Abstract
(1) Background: Current studies show conflicting results regarding the relationship between dietary acid load (DAL) and blood pressure. (2) Methods: The study used data from the Chinese Health and Nutrition Survey (CHNS) 2009. DAL was assessed on the basis of potential renal acid load (PRAL) and net endogenous acid production (NEAP). To examine the link between DAL and the risk of hypertension, a multivariate logistic regression model was utilized. (3) Results: A total of 7912 subjects were enrolled in the study, of whom 2133 participants had hypertension, a prevalence of 27.0%. After accounting for potential covariates, higher PRAL and NEAP scores were associated with a greater likelihood of developing hypertension, with ORs of 1.34 (95% CI, 1.10–1.62) and 1.29 (95% CI, 1.09–1.53) for PRAL and NEAP scores in Q4, respectively, compared with Q1. In the male group, PRAL and NEAP scores were positively linked to hypertension risk, with ORs of 1.33 (95% CI, 1.06–1.67) and 1.46 (95% CI, 1.14–1.85) for PRAL and NEAP scores in Q4, respectively, compared with Q1, while no significant associations were observed in the female group. Correlations between PRAL scores and hypertension risk lacked significance in the subgroup analyses for participants aged <60 years. There was a significant nonlinear connection observed in the dose–response relationship between DAL (based on PRAL) and hypertension; (4) Conclusions: In Chinese adults, higher PRAL and NEAP scores were positively linked to hypertension risk. This implies that a diet with a low DAL may be a favorable dietary pattern for lowering blood pressure.
1. Introduction
Worldwide, hypertension is a crucial risk factor for chronic kidney disease, cardiovascular disease, stroke, and premature death, and it has become an important public health problem affecting human health [1]. The worldwide occurrence of high blood pressure in individuals between the ages of 30 and 79 was 32% for females and 34% for males in 2019, indicating that it is a significant factor in the overall impact of illness worldwide [2]. Although the prevalence of hypertension is generally on the rise, the rate of achieving blood pressure control remains low, with more than 40% of individuals treated for hypertension failing to achieve satisfactory blood pressure control, particularly in low- and middle-income countries [3]. Besides genetic factors, the main causes of hypertension are largely attributable to modifiable environmental factors, among which is a poor diet, which has long been recognized to increase the risk of developing hypertension [4]. Dietary intake of meat, fruit, vegetables, sodium, magnesium, and potassium has an impact on blood pressure values, which can be negative or beneficial. The World Health Organization guidelines for the prevention and treatment of hypertension indicate that dietary changes can reduce blood pressure, and a healthy diet plays a major role in preventing cardiovascular disease [1].
Numerous prior studies have indicated that adopting nutritious eating habits, like the Mediterranean diet and the DASH diet, can decrease the likelihood of hypertension by providing an abundance of anti-inflammatory and antioxidant substances [5,6]. However, in recent years, imbalances in endogenous acid–base homeostasis have been recognized as a driver of many chronic diseases, with potentially significant implications for cardiometabolic risk factors [7]. While food is being digested, it can produce either acid or base precursors (like sulfate or organic anions, such as citrate, malate, etc.), which can have a significant impact on endogenous acid–base homeostasis [8]. Data on food intake can be used to measure DAL and thus estimate the body acid–base changes induced by food intake [9]. It has been suggested that chronic DAL can result in mild metabolic acidosis, potentially leading to elevated cortisol release and impacting blood pressure levels [10]. Frassetto and Remer et al. suggested that DAL could be estimated using mathematical equations, with NEAP and PRAL being the two frequently employed methods for assessment [8,11]. The NEAP score is determined by considering the intake of dietary protein and potassium, while the PRAL score considers the intake of dietary protein, potassium, magnesium, calcium, and phosphorus [12]. Higher PRAL and NEAP scores indicate greater acidogenic potential and correlate with higher dietary acid load [12,13]. Generally, foods that are abundant in protein and phosphorus, primarily those derived from animal sources, like meat, seafood, and cheese, as well as plant sources, like grains, have the potential to increase dietary acid load (DAL) as acidic precursors [14], whereas foods rich in potassium, calcium, and magnesium, such as vegetables and fruits, are prone to alkalizing effects with the exchange of hydrogen ions, which reduce DAL [15].
