The Role of Methionine-Rich Diet in Unhealthy Cerebrovascular and Brain Aging: Mechanisms and Implications for Cognitive Impairment
Abstract
1. Introduction
2. Methionine-Rich Diet, Hyperhomocysteinemia, and Aging
2.1. Dietary Sources of Methionine
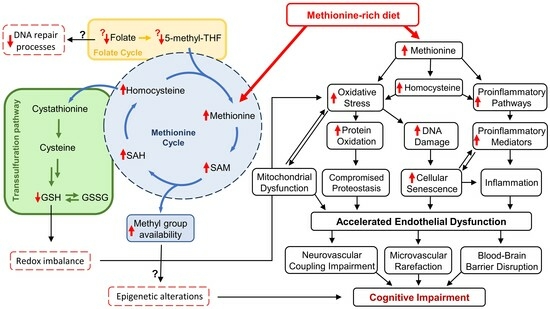
2.2. Methionine Metabolism
2.3. Hyperhomocysteinemia
2.4. Effects of Methionine-Rich Diet and Hyperhomocysteinemia on Cellular Mechanisms of Aging
2.4.1. Oxidative Stress and Mitochondrial Dysfunction
2.4.2. Inflammation
2.4.3. Epigenetic Regulation of Aging Processes
2.4.4. DNA Damage and Repair Mechanisms
2.4.5. Cellular Senescence
2.4.6. Altered Protein Homeostasis and Proteostasis Network
3. Methionine-Rich Diet and Vascular Contributions to Cognitive Impairment
3.1. Atherosclerosis and Stroke
3.2. Endothelial Dysfunction and Dysregulation of Cerebral Blood Flow
3.3. Microvascular Rarefaction
3.4. Blood–Brain Barrier Disruption and Neuroinflammation
4. Methionine-Rich Diet and Synaptic Function/Neuronal Health
4.1. Effects of High Methionine Intake and Hyperhomocysteinemia on Synaptic Plasticity and Neurotransmitter Systems
4.2. Neuronal Damage and Neurodegenerative Processes Induced by High Methionine Intake and Hyperhomocysteinemia
4.3. Methionine-Rich Diet, Hyperhomocysteinemia, and Altered Functional Connectivity
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ungvari, Z.; Adany, R. The future of healthy aging: Translation of geroscience discoveries to public health practice. Eur. J. Public Health 2021, 31, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Ortman, J.M.; Velkoff, V.A.; Hogan, H. An Aging Nation: The Older Population in the United States, Current Population Reports; U.S. Census Bureau: Washington, DC, USA, 2014; pp. 25–1140.
- Malhotra, R.; Bautista, M.A.C.; Muller, A.M.; Aw, S.; Koh, G.C.H.; Theng, Y.L.; Hoskins, S.J.; Wong, C.H.; Miao, C.; Lim, W.S.; et al. The Aging of a Young Nation: Population Aging in Singapore. Gerontologist 2019, 59, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.L.; Simonsick, E.M.; Dong, B.R.; Kasper, J.D.; Xue, Q.L. Frailty, with or without Cognitive Impairment, Is a Strong Predictor of Recurrent Falls in a US Population-Representative Sample of Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e354–e360. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Chen, X.; Tang, D.; Kelley, A.S.; Li, J. Prevalence of Memory-Related Diagnoses among U.S. Older Adults with Early Symptoms of Cognitive Impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1846–1853. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, J.; Roman, R.J.; Fan, F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? Geroscience 2022, 44, 1879–1883. [Google Scholar] [CrossRef]
- Enroth, L.; Raitanen, J.; Halonen, P.; Tiainen, K.; Jylha, M. Trends of Physical Functioning, Morbidity, and Disability-Free Life Expectancy among the Oldest Old: Six Repeated Cross-Sectional Surveys Between 2001 and 2018 in the Vitality 90+ Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1227–1233. [Google Scholar] [CrossRef]
- Ullrich, P.; Werner, C.; Bongartz, M.; Eckert, T.; Abel, B.; Schonstein, A.; Kiss, R.; Hauer, K. Increasing Life-Space Mobility in Community-Dwelling Older Persons with Cognitive Impairment Following Rehabilitation: A Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1988–1996. [Google Scholar] [CrossRef]
- Wadley, V.G.; Bull, T.P.; Zhang, Y.; Barba, C.; Bryan, R.N.; Crowe, M.; Desiderio, L.; Deutsch, G.; Erus, G.; Geldmacher, D.S.; et al. Cognitive Processing Speed Is Strongly Related to Driving Skills, Financial Abilities, and Other Instrumental Activities of Daily Living in Persons with Mild Cognitive Impairment and Mild Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1829–1838. [Google Scholar] [CrossRef]
- Akushevich, I.; Yashkin, A.; Kovtun, M.; Kravchenko, J.; Arbeev, K.; Yashin, A.I. Forecasting prevalence and mortality of Alzheimer’s disease using the partitioning models. Exp. Gerontol. 2023, 174, 112133. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Breitner, J.C.; Welsh, K.A.; Gau, B.A.; McDonald, W.M.; Steffens, D.C.; Saunders, A.M.; Magruder, K.M.; Helms, M.J.; Plassman, B.L.; Folstein, M.F.; et al. Alzheimer’s disease in the National Academy of Sciences-National Research Council Registry of Aging Twin Veterans. III. Detection of cases, longitudinal results, and observations on twin concordance. Arch. Neurol. 1995, 52, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Montenigro, P.; Nejati, M.; Maserejian, N. Estimating prevalence of early Alzheimer’s disease in the United States, accounting for racial and ethnic diversity. Alzheimers Dement. 2023, 19, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Bendayan, R.; Zhu, Y.; Federman, A.D.; Dobson, R.J.B. Multimorbidity Patterns and Memory Trajectories in Older Adults: Evidence From the English Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 867–875. [Google Scholar] [CrossRef]
- Fassier, P.; Kang, J.H.; Lee, I.M.; Grodstein, F.; Vercambre, M.N. Vigorous Physical Activity and Cognitive Trajectory Later in Life: Prospective Association and Interaction by Apolipoprotein E e4 in the Nurses’ Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.C.; Stoner, J.A.; Donna-Ferreira, F.; Malatinszky, G.C.; Guthery, L.D.; Scott, J.; Prodan, C.I. High rates of undiagnosed vascular cognitive impairment among American Indian veterans. Geroscience 2019, 41, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Skoog, I.; Schneider, J.A.; Pantoni, L.; Mok, V.; Chen, C.L.H.; Scheltens, P. Vascular cognitive impairment. Nat. Rev. Dis. Primers 2018, 4, 18003. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Kiss, T.; Tarantini, S.; Nyul-Toth, A.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Tabak, A.; Institoris, A.; et al. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H740–H761. [Google Scholar] [CrossRef]
- Csipo, T.; Lipecz, A.; Fulop, G.A.; Hand, R.A.; Ngo, B.N.; Dzialendzik, M.; Tarantini, S.; Balasubramanian, P.; Kiss, T.; Yabluchanska, V.; et al. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience 2019, 41, 125–136. [Google Scholar] [CrossRef]
- Csipo, T.; Mukli, P.; Lipecz, A.; Tarantini, S.; Bahadli, D.; Abdulhussein, O.; Owens, C.; Kiss, T.; Balasubramanian, P.; Nyul-Toth, A.; et al. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience 2019, 41, 495–509. [Google Scholar] [CrossRef]
- Gardner, A.W.; Montgomery, P.S.; Wang, M.; Shen, B.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Yabluchanskiy, A.; Csiszar, A.; Waldstein, S.R. Cognitive decrement in older adults with symptomatic peripheral artery disease. Geroscience 2021, 43, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Nyul-Toth, A.; Fulop, G.A.; Tarantini, S.; Kiss, T.; Ahire, C.; Faakye, J.A.; Ungvari, A.; Toth, P.; Toth, A.; Csiszar, A.; et al. Cerebral venous congestion exacerbates cerebral microhemorrhages in mice. Geroscience 2022, 44, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Mukli, P.; Csipo, T.; Lipecz, A.; Silva-Palacios, F.; Dasari, T.W.; Tarantini, S.; Gardner, A.W.; Montgomery, P.S.; Waldstein, S.R.; et al. Microvascular dysfunction and neurovascular uncoupling are exacerbated in peripheral artery disease, increasing the risk of cognitive decline in older adults. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H924–H935. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kirkpatrick, A.C.; Csiszar, A.; Prodan, C.I. Cerebral microhemorrhages: Mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1128–H1143. [Google Scholar] [CrossRef]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Nation, D.A.; Kisler, K.; Toga, A.W.; Zlokovic, B.V. Imaging subtle leaks in the blood-brain barrier in the aging human brain: Potential pitfalls, challenges, and possible solutions. Geroscience 2022, 44, 1339–1351. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Gottesman, R.F.; Bernstein, K.E.; Seshadri, S.; McKee, A.; Snyder, H.; Greenberg, S.M.; Yaffe, K.; Schaffer, C.B.; Yuan, C.; et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020, 16, 1714–1733. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Fitzgibbon-Collins, L.K.; Heckman, G.A.; Bains, I.; Noguchi, M.; McIlroy, W.E.; Hughson, R.L. Older Adults’ Drop in Cerebral Oxygenation on Standing Correlates with Postural Instability and May Improve with Sitting Prior to Standing. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1124–1133. [Google Scholar] [CrossRef]
- Beason-Held, L.L.; Fournier, D.; Shafer, A.T.; Fabbri, E.; An, Y.; Huang, C.W.; Bilgel, M.; Wong, D.F.; Ferrucci, L.; Resnick, S.M. Disease Burden Affects Aging Brain Function. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Jacob, M.A.; Norris, D.G.; de Leeuw, F.E.; Tuladhar, A.M. Longitudinal Relation Between Structural Network Efficiency, Cognition, and Gait in Cerebral Small Vessel Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Siejka, T.P.; Srikanth, V.K.; Hubbard, R.E.; Moran, C.; Beare, R.; Wood, A.G.; Collyer, T.A.; Gujjari, S.; Phan, T.G.; Callisaya, M.L. Frailty Is Associated with Cognitive Decline Independent of Cerebral Small Vessel Disease and Brain Atrophy. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Toth, L.; Czigler, A.; Hegedus, E.; Komaromy, H.; Amrein, K.; Czeiter, E.; Yabluchanskiy, A.; Koller, A.; Orsi, G.; Perlaki, G.; et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022, 44, 2771–2783. [Google Scholar] [CrossRef]
- Kiss, T.; Nyúl-Tóth, Á.; Gulej, R.; Tarantini, S.; Csipo, T.; Mukli, P.; Ungvari, A.; Balasubramanian, P.; Yabluchanskiy, A.; Benyo, Z.; et al. Old blood from heterochronic parabionts accelerates vascular aging in young mice: Transcriptomic signature of pathologic smooth muscle remodeling. Geroscience 2022, 44, 953–981. [Google Scholar] [CrossRef]
- Kiss, T.; Nyul-Toth, A.; DelFavero, J.; Balasubramanian, P.; Tarantini, S.; Faakye, J.; Gulej, R.; Ahire, C.; Ungvari, A.; Yabluchanskiy, A.; et al. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience 2022, 44, 661–681. [Google Scholar] [CrossRef]
- Gosalia, J.; Montgomery, P.S.; Zhang, S.; Pomilla, W.A.; Wang, M.; Liang, M.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A.; Proctor, D.N.; et al. Increased pulse wave velocity is related to impaired working memory and executive function in older adults with metabolic syndrome. Geroscience 2022, 44, 2831–2844. [Google Scholar] [CrossRef]
- Whitehead, S.N.; Bruno, A.; Burns, J.M.; Carmichael, S.T.; Csiszar, A.; Edwards, J.D.; Elahi, F.M.; Faraco, G.; Gould, D.B.; Gustafson, D.R.; et al. Expanding the horizon of research into the pathogenesis of the white matter diseases: Proceedings of the 2021 Annual Workshop of the Albert Research Institute for White Matter and Cognition. Geroscience 2021, 16, 1714–1733. [Google Scholar] [CrossRef]
- Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Mukli, P.; Balasubramanian, P.; Ungvari, A.; Toth, P.; Benyo, Z.; Sonntag, W.E.; et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience 2021, 43, 2387–2394. [Google Scholar] [CrossRef]
- Tarantini, S.; Balasubramanian, P.; Yabluchanskiy, A.; Ashpole, N.M.; Logan, S.; Kiss, T.; Ungvari, A.; Nyul-Toth, A.; Schwartzman, M.L.; Benyo, Z.; et al. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: Implications for brain aging. Geroscience 2021, 43, 901–911. [Google Scholar] [CrossRef]
- Tarantini, S.; Balasubramanian, P.; Delfavero, J.; Csipo, T.; Yabluchanskiy, A.; Kiss, T.; Nyul-Toth, A.; Mukli, P.; Toth, P.; Ahire, C.; et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 2021, 43, 2427–2440. [Google Scholar] [CrossRef] [PubMed]
