.; Chang, H. Folate intake and the risk of breast cancer: An up-to-date meta-analysis of prospective studies. Eur. J. Clin. Nutr. 2019, 73, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, M.; Bønaa, K.H.; Nygård, O.; Arnesen, E.; Ueland, P.M.; Nordrehaug, J.E.; Rasmussen, K.; Njølstad, I.; Refsum, H.; Nilsen, D.W.; et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA 2009, 302, 2119–2126. [Google Scholar] [CrossRef]
- Song, J.; Sohn, K.J.; Medline, A.; Ash, C.; Gallinger, S.; Kim, Y.I. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/− Msh2−/− Mice. Cancer Res. 2000, 60, 3191–3199. [Google Scholar] [PubMed]
- Le Leu, R.K.; Young, G.P.; McIntosh, G.H. Folate deficiency reduces the development of colorectal cancer in rats. Carcinogenesis 2000, 21, 2261–2265. [Google Scholar] [CrossRef]
- Lindzon, G.M.; Medline, A.; Sohn, K.J.; Depeint, F.; Croxford, R.; Kim, Y.I. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis 2009, 30, 1536–1543. [Google Scholar] [CrossRef]
- Galeone, C.; Edefonti, V.; Parpinel, M.; Leoncini, E.; Matsuo, K.; Talamini, R.; Olshan, A.F.; Zevallos, J.P.; Winn, D.M.; Jayaprakash, V.; et al. Folate intake and the risk of oral cavity and pharyngeal cancer: A pooled analysis within the International Head and Neck Cancer Epidemiology Consortium. Int. J. Cancer 2015, 136, 904–914. [Google Scholar] [CrossRef]
- Tio, M.; Andrici, J.; Cox, M.R.; Eslick, G.D. Folate intake and the risk of upper gastrointestinal cancers: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2014, 29, 250–258. [Google Scholar] [CrossRef]
- Fu, H.; Zeng, J.; Liu, C.; Gu, Y.; Zou, Y.; Chang, H. Folate Intake and Risk of Pancreatic Cancer: A Systematic Review and Updated Meta-Analysis of Epidemiological Studies. Dig. Dis. Sci. 2021, 66, 2368–2379. [Google Scholar] [CrossRef]
- Gu, Y.; Zeng, J.; Zou, Y.; Liu, C.; Fu, H.; Chang, H. Folate Intake and Risk of Urothelial Carcinoma: A Systematic Review and Meta-Analysis of Epidemiological Studies. Nutr. Cancer 2022, 74, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Shui, B. Folate intake and risk of bladder cancer: A meta-analysis of epidemiological studies. Int. J. Food Sci. Nutr. 2014, 65, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Zhou, L.; Zhang, H.W.; Hou, A.J.; Gao, H.F.; Zhou, Y.H. Association between folate intake and the risk of lung cancer: A dose-response meta-analysis of prospective studies. PLoS ONE 2014, 9, e93465. [Google Scholar] [CrossRef]
- Stanisławska-Sachadyn, A.; Borzyszkowska, J.; Krzemiński, M.; Janowicz, A.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Limon, J. Folate/homocysteine metabolism and lung cancer risk among smokers. PLoS ONE 2019, 14, e0214462. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, P.; Hu, P.; Li, M.; Li, X.; Guo, H.; Li, J.; Chu, R.; Zhang, W.; Wang, H. Folate intake and MTHFR polymorphism C677T is not associated with ovarian cancer risk: Evidence from the meta-analysis. Mol. Biol. Rep. 2013, 40, 6547–6560. [Google Scholar] [CrossRef]
- Du, L.; Wang, Y.; Zhang, H.; Zhang, H.; Gao, Y. Folate intake and the risk of endometrial cancer: A meta-analysis. Oncotarget 2016, 7, 85176–85184. [Google Scholar] [CrossRef]