Recently, many scholars have begun to focus on the correlation between DAL and the likelihood of high blood pressure. However, the existing studies are inconsistent and contradictory. An observational study from Germany involving 6788 subjects showed that elevated DAL scores were linked to higher systolic blood pressure and a greater prevalence of hypertension, with individuals in the highest PRAL score category having a 45% higher likelihood of developing hypertension compared with those in the group with the lowest score group [16]. However, Rotterdam’s study showed no significant association [17]. The majority of the existing proof comes from high-income countries, and the findings of international research might not be relevant to the Chinese population because of variances in geographical regions, ethnicities, and dietary habits. Until now, there has been minimal investigation into this matter in China, with only one cross-sectional study published on a population in southern China, which showed that PRAL was positively associated with hypertension risk among males [18]. However, this study lacked national representation, and the extrapolation of the results was limited. Considering the current high prevalence of hypertension, it is imperative to carry out additional research on the correlation between DAL and the likelihood of developing hypertension to gather more epidemiological proof among the Chinese population and offer scientific recommendations for the prevention and treatment of hypertension. Consequently, we employed the Chinese Health and Nutrition Survey (CHNS) to evaluate whether DAL is linked to the likelihood of hypertension in Chinese adults.
2. Materials and Methods
2.1. Data Sources and Study Population
This research used open data from the CHNS, a prospective longitudinal study based on household surveys [19]. The first survey was in 1989, and so far, nine rounds of data collection have been conducted, including basic information, disease and health information, socioeconomic status, and dietary and exercise status. Since biological information was only collected in 2009, our study used 2009 data.
A total of 18,805 subjects participated in the CHNS in 2009. The study ultimately included 7912 participants with complete recorded information on anthropometric indices, lifestyle habits, dietary data, and blood serologic tests. We excluded 7541 subjects without dietary information, 34 participants with unreasonable energy intake, 2232 participants with missing serologic examination data, 58 pregnant women, 773 participants aged <18 years, and 255 participants with severe cardiovascular and cerebrovascular diseases (Figure 1).
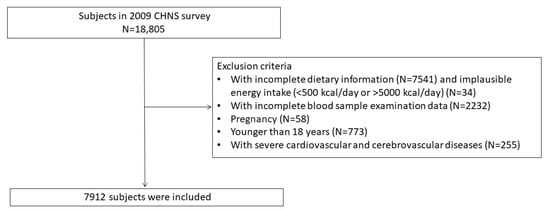
Figure 1.
Flowchart outlining the process of selecting participants.
A consent form was filled out by each participant before the study. This study was ethically reviewed by the Carolina Population Center at the University of North Carolina at Chapel Hill and the Institute of Nutrition and Health at the Chinese Center for Disease Control and Prevention (CCDC) (2015017).
2.2. Evaluation of Diet, PRAL and NEAP
Data on the dietary intake of participants was gathered through a reliable 24 h dietary survey, and dietary consumption information was collected for a total of 3 days (2 weekdays and 1 weekend) [20]. According to the Chinese Food Composition Table (2002 and 2004), daily intakes of energy and nutrients were calculated.
The formulae used to calculate PRAL and NEAP scores were as follows [8,11].
PRAL (mEq/d) = 0.4888 × protein intake (g/d) + 0.0366 × phosphorus (mg/d) − 0.0205 × potassium (mg/d) − 0.0125 × calcium (mg/d) − 0.0263 × magnesium (mg/d)
NEAP (mEq/d) = (54.5 × protein intake (g/d) ÷ potassium intake (mEq/d)) − 10.2.