- Nyul-Toth, A.; Tarantini, S.; DelFavero, J.; Yan, F.; Balasubramanian, P.; Yabluchanskiy, A.; Ahire, C.; Kiss, T.; Csipo, T.; Lipecz, A.; et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1370–H1392. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Tarantini, S.; Csipo, T.; Balasubramanian, P.; Nyul-Toth, A.; Yabluchanskiy, A.; Wren, J.D.; Garman, L.; Huffman, D.M.; Csiszar, A.; et al. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: Transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience 2020, 42, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Nyul-Toth, A.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wren, J.D.; Garman, L.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: Transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 2020, 42, 527–546. [Google Scholar] [CrossRef]
- Kiss, T.; Nyul-Toth, A.; Balasubramanian, P.; Tarantini, S.; Ahire, C.; DelFavero, J.; Yabluchanskiy, A.; Csipo, T.; Farkas, E.; Wiley, G.; et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 2020, 42, 429–444. [Google Scholar] [CrossRef]
- Tarantini, S.; Yabluchanskiy, A.; Csipo, T.; Fulop, G.; Kiss, T.; Balasubramanian, P.; DelFavero, J.; Ahire, C.; Ungvari, A.; Nyul-Toth, A.; et al. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience 2019, 41, 533–542. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Sule, Z.; et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019, 24, 101192. [Google Scholar] [CrossRef]
- Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Csipo, T.; Nyul-Toth, A.; Lipecz, A.; Szabo, C.; et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience 2019, 41, 419–439. [Google Scholar] [CrossRef]
- Farias Quipildor, G.E.; Mao, K.; Hu, Z.; Novaj, A.; Cui, M.H.; Gulinello, M.; Branch, C.A.; Gubbi, S.; Patel, K.; Moellering, D.R.; et al. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience 2019, 41, 185–208. [Google Scholar] [CrossRef]
- Csiszar, A.; Yabluchanskiy, A.; Ungvari, A.; Ungvari, Z.; Tarantini, S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. Geroscience 2019, 41, 609–617. [Google Scholar] [CrossRef]
- Csiszar, A.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Kiss, T.; Farkas, E.; Baur, J.A.; Ungvari, Z.I. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1253–H1266. [Google Scholar] [CrossRef] [PubMed]
- Van Skike, C.E.; Jahrling, J.B.; Olson, A.B.; Sayre, N.L.; Hussong, S.A.; Ungvari, Z.; Lechleiter, J.D.; Galvan, V. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H693–H703. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Valcarcel-Ares, N.M.; Yabluchanskiy, A.; Fulop, G.A.; Hertelendy, P.; Gautam, T.; Farkas, E.; Perz, A.; Rabinovitch, P.S.; Sonntag, W.E.; et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 2018, 17, e12731. [Google Scholar] [CrossRef] [PubMed]
- Fulop, G.A.; Ramirez-Perez, F.I.; Kiss, T.; Tarantini, S.; Valcarcel Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Conley, S.M.; Ballabh, P.; Martinez-Lemus, L.A.; et al. IGF-1 deficiency Promotes Pathological Remodeling of Cerebral Arteries: A Potential Mechanism Contributing to the Pathogenesis of Intracerebral Hemorrhages in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 74, 446–454. [Google Scholar] [CrossRef]
- Fulop, G.A.; Kiss, T.; Tarantini, S.; Balasubramanian, P.; Yabluchanskiy, A.; Farkas, E.; Bari, F.; Ungvari, Z.; Csiszar, A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 2018, 40, 513–521. [Google Scholar] [CrossRef]
- Tarantini, S.; Tucsek, Z.; Valcarcel-Ares, M.N.; Toth, P.; Gautam, T.; Giles, C.B.; Ballabh, P.; Wei, J.Y.; Wren, J.D.; Ashpole, N.M.; et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: Implications for cerebromicrovascular and brain aging. Age 2016, 38, 273–289. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Ashpole, N.M.; Tucsek, Z.; Milne, G.L.; Valcarcel-Ares, N.M.; Menyhart, A.; Farkas, E.; Sonntag, W.E.; Csiszar, A.; et al. IGF-1 deficiency impairs neurovascular coupling in mice: Implications for cerebromicrovascular aging. Aging Cell 2015, 14, 1034–1044. [Google Scholar] [CrossRef]
- Springo, Z.; Tarantini, S.; Toth, P.; Tucsek, Z.; Koller, A.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging Exacerbates Pressure-Induced Mitochondrial Oxidative Stress in Mouse Cerebral Arteries. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1355–1359. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.; Cogger, V.C.; Mitchell, S.J.; Senior, A.; de Cabo, R.; Raubenheimer, D.; Simpson, S.J. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell. Mol. Life Sci. 2016, 73, 1237–1252. [Google Scholar] [CrossRef]
- Minor, R.K.; Allard, J.S.; Younts, C.M.; Ward, T.M.; de Cabo, R. Dietary interventions to extend life span and health span based on calorie restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 695–703. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Pomatto-Watson, L.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent fasting: From calories to time restriction. Geroscience 2021, 43, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Montegut, L.; de Cabo, R.; Zitvogel, L.; Kroemer, G. Science-Driven Nutritional Interventions for the Prevention and Treatment of Cancer. Cancer Discov. 2022, 12, 2258–2279. [Google Scholar] [CrossRef]
- Guo, J.; Schupf, N.; Cruz, E.; Stern, Y.; Mayeux, R.P.; Gu, Y. Association Between Mediterranean Diet and Functional Status in Older Adults: A Longitudinal Study Based on the Washington Heights-Inwood Columbia Aging Project. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1873–1881. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Sternberg, A.L.; Mitchell, C.M.; Blackford, A.L.; Schrack, J.; Wanigatunga, A.A.; Michos, E.; Juraschek, S.P.; Szanton, S.; Kalyani, R.; et al. Effects of Vitamin D on Physical Function: Results from the STURDY Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1585–1592. [Google Scholar] [CrossRef]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortola, R.; Garcia-Esquinas, E.; Martinez-Gomez, D.; Struijk, E.A.; Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Sotos-Prieto, M. A Mediterranean Lifestyle and Frailty Incidence in Older Adults: The Seniors-ENRICA-1 Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1845–1852. [Google Scholar] [CrossRef]
- Promislow, D.E.L. A New Concept in Diet Restriction Is Cleaning Up! J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 599–600. [Google Scholar] [CrossRef]
- Ramaker, M.E.; Corcoran, D.L.; Apsley, A.T.; Kobor, M.S.; Kraus, V.B.; Kraus, W.E.; Lin, D.T.S.; Orenduff, M.C.; Pieper, C.F.; Waziry, R.; et al. Epigenome-wide Association Study Analysis of Calorie Restriction in Humans, CALERIETM Trial Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2395–2401. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Ortola, R.; Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Garcia-Esquinas, E. Adherence to the Mediterranean Diet and Physical Resilience in Older Adults: The Seniors-ENRICA Cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 505–512. [Google Scholar] [CrossRef]
- Stephen, R.; Ngandu, T.; Liu, Y.; Peltonen, M.; Antikainen, R.; Kemppainen, N.; Laatikainen, T.; Lotjonen, J.; Rinne, J.; Strandberg, T.; et al. Change in CAIDE Dementia Risk Score and Neuroimaging Biomarkers During a 2-Year Multidomain Lifestyle Randomized Controlled Trial: Results of a Post-Hoc Subgroup Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Talegawkar, S.A.; Jin, Y.; Xue, Q.L.; Tanaka, T.; Simonsick, E.M.; Tucker, K.L.; Ferrucci, L. Dietary Pattern Trajectories in Middle Age and Physical Function in Older Age. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.J.; Presse, N.; Rahme, E.; Ferland, G.; Bherer, L.; Chevalier, S. Milk, Yogurt, and Cheese Intake Is Positively Associated with Cognitive Executive Functions in Older Adults of the Canadian Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Power, R.; Prado-Cabrero, A.; Mulcahy, R.; Howard, A.; Nolan, J.M. The Role of Nutrition for the Aging Population: Implications for Cognition and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2019, 10, 619–639. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhou, Y.; Liu, Q.; Chen, X.; Liu, X.; Grune, T.; Shi, L.; Hou, M.; Liu, Z. Effects of methionine intake on cognitive function in mild cognitive impairment patients and APP/PS1 Alzheimer’s Disease model mice: Role of the cystathionine-beta-synthase/H(2)S pathway. Redox Biol. 2023, 59, 102595. [Google Scholar] [CrossRef]
- Troen, A.M.; Shea-Budgell, M.; Shukitt-Hale, B.; Smith, D.E.; Selhub, J.; Rosenberg, I.H. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12474–12479. [Google Scholar] [CrossRef]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, Y.; Li, S.; Ge, Y.; Shi, Y.; Tang, X.; Le, G. Methionine Restriction Improves Cognitive Ability by Alleviating Hippocampal Neuronal Apoptosis through H19 in Middle-Aged Insulin-Resistant Mice. Nutrients 2022, 14, 4503. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, Y.; Sun, Y.; Liu, Q.; Duan, H.; Huang, L.; Zhou, D.; Wang, Z.; Zhao, J.; Li, Z.; et al. Circulating Amyloid-beta and Methionine-Related Metabolites to Predict the Risk of Mild Cognitive Impairment: A Nested Case-Control Study. J. Alzheimers Dis. 2022, 90, 389–404. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Tabibzadeh, S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019, 49, 144–164. [Google Scholar] [CrossRef]
- Silberstein, R.B.; Pipingas, A.; Scholey, A.B. Homocysteine Modulates Brain Functional Connectivity in a Memory Retrieval Task. J. Alzheimers Dis. 2022, 90, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, Y.; Yuan, S.; Xie, M.; Dong, Y.; Li, J.; He, Q.; Ye, X.; Lv, Y.; Hocher, C.F.; et al. Prevalence and risk factors for hyperhomocysteinemia: A population-based cross-sectional study from Hunan, China. BMJ Open 2021, 11, e048575. [Google Scholar] [CrossRef] [PubMed]
- Aissa, A.F.; Tryndyak, V.P.; de Conti, A.; Rita Thomazela Machado, A.; Tuttis, K.; da Silva Machado, C.; Hernandes, L.C.; Wellington da Silva Santos, P.; Mara Serpeloni, J.; Pogribny, I.P.; et al. Epigenetic changes induced in mice liver by methionine-supplemented and methionine-deficient diets. Food Chem. Toxicol. 2022, 163, 112938. [Google Scholar] [CrossRef] [PubMed]
- Navik, U.; Sheth, V.G.; Kabeer, S.W.; Tikoo, K. Dietary Supplementation of Methyl Donor l-Methionine Alters Epigenetic Modification in Type 2 Diabetes. Mol. Nutr. Food Res. 2019, 63, e1801401. [Google Scholar] [CrossRef]
- Singh, M.; George, A.K.; Eyob, W.; Homme, R.P.; Stansic, D.; Tyagi, S.C. High-methionine diet in skeletal muscle remodeling: Epigenetic mechanism of homocysteine-mediated growth retardation. Can. J. Physiol. Pharmacol. 2021, 99, 56–63. [Google Scholar] [CrossRef]
- Troen, A.M.; Lutgens, E.; Smith, D.E.; Rosenberg, I.H.; Selhub, J. The atherogenic effect of excess methionine intake. Proc. Natl. Acad. Sci. USA 2003, 100, 15089–15094. [Google Scholar] [CrossRef]
- Caro, P.; Gomez, J.; Sanchez, I.; Naudi, A.; Ayala, V.; Lopez-Torres, M.; Pamplona, R.; Barja, G. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenation Res. 2009, 12, 421–434. [Google Scholar] [CrossRef]
- McCampbell, A.; Wessner, K.; Marlatt, M.W.; Wolffe, C.; Toolan, D.; Podtelezhnikov, A.; Yeh, S.; Zhang, R.; Szczerba, P.; Tanis, K.Q.; et al. Induction of Alzheimer’s-like changes in brain of mice expressing mutant APP fed excess methionine. J. Neurochem. 2011, 116, 82–92. [Google Scholar] [CrossRef]
- Bagi, Z.; Ungvari, Z.; Koller, A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: Possible role of peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 28–33. [Google Scholar] [CrossRef][Green Version]
- Bagi, Z.; Ungvari, Z.; Szollar, L.; Koller, A. Flow-Induced Constriction in Arterioles of Hyperhomocysteinemic Rats Is Due to Impaired Nitric Oxide and Enhanced Thromboxane A(2) Mediation. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 233–237. [Google Scholar] [CrossRef]