- Wien, T.N.; Pike, E.; Wisloff, T.; Staff, A.; Smeland, S.; Klemp, M. Cancer risk with folic acid supplements: A systematic review and meta-analysis. BMJ Open 2012, 2, e000653. [Google Scholar] [CrossRef] [PubMed]
- Oliai Araghi, S.; Kiefte-de Jong, J.C.; van Dijk, S.C.; Swart, K.M.A.; van Laarhoven, H.W.; van Schoor, N.M.; de Groot, L.; Lemmens, V.; Stricker, B.H.; Uitterlinden, A.G.; et al. Folic Acid and Vitamin B12 Supplementation and the Risk of Cancer: Long-term Follow-up of the B Vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol. Biomark. Prev. 2019, 28, 275–282. [Google Scholar] [CrossRef]
- Rosati, R.; Ma, H.; Cabelof, D.C. Folate and colorectal cancer in rodents: A model of DNA repair deficiency. J. Oncol. 2012, 2012, 105949. [Google Scholar] [CrossRef]
- Hansen, M.F.; Jensen, S.Ø.; Füchtbauer, E.-M.; Martensen, P.M. High folic acid diet enhances tumour growth in PyMT-induced breast cancer. Br. J. Cancer 2017, 116, 752–761. [Google Scholar] [CrossRef]
- Ly, A.; Lee, H.; Chen, J.; Sie, K.K.Y.; Renlund, R.; Medline, A.; Sohn, K.J.; Croxford, R.; Thompson, L.U.; Kim, Y.I. Effect of maternal and postweaning folic acid supplementation on mammary tumor risk in the offspring. Cancer Res. 2011, 71, 988–997. [Google Scholar] [CrossRef]
- Deghan Manshadi, S.; Ishiguro, L.; Sohn, K.-J.; Medline, A.; Renlund, R.; Croxford, R.; Kim, Y.-I. Folic Acid Supplementation Promotes Mammary Tumor Progression in a Rat Model. PLoS ONE 2014, 9, e84635. [Google Scholar] [CrossRef]
- Rycyna, K.J.; Bacich, D.J.; O’Keefe, D.S. Opposing roles of folate in prostate cancer. Urology 2013, 82, 1197–1203. [Google Scholar] [CrossRef]
- Savini, C.; Yang, R.; Savelyeva, L.; Göckel-Krzikalla, E.; Hotz-Wagenblatt, A.; Westermann, F.; Rösl, F. Folate Repletion after Deficiency Induces Irreversible Genomic and Transcriptional Changes in Human Papillomavirus Type 16 (HPV16)-Immortalized Human Keratinocytes. Int. J. Mol. Sci. 2019, 20, 1100. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Tang, Y.S.; Kusumanchi, P.; Stabler, S.P.; Zhang, Y.; Antony, A.C. Folate Deficiency Facilitates Genomic Integration of Human Papillomavirus Type 16 DNA In Vivo in a Novel Mouse Model for Rapid Oncogenic Transformation of Human Keratinocytes. J. Nutr. 2018, 148, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ali, T.; Kaur, J. Folic acid depletion as well as oversupplementation helps in the progression of hepatocarcinogenesis in HepG2 cells. Sci. Rep. 2022, 12, 16617. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, H.; Xu, Y.; Xu, D.; Sun, H.; Han, L. Associations of dietary folate, vitamin B6 and B12 intake with cardiovascular outcomes in 115664 participants: A large UK population-based cohort. Eur. J. Clin. Nutr. 2022, 77, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Xu, H.; Zhang, H.; Zhang, J.; Wan, Z.; Zhao, X.; Yu, Z. Intakes of Folate, Vitamin B6, and Vitamin B12 in Relation to All-Cause and Cause-Specific Mortality: A National Population-Based Cohort. Nutrients 2022, 14, 2253. [Google Scholar] [CrossRef]
- Li, Y.; Huang, T.; Zheng, Y.; Muka, T.; Troup, J.; Hu, F.B. Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e003768. [Google Scholar] [CrossRef] [PubMed]