2.3. Definition of Hypertension
Trained physicians used a standard mercury sphygmomanometer to take blood pressure measurements on the subject’s right arm and completed three measurements at 30 s intervals if each measurement was performed normally. Otherwise, subjects were asked to rest for 10–30 min before the next measurement [21]. In our study, the average of three measurements of systolic and diastolic blood pressure was used. Hypertension was defined as an SBP of ≥140 mm Hg and/or a diastolic blood pressure of ≥90 mm Hg, either previously diagnosed by a physician or currently on medication to lower blood pressure [22].
2.4. Other Variables
Trained researchers collected sociodemographic information, behavioral lifestyle information, anthropometric indicators, and medical history through the CHNS questionnaire. Sociodemographic information included age (continuous variable), gender, marital status (single, married, or other), region (urban or rural), and education level categorized into 3 levels: low (elementary school and below), medium (middle school, high school, and technical school), and high (college and above). Behavioral lifestyle included smoking status (yes or no), alcohol intake (yes or no), sleep duration (6–9 h, ≤6 h, or ≥9 h), and physical activity (continuous variable). Physical activity was calculated by multiplying subjects’ occupation-related activity, transportation-related activity, and daily physical activity by specific metabolic equivalents (METs) to calculate the corresponding energy metabolism level and then multiplying the MET value of individual activities by the duration of the individual activities to calculate the total metabolic equivalent hours per week. The final unit was MET-hours/week. The calculation of METs was based on the 2011 update of the Physical Activity Compendium and previous studies with data from China [23,24]. Body mass index (BMI, continuous variable) was calculated according to a specific formula based on height (m) and weight (kg). Blood samples were obtained after fasting to measure total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), and triglycerides (TG), and all of the above were statistically analyzed as continuous variables. Hyperuricemia was classified into two groups, “yes” and “no”, on the basis of serum uric acid levels of ≥420 µmol/L (7 mg/dL) in men and ≥360 µmol/L (6 mg/dL) in women [25,26]. Diabetes mellitus was defined as fasting plasma glucose (FPG) ≥7.0 mmol/L, glycosylated hemoglobin ≥6.5%, or self-reported diabetes mellitus and was categorized into “yes” and “no” groups [27]. On the basis of serum creatinine values, the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [28].
2.5. Statistical Analysis
A descriptive analysis of baseline characteristics was performed using the mean ± standard deviation (SD) or median (interquartile range) for continuous variables and counts (percentages) for categorical variables. In the analysis of variance, Student’s t-tests were used for normally distributed continuous variables, Wilcoxon rank-sum tests were used for non-normally distributed variables, and chi-square tests were used for categorical variables.
We used multilevel logistic regression models to explore the relationship between DAL (PRAL and NEAP) and hypertension risk. The odds ratio (OR) was computed by utilizing the quartiles of the DAL scores, with Q1, the group with the lowest scores, serving as the baseline reference group. Since sodium intake affects blood pressure values, it was included as a covariate for statistical analysis. The calculation of sodium in this study included only the contents of sodium in food. Three models were set up in this study to analyze the exposure factors and outcome variables. Model 1 included no covariates; model 2 adjusted for age, sex, education, region, marital status, smoking status, alcohol intake, sleep duration, BMI, TC, TG, HDL-C, LDL-C, hyperuricemia, diabetes mellitus, and eGFR; and model 3 was further adjusted for energy, dietary fiber, and sodium intake. Dividing the population by age (<60 and ≥60 years) and gender, we conducted subgroup analyses. Meanwhile, considering that physical activity can affect blood pressure, the CHNS gathered information on the physical activity of the participants, and physical activity was examined with post hoc analyses because 2937 (37%) of the study subjects were missing data on physical activity in our study. After excluding subjects with missing data, physical activity was used as a covariate to investigate the potential impact of this covariate with model 3. Taking into account that subjects may have changed their existing diets because of disease conditions, we also performed sensitivity analyses, excluding participants with diabetes and an eGFR of <60 mL/min/1.73 m2.
To investigate the dose–response correlation between DAL and hypertension prevalence, we examined the nonlinear relationship using a multivariate-adjusted restricted cubic spline (model 3).