- Ungvari, Z.; Csiszar, A.; Bagi, Z.; Koller, A. Impaired nitric oxide-mediated flow-induced coronary dilation in hyperhomocysteinemia: Morphological and functional evidence for increased peroxynitrite formation. Am. J. Pathol. 2002, 161, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A.; Edwards, J.G.; Kaminski, P.M.; Wolin, M.S.; Kaley, G.; Koller, A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: Role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Pacher, P.; Rischak, K.; Szollar, L.; Koller, A. Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Sarkadi-Nagy, E.; Bagi, Z.; Szollar, L.; Koller, A. Simultaneously Increased TxA2 Activity in Isolated Arterioles and Platelets of Rats with Hyperhomocysteinemia. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1203–1208. [Google Scholar] [CrossRef]
- Ables, G.P.; Ouattara, A.; Hampton, T.G.; Cooke, D.; Perodin, F.; Augie, I.; Orentreich, D.S. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci. Rep. 2015, 5, 8886. [Google Scholar] [CrossRef]
- Ables, G.P.; Perrone, C.E.; Orentreich, D.; Orentreich, N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS ONE 2012, 7, e51357. [Google Scholar] [CrossRef]
- Barcena, C.; Quiros, P.M.; Durand, S.; Mayoral, P.; Rodriguez, F.; Caravia, X.M.; Marino, G.; Garabaya, C.; Fernandez-Garcia, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef]
- Ghosh, S.; Wanders, D.; Stone, K.P.; Van, N.T.; Cortez, C.C.; Gettys, T.W. A systems biology analysis of the unique and overlapping transcriptional responses to caloric restriction and dietary methionine restriction in rats. FASEB J. 2014, 28, 2577–2590. [Google Scholar] [CrossRef]
- Gomez, J.; Caro, P.; Sanchez, I.; Naudi, A.; Jove, M.; Portero-Otin, M.; Lopez-Torres, M.; Pamplona, R.; Barja, G. Effect of methionine dietary supplementation on mitochondrial oxygen radical generation and oxidative DNA damage in rat liver and heart. J. Bioenerg. Biomembr. 2009, 41, 309–321. [Google Scholar] [CrossRef]
- Hine, C.; Mitchell, J.R. Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp. Gerontol. 2015, 68, 26–32. [Google Scholar] [CrossRef]
- Hipkiss, A.R. On methionine restriction, suppression of mitochondrial dysfunction and aging. Rejuvenation Res. 2008, 11, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Johnson, F.B. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS ONE 2014, 9, e97729. [Google Scholar] [CrossRef] [PubMed]
- Jove, M.; Ayala, V.; Ramirez-Nunez, O.; Naudi, A.; Cabre, R.; Spickett, C.M.; Portero-Otin, M.; Pamplona, R. Specific lipidome signatures in central nervous system from methionine-restricted mice. J. Proteome Res. 2013, 12, 2679–2689. [Google Scholar] [CrossRef] [PubMed]
- Komninou, D.; Malloy, V.L.; Zimmerman, J.A.; Sinha, R.; Richie, J.P., Jr. Methionine restriction delays aging-related urogenital diseases in male Fischer 344 rats. Geroscience 2019, 42, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Lees, E.K.; Krol, E.; Grant, L.; Shearer, K.; Wyse, C.; Moncur, E.; Bykowska, A.S.; Mody, N.; Gettys, T.W.; Delibegovic, M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 2014, 13, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, S.; Nichenametla, S.; Sinha, R.; Wilson, R.P.; Richie, J.P., Jr. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med. 2013, 238, 392–399. [Google Scholar] [CrossRef]
- Perrone, C.E.; Malloy, V.L.; Orentreich, D.S.; Orentreich, N. Metabolic adaptations to methionine restriction that benefit health and lifespan in rodents. Exp. Gerontol. 2013, 48, 654–660. [Google Scholar] [CrossRef]
- Sanchez-Roman, I.; Barja, G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013, 48, 1030–1042. [Google Scholar] [CrossRef]
- Sanz, A.; Caro, P.; Ayala, V.; Portero-Otin, M.; Pamplona, R.; Barja, G. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006, 20, 1064–1073. [Google Scholar] [CrossRef]
- Stone, K.P.; Wanders, D.; Orgeron, M.; Cortez, C.C.; Gettys, T.W. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 2014, 63, 3721–3733. [Google Scholar] [CrossRef]
- Sun, L.; Sadighi Akha, A.A.; Miller, R.A.; Harper, J.M. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Tyrovolas, S.; Panagiotakos, D.B. Red meat consumption and healthy ageing: A review. Maturitas 2016, 84, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in meat consumption in the USA. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371, m4141. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zhuang, Z.; Zhao, Y.; Song, Z.; Xiao, W.; Wang, W.; Li, Y.; Huang, N.; Jia, J.; Liu, Z.; et al. Unprocessed Red Meat and Processed Meat Consumption, Plasma Metabolome, and Risk of Ischemic Heart Disease: A Prospective Cohort Study of UK Biobank. J. Am. Heart Assoc. 2023, 12, e027934. [Google Scholar] [CrossRef]
- Fan, B.; Huang, X.; Zhao, J.V. Exploration of Metabolic Biomarkers Linking Red Meat Consumption to Ischemic Heart Disease Mortality in the UK Biobank. Nutrients 2023, 15, 1865. [Google Scholar] [CrossRef]
- Johnston, B.C.; Zeraatkar, D.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Marshall, C.; Stover, P.J.; Fairweather-Taitt, S.; Wojcik, G.; et al. Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations From the Nutritional Recommendations (NutriRECS) Consortium. Ann. Intern. Med. 2019, 171, 756–764. [Google Scholar] [CrossRef]
- Mota, J.O.; Guillou, S.; Pierre, F.; Membre, J.M. Public health risk-benefit assessment of red meat in France: Current consumption and alternative scenarios. Food Chem. Toxicol. 2021, 149, 111994. [Google Scholar] [CrossRef]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red meat consumption, cardiovascular diseases, and diabetes: A systematic review and meta-analysis. Eur. Heart J. 2023, 44, 2626–2635. [Google Scholar] [CrossRef]
- Singh, B.; Khan, A.A.; Anamika, F.; Munjal, R.; Munjal, J.; Jain, R. Red Meat Consumption and its Relationship with Cardiovascular Health: A Review of Pathophysiology and Literature. Cardiol. Rev. 2023. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, J.L.; Chen, Q.C.; Xiao, W.K.; Ma, G.P.; Liang, J.H.; Chen, X.K.; Wang, S.; Zhou, X.X.; Wu, H.; et al. Red meat consumption and risk for dyslipidaemia and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 996467. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ma, H.; Song, Q.; Zhou, T.; Hu, Y.; Heianza, Y.; Manson, J.E.; Qi, L. Red meat consumption and all-cause and cardiovascular mortality: Results from the UK Biobank study. Eur. J. Nutr. 2022, 61, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Guyatt, G.H.; Alonso-Coello, P.; Bala, M.M.; Rabassa, M.; Han, M.A.; Vernooij, R.W.M.; Valli, C.; El Dib, R.; Johnston, B.C. Red and Processed Meat Consumption and Risk for All-Cause Mortality and Cardiometabolic Outcomes. Ann. Intern. Med. 2020, 172, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Zeraatkar, D.; Johnston, B.C.; Bartoszko, J.; Cheung, K.; Bala, M.M.; Valli, C.; Rabassa, M.; Sit, D.; Milio, K.; Sadeghirad, B.; et al. Effect of Lower Versus Higher Red Meat Intake on Cardiometabolic and Cancer Outcomes: A Systematic Review of Randomized Trials. Ann. Intern. Med. 2019, 171, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hayden, K.; Jackson, R.; Schutte, R. Association of red and processed meat consumption with cardiovascular morbidity and mortality in participants with and without obesity: A prospective cohort study. Clin. Nutr. 2021, 40, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Saadatnia, M.; Shakeri, F.; Beykverdi, M.; Keshteli, A.H.; Esmaillzadeh, A. A case-control study on red meat consumption and risk of stroke among a group of Iranian adults. Public Health Nutr. 2015, 18, 1084–1090. [Google Scholar] [CrossRef]
- Amiano, P.; Chamosa, S.; Etxezarreta, N.; Arriola, L.; Sanchez, M.J.; Ardanaz, E.; Molina-Montes, E.; Chirlaque, M.D.; Moreno-Iribas, C.; Huerta, J.M.; et al. Unprocessed red meat and processed meat consumption and risk of stroke in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Clin. Nutr. 2016, 70, 313–319. [Google Scholar] [CrossRef]
- Chen, F.; Hu, W.; Chen, S.; Si, A.; Zhang, Y.; Ma, J. Stroke mortality attributable to high red meat intake in China and South Korea: An age-period-cohort and joinpoint analysis. Front. Nutr. 2022, 9, 921592. [Google Scholar] [CrossRef]
- Chen, G.C.; Lv, D.B.; Pang, Z.; Liu, Q.F. Red and processed meat consumption and risk of stroke: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2013, 67, 91–95. [Google Scholar] [CrossRef]
- Kaluza, J.; Wolk, A.; Larsson, S.C. Red meat consumption and risk of stroke: A meta-analysis of prospective studies. Stroke 2012, 43, 2556–2560. [Google Scholar] [CrossRef]
- Kim, K.; Hyeon, J.; Lee, S.A.; Kwon, S.O.; Lee, H.; Keum, N.; Lee, J.K.; Park, S.M. Role of Total, Red, Processed, and White Meat Consumption in Stroke Incidence and Mortality: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005983. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Red meat consumption and risk of stroke in Swedish men. Am. J. Clin. Nutr. 2011, 94, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Red meat consumption and risk of stroke in Swedish women. Stroke 2011, 42, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef]
- Veno, S.K.; Bork, C.S.; Jakobsen, M.U.; Lundbye-Christensen, S.; Bach, F.W.; McLennan, P.L.; Tjonneland, A.; Schmidt, E.B.; Overvad, K. Substitution of Fish for Red Meat or Poultry and Risk of Ischemic Stroke. Nutrients 2018, 10, 1648. [Google Scholar] [CrossRef]
- Yang, C.; Pan, L.; Sun, C.; Xi, Y.; Wang, L.; Li, D. Red Meat Consumption and the Risk of Stroke: A Dose-Response Meta-analysis of Prospective Cohort Studies. J. Stroke Cerebrovasc. Dis. 2016, 25, 1177–1186. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J.; National, H.; Nutrition Examination, S. Hyperhomocysteinemia associated with poor recall in the third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 2001, 73, 927–933. [Google Scholar] [CrossRef]
- Selhub, J. The many facets of hyperhomocysteinemia: Studies from the Framingham cohorts. J. Nutr. 2006, 136, 1726S–1730S. [Google Scholar] [CrossRef]
- Holmen, M.; Hvas, A.M.; Arendt, J.F.H. Hyperhomocysteinemia and Ischemic Stroke: A Potential Dose-Response Association-A Systematic Review and Meta-analysis. TH Open 2021, 5, e420–e437. [Google Scholar] [CrossRef]
- Kumral, E.; Saruhan, G.; Aktert, D.; Orman, M. Association of Hyperhomocysteinemia with Stroke Recurrence after Initial Stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2047–2054. [Google Scholar] [CrossRef]
- Lu, Z.H.; Li, J.; Li, X.L.; Ding, M.; Mao, C.J.; Zhu, X.Y.; Liu, C.F. Hypertension with Hyperhomocysteinemia Increases the Risk of Early Cognitive Impairment after First-Ever Ischemic Stroke. Eur. Neurol. 2019, 82, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Hoshino, T.; Ishizuka, K.; Toi, S.; Takahashi, S.; Wako, S.; Arai, S.; Kitagawa, K. Hyperhomocysteinemia Increases Vascular Risk in Stroke Patients with Chronic Kidney Disease. J. Atheroscler. Thromb. 2022, 30, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Fu, Q.; Cao, Q.; Hao, L.; Zong, Z. Sex differences in risk factors for stroke in patients with hypertension and hyperhomocysteinemia. Sci. Rep. 2019, 9, 14313. [Google Scholar] [CrossRef] [PubMed]
- Poddar, R. Hyperhomocysteinemia is an emerging comorbidity in ischemic stroke. Exp. Neurol. 2021, 336, 113541. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, S.I.; Al-Mistarehi, A.H.; Yassin, A.; Rabab’ah, W.; Skaff, H.; Ibdah, R. A Concurrent Ischemic Stroke, Myocardial Infarction, and Aortic Thrombi in a Young Patient with Hyperhomocysteinemia: A Case Report. Int. Med. Case Rep. J. 2020, 13, 581–590. [Google Scholar] [CrossRef]
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef]
- Refsum, H.; Ueland, P.M.; Nygard, O.; Vollset, S.E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998, 49, 31–62. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.S.; Neuman, M. Methionine deficiency and hepatic injury in a dietary steatohepatitis model. Dig. Dis. Sci. 2008, 53, 767–776. [Google Scholar] [CrossRef]
- Chao, C.L.; Kuo, T.L.; Lee, Y.T. Effects of methionine-induced hyperhomocysteinemia on endothelium- dependent vasodilation and oxidative status in healthy adults. Circulation 2000, 101, 485–490. [Google Scholar] [CrossRef]