- Bønaa, K.H.; Njølstad, I.; Ueland, P.M.; Schirmer, H.; Tverdal, A.; Steigen, T.; Wang, H.; Nordrehaug, J.E.; Arnesen, E.; Rasmussen, K. Homocysteine Lowering and Cardiovascular Events after Acute Myocardial Infarction. N. Engl. J. Med. 2006, 354, 1578–1588. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Zhou, C.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Liu, C.; Liang, M.; Wang, X.; et al. Relationship of several serum folate forms with the risk of mortality: A prospective cohort study. Clin. Nutr. 2021, 40, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Dong, B.; Wang, Z. Serum folate concentrations and all-cause, cardiovascular disease and cancer mortality: A cohort study based on 1999–2010 National Health and Nutrition Examination Survey (NHANES). Int. J. Cardiol. 2016, 219, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.V.; Schooling, C.M.; Zhao, J.X. The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Ann. Epidemiol. 2018, 28, 249–257. [Google Scholar] [CrossRef]
- Yang, H.T.; Lee, M.; Hong, K.S.; Ovbiagele, B.; Saver, J.L. Efficacy of folic acid supplementation in cardiovascular disease prevention: An updated meta-analysis of randomized controlled trials. Eur. J. Intern. Med. 2012, 23, 745–754. [Google Scholar] [CrossRef]
- Pannia, E.; Hammoud, R.; Simonian, R.; Arning, E.; Ashcraft, P.; Wasek, B.; Bottiglieri, T.; Pausova, Z.; Kubant, R.; Anderson, G.H. [6S]-5-Methyltetrahydrofolic Acid and Folic Acid Pregnancy Diets Differentially Program Metabolic Phenotype and Hypothalamic Gene Expression of Wistar Rat Dams Post-Birth. Nutrients 2020, 13, 48. [Google Scholar] [CrossRef]
- Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am. J. Clin. Nutr. 2005, 82, 806–812. [CrossRef] [PubMed]
- Chen, Y.; Liu, R.; Zhang, G.; Yu, Q.; Jia, M.; Zheng, C.; Wang, Y.; Xu, C.; Zhang, Y.; Liu, E. Hypercysteinemia promotes atherosclerosis by reducing protein S-nitrosylation. Biomed. Pharmacother. 2015, 70, 253–259. [Google Scholar] [CrossRef]
- Kalita, J.; Kumar, G.; Bansal, V.; Misra, U.K. Relationship of homocysteine with other risk factors and outcome of ischemic stroke. Clin. Neurol. Neurosurg. 2009, 111, 364–367. [Google Scholar] [CrossRef]
- Bonetti, F.; Brombo, G.; Magon, S.; Zuliani, G. Cognitive Status According to Homocysteine and B-Group Vitamins in Elderly Adults. J. Am. Geriatr. Soc. 2015, 63, 1158–1163. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Chandak, G.R.; Joglekar, C.; Katre, P.; Bhat, D.S.; Singh, S.N.; Janipalli, C.S.; Refsum, H.; Krishnaveni, G.; Veena, S.; et al. Maternal homocysteine in pregnancy and offspring birthweight: Epidemiological associations and Mendelian randomization analysis. Int. J. Epidemiol. 2014, 43, 1487–1497. [Google Scholar] [CrossRef]
- den Heijer, M.; Brouwer, I.A.; Bos, G.M.; Blom, H.J.; van der Put, N.M.; Spaans, A.P.; Rosendaal, F.R.; Thomas, C.M.; Haak, H.L.; Wijermans, P.W.; et al. Vitamin supplementation reduces blood homocysteine levels: A controlled trial in patients with venous thrombosis and healthy volunteers. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 356–361. [Google Scholar] [CrossRef]