A statistically significant value was considered when p < 0.05 on both sides. Statistical analysis was performed on all data using SPSS 23. The figures were generated by GraphPad Prism 9.4.1 and R version 4.2.2.
3. Results
3.1. Participants Characteristics
Our study enrolled 7912 subjects with a mean age of 50.2 ± 14.9 years, of whom 4187 (52.9%) were females and 3725 (47.1%) were males. The study included 2133 individuals with hypertension, indicating a hypertension prevalence rate of 27.0%. A total of 5496 participants (69.5%) lived in rural areas, 5367 (67.8%) had a sleep duration of 6–9 h, 2450 (31.0%) and 1678 (21.2%) history of smoking and alcohol intake, respectively, 1195 (15.1%) had hyperuricemia, and 827 (10.5%) had diabetes mellitus. The median PRAL and NEAP were 24.7 mEq/d and 76.6 mEq/d, respectively. Subjects were divided into two groups according to whether they had hypertension or not, and the distribution of their characteristics is described in Table 1. The hypertension group was older and had higher TC, TG, LDL-C, and BMI and lower eGFR and physical activity levels compared with the non-hypertension group.

Table 1.
Characteristics of subjects categorized by their hypertension status.
The nutrient intake of the subjects was expressed using energy adjustment, as shown in Table 2. The hypertension group consumed a higher amount of energy-adjusted total protein, plant protein, calcium, phosphorus, and sodium, and the non-hypertension group demonstrated more energy intake. There were no differences in energy-adjusted carbohydrate, fat, dietary fiber, animal protein, potassium, or magnesium consumption between the two groups.

Table 2.
Intakes of energy and nutrients (energy-adjusted, per 1000 kcal).
3.2. The Correlation between DAL and Hypertension
3.2.1. PRAL and Hypertension
As shown in Table 3, in the logistic regression statistics of model 1, we found no correlation between the PRAL score and hypertension risk. The reference group comprised the data of the first quartile (Q1), and the OR values of Q2, Q3, and Q4 were 0.92 (0.81–1.07), 0.88 (0.76–1.01), and 1.03 (0.90–1.19), respectively, which were not statistically different. By contrast, in model 2 and model 3, there was a correlation between elevated PRAL scores and an increased risk of hypertension. Refer to Table S1 for complete information. When PRAL was used as a continuous variable in model 3, positive correlations were also found; these results are not shown.

Table 3.
ORs (and 95%CIs) based on PRAL for hypertension risk.
3.2.2. NEAP and Hyperuricemia
Consistent with the PRAL statistics, the NEAP scores and the risk of hypertension were not statistically significant in model 1. However, after further adjustment for covariates in model 2 and model 3, the findings indicated that increased NEAP scores were linked to an elevated risk of hypertension (Table 4). Model 3 showed ORs of 1.09 (0.93–1.28), 1.12 (1.03–1.43), and 1.29 (1.09–1.53) in Q2, Q3, and Q4, respectively, compared with Q1. The detailed results are shown in Table S2. Significant positive correlations also existed when NEAP was used as a continuous variable in model 3; these results are not shown.

Table 4.
ORs (and 95%CIs) based on NEAP for hypertension risk.
3.2.3. Stratification Analysis Based on Gender and Age
In the female subgroup, there was no correlation between PRAL or NEAP scores and a higher likelihood of developing hypertension. In the male group, after controlling for confounding variables in model 3, there was an increased risk of hypertension in Q4 compared with Q1 for both the PRAL and NEAP scores, with ORs of 1.33 (1.06–1.67), and 1.46 (1.14–1.85), respectively. Subgroup analysis based on age grouping showed that elevated PRAL was associated with an increased risk of hypertension in participants aged <60 years, with an OR of 1.31 (1.03–1.66) in Q4 compared with Q1, which was a statistically significant difference. However, in participants aged ≥60 years, there was no correlation between the above two after correcting for potential confounders. In participants aged <60 years group and ≥60 years, higher NEAP scores were significantly and positively correlated with a higher hypertension prevalence, with ORs of 1.35 (1.08–1.70) and 1.37 (1.03–1.82) in Q4, respectively, as shown in Figure 2a,b.