- Di Minno, M.N.; Pezzullo, S.; Palmieri, V.; Coppola, A.; D’Angelo, A.; Sampietro, F.; Cavalca, V.; Tremoli, E.; Di Minno, G. Genotype-independent in vivo oxidative stress following a methionine loading test: Maximal platelet activation in subjects with early-onset thrombosis. Thromb. Res. 2011, 128, e43–e48. [Google Scholar] [CrossRef]
- Yalcinkaya-Demirsoz, S.; Depboylu, B.; Dogru-Abbasoglu, S.; Unlucerci, Y.; Uysal, M. Effects of high methionine diet on oxidative stress in serum, apo-B containing lipoproteins, heart, and aorta in rabbits. Ann. Clin. Lab. Sci. 2009, 39, 386–391. [Google Scholar] [PubMed]
- Zhang, R.; Ma, J.; Xia, M.; Zhu, H.; Ling, W. Mild hyperhomocysteinemia induced by feeding rats diets rich in methionine or deficient in folate promotes early atherosclerotic inflammatory processes. J. Nutr. 2004, 134, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Valcarcel-Ares, M.N.; Yabluchanskiy, A.; Tucsek, Z.; Hertelendy, P.; Kiss, T.; Gautam, T.; Zhang, X.A.; Sonntag, W.E.; de Cabo, R.; et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Sosnowska, D.; Wang, M.; Monticone, R.E.; Telljohann, R.; Pinto, J.T.; de Cabo, R.; Sonntag, W.E.; et al. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bailey-Downs, L.; Sosnowska, D.; Gautam, T.; Koncz, P.; Losonczy, G.; Ballabh, P.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Vascular oxidative stress in aging: A homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H363–H372. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Nyul-Toth, A.; Kiss, T.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Benyo, Z.; Csiszar, A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: From increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019, 41, 727–738. [Google Scholar] [CrossRef]
- Addabbo, F.; Ratliff, B.; Park, H.C.; Kuo, M.C.; Ungvari, Z.; Csiszar, A.; Krasnikov, B.; Sodhi, K.; Zhang, F.; Nasjletti, A.; et al. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: Proteomic approach. Am. J. Pathol. 2009, 174, 34–43. [Google Scholar] [CrossRef][Green Version]
- Csiszar, A.; Gautam, T.; Sosnowska, D.; Tarantini, S.; Banki, E.; Tucsek, Z.; Toth, P.; Losonczy, G.; Koller, A.; Reglodi, D.; et al. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H292–H306. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Orosz, Z.; Ungvari, Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med. Hypotheses 2006, 67, 904–908. [Google Scholar] [CrossRef]
- Csiszar, A.; Sosnowska, D.; Wang, M.; Lakatta, E.G.; Sonntag, W.E.; Ungvari, Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: Reversal by resveratrol treatment. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 811–820. [Google Scholar] [CrossRef]
- Csiszar, A.; Wang, M.; Lakatta, E.G.; Ungvari, Z.I. Inflammation and endothelial dysfunction during aging: Role of NF-κB. J. Appl. Physiol. 2008, 105, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Rabinovitch, P.S.; Ungvari, Z. Mitochondria and cardiovascular aging. Circ. Res. 2012, 110, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Orosz, Z.; Labinskyy, N.; Rivera, A.; Xiangmin, Z.; Smith, K.; Csiszar, A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H37–H47. [Google Scholar] [CrossRef]
- Dayal, S.; Arning, E.; Bottiglieri, T.; Boger, R.H.; Sigmund, C.D.; Faraci, F.M.; Lentz, S.R. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 2004, 35, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kertowidjojo, E.; Ojaimi, C.; Martin-Fernandez, B.; Kandhi, S.; Wolin, M.; Hintze, T.H. Long-term methionine-diet induced mild hyperhomocysteinemia associated cardiac metabolic dysfunction in multiparous rats. Physiol. Rep. 2015, 3, e12292. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.; Durant, R.; Jaussent, A.; Picot, M.C.; Morena, M.; Badiou, S.; Dupuy, A.M.; Jeandel, C.; Cristol, J.P. Homocysteine and inflammation as main determinants of oxidative stress in the elderly. Free. Radic. Biol. Med. 2009, 46, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Labinskyy, N.; Jimenez, R.; Pinto, J.T.; Ballabh, P.; Losonczy, G.; Pearson, K.J.; de Cabo, R.; Ungvari, Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: Role of circulating factors and SIRT1. Mech. Ageing Dev. 2009, 130, 518–527. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, N.M.; Yabluchanskiy, A.; Springo, Z.; Fulop, G.A.; Ashpole, N.; Gautam, T.; Giles, C.B.; Wren, J.D.; Sonntag, W.E.; et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 2017, 16, 469–479. [Google Scholar] [CrossRef]
- Bailey-Downs, L.C.; Mitschelen, M.; Sosnowska, D.; Toth, P.; Pinto, J.T.; Ballabh, P.; Valcarcel-Ares, M.N.; Farley, J.; Koller, A.; Henthorn, J.C.; et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: A novel model of vascular aging. J. Gerontol. Biol. Med. Sci. 2012, 67, 313–329. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Smith, K.; Rivera, A.; Orosz, Z.; Ungvari, Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am. J. Pathol. 2007, 170, 388–698. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Ungvari, Z.; Zhang, C. Resveratrol improves endothelial function: Role of TNF{alpha} and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, T.L.; Powell, D.K.; Smith, C.D.; Greenstein, A.; Wilcock, D.M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dong, Z.; Zhao, Y.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine induces mitochondrial dysfunction involving the crosstalk between oxidative stress and mitochondrial pSTAT3 in rat ischemic brain. Sci. Rep. 2017, 7, 6932. [Google Scholar] [CrossRef]
- Kaplan, P.; Tatarkova, Z.; Sivonova, M.K.; Racay, P.; Lehotsky, J. Homocysteine and Mitochondria in Cardiovascular and Cerebrovascular Systems. Int. J. Mol. Sci. 2020, 21, 7698. [Google Scholar] [CrossRef] [PubMed]
- Mahaman, Y.A.R.; Huang, F.; Wu, M.; Wang, Y.; Wei, Z.; Bao, J.; Salissou, M.T.M.; Ke, D.; Wang, Q.; Liu, R.; et al. Moringa Oleifera Alleviates Homocysteine-Induced Alzheimer’s Disease-Like Pathology and Cognitive Impairments. J. Alzheimers Dis. 2018, 63, 1141–1159. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Giuffre, M.; Caruso, P.; Gazzin, S.; Tiribelli, C. Homocysteine in Neurology: A Possible Contributing Factor to Small Vessel Disease. Int. J. Mol. Sci. 2021, 22, 2051. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.L.; Chen, Y.; Ren, F.; Wang, L.; Zhu, M.L.; Lu, J.X.; Wang, Q.Q.; Lu, C.B.; Liu, C.; Bai, Y.P.; et al. Nitrosative stress induced by homocysteine thiolactone drives vascular cognitive impairments via GTP cyclohydrolase 1 S-nitrosylation in vivo. Redox Biol. 2022, 58, 102540. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Huffman, D.M.; Vijg, J.; Milman, S.; Singh, R.; Barzilai, N. Einstein-Nathan Shock Center: Translating the hallmarks of aging to extend human health span. Geroscience 2021, 43, 2167–2182. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Meszaros, A.; Molnar, K.; Nogradi, B.; Hernadi, Z.; Nyul-Toth, A.; Wilhelm, I.; Krizbai, I.A. Neurovascular Inflammaging in Health and Disease. Cells 2020, 9, 1614. [Google Scholar] [CrossRef]
- Royce, G.H.; Brown-Borg, H.M.; Deepa, S.S. The potential role of necroptosis in inflammaging and aging. Geroscience 2019, 41, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.R. Role of heat shock proteins in aging and chronic inflammatory diseases. Geroscience 2021, 43, 2515–2532. [Google Scholar] [CrossRef] [PubMed]
- Trial, J.; Diaz Lankenau, R.; Angelini, A.; Tovar Perez, J.E.; Taffet, G.E.; Entman, M.L.; Cieslik, K.A. Treatment with a DC-SIGN ligand reduces macrophage polarization and diastolic dysfunction in the aging female but not male mouse hearts. Geroscience 2021, 43, 881–899. [Google Scholar] [CrossRef]
- Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Bhat, A.; Bishir, M.; Bolla, S.R.; Yang, J.; Essa, M.M.; Chidambaram, S.B.; Guillemin, G.J.; et al. Sleep, brain vascular health and ageing. Geroscience 2020, 42, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Huffman, D.M.; Csiszar, A.; Ungvari, Z. Heterochronic blood exchange attenuates age-related neuroinflammation and confers cognitive benefits: Do microvascular protective effects play a role? Geroscience 2021, 43, 111–113. [Google Scholar] [CrossRef]
- Mehdipour, M.; Mehdipour, T.; Skinner, C.M.; Wong, N.; Liu, C.; Chen, C.C.; Jeon, O.H.; Zuo, Y.; Conboy, M.J.; Conboy, I.M. Plasma dilution improves cognition and attenuates neuroinflammation in old mice. Geroscience 2021, 43, 1–18. [Google Scholar] [CrossRef]
- Thadathil, N.; Nicklas, E.H.; Mohammed, S.; Lewis, T.L., Jr.; Richardson, A.; Deepa, S.S. Necroptosis increases with age in the brain and contributes to age-related neuroinflammation. Geroscience 2021, 43, 2345–2361. [Google Scholar] [CrossRef]
- Towner, R.A.; Gulej, R.; Zalles, M.; Saunders, D.; Smith, N.; Lerner, M.; Morton, K.A.; Richardson, A. Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience 2021, 43, 563–578. [Google Scholar] [CrossRef]
- Dorigatti, A.O.; Riordan, R.; Yu, Z.; Ross, G.; Wang, R.; Reynolds-Lallement, N.; Magnusson, K.; Galvan, V.; Perez, V.I. Brain cellular senescence in mouse models of Alzheimer’s disease. Geroscience 2022, 44, 1157–1168. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Li, Y.; Lei, Z.; Carter, J.; He, J.; Choi, H.M.C.; Khan, N.; Li, H.; Allen, S.; Lipinski, M.M.; et al. Functional and transcriptional profiling of microglial activation during the chronic phase of TBI identifies an age-related driver of poor outcome in old mice. Geroscience 2022, 44, 1407–1440. [Google Scholar] [CrossRef]
- Cribb, L.; Hodge, A.M.; Yu, C.; Li, S.X.; English, D.R.; Makalic, E.; Southey, M.C.; Milne, R.L.; Giles, G.G.; Dugue, P.A. Inflammation and Epigenetic Aging Are Largely Independent Markers of Biological Aging and Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Dugue, P.A.; Hodge, A.M.; Ulvik, A.; Ueland, P.M.; Midttun, O.; Rinaldi, S.; MacInnis, R.J.; Li, S.X.; Meyer, K.; Navionis, A.S.; et al. Association of Markers of Inflammation, the Kynurenine Pathway and B Vitamins with Age and Mortality, and a Signature of Inflammaging. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, Y.; Wei, H.J.; Dong, J.F.; Zhang, J.N. Methionine diet-induced hyperhomocysteinemia accelerates cerebral aneurysm formation in rats. Neurosci. Lett. 2011, 494, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bagi, Z.; Kroenke, C.D.; Fopiano, K.A.; Tian, Y.; Filosa, J.A.; Sherman, L.S.; Larson, E.B.; Keene, C.D.; Degener O’Brien, K.; Adeniyi, P.A.; et al. Association of cerebral microvascular dysfunction and white matter injury in Alzheimer’s disease. Geroscience 2022, 44, 1–14. [Google Scholar] [CrossRef]
- Custodero, C.; Ciavarella, A.; Panza, F.; Gnocchi, D.; Lenato, G.M.; Lee, J.; Mazzocca, A.; Sabba, C.; Solfrizzi, V. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: A systematic review and meta-analysis. Geroscience 2022, 44, 1373–1392. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Castrogiovanni, P.; Vinciguerra, M.; Imbesi, R.; Ulivieri, M.; Fazio, F.; Blennow, K.; Zetterberg, H.; Di Rosa, M. A sex-stratified analysis of neuroimmune gene expression signatures in Alzheimer’s disease brains. Geroscience 2023, 45, 523–541. [Google Scholar] [CrossRef]
- Wan, Y.W.; Al-Ouran, R.; Mangleburg, C.G.; Perumal, T.M.; Lee, T.V.; Allison, K.; Swarup, V.; Funk, C.C.; Gaiteri, C.; Allen, M.; et al. Meta-Analysis of the Alzheimer’s Disease Human Brain Transcriptome and Functional Dissection in Mouse Models. Cell Rep. 2020, 32, 107908. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Fatima, M.; Mondal, A.C. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: Rational insights for the therapeutic approaches. J. Clin. Neurosci. 2019, 59, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L.; Dayon, L.; Kirkland, R.; Wojcik, J.; Peyratout, G.; Severin, I.C.; Henry, H.; Oikonomidi, A.; Migliavacca, E.; Bacher, M.; et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018, 14, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-B interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Garagnani, P.; Santoro, A.; Giuliani, C.; Ostan, R.; Fabbri, C.; Milazzo, M.; Gentilini, D.; di Blasio, A.M.; Pietruszka, B.; et al. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: A pilot study from the NU-AGE project. Geroscience 2020, 42, 687–701. [Google Scholar] [CrossRef]