- van der Griend, R.; Biesma, D.H.; Haas, F.J.; Faber, J.A.; Duran, M.; Meuwissen, O.J.; Banga, J.D. The effect of different treatment regimens in reducing fasting and postmethionine-load homocysteine concentrations. J. Intern. Med. 2000, 248, 223–229. [Google Scholar] [CrossRef]
- Tighe, P.; Ward, M.; McNulty, H.; Finnegan, O.; Dunne, A.; Strain, J.; Molloy, A.M.; Duffy, M.; Pentieva, K.; Scott, J.M. A dose-finding trial of the effect of long-term folic acid intervention: Implications for food fortification policy. Am. J. Clin. Nutr. 2011, 93, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Tian, D.; Zhang, C.; Wang, W.; Wang, L.; Ge, M.; Hou, Q.; Zhang, W. Efficacy of Folic Acid Therapy in Patients with Hyperhomocysteinemia. J. Am. Coll. Nutr. 2017, 36, 528–532. [Google Scholar] [CrossRef]
- Reynolds, E.H. What is the safe upper intake level of folic acid for the nervous system? Implications for folic acid fortification policies. Eur. J. Clin. Nutr. 2016, 70, 537–540. [Google Scholar] [CrossRef]
- Bailey, R.L.; Jun, S.; Murphy, L.; Green, R.; Gahche, J.J.; Dwyer, J.T.; Potischman, N.; McCabe, G.P.; Miller, J.W. High folic acid or folate combined with low vitamin B-12 status: Potential but inconsistent association with cognitive function in a nationally representative cross-sectional sample of US older adults participating in the NHANES. Am. J. Clin. Nutr. 2020, 112, 1547–1557. [Google Scholar] [CrossRef]
- Paul, L.; Selhub, J. Interaction between excess folate and low vitamin B12 status. Mol. Asp. Med. 2017, 53, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Will, J.J.; Mueller, J.F.; Brodine, C.; Kiely, C.E.; Friedman, B.; Hawkins, V.R.; Dutra, J.; Vilter, R.W. Folic acid and vitamin B12 in pernicious anemia; studies on patients treated with these substances over a ten year period. J. Lab. Clin. Med. 1959, 53, 22–38. [Google Scholar]
- Lear, A.A.; Castle, W.B. Supplemental folic acid therapy in pernicious anemia: The effect on erythropoiesis and serum vitamin B12 concentrations in selected cases. J. Lab. Clin. Med. 1956, 47, 88–97. [Google Scholar]
- Bok, J.; Faber, J.G.; De Vries, J.A.; Kroese, W.F.; Nieweg, H.O. The effect of pteroylglutamic acid administration on the serum vitamin B12 concentration in pernicious anemia in relapse. J. Lab. Clin. Med. 1958, 51, 667–671. [Google Scholar]
- Moore, E.M.; Ames, D.; Mander, A.G.; Carne, R.P.; Brodaty, H.; Woodward, M.C.; Boundy, K.; Ellis, K.A.; Bush, A.I.; Faux, N.G.; et al. Among vitamin B12 deficient older people, high folate levels are associated with worse cognitive function: Combined data from three cohorts. J. Alzheimers Dis. 2014, 39, 661–668. [Google Scholar] [CrossRef]
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Hebert, L.E.; Scherr, P.A.; Schneider, J.A. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch. Neurol. 2005, 62, 641–645. [Google Scholar] [CrossRef]
- van Gool, J.D.; Hirche, H.; Lax, H.; Schaepdrijver, L. Fallacies of clinical studies on folic acid hazards in subjects with a low vitamin B(12) status. Crit. Rev. Toxicol. 2020, 50, 177–187. [Google Scholar] [CrossRef]