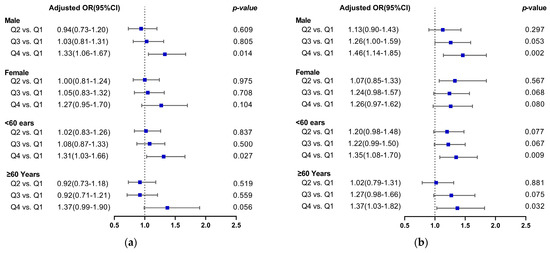
Figure 2.
Forest plot of DAL versus hypertension risk after age and sex stratification. (a) PRAL and the risk of hypertension; (b) NEAP and the risk of hypertension. The blue squares represent multivariate-adjusted odds ratio.
3.3. Sensitivity Analysis
To determine the stability of the results, we performed sensitivity analyses. Considering that exercise and physical activity can affect blood pressure, questionnaires were used to determine subjects’ exercise and physical activity in the CHNS; however, physical activity data were missing for 2937 study subjects (37%) in our study, so after excluding subjects with missing data, physical activity was included as a covariate, and the potential effect of this variable was further investigated with model 3. The findings of the research were in line with the outcomes of excluding physical activity. In addition, when the sensitivity analyses excluded subjects with diabetes mellitus and an eGFR of <60 mL/min/1.73 m2, the correlation between PRAL, NEAP, and hypertension remained significant, and our findings were not altered (Tables S3–S5).
3.4. DAL and Hypertension Risk Based on Restricted Cubic Spline Analysis (RCS)
The analysis using the model 3-adjusted restricted cubic spline demonstrated a noteworthy nonlinear correlation between PRAL and hypertension risk (P for nonlinear trend = 0.0037), with an overall U-shaped relationship, as shown in Figure 3a. For NEAP, a significant nonlinear association was not observed (P for nonlinear trend = 0.0827), as depicted in Figure 3b.
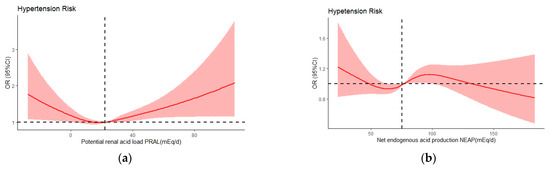
Figure 3.
Odds ratios (ORs) of PRAL (a) and NEAP (b) versus hypertension risk adjusted by model 3. The red lines indicate multivariate-adjusted odds ratio and the red shadows indicate the 95%CIs derived from restricted cubic splineregression.The median intakes were set as references (black dashed lines) (OR = 1.00). CI, confidence limit.
4. Discussion
This is the first large cross-sectional study on the association between DAL and hypertension in Chinese adults that is geographically diverse, contains extensive data, and corrects for many potential confounders. When multiple covariates were adjusted in model 3, our study discovered that dietary acid load levels, as evaluated by PRAL and NEAP, were linked to the prevalence of hypertension, with higher scores associated with a higher risk of hypertension. This positive association was statistically significant among males and participants aged <60 years; moreover, the positive association between NEAP and hypertension persisted in participants aged ≥60 years. The sensitivity analyses yield consistent results with the statistical findings of the overall population.