- de Lima Camillo, L.P.; Quinlan, R.B.A. A ride through the epigenetic landscape: Aging reversal by reprogramming. Geroscience 2021, 43, 463–485. [Google Scholar] [CrossRef]
- Horvath, S.; Zoller, J.A.; Haghani, A.; Jasinska, A.J.; Raj, K.; Breeze, C.E.; Ernst, J.; Vaughan, K.L.; Mattison, J.A. Epigenetic clock and methylation studies in the rhesus macaque. Geroscience 2021, 43, 2441–2453. [Google Scholar] [CrossRef]
- Horvath, S.; Zoller, J.A.; Haghani, A.; Lu, A.T.; Raj, K.; Jasinska, A.J.; Mattison, J.A.; Salmon, A.B. DNA methylation age analysis of rapamycin in common marmosets. Geroscience 2021, 43, 2413–2425. [Google Scholar] [CrossRef]
- Mendenhall, A.R.; Martin, G.M.; Kaeberlein, M.; Anderson, R.M. Cell-to-cell variation in gene expression and the aging process. Geroscience 2021, 43, 181–196. [Google Scholar] [CrossRef]
- Schachtschneider, K.M.; Schook, L.B.; Meudt, J.J.; Shanmuganayagam, D.; Zoller, J.A.; Haghani, A.; Li, C.Z.; Zhang, J.; Yang, A.; Raj, K.; et al. Epigenetic clock and DNA methylation analysis of porcine models of aging and obesity. Geroscience 2021, 43, 2467–2483. [Google Scholar] [CrossRef]
- Fraszczyk, E.; Thio, C.H.L.; Wackers, P.; Dolle, M.E.T.; Bloks, V.W.; Hodemaekers, H.; Picavet, H.S.; Stynenbosch, M.; Verschuren, W.M.M.; Snieder, H.; et al. DNA methylation trajectories and accelerated epigenetic aging in incident type 2 diabetes. Geroscience 2022, 44, 2671–2684. [Google Scholar] [CrossRef] [PubMed]
- Milicic, L.; Vacher, M.; Porter, T.; Dore, V.; Burnham, S.C.; Bourgeat, P.; Shishegar, R.; Doecke, J.; Armstrong, N.J.; Tankard, R.; et al. Comprehensive analysis of epigenetic clocks reveals associations between disproportionate biological ageing and hippocampal volume. Geroscience 2022, 44, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.Y.; Sheng, D.; Bae, H.G.; Kang, S.W.; Fann, D.Y.; Park, J.; Kim, J.; Alli-Shaik, A.; Lee, J.; Kim, E.; et al. Integrative epigenomic and transcriptomic analyses reveal metabolic switching by intermittent fasting in brain. Geroscience 2022, 44, 2171–2194. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.E.; Alsaggaf, R.; Katta, S.; Dagnall, C.; Aubert, G.; Hicks, B.D.; Spellman, S.R.; Savage, S.A.; Horvath, S.; Gadalla, S.M. Telomere length and epigenetic clocks as markers of cellular aging: A comparative study. Geroscience 2022, 44, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.M.; Sommerer, Y.; Kalies, C.H.; Spira, D.; Bertram, L.; Demuth, I. Vitamin D supplementation is associated with slower epigenetic aging. Geroscience 2022, 44, 1847–1859. [Google Scholar] [CrossRef]
- Yusipov, I.; Kondakova, E.; Kalyakulina, A.; Krivonosov, M.; Lobanova, N.; Bacalini, M.G.; Franceschi, C.; Vedunova, M.; Ivanchenko, M. Accelerated epigenetic aging and inflammatory/immunological profile (ipAGE) in patients with chronic kidney disease. Geroscience 2022, 44, 817–834. [Google Scholar] [CrossRef]
- Horvath, S.; Lin, D.T.S.; Kobor, M.S.; Zoller, J.A.; Said, J.W.; Morgello, S.; Singer, E.; Yong, W.H.; Jamieson, B.D.; Levine, A.J. HIV, pathology and epigenetic age acceleration in different human tissues. Geroscience 2022, 44, 1609–1620. [Google Scholar] [CrossRef]
- Nwanaji-Enwerem, J.C.; Colicino, E.; Gao, X.; Wang, C.; Vokonas, P.; Boyer, E.W.; Baccarelli, A.A.; Schwartz, J. Associations of Plasma Folate and Vitamin B6 with Blood DNA Methylation Age: An Analysis of One-Carbon Metabolites in the VA Normative Aging Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 760–769. [Google Scholar] [CrossRef]
- Shadyab, A.H.; McEvoy, L.K.; Horvath, S.; Whitsel, E.A.; Rapp, S.R.; Espeland, M.A.; Resnick, S.M.; Manson, J.E.; Chen, J.C.; Chen, B.H.; et al. Association of Epigenetic Age Acceleration with Incident Mild Cognitive Impairment and Dementia among Older Women. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1239–1244. [Google Scholar] [CrossRef]
- Liu, H.; Lutz, M.; Luo, S.; Alzheimer’s Disease Neuroimaging, I. Genetic Association Between Epigenetic Aging-Acceleration and the Progression of Mild Cognitive Impairment to Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1734–1742. [Google Scholar] [CrossRef]
- Vaccarino, V.; Huang, M.; Wang, Z.; Hui, Q.; Shah, A.J.; Goldberg, J.; Smith, N.; Kaseer, B.; Murrah, N.; Levantsevych, O.M.; et al. Epigenetic Age Acceleration and Cognitive Decline: A Twin Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Chaturvedi, P.; Kalani, K.; Kamat, P.K.; Chaturvedi, P. A high methionine, low folate and vitamin B(6)/B(12) containing diet can be associated with memory loss by epigenetic silencing of netrin-1. Neural Regen. Res. 2019, 14, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A. Assessing the effects of high methionine intake on DNA methylation. J. Nutr. 2006, 136, 1706S–1710S. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.L.; Bueno Rde, B.; Burim, R.V.; Queiroz, R.H.; Bianchi Mde, L.; Antunes, L.M. The effects of dietary supplementation of methionine on genomic stability and p53 gene promoter methylation in rats. Mutat. Res. 2011, 722, 78–83. [Google Scholar] [CrossRef]
- Grasselli, C.; Bombelli, S.; Eriani, S.; Domenici, G.; Galluccio, R.; Tropeano, C.; De Marco, S.; Bolognesi, M.M.; Torsello, B.; Bianchi, C.; et al. DNA Damage in Circulating Hematopoietic Progenitor Stem Cells as Promising Biological Sensor of Frailty. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1279–1286. [Google Scholar] [CrossRef]
- Ognik, K.; Konieczka, P.; Mikulski, D.; Jankowski, J. The effect of different dietary ratios of lysine and arginine in diets with high or low methionine levels on oxidative and epigenetic DNA damage, the gene expression of tight junction proteins and selected metabolic parameters in Clostridium perfringens-challenged turkeys. Vet. Res. 2020, 51, 50. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Folate, DNA damage and the aging brain. Mech. Ageing Dev. 2010, 131, 236–241. [Google Scholar] [CrossRef]
- Hense, J.D.; Garcia, D.N.; Isola, J.V.; Alvarado-Rincon, J.A.; Zanini, B.M.; Prosczek, J.B.; Stout, M.B.; Mason, J.B.; Walsh, P.T.; Brieno-Enriquez, M.A.; et al. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. Geroscience 2022, 44, 1747–1759. [Google Scholar] [CrossRef]
- Fielding, R.A.; Atkinson, E.J.; Aversa, Z.; White, T.A.; Heeren, A.A.; Achenbach, S.J.; Mielke, M.M.; Cummings, S.R.; Pahor, M.; Leeuwenburgh, C.; et al. Associations between biomarkers of cellular senescence and physical function in humans: Observations from the lifestyle interventions for elders (LIFE) study. Geroscience 2022, 44, 2757–2770. [Google Scholar] [CrossRef]
- Dungan, C.M.; Figueiredo, V.C.; Wen, Y.; VonLehmden, G.L.; Zdunek, C.J.; Thomas, N.T.; Mobley, C.B.; Murach, K.A.; Brightwell, C.R.; Long, D.E.; et al. Senolytic treatment rescues blunted muscle hypertrophy in old mice. Geroscience 2022, 44, 1925–1940. [Google Scholar] [CrossRef]
- Bloom, S.I.; Tucker, J.R.; Lim, J.; Thomas, T.G.; Stoddard, G.J.; Lesniewski, L.A.; Donato, A.J. Aging results in DNA damage and telomere dysfunction that is greater in endothelial versus vascular smooth muscle cells and is exacerbated in atheroprone regions. Geroscience 2022, 44, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Karin, O.; Alon, U. Senescent cell accumulation mechanisms inferred from parabiosis. Geroscience 2021, 43, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Wilkinson, J.E.; Hughes, B.; Gadela, N.; Ladiges, W.C.; Vo, N.; Niedernhofer, L.J.; Huffman, D.M.; Robbins, P.D. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience 2020, 42, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Petersen, R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018, 128, 1208–1216. [Google Scholar] [CrossRef]
- Csipo, T.; Lipecz, A.; Ashpole, N.M.; Balasubramanian, P.; Tarantini, S. Astrocyte senescence contributes to cognitive decline. Geroscience 2019, 42, 51–55. [Google Scholar] [CrossRef]
- Lawrence, I.; Bene, M.; Nacarelli, T.; Azar, A.; Cohen, J.Z.; Torres, C.; Johannes, G.; Sell, C. Correlations between age, functional status, and the senescence-associated proteins HMGB2 and p16(INK4a). Geroscience 2018, 40, 193–199. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Chinta, S.J.; Woods, G.; Rane, A.; Demaria, M.; Campisi, J.; Andersen, J.K. Cellular senescence and the aging brain. Exp. Gerontol. 2014, 68, 3–7. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Baker, D.J.; Tachibana, M.; Liu, C.C.; van Deursen, J.M.; Brott, T.G.; Bu, G.; Kanekiyo, T. Vascular Cell Senescence Contributes to Blood-Brain Barrier Breakdown. Stroke 2016, 47, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lei, Q.; Xie, J.; Liu, H.; Li, J.; Zhang, X.; Zhang, T.; Gou, X. Potential Regulators of the Senescence-Associated Secretory Phenotype During Senescence and Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 2207–2218. [Google Scholar] [CrossRef]
- Romashkan, S.; Chang, H.; Hadley, E.C. National Institute on Aging Workshop: Repurposing Drugs or Dietary Supplements for Their Senolytic or Senomorphic Effects: Considerations for Clinical Trials. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1144–1152. [Google Scholar] [CrossRef]
- Baar, M.P.; Brandt, R.M.C.; Putavet, D.A.; Klein, J.D.D.; Derks, K.W.J.; Bourgeois, B.R.M.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.A.; et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017, 169, 132–147.e116. [Google Scholar] [CrossRef]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.G.; Ogrodnik, M.B.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Patil, P.; Dong, Q.; Wang, D.; Chang, J.; Wiley, C.; Demaria, M.; Lee, J.; Kang, J.; Niedernhofer, L.J.; Robbins, P.D.; et al. Systemic clearance of p16. Aging Cell 2019, 18, e12927. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Ahire, C.; Nyul-Toth, A.; DelFavero, J.; Gulej, R.; Faakye, J.A.; Tarantini, S.; Kiss, T.; Kuan-Celarier, A.; Balasubramanian, P.; Ungvari, A.; et al. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell 2023, 22, e13832. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanksiy, A.; Tarantini, S.; Balasubramaniam, P.; Kiss, T.; Csipo, T.; Fulop, G.A.; Lipecz, A.; delFavero, J.; Nyul-Toth, A.; Sonntag, W.E.; et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.S.; Li, J.; Chen, L.; Yang, Y.F.; He, P.L.; Li, J.; Yang, J. Salidroside attenuates endothelial cellular senescence via decreasing the expression of inflammatory cytokines and increasing the expression of SIRT3. Mech. Ageing Dev. 2018, 175, 1–6. [Google Scholar] [CrossRef]
- Albertini, E.; Koziel, R.; Durr, A.; Neuhaus, M.; Jansen-Durr, P. Cystathionine beta synthase modulates senescence of human endothelial cells. Aging 2012, 4, 664–673. [Google Scholar] [CrossRef][Green Version]
- Koziel, R.; Ruckenstuhl, C.; Albertini, E.; Neuhaus, M.; Netzberger, C.; Bust, M.; Madeo, F.; Wiesner, R.J.; Jansen-Durr, P. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell 2014, 13, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Auzmendi-Iriarte, J.; Matheu, A. Impact of Chaperone-Mediated Autophagy in Brain Aging: Neurodegenerative Diseases and Glioblastoma. Front. Aging Neurosci. 2020, 12, 630743. [Google Scholar] [CrossRef] [PubMed]