- Selhub, J.; Miller, J.W.; Troen, A.M.; Mason, J.B.; Jacques, P.F. Perspective: The High-Folate-Low-Vitamin B-12 Interaction Is a Novel Cause of Vitamin B-12 Depletion with a Specific Etiology—A Hypothesis. Adv. Nutr. 2022, 13, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.; Sobczyńska-Malefora, A. The Relationship Between Folate, Vitamin B12 and Gestational Diabetes Mellitus With Proposed Mechanisms and Foetal Implications. J. Fam. Reprod. Health 2021, 15, 141–149. [Google Scholar] [CrossRef]
- Roy, S.; Kale, A.; Dangat, K.; Sable, P.; Kulkarni, A.; Joshi, S. Maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids: Implications for neurodevelopmental risk in the rat offspring. Brain Dev. 2012, 34, 64–71. [Google Scholar] [CrossRef]
- Henderson, A.M.; Tai, D.C.; Aleliunas, R.E.; Aljaadi, A.M.; Glier, M.B.; Xu, E.E.; Miller, J.W.; Verchere, C.B.; Green, T.J.; Devlin, A.M. Maternal folic acid supplementation with vitamin B(12) deficiency during pregnancy and lactation affects the metabolic health of adult female offspring but is dependent on offspring diet. FASEB J. 2018, 32, 5039–5050. [Google Scholar] [CrossRef]
- Kulkarni, A.; Dangat, K.; Kale, A.; Sable, P.; Chavan-Gautam, P.; Joshi, S. Effects of Altered Maternal Folic Acid, Vitamin B12 and Docosahexaenoic Acid on Placental Global DNA Methylation Patterns in Wistar Rats. PLoS ONE 2011, 6, e17706. [Google Scholar] [CrossRef]
- Sable, P.; Dangat, K.; Kale, A.; Joshi, S. Altered brain neurotrophins at birth: Consequence of imbalance in maternal folic acid and vitamin B12 metabolism. Neuroscience 2011, 190, 127–134. [Google Scholar] [CrossRef]
- Sweeney, M.; McPartlin, J.; Scott, J. Folic acid fortification and public health: Report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.W.; Ayling, J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15424–15429. [Google Scholar] [CrossRef]
- Patanwala, I.; King, M.J.; Barrett, D.A.; Rose, J.; Jackson, R.; Hudson, M.; Philo, M.; Dainty, J.R.; Wright, A.J.; Finglas, P.M.; et al. Folic acid handling by the human gut: Implications for food fortification and supplementation. Am. J. Clin. Nutr. 2014, 100, 593–599. [Google Scholar] [CrossRef]
- Mohanty, V.; Shah, A.; Allender, E.; Siddiqui, M.R.; Monick, S.; Ichi, S.; Mania-Farnell, B.; McLone, D.G.; Tomita, T.; Mayanil, C.S. Folate Receptor Alpha Upregulates Oct4, Sox2 and Klf4 and Downregulates miR-138 and miR-let-7 in Cranial Neural Crest Cells. Stem Cells 2016, 34, 2721–2732. [Google Scholar] [CrossRef]
- Boshnjaku, V.; Shim, K.W.; Tsurubuchi, T.; Ichi, S.; Szany, E.V.; Xi, G.; Mania-Farnell, B.; McLone, D.G.; Tomita, T.; Mayanil, C.S. Nuclear localization of folate receptor alpha: A new role as a transcription factor. Sci. Rep. 2012, 2, 980. [Google Scholar] [CrossRef]
- Mohanty, V.; Siddiqui, M.R.; Tomita, T.; Mayanil, C.S. Folate receptor alpha is more than just a folate transporter. Neurogenesis 2017, 4, e1263717. [Google Scholar] [CrossRef]
- Frigerio, B.; Bizzoni, C.; Jansen, G.; Leamon, C.P.; Peters, G.J.; Low, P.S.; Matherly, L.H.; Figini, M. Folate receptors and transporters: Biological role and diagnostic/therapeutic targets in cancer and other diseases. J. Exp. Clin. Cancer Res. 2019, 38, 125. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.F.; Greibe, E.; Skovbjerg, S.; Rohde, S.; Kristensen, A.C.; Jensen, T.R.; Stentoft, C.; Kjær, K.H.; Kronborg, C.S.; Martensen, P.M. Folic acid mediates activation of the pro-oncogene STAT3 via the Folate Receptor alpha. Cell Signal. 2015, 27, 1356–1368. [Google Scholar] [CrossRef]