Regarding the correlation between DAL and the likelihood of high blood pressure, our findings align with the outcomes of multiple prior observational and prospective investigations. A study on a large representative sample of Japanese adults revealed that higher PRAL and NEAP scores were positively linked to higher systolic and diastolic blood pressure in males. Conversely, in females, only systolic blood pressure showed a positive association with PRAL and NEAP scores, while diastolic blood pressure did not. Importantly, these results were unaffected by the influence of BMI on blood pressure [29]. According to a recent systematic review and meta-analysis, adult populations with higher DAL scores have a correspondingly increased risk of hypertension. A high PRAL is associated with a 14% increase in the risk of hypertension, while a high NEAP is associated with a 35% increase [30]. In a study conducted in the United States, 87,293 women with no prior hypertension were examined. The findings revealed a significant association between NEAP and the development of hypertension, with a 14% increased risk of hypertension in the group with the highest scores compared with the group with the lowest scores (HR: 1.14; 95% CI: 1.05, 1.24) after adjusting for the effects of potential covariates [31]. However, in our subgroup analyses according to gender, we did not find consistent results. Specifically, we found a positive association between PRAL and NEAP and hypertension risk in males, whereas no significant correlation was detected in females. In a cross-sectional study in China’s Guangdong Province, a higher PRAL was found to be associated with hypertension risk in the male population, but this association was not statistically significant in the female group. Additionally, there was no association between NEAP and hypertension risk in males or females [18]. A meta-analysis of DAL and hypertension risk found conflicting results for gender-stratified analyses, and further validation is needed for the combined effects across genders [30]. The different findings considered may be related to the research design and subject exclusion and inclusion criteria, or they may have resulted from differences in geographic characteristics, genetic factors, behavioral habits, and diet quality. In addition, studies have reported that some biological factors in females are protective, such as estrogen [32]. This study offers epidemiological evidence to examine whether there are disparities between genders in the occurrence of DAL and hypertension risk among the Chinese population.
In subgroup analyses based on age grouping, we found no correlation between PRAL scores and risk of hypertension in those aged ≥60 years. Results from both cross-sectional and longitudinal studies in older populations from Sweden have shown no correlations between DAL assessed using PRAL and NEAP and various blood pressure indices, which is consistent with our findings on PRAL [33]. The Rotterdam Study involved participants with an average age of 65 years, which did not find any proof to back the claim that PRAL, NEAP, and the likelihood of hypertension in elderly individuals are connected [17]. Nevertheless, in our research, Q4 of NEAP was found to have a greater risk of developing hypertension than Q1 in people aged ≥60 years. This discrepancy should be interpreted with caution given the different calculation formulas for estimating dietary acid load for PRAL and NEAP. The PRAL calculation includes other nutrients in addition to dietary protein intake and potassium, taking into account the ionic balance of magnesium and calcium as well as the dissociation of phosphate, whereas the NEAP calculation assumes that all positive minerals can be ignored except potassium, and it considers only dietary protein and potassium involvement, which may affect accuracy [34]. In addition, Remer et al. indicated that compared with NEAP, PRAL exhibited a stronger correlation with net acid excretion in 24 h urine [11]. Currently, there is a lack of clarity on the reasons why no association has been found between DAL and hypertension in the elderly population. Research indicates that hypertension in elderly individuals may be primarily attributable to other causes, such as atherosclerosis, attenuating the effect of dietary factors, such as DAL, on blood pressure [35]. In addition, renal function affects endogenous acid elimination with a corresponding decline in eGFR with increasing age, leading to the limitation of acid elimination, and therefore, older adults may be better able to tolerate diets with a high acid-forming potential [17].