- Chocron, E.S.; Munkacsy, E.; Kim, H.S.; Karpowicz, P.; Jiang, N.; Van Skike, C.E.; DeRosa, N.; Banh, A.Q.; Palavicini, J.P.; Wityk, P.; et al. Genetic and pharmacologic proteasome augmentation ameliorates Alzheimer’s-like pathology in mouse and fly APP overexpression models. Sci. Adv. 2022, 8, eabk2252. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Taylor, R.C.; Hetz, C. Mastering organismal aging through the endoplasmic reticulum proteostasis network. Aging Cell 2020, 19, e13265. [Google Scholar] [CrossRef]
- Cozachenco, D.; Ribeiro, F.C.; Ferreira, S.T. Defective proteostasis in Alzheimer’s disease. Ageing Res. Rev. 2023, 85, 101862. [Google Scholar] [CrossRef]
- Kulkarni, A.; Preeti, K.; Tryphena, K.P.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Proteostasis in Parkinson’s disease: Recent development and possible implication in diagnosis and therapeutics. Ageing Res. Rev. 2023, 84, 101816. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mishra, A.K.; Peer, G.D.G.; Bagabir, S.A.; Haque, S.; Pandey, R.P.; Raj, V.S.; Jain, N.; Pandey, A.; Kar, S.K. The Interplay of the Unfolded Protein Response in Neurodegenerative Diseases: A Therapeutic Role of Curcumin. Front. Aging Neurosci. 2021, 13, 767493. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.L.; He, L. Disrupted ubiquitin proteasome system underlying tau accumulation in Alzheimer’s disease. Neurobiol. Aging 2021, 99, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Nichenametla, S.N.; Mattocks, D.A.L.; Malloy, V.L.; Pinto, J.T. Sulfur amino acid restriction-induced changes in redox-sensitive proteins are associated with slow protein synthesis rates. Ann. N. Y. Acad. Sci. 2018, 1418, 80–94. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Wu, G.; Sun, J.; Wang, Y.; Guo, H.; Shi, Y.; Cheng, X.; Tang, X.; Le, G. Dietary methionine restriction regulated energy and protein homeostasis by improving thyroid function in high fat diet mice. Food Funct. 2018, 9, 3718–3731. [Google Scholar] [CrossRef]
- Jakubowski, H. Proteomic exploration of cystathionine beta-synthase deficiency: Implications for the clinic. Expert Rev. Proteom. 2020, 17, 751–765. [Google Scholar] [CrossRef]
- Reddy, V.S.; Trinath, J.; Reddy, G.B. Implication of homocysteine in protein quality control processes. Biochimie 2019, 165, 19–31. [Google Scholar] [CrossRef]
- Wiedenhoeft, T.; Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Balasubramanian, P.; Lipecz, A.; Kiss, T.; Csiszar, A.; Csiszar, A.; et al. Fusogenic liposomes effectively deliver resveratrol to the cerebral microcirculation and improve endothelium-dependent neurovascular coupling responses in aged mice. Geroscience 2019, 41, 711–725. [Google Scholar] [CrossRef]
- Lipecz, A.; Csipo, T.; Tarantini, S.; Hand, R.A.; Ngo, B.N.; Conley, S.; Nemeth, G.; Tsorbatzoglou, A.; Courtney, D.L.; Yabluchanska, V.; et al. Age-related impairment of neurovascular coupling responses: A dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience 2019, 41, 341–349. [Google Scholar] [CrossRef]
- Kiss, T.; Balasubramanian, P.; Valcarcel-Ares, M.N.; Tarantini, S.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Reglodi, D.; Zhang, X.A.; Bari, F.; et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for prevention of vascular cognitive impairment. Geroscience 2019, 41, 619–630. [Google Scholar] [CrossRef]
- Fulop, G.A.; Ahire, C.; Csipo, T.; Tarantini, S.; Kiss, T.; Balasubramanian, P.; Yabluchanskiy, A.; Farkas, E.; Toth, A.; Nyul-Toth, A.; et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 2019, 41, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Gottesman, R.F. Cerebrovascular Alterations in Alzheimer Disease. Circ. Res. 2018, 123, 406–408. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Iadecola, C.; Gottesman, R.F. Neurovascular and Cognitive Dysfunction in Hypertension. Circ. Res. 2019, 124, 1025–1044. [Google Scholar] [CrossRef]
- Iadecola, C.; Park, L.; Capone, C. Threats to the mind: Aging, amyloid, and hypertension. Stroke 2009, 40, S40–S44. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Csiszar, A.; Ungvari, Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H1–H20. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Ma, X.; Ding, D.; Zhu, X.; Zhang, H.; Liu, J.; Mu, J.; Zhang, M. Reversal of neurovascular decoupling and cognitive impairment in patients with end-stage renal disease during a hemodialysis session: Evidence from a comprehensive fMRI analysis. Hum. Brain Mapp. 2023, 44, 989–1001. [Google Scholar] [CrossRef]
- Rosengarten, B.; Osthaus, S.; Auch, D.; Kaps, M. Effects of acute hyperhomocysteinemia on the neurovascular coupling mechanism in healthy young adults. Stroke 2003, 34, 446–451. [Google Scholar] [CrossRef]
- Toda, N.; Okamura, T. Hyperhomocysteinemia impairs regional blood flow: Involvements of endothelial and neuronal nitric oxide. Pflugers Arch. 2016, 468, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- van Laar, P.J.; van der Graaf, Y.; Mali, W.P.; van der Grond, J.; Hendrikse, J.; Group, S.S. Effect of cerebrovascular risk factors on regional cerebral blood flow. Radiology 2008, 246, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.J.; Abner, E.; Bakshi, V.; Goulding, D.S.; Grau, E.M.; Lin, A.L.; Norris, C.M.; Sudduth, T.L.; Webster, S.J.; Wilcock, D.M.; et al. Blood Flow Deficits and Cerebrovascular Changes in a Dietary Model of Hyperhomocysteinemia. ASN Neuro 2019, 11, 1759091419865788. [Google Scholar] [CrossRef] [PubMed]
- Giusti, B.; Saracini, C.; Bolli, P.; Magi, A.; Martinelli, I.; Peyvandi, F.; Rasura, M.; Volpe, M.; Lotta, L.A.; Rubattu, S.; et al. Early-onset ischaemic stroke: Analysis of 58 polymorphisms in 17 genes involved in methionine metabolism. Thromb. Haemost. 2010, 104, 231–242. [Google Scholar] [CrossRef]
- Wu, G.H.; Kong, F.Z.; Dong, X.F.; Wu, D.F.; Guo, Q.Z.; Shen, A.R.; Cheng, Q.Z.; Luo, W.F. Association between hyperhomocysteinemia and stroke with atherosclerosis and small artery occlusion depends on homocysteine metabolism-related vitamin levels in Chinese patients with normal renal function. Metab. Brain Dis. 2017, 32, 859–865. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mannisto, S.; Virtanen, M.J.; Kontto, J.; Albanes, D.; Virtamo, J. Folate, vitamin B6, vitamin B12, and methionine intakes and risk of stroke subtypes in male smokers. Am. J. Epidemiol. 2008, 167, 954–961. [Google Scholar] [CrossRef]
- Williams, S.R.; Yang, Q.; Chen, F.; Liu, X.; Keene, K.L.; Jacques, P.; Chen, W.M.; Weinstein, G.; Hsu, F.C.; Beiser, A.; et al. Genome-wide meta-analysis of homocysteine and methionine metabolism identifies five one carbon metabolism loci and a novel association of ALDH1L1 with ischemic stroke. PLoS Genet. 2014, 10, e1004214. [Google Scholar] [CrossRef]
- Kim, O.J.; Hong, S.P.; Ahn, J.Y.; Hong, S.H.; Hwang, T.S.; Kim, S.O.; Yoo, W.; Oh, D.; Kim, N.K. Influence of combined methionine synthase (MTR 2756A > G) and methylenetetrahydrofolate reductase (MTHFR 677C > T) polymorphisms to plasma homocysteine levels in Korean patients with ischemic stroke. Yonsei Med. J. 2007, 48, 201–209. [Google Scholar] [CrossRef][Green Version]
- Selhub, J.; Troen, A.M. Sulfur amino acids and atherosclerosis: A role for excess dietary methionine. Ann. N. Y. Acad. Sci. 2016, 1363, 18–25. [Google Scholar] [CrossRef]
- Toborek, M.; Kopieczna-Grzebieniak, E.; Drozdz, M.; Wieczorek, M. Increased lipid peroxidation as a mechanism of methionine-induced atherosclerosis in rabbits. Atherosclerosis 1995, 115, 217–224. [Google Scholar] [CrossRef]
- Yang, A.N.; Zhang, H.P.; Sun, Y.; Yang, X.L.; Wang, N.; Zhu, G.; Zhang, H.; Xu, H.; Ma, S.C.; Zhang, Y.; et al. High-methionine diets accelerate atherosclerosis by HHcy-mediated FABP4 gene demethylation pathway via DNMT1 in ApoE(−/−) mice. FEBS Lett. 2015, 589, 3998–4009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Moller, J.; Danielsen, C.C.; Bentzon, J.; Ravn, H.B.; Austin, R.C.; Falk, E. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Hare, D.L. High dietary methionine plus cholesterol stimulates early atherosclerosis and late fibrous cap development which is associated with a decrease in GRP78 positive plaque cells. Int. J. Exp. Pathol. 2009, 90, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Hare, D.L.; Buxton, B.F.; Black, M.J. High dietary methionine plus cholesterol exacerbates atherosclerosis formation in the left main coronary artery of rabbits. Atherosclerosis 2004, 176, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, I.T.; Luchakov, Y.I.; Moskvin, A.N.; Gutsaeva, D.R.; Allen, B.W.; Thalmann, E.D.; Piantadosi, C.A. Cerebral blood flow and brain oxygenation in rats breathing oxygen under pressure. J. Cereb. Blood Flow Metab. 2005, 25, 1288–1300. [Google Scholar] [CrossRef]
- Tarantini, S.; Fulop, G.A.; Kiss, T.; Farkas, E.; Zolei-Szenasi, D.; Galvan, V.; Toth, P.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience 2017, 39, 465–473. [Google Scholar] [CrossRef]
- Tarantini, S.; Yabluchanksiy, A.; Fulop, G.A.; Hertelendy, P.; Valcarcel-Ares, M.N.; Kiss, T.; Bagwell, J.M.; O’Connor, D.; Farkas, E.; Sorond, F.; et al. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience 2017, 39, 601–614. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Davila, A.; Valcarcel-Ares, M.N.; Tucsek, Z.; Varamini, B.; Ballabh, P.; Sonntag, W.E.; Baur, J.A.; Csiszar, A.; et al. Purinergic glio-endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1837–H1845. [Google Scholar] [CrossRef]
- Toth, P.; Tarantini, S.; Tucsek, Z.; Ashpole, N.M.; Sosnowska, D.; Gautam, T.; Ballabh, P.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Resveratrol treatment rescues neurovascular coupling in aged mice: Role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H299–H308. [Google Scholar] [CrossRef]
- Tucsek, Z.; Toth, P.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Warrington, J.P.; Giles, C.B.; Wren, J.D.; Koller, A.; Ballabh, P.; et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1339–1352. [Google Scholar] [CrossRef]
- Vendemiale, G.; Romano, A.D.; Dagostino, M.; de Matthaeis, A.; Serviddio, G. Endothelial dysfunction associated with mild cognitive impairment in elderly population. Aging Clin. Exp. Res. 2013, 25, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Bhayadia, R.; Schmidt, B.M.; Melk, A.; Homme, M. Senescence-Induced Oxidative Stress Causes Endothelial Dysfunction. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 161–169. [Google Scholar] [CrossRef] [PubMed]
- de Picciotto, N.E.; Gano, L.B.; Johnson, L.C.; Martens, C.R.; Sindler, A.L.; Mills, K.F.; Imai, S.; Seals, D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016, 15, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Ungvari, Z.; Edwards, J.G.; Kaminski, P.M.; Wolin, M.S.; Koller, A.; Kaley, G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 2002, 90, 1159–1166. [Google Scholar] [CrossRef]
- Istvan, L.; Czako, C.; Elo, A.; Mihaly, Z.; Sotonyi, P.; Varga, A.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef]
- Tarantini, S.; Yabluchanskiy, A.; Lindsey, M.L.; Csiszar, A.; Ungvari, Z. Effect of genetic depletion of MMP-9 on neurological manifestations of hypertension-induced intracerebral hemorrhages in aged mice. Geroscience 2021, 43, 2611–2619. [Google Scholar] [CrossRef]
- Montagne, A.; Barnes, S.R.; Sweeney, M.D.; Halliday, M.R.; Sagare, A.P.; Zhao, Z.; Toga, A.W.; Jacobs, R.E.; Liu, C.Y.; Amezcua, L.; et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015, 85, 296–302. [Google Scholar] [CrossRef]
- Bellamy, M.F.; McDowell, I.F.; Ramsey, M.W.; Brownlee, M.; Bones, C.; Newcombe, R.G.; Lewis, M.J. Hyperhomocysteinemia after an oral methionine load acutely impairs endothelial function in healthy adults. Circulation 1998, 98, 1848–1852. [Google Scholar] [CrossRef]
- Bellamy, M.F.; McDowell, I.F.; Ramsey, M.W.; Brownlee, M.; Newcombe, R.G.; Lewis, M.J. Oral folate enhances endothelial function in hyperhomocysteinaemic subjects. Eur. J. Clin. Investig. 1999, 29, 659–662. [Google Scholar] [CrossRef]