- Zhang, X.M.; Huang, G.W.; Tian, Z.H.; Ren, D.L.; Wilson, J.X. Folate stimulates ERK1/2 phosphorylation and cell proliferation in fetal neural stem cells. Nutr. Neurosci. 2009, 12, 226–232. [Google Scholar] [CrossRef]
- Kuo, C.T.; Chang, C.; Lee, W.S. Folic acid inhibits COLO-205 colon cancer cell proliferation through activating the FRα/c-SRC/ERK1/2/NFκB/TP53 pathway: In vitro and in vivo studies. Sci. Rep. 2015, 5, 11187. [Google Scholar] [CrossRef]
- Kalmbach, R.D.; Choumenkovitch, S.F.; Troen, A.M.; D’Agostino, R.; Jacques, P.F.; Selhub, J. Circulating folic acid in plasma: Relation to folic acid fortification. Am. J. Clin. Nutr. 2008, 88, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [CrossRef]
- Wu, S.; Guo, W.; Li, X.; Liu, Y.; Li, Y.; Lei, X.; Yao, J.; Yang, X. Paternal chronic folate supplementation induced the transgenerational inheritance of acquired developmental and metabolic changes in chickens. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191653. [Google Scholar] [CrossRef]
- Tam, C.; O’Connor, D.; Koren, G. Circulating unmetabolized folic Acid: Relationship to folate status and effect of supplementation. Obstet. Gynecol. Int. 2012, 2012, 485179. [Google Scholar] [CrossRef]
- Kao, T.T.; Wang, K.C.; Chang, W.N.; Lin, C.Y.; Chen, B.H.; Wu, H.L.; Shi, G.Y.; Tsai, J.N.; Fu, T.F. Characterization and comparative studies of zebrafish and human recombinant dihydrofolate reductases—Inhibition by folic acid and polyphenols. Drug Metab. Dispos. 2008, 36, 508–516. [Google Scholar] [CrossRef]
- Palchetti, C.Z.; Paniz, C.; De Carli, E.; Marchioni, D.M.; Colli, C.; Steluti, J.; Pfeiffer, C.M.; Fazili, Z.; Guerra-Shinohara, E.M. Association between Serum Unmetabolized Folic Acid Concentrations and Folic Acid from Fortified Foods. J. Am. Coll. Nutr. 2017, 36, 572–578. [Google Scholar] [CrossRef]
- Williams, B.A.; Mayer, C.; McCartney, H.; Devlin, A.M.; Lamers, Y.; Vercauteren, S.M.; Wu, J.K.; Karakochuk, C.D. Detectable Unmetabolized Folic Acid and Elevated Folate Concentrations in Folic Acid-Supplemented Canadian Children with Sickle Cell Disease. Front. Nutr. 2021, 8, 642306. [Google Scholar] [CrossRef]
- Christensen, K.E.; Mikael, L.G.; Leung, K.Y.; Levesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef]
- Bahous, R.H.; Jadavji, N.M.; Deng, L.; Cosin-Tomas, M.; Lu, J.; Malysheva, O.; Leung, K.Y.; Ho, M.K.; Pallas, M.; Kaliman, P.; et al. High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring. Hum. Mol. Genet. 2017, 26, 888–900. [Google Scholar] [CrossRef]
- Cornet, D.; Clement, A.; Clement, P.; Menezo, Y. High doses of folic acid induce a pseudo-methylenetetrahydrofolate syndrome. SAGE Open Med. Case Rep. 2019, 7, 2050313X19850435. [Google Scholar] [CrossRef]
- Jennings, B.A.; Willis, G. How folate metabolism affects colorectal cancer development and treatment; a story of heterogeneity and pleiotropy. Cancer Lett. 2015, 356, 224–230. [Google Scholar] [CrossRef]
- Pufulete, M.; Emery, P.W.; Sanders, T.A.B. Folate, DNA methylation and colo-rectal cancer. Proc. Nutr. Soc. 2003, 62, 437–445. [Google Scholar] [CrossRef]
- Hanley, M.P.; Aladelokun, O.; Kadaveru, K.; Rosenberg, D.W. Methyl donor deficiency blocks colorectal cancer development by affecting key metabolic pathways. Cancer Prev. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Ryan, B.M.; Weir, D.G. Relevance of folate metabolism in the pathogenesis of colorectal cancer. J. Lab. Clin. Med. 2001, 138, 164–176. [Google Scholar] [CrossRef]
- Charles, M.A.; Johnson, I.T.; Belshaw, N.J. Supra-physiological folic acid concentrations induce aberrant DNA methylation in normal human cells in vitro. Epigenetics 2012, 7, 689–694. [Google Scholar] [CrossRef]
- Ortbauer, M.; Ripper, D.; Fuhrmann, T.; Lassi, M.; Auernigg-Haselmaier, S.; Stiegler, C.; Konig, J. Folate deficiency and over-supplementation causes impaired folate metabolism: Regulation and adaptation mechanisms in Caenorhabditis elegans. Mol. Nutr. Food Res. 2016, 60, 949–956. [Google Scholar] [CrossRef]
- Han, X.; Wang, B.; Jin, D.; Liu, K.; Wang, H.; Chen, L.; Zu, Y. Precise Dose of Folic Acid Supplementation Is Essential for Embryonic Heart Development in Zebrafish. Biology 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, R.; Battaglia-Hsu, S.F.; Hergalant, S.; Quéré, M.; Alberto, J.M.; Chéry, C.; Rouyer, P.; Gauchotte, G.; Guéant, J.L.; Namour, F. Folate can promote the methionine-dependent reprogramming of glioblastoma cells towards pluripotency. Cell Death Dis. 2019, 10, 596. [Google Scholar] [CrossRef]
- Murray, L.K.; Smith, M.J.; Jadavji, N.M. Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr. Rev. 2018, 76, 708–721. [Google Scholar] [CrossRef]
- Ondičová, M.; Irwin, R.E.; Thursby, S.J.; Hilman, L.; Caffrey, A.; Cassidy, T.; McLaughlin, M.; Lees-Murdock, D.J.; Ward, M.; Murphy, M.; et al. Folic acid intervention during pregnancy alters DNA methylation, affecting neural target genes through two distinct mechanisms. Clin. Epigenet. 2022, 14, 63. [Google Scholar] [CrossRef]
- Richmond, R.C.; Sharp, G.C.; Herbert, G.; Atkinson, C.; Taylor, C.; Bhattacharya, S.; Campbell, D.; Hall, M.; Kazmi, N.; Gaunt, T.; et al. The long-term impact of folic acid in pregnancy on offspring DNA methylation: Follow-up of the Aberdeen Folic Acid Supplementation Trial (AFAST). Int. J. Epidemiol. 2018, 47, 928–937. [Google Scholar] [CrossRef]
- Li, Z.; Gueant-Rodriguez, R.M.; Quilliot, D.; Sirveaux, M.A.; Meyre, D.; Gueant, J.L.; Brunaud, L. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin. Nutr. 2018, 37, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef]
- Smith, D.E.C.; Hornstra, J.M.; Kok, R.M.; Blom, H.J.; Smulders, Y.M. Folic acid supplementation does not reduce intracellular homocysteine, and may disturb intracellular one-carbon metabolism. Clin. Chem. Lab. Med. (CCLM) 2013, 51, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Ted Brown, W.; Junaid, M.A. High Gestational Folic Acid Supplementation Alters Expression of Imprinted and Candidate Autism Susceptibility Genes in a sex-Specific Manner in Mouse Offspring. J. Mol. Neurosci. 2016, 58, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Kuizon, S.; Brown, W.T.; Junaid, M.A. DNA Methylation Profiling at Single-Base Resolution Reveals Gestational Folic Acid Supplementation Influences the Epigenome of Mouse Offspring Cerebellum. Front. Neurosci. 2016, 10, 168. [Google Scholar] [CrossRef]