- Chambers, J.C.; McGregor, A.; Jean-Marie, J.; Obeid, O.A.; Kooner, J.S. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: An effect reversible with vitamin C therapy. Circulation 1999, 99, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Obeid, O.A.; Kooner, J.S. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Ueland, P.M.; Obeid, O.A.; Wrigley, J.; Refsum, H.; Kooner, J.S. Improved vascular endothelial function after oral B vitamins: An effect mediated through reduced concentrations of free plasma homocysteine. Circulation 2000, 102, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Chook, P.; Lolin, Y.I.; Cheung, A.S.; Chan, L.T.; Sun, Y.Y.; Sanderson, J.E.; Metreweli, C.; Celermajer, D.S. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation 1997, 96, 2542–2544. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.S.; Chook, P.; Lolin, Y.I.; Sanderson, J.E.; Metreweli, C.; Celermajer, D.S. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J. Am. Coll. Cardiol. 1999, 34, 2002–2006. [Google Scholar] [CrossRef]
- Weiss, N.; Zhang, Y.Y.; Heydrick, S.; Bierl, C.; Loscalzo, J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 12503–12508. [Google Scholar] [CrossRef]
- Kamat, P.K.; Vacek, J.C.; Kalani, A.; Tyagi, N. Homocysteine Induced Cerebrovascular Dysfunction: A Link to Alzheimer’s Disease Etiology. Open Neurol. J. 2015, 9, 9–14. [Google Scholar] [CrossRef]
- Carey, A.; Fossati, S. Hypertension and hyperhomocysteinemia as modifiable risk factors for Alzheimer’s disease and dementia: New evidence, potential therapeutic strategies, and biomarkers. Alzheimers Dement. 2023, 19, 671–695. [Google Scholar] [CrossRef]
- Faber, J.E.; Zhang, H.; Lassance-Soares, R.M.; Prabhakar, P.; Najafi, A.H.; Burnett, M.S.; Epstein, S.E. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1748–1756. [Google Scholar] [CrossRef]
- Riddle, D.R.; Sonntag, W.E.; Lichtenwalner, R.J. Microvascular plasticity in aging. Ageing Res. Rev. 2003, 2, 149–168. [Google Scholar] [CrossRef]
- Chantler, P.D.; Shrader, C.D.; Tabone, L.E.; d’Audiffret, A.C.; Huseynova, K.; Brooks, S.D.; Branyan, K.W.; Grogg, K.A.; Frisbee, J.C. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation 2015, 22, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Frisbee, J.C. Remodeling of the skeletal muscle microcirculation increases resistance to perfusion in obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H104–H111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frisbee, J.C. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R307–R316. [Google Scholar] [CrossRef] [PubMed]
- Frisbee, J.C. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation 2005, 12, 383–392. [Google Scholar] [CrossRef]
- Hoffmann, J.; Haendeler, J.; Aicher, A.; Rössig, L.; Vasa, M.; Zeiher, A.M.; Dimmeler, S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: Important role of nitric oxide. Circ. Res. 2001, 89, 709–715. [Google Scholar] [CrossRef]
- Kobayashi, N.; DeLano, F.A.; Schmid-Schönbein, G.W. Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2114–2121. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Sousa-Ribeiro, D.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson’s disease. J. Cereb. Blood Flow Metab. 2009, 29, 230–234. [Google Scholar] [CrossRef]
- Galli, G.; Pinchera, A.; Piaggi, P.; Fierabracci, P.; Giannetti, M.; Querci, G.; Scartabelli, G.; Manetti, L.; Ceccarini, G.; Martinelli, S.; et al. Serum insulin-like growth factor-1 concentrations are reduced in severely obese women and raise after weight loss induced by laparoscopic adjustable gastric banding. Obes. Surg. 2012, 22, 1276–1280. [Google Scholar] [CrossRef]
- Bancu, I.; Navarro Díaz, M.; Serra, A.; Granada, M.; Lopez, D.; Romero, R.; Bonet, J. Low Insulin-Like Growth Factor-1 Level in Obesity Nephropathy: A New Risk Factor? PLoS ONE 2016, 11, e0154451. [Google Scholar] [CrossRef][Green Version]
- Breese, C.R.; Ingram, R.L.; Sonntag, W.E. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J. Gerontol. 1991, 46, B180–B187. [Google Scholar] [CrossRef]
- Csiszar, A.; Sosnowska, D.; Tucsek, Z.; Gautam, T.; Toth, P.; Losonczy, G.; Colman, R.J.; Weindruch, R.; Anderson, R.M.; Sonntag, W.E.; et al. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Kiss, T.; Wren, J.D.; Giles, C.B.; Griffin, C.T.; Murfee, W.L.; Pacher, P.; Csiszar, A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018, 15, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tucsek, Z.; Sosnowska, D.; Toth, P.; Gautam, T.; Podlutsky, A.; Csiszar, A.; Losonczy, G.; Valcarcel-Ares, M.N.; Sonntag, W.E. Aging-Induced Dysregulation of Dicer1-Dependent MicroRNA Expression Impairs Angiogenic Capacity of Rat Cerebromicrovascular Endothelial Cells. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel-Ares, M.N.; Gautam, T.; Warrington, J.P.; Bailey-Downs, L.; Sosnowska, D.; de Cabo, R.; Losonczy, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: Implications for microvascular aging. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 821–829. [Google Scholar] [CrossRef]
- Bohannon, D.G.; Okhravi, H.R.; Kim, J.; Kuroda, M.J.; Didier, E.S.; Kim, W.K. A subtype of cerebrovascular pericytes is associated with blood-brain barrier disruption that develops during normal aging and simian immunodeficiency virus infection. Neurobiol. Aging 2020, 96, 128–136. [Google Scholar] [CrossRef]
- Costea, L.; Meszaros, A.; Bauer, H.; Bauer, H.C.; Traweger, A.; Wilhelm, I.; Farkas, A.E.; Krizbai, I.A. The Blood-Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int. J. Mol. Sci. 2019, 20, 5472. [Google Scholar] [CrossRef]
- Erdo, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef]
- Farrall, A.J.; Wardlaw, J.M. Blood-brain barrier: Ageing and microvascular disease--systematic review and meta-analysis. Neurobiol. Aging 2009, 30, 337–352. [Google Scholar] [CrossRef]
- Chu, M.; Teng, J.; Guo, L.; Wang, Y.; Zhang, L.; Gao, J.; Liu, L. Mild hyperhomocysteinemia induces blood-brain barrier dysfunction but not neuroinflammation in the cerebral cortex and hippocampus of wild-type mice. Can. J. Physiol. Pharmacol. 2021, 99, 847–856. [Google Scholar] [CrossRef]
- Tchantchou, F.; Hsia, R.C.; Puche, A.; Fiskum, G. Hippocampal vulnerability to hyperhomocysteinemia worsens pathological outcomes of mild traumatic brain injury in rats. J. Cent. Nerv. Syst. Dis. 2023, 15, 11795735231160025. [Google Scholar] [CrossRef]
- Tawfik, A.; Elsherbiny, N.M.; Zaidi, Y.; Rajpurohit, P. Homocysteine and Age-Related Central Nervous System Diseases: Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 6259. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules 2020, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, H.; Chang, N. Hyperhomocysteinemia due to short-term folate deprivation is related to electron microscopic changes in the rat brain. J. Nutr. 2002, 132, 3418–3421. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Markand, S.; Al-Shabrawey, M.; Mayo, J.N.; Reynolds, J.; Bearden, S.E.; Ganapathy, V.; Smith, S.B. Alterations of retinal vasculature in cystathionine-beta-synthase heterozygous mice: A model of mild to moderate hyperhomocysteinemia. Am. J. Pathol. 2014, 184, 2573–2585. [Google Scholar] [CrossRef] [PubMed]
- Gulej, R.; Nyul-Toth, A.; Ahire, C.; DelFavero, J.; Balasubramanian, P.; Kiss, T.; Tarantini, S.; Benyo, Z.; Pacher, P.; Csik, B.; et al. Elimination of senescent cells by treatment with Navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. Geroscience 2023. [Google Scholar] [CrossRef]
- Preininger, M.K.; Kaufer, D. Blood-Brain Barrier Dysfunction and Astrocyte Senescence as Reciprocal Drivers of Neuropathology in Aging. Int. J. Mol. Sci. 2022, 23, 6217. [Google Scholar] [CrossRef]
- Tucsek, Z.; Toth, P.; Sosnowsk, D.; Gautam, T.; Mitschelen, M.; Koller, A.; Szalai, G.; Sonntag, W.E.; Ungvari, Z.; Csiszar, A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: Effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1212–1226. [Google Scholar] [CrossRef]
- Valcarcel-Ares, M.N.; Tucsek, Z.; Kiss, T.; Giles, C.B.; Tarantini, S.; Yabluchanskiy, A.; Balasubramanian, P.; Gautam, T.; Galvan, V.; Ballabh, P.; et al. Obesity in Aging Exacerbates Neuroinflammation, Dysregulating Synaptic Function-related Genes and Altering Eicosanoid Synthesis in the Mouse Hippocampus: Potential Role in Impaired Synaptic Plasticity and Cognitive Decline. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 74, 290–298. [Google Scholar] [CrossRef]
- Baydas, G.; Ozer, M.; Yasar, A.; Tuzcu, M.; Koz, S.T. Melatonin improves learning and memory performances impaired by hyperhomocysteinemia in rats. Brain Res. 2005, 1046, 187–194. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Amakhin, D.V.; Trofimova, A.M.; Tumanova, N.L.; Dubrovskaya, N.M.; Kalinina, D.S.; Kovalenko, A.A.; Shcherbitskaia, A.D.; Vasilev, D.S.; Zaitsev, A.V. Maternal Hyperhomocysteinemia Produces Memory Deficits Associated with Impairment of Long-Term Synaptic Plasticity in Young Rats. Cells 2022, 12, 58. [Google Scholar] [CrossRef]
- Suszynska-Zajczyk, J.; Luczak, M.; Marczak, L.; Jakubowski, H. Hyperhomocysteinemia and bleomycin hydrolase modulate the expression of mouse brain proteins involved in neurodegeneration. J. Alzheimers Dis. 2014, 40, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, X.; Zhang, Q.; Luo, S.; Liu, H.; Wang, X.; Sai, N.; Zhang, X. Homocysteine can aggravate depressive like behaviors in a middle cerebral artery occlusion/reperfusion rat model: A possible role for NMDARs-mediated synaptic alterations. Nutr. Neurosci. 2023, 26, 483–495. [Google Scholar] [CrossRef]
- Viggiano, A.; Viggiano, E.; Monda, M.; Ingrosso, D.; Perna, A.F.; De Luca, B. Methionine-enriched diet decreases hippocampal antioxidant defences and impairs spontaneous behaviour and long-term potentiation in rats. Brain Res. 2012, 1471, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.S.; Jiang, X.; Ni, Z.F.; Ma, Z.W.; Xie, A.J.; Cheng, X.S.; Wang, Q.; Wang, J.Z.; Liu, G.P. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J. Neurochem. 2013, 124, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zeng, X.N.; Guo, H.M.; Wu, X.M.; Chen, H.J.; Di, R.K.; Wu, Y. Cognitive and neurochemical alterations in hyperhomocysteinemic rat. Neurol. Sci. 2012, 33, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Mabasa, L.; Crane, C.; Park, C.S.; Venancio, V.P.; Bianchi, M.L.; Antunes, L.M. Maternal vitamin B6 deficient or supplemented diets on expression of genes related to GABAergic, serotonergic, or glutamatergic pathways in hippocampus of rat dams and their offspring. Mol. Nutr. Food Res. 2016, 60, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Kulminski, A.M.; Loiko, E.; Loika, Y.; Culminskaya, I. Pleiotropic predisposition to Alzheimer’s disease and educational attainment: Insights from the summary statistics analysis. Geroscience 2022, 44, 265–280. [Google Scholar] [CrossRef]
- Lu, W.H.; de Souto Barreto, P.; Rolland, Y.; Bouyahia, A.; Fischer, C.; Mangin, J.F.; Giudici, K.V.; Vellas, B.; Group, M.D. Biological and Neuroimaging Markers as Predictors of 5-Year Incident Frailty in Older Adults: A Secondary Analysis of the MAPT Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, e361–e369. [Google Scholar] [CrossRef]
- Guillotin, S.; Vallet, A.; Lorthois, S.; Cestac, P.; Schmidt, E.; Delcourt, N. Association Between Homocysteine, Frailty and Biomechanical Response of the CNS in NPH-Suspected Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1335–1343. [Google Scholar] [CrossRef]
- Kovalska, M.; Baranovicova, E.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Kovalska, L.; Lehotsky, J. Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats. Int. J. Mol. Sci. 2021, 22, 4961. [Google Scholar] [CrossRef]
- Gallucci, M.; Zanardo, A.; Bendini, M.; Di Paola, F.; Boldrini, P.; Grossi, E. Serum folate, homocysteine, brain atrophy, and auto-CM system: The Treviso Dementia (TREDEM) study. J. Alzheimers Dis. 2014, 38, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.J.; Dimayuga, E.; Morganti, J.M.; Van Eldik, L.J. Microglial-associated responses to comorbid amyloid pathology and hyperhomocysteinemia in an aged knock-in mouse model of Alzheimer’s disease. J. Neuroinflamm. 2020, 17, 274. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.B.; Han, J.W.; Song, J.; Lee, K.; Kim, T.H.; Kwak, K.P.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Moon, S.W.; et al. Hypohomocysteinemia may increases the risk of dementia and Alzheimer’s disease: A nationwide population-based prospective cohort study. Clin. Nutr. 2021, 40, 4579–4584. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, N.M.; Sharma, I.; Kira, D.; Alhusban, S.; Samra, Y.A.; Jadeja, R.; Martin, P.; Al-Shabrawey, M.; Tawfik, A. Homocysteine Induces Inflammation in Retina and Brain. Biomolecules 2020, 10, 393. [Google Scholar] [CrossRef]
- Farina, N.; Jerneren, F.; Turner, C.; Hart, K.; Tabet, N. Homocysteine concentrations in the cognitive progression of Alzheimer’s disease. Exp. Gerontol. 2017, 99, 146–150. [Google Scholar] [CrossRef]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Adamkov, M.; Kovalska, L.; Lehotsky, J. Alzheimer’s Disease-like Pathological Features in the Dorsal Hippocampus of Wild-Type Rats Subjected to Methionine-Diet-Evoked Mild Hyperhomocysteinaemia. Cells 2023, 12, 2087. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsu, S.W.; Huang, C.W.; Chang, W.N.; Chen, S.F.; Wu, M.K.; Chang, C.C.; Hwang, L.C.; Chen, P.C. Effects of Homocysteine on white matter diffusion parameters in Alzheimer’s disease. BMC Neurol. 2017, 17, 192. [Google Scholar] [CrossRef]
- Luzzi, S.; Cherubini, V.; Falsetti, L.; Viticchi, G.; Silvestrini, M.; Toraldo, A. Homocysteine, Cognitive Functions, and Degenerative Dementias: State of the Art. Biomedicines 2022, 10, 2741. [Google Scholar] [CrossRef]
- Shirafuji, N.; Hamano, T.; Yen, S.H.; Kanaan, N.M.; Yoshida, H.; Hayashi, K.; Ikawa, M.; Yamamura, O.; Kuriyama, M.; Nakamoto, Y. Homocysteine Increases Tau Phosphorylation, Truncation and Oligomerization. Int. J. Mol. Sci. 2018, 19, 891. [Google Scholar] [CrossRef]
- Song, Y.; Quan, M.; Li, T.; Jia, J. Serum Homocysteine, Vitamin B12, Folate, and Their Association with Mild Cognitive Impairment and Subtypes of Dementia. J. Alzheimers Dis. 2022, 90, 681–691. [Google Scholar] [CrossRef]
- Weekman, E.M.; Johnson, S.N.; Rogers, C.B.; Sudduth, T.L.; Xie, K.; Qiao, Q.; Fardo, D.W.; Bottiglieri, T.; Wilcock, D.M. Atorvastatin rescues hyperhomocysteinemia-induced cognitive deficits and neuroinflammatory gene changes. J. Neuroinflamm. 2023, 20, 199. [Google Scholar] [CrossRef]
- Weekman, E.M.; Sudduth, T.L.; Price, B.R.; Woolums, A.E.; Hawthorne, D.; Seaks, C.E.; Wilcock, D.M. Time course of neuropathological events in hyperhomocysteinemic amyloid depositing mice reveals early neuroinflammatory changes that precede amyloid changes and cerebrovascular events. J. Neuroinflamm. 2019, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Weekman, E.M.; Woolums, A.E.; Sudduth, T.L.; Wilcock, D.M. Hyperhomocysteinemia-Induced Gene Expression Changes in the Cell Types of the Brain. ASN Neuro 2017, 9, 1759091417742296. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Cervellati, C.; Brombo, G.; Trentini, A.; Roncon, L.; Zuliani, G. Elevated Blood Homocysteine and Risk of Alzheimer’s Dementia: An Updated Systematic Review and Meta-Analysis Based on Prospective Studies. J. Prev. Alzheimers Dis. 2021, 8, 329–334. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Judica, E.; Cotelli, M.; Alu, F.; Rossini, P.M. Human brain networks: A graph theoretical analysis of cortical connectivity normative database from EEG data in healthy elderly subjects. Geroscience 2020, 42, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.W.; Pieruccini-Faria, F.; Witt, S.T.; Bartha, R.; Doherty, T.J.; Nagamatsu, L.S.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; Bherer, L.; et al. Combining exercise with cognitive training and vitamin D(3) to improve functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI). Results from the SYNERGIC trial. Geroscience 2023, 45, 1967–1985. [Google Scholar] [CrossRef]
- Bray, N.W.; Pieruccini-Faria, F.; Witt, S.T.; Rockwood, K.; Bartha, R.; Doherty, T.J.; Nagamatsu, L.S.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; et al. Frailty and functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI): Baseline results from the SYNERGIC Trial. Geroscience 2023, 45, 1033–1048. [Google Scholar] [CrossRef]
- Czoch, A.; Kaposzta, Z.; Mukli, P.; Stylianou, O.; Eke, A.; Racz, F.S. Resting-state fractal brain connectivity is associated with impaired cognitive performance in healthy aging. Geroscience 2023. [Google Scholar] [CrossRef]
- Hardcastle, C.; Hausman, H.K.; Kraft, J.N.; Albizu, A.; Evangelista, N.D.; Boutzoukas, E.M.; O’Shea, A.; Langer, K.; Van Van Etten, E.; Bharadwaj, P.K.; et al. Higher-order resting state network association with the useful field of view task in older adults. Geroscience 2022, 44, 131–145. [Google Scholar] [CrossRef]
- Hausman, H.K.; Hardcastle, C.; Albizu, A.; Kraft, J.N.; Evangelista, N.D.; Boutzoukas, E.M.; Langer, K.; O’Shea, A.; Van Etten, E.J.; Bharadwaj, P.K.; et al. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. Geroscience 2022, 44, 847–866. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, L.C.; Wu, R.M.; Hwang, I.S. Effects of task prioritization on a postural-motor task in early-stage Parkinson’s disease: EEG connectivity and clinical implication. Geroscience 2022, 44, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.; Stumme, J.; da Costa Campos, L.; Dellani, P.; Rubbert, C.; Caspers, J.; Caspers, S.; Jockwitz, C. Prediction of cognitive performance differences in older age from multimodal neuroimaging data. Geroscience 2023. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, F.; Pappalettera, C.; Guglielmi, V.; Cacciotti, A.; Manenti, R.; Judica, E.; Vecchio, F.; Rossini, P.M. The combination of hyperventilation test and graph theory parameters to characterize EEG changes in mild cognitive impairment (MCI) condition. Geroscience 2023, 45, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Pappalettera, C.; Miraglia, F.; Cotelli, M.; Rossini, P.M.; Vecchio, F. Analysis of complexity in the EEG activity of Parkinson’s disease patients by means of approximate entropy. Geroscience 2022, 44, 1599–1607. [Google Scholar] [CrossRef]
- Ren, P.; Ma, M.; Zhuang, Y.; Huang, J.; Tan, M.; Wu, D.; Luo, G. Dorsal and ventral fronto-amygdala networks underlie risky decision-making in age-related cognitive decline. Geroscience 2023. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, L.; He, C.; Feng, H.; Xie, C.; Depression Imaging, R.C. Age- and gender-related dispersion of brain networks across the lifespan. Geroscience 2023. [Google Scholar] [CrossRef]
- Williamson, J.; James, S.A.; Mukli, P.; Yabluchanskiy, A.; Wu, D.H.; Sonntag, W.; Ciro, C.; Yang, Y. Sex difference in brain functional connectivity of hippocampus in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 959394. [Google Scholar] [CrossRef]
- Garcia-Casares, N.; Bernal-Lopez, M.R.; Roe-Vellve, N.; Gutierrez-Bedmar, M.; Fernandez-Garcia, J.C.; Garcia-Arnes, J.A.; Ramos-Rodriguez, J.R.; Alfaro, F.; Santamaria-Fernandez, S.; Steward, T.; et al. Brain Functional Connectivity Is Modified by a Hypocaloric Mediterranean Diet and Physical Activity in Obese Women. Nutrients 2017, 9, 685. [Google Scholar] [CrossRef]
- Gaynor, A.M.; Varangis, E.; Song, S.; Gazes, Y.; Noofoory, D.; Babukutty, R.S.; Habeck, C.; Stern, Y.; Gu, Y. Diet moderates the effect of resting state functional connectivity on cognitive function. Sci. Rep. 2022, 12, 16080. [Google Scholar] [CrossRef]
- Li, T.; Willette, A.A.; Wang, Q.; Pollpeter, A.; Larsen, B.A.; Mohammadiarvejeh, P.; Fili, M. Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank. Nutrients 2023, 15, 3390. [Google Scholar] [CrossRef]
- Jiang, L.; Geng, W.; Chen, H.; Zhang, H.; Bo, F.; Mao, C.N.; Chen, Y.C.; Yin, X. Decreased functional connectivity within the default-mode network in acute brainstem ischemic stroke. Eur. J. Radiol. 2018, 105, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Li, X.; Chang, L.; Liu, Y.; Jia, L.; Gao, L.; Ren, L. Hypertension with High Homocysteine Is Associated with Default Network Gray Matter Loss. Front. Neurol. 2021, 12, 740819. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Lehoczki, A.; Tarantini, S.; Varga, J.T. Effects of omega-3 supplementation on quality of life, nutritional status, inflammatory parameters, lipid profile, exercise tolerance and inhaled medications in chronic obstructive pulmonary disease. Ann. Palliat. Med. 2022, 11, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yabluchanskiy, A.; Tarantini, S.; Allu, S.R.; Sencan-Egilmez, I.; Leng, J.; Alfadhel, M.A.H.; Porter, J.E.; Fu, B.; Ran, C.; et al. Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: Evidence for microvascular injury in the cerebral white matter. Geroscience 2023, 45, 1491–1510. [Google Scholar] [CrossRef]
- Negri, S.; Sanford, M.; Shi, H.; Tarantini, S. The role of endothelial TRP channels in age-related vascular cognitive impairment and dementia. Front. Aging Neurosci. 2023, 15, 1149820. [Google Scholar] [CrossRef]
- Sanford, M.; Negri, S.; Tarantini, S. Editorial: New developments in understanding brain and cerebromicrovascular aging: Toward prevention of vascular cognitive impairment and Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1020271. [Google Scholar] [CrossRef]
- Tarantini, S.; Subramanian, M.; Butcher, J.T.; Yabluchanskiy, A.; Li, X.; Miller, R.A.; Balasubramanian, P. Revisiting adipose thermogenesis for delaying aging and age-related diseases: Opportunities and challenges. Ageing Res. Rev. 2023, 87, 101912. [Google Scholar] [CrossRef]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortola, R.; Carballo-Casla, A.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Sotos-Prieto, M. Plant-based diets and risk of frailty in community-dwelling older adults: The Seniors-ENRICA-1 cohort. Geroscience 2023, 45, 221–232. [Google Scholar] [CrossRef]
- Dobreva, I.; Marston, L.; Mukadam, N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience 2022, 44, 2541–2554. [Google Scholar] [CrossRef]
- Raffin, J.; Rolland, Y.; Aggarwal, G.; Nguyen, A.D.; Morley, J.E.; Li, Y.; Bateman, R.J.; Vellas, B.; Barreto, P.S.; Group, M.D. Associations Between Physical Activity, Blood-Based Biomarkers of Neurodegeneration, and Cognition in Healthy Older Adults: The MAPT Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungvari, A.; Gulej, R.; Csik, B.; Mukli, P.; Negri, S.; Tarantini, S.; Yabluchanskiy, A.; Benyo, Z.; Csiszar, A.; Ungvari, Z. The Role of Methionine-Rich Diet in Unhealthy Cerebrovascular and Brain Aging: Mechanisms and Implications for Cognitive Impairment. Nutrients 2023, 15, 4662. https://doi.org/10.3390/nu15214662
Ungvari A, Gulej R, Csik B, Mukli P, Negri S, Tarantini S, Yabluchanskiy A, Benyo Z, Csiszar A, Ungvari Z. The Role of Methionine-Rich Diet in Unhealthy Cerebrovascular and Brain Aging: Mechanisms and Implications for Cognitive Impairment. Nutrients. 2023; 15(21):4662. https://doi.org/10.3390/nu15214662
Chicago/Turabian StyleUngvari, Anna, Rafal Gulej, Boglarka Csik, Peter Mukli, Sharon Negri, Stefano Tarantini, Andriy Yabluchanskiy, Zoltan Benyo, Anna Csiszar, and Zoltan Ungvari. 2023. "The Role of Methionine-Rich Diet in Unhealthy Cerebrovascular and Brain Aging: Mechanisms and Implications for Cognitive Impairment" Nutrients 15, no. 21: 4662. https://doi.org/10.3390/nu15214662
APA StyleUngvari, A., Gulej, R., Csik, B., Mukli, P., Negri, S., Tarantini, S., Yabluchanskiy, A., Benyo, Z., Csiszar, A., & Ungvari, Z. (2023). The Role of Methionine-Rich Diet in Unhealthy Cerebrovascular and Brain Aging: Mechanisms and Implications for Cognitive Impairment. Nutrients, 15(21), 4662. https://doi.org/10.3390/nu15214662