- Barua, S.; Kuizon, S.; Chadman, K.K.; Flory, M.J.; Brown, W.T.; Junaid, M.A. Single-base resolution of mouse offspring brain methylome reveals epigenome modifications caused by gestational folic acid. Epigenet. Chromatin 2014, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Cosín-Tomás, M.; Leclerc, D.; Malysheva, O.V.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Sex-Specific Gene Expression in Brain of Young Mice and Embryos. Nutrients 2022, 14, 1051. [Google Scholar] [CrossRef]
- Henry, C.J.; Nemkov, T.; Casas-Selves, M.; Bilousova, G.; Zaberezhnyy, V.; Higa, K.C.; Serkova, N.J.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J. Folate dietary insufficiency and folic acid supplementation similarly impair metabolism and compromise hematopoiesis. Haematologica 2017, 102, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. Cell Metabolism Review One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Moran, R.G. Roles of folylpoly-gamma-glutamate synthetase in therapeutics with tetrahydrofolate antimetabolites: An overview. Semin. Oncol. 1999, 26, 24–32. [Google Scholar] [PubMed]
- Taylor, S.Y.; Dixon, H.M.; Yoganayagam, S.; Price, N.; Lang, D. Folic acid modulates eNOS activity via effects on posttranslational modifications and protein-protein interactions. Eur. J. Pharmacol. 2013, 714, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Madore, J.; Ramus, S.J.; Clarke, B.A.; Pharoah, P.D.; Deen, S.; Bowtell, D.D.; Odunsi, K.; Menon, U.; Morrison, C.; et al. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: Implications for clinical testing. An Ovarian Tumour Tissue Analysis consortium study. Br. J. Cancer 2014, 111, 2297–2307. [Google Scholar] [CrossRef]
- Qiu, W.; Gobinath, A.R.; Wen, Y.; Austin, J.; Galea, L.A.M. Folic acid, but not folate, regulates different stages of neurogenesis in the ventral hippocampus of adult female rats. J. Neuroendocrinol. 2019, 31, e12787. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Yu, B.; Mao, X.; Huang, Z.; Chen, D. Effect of maternal folic acid supplementation on hepatic proteome in newborn piglets. Nutrition 2013, 29, 230–234. [Google Scholar] [CrossRef]
- Pannia, E.; Hammoud, R.; Kubant, R.; Sa, J.Y.; Simonian, R.; Wasek, B.; Ashcraft, P.; Bottiglieri, T.; Pausova, Z.; Anderson, G.H. High Intakes of [6S]-5-Methyltetrahydrofolic Acid Compared with Folic Acid during Pregnancy Programs Central and Peripheral Mechanisms Favouring Increased Food Intake and Body Weight of Mature Female Offspring. Nutrients 2021, 13, 1477. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef]
- Annibal, A.; Tharyan, R.G.; Schonewolff, M.F.; Tam, H.; Latza, C.; Auler, M.M.K.; Antebi, A. Regulation of the one carbon folate cycle as a shared metabolic signature of longevity. Nat. Commun. 2021, 12, 3486. [Google Scholar] [CrossRef]
- Liu, Y.J.; Janssens, G.E.; McIntyre, R.L.; Molenaars, M.; Kamble, R.; Gao, A.W.; Jongejan, A.; Weeghel, M.V.; MacInnes, A.W.; Houtkooper, R.H. Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet. 2019, 15, e1007633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).