The underlying mechanisms of the association between DAL and the risk of hypertension are not clearly defined, but several studies have proposed reasonable mechanisms to explain the positive association between them. First, low-grade metabolic acidosis due to diet can increase the proton load and decreases blood pH, and the body’s acid–base homeostasis affects renal absorption of calcium and magnesium [36]. Some research evidence suggests that a high acid load can cause an increase in calcium and magnesium excretion and altered calcium homeostasis, which is associated with elevated blood pressure [37,38]. Second, low levels of metabolic acids can stimulate increased cortisol secretion and decreased inactivation, which can lead to increased blood pressure [10]. Third, the effect of DAL on renal function may also indirectly affect blood pressure. Studies have demonstrated that an elevated DAL can lead to a rise in hydrogen ion concentration in the renal tubules, along with an increase in angiotensin II and aldosterone. This slight acidosis prompts the generation of ammonia by tubular cells, which is utilized to counterbalance the hydrogen ion burden. A prolonged elevation of ammonia concentration in the kidneys can have adverse effects on renal well-being, potentially impacting blood pressure in the kidneys and the renin–angiotensin–aldosterone system [39]. In the analysis of our subgroups, the inclusion of eGFR in the adjustments did not weaken the positive associations between NEAP, PRAL, and the occurrence of hypertension in males and participants aged <60 years. Finally, fruits and vegetables, which are characterized by a low DAL, contain ample amounts of potassium, magnesium, and calcium and their intake has a negative correlation with the likelihood of developing hypertension [40]. Meanwhile, a diet with a low DAL is rich in dietary fiber, which facilitates the cultivation of diverse intestinal microbiota, leading to the promotion of intestinal ecological balance, which may have a positive effect on blood pressure [41,42].
Studies have demonstrated that a higher dietary sodium intake is positively associated with the risk of hypertension [43] and that dietary sodium intake can be measured by a variety of methods, including dietary and urinary assessments [44]. The 24 h urinary sodium excretion test is considered the gold standard for determining dietary sodium. However, it has been suggested that daily dietary sodium intake fluctuates and that the best way to assess sodium intake is to collect multiple complete 24 h urine samples during nonconsecutive 3- to 10-day periods to reflect sodium excretion [45]; the complexity of such measurements is more difficult to generalize and apply in large sample population surveys. Dietary assessment methods (3-day, 24-h, and food frequency methods) can provide valid estimates of dietary nutrient intake and are recommended for assessing dietary nutrient intake [46,47]. A meta-analysis by McLean et al. found that the 24 h dietary recall method tended to underestimate dietary sodium intake compared with the 24 h urinary sodium excretion [48]. Now, more studies have begun to use dietary sodium as a substitute for urinary sodium to assess the relationship between sodium intake and disease, especially some large studies. A study using data from the National Health and Nutrition Examination Survey (NHANES) showed that excessive dietary sodium intake was associated with high systolic blood pressure [49]. A meta-analysis of dietary sodium and cardiovascular disease risk examining both 24 h urinary sodium assessments and dietary questionnaires assessing sodium intake showed that high sodium intake is an important risk factor for cardiovascular disease, with a 6% increase in cardiovascular disease risk for each additional 1 g of sodium in the diet [50]. We used data from a large-sample study conducted in a wide geographical area of China in which sodium intake was calculated by a 3-day 24 h dietary survey, and it has been shown that the use of multiple 24 h dietary recalls can help to narrow the discrepancy between dietary sodium assessments and 24 h urinary sodium assessments [48]. Therefore, even though the CHNS did not collect indicators for urinary sodium assessment, we still consider the results of the study to be representative, valuable, and interesting. In our study, sodium was adjusted as a covariate, and we were more concerned about the effect of DAL on blood pressure. In an investigative study in Japan on DAL and hypertension that adjusted for dietary sodium as a covariate, a high DAL was found to be associated with an increased prevalence of hypertension [51], which is consistent with our findings. In future studies, we can further explore the relationship between DAL and the risk of hypertension by using urinary sodium excretion as a covariate. There were certain advantages in the present investigation. First, we employed various parameters to define hypertension in our study. Besides diagnosing hypertension by averaging three repeated measurements of blood pressure, we also included subjects who had been previously diagnosed by a physician, were taking antihypertensive medication, and self-reported their diagnosis, which allowed for a more accurate reflection of the reality of an individual’s blood pressure. Second, we used a large geographically representative sample and controlled for a wide range of potentially confounding variables to examine the relationship between DAL and the prevalence of hypertension, and the findings were reliable and representative. The findings of our study provide supporting evidence for investigating the correlation between DAL and the likelihood of developing hypertension in Chinese populations, and theyindicate that adopting a diet with a low DAL may serve as an efficient dietary approach to managing and decreasing hypertension risk.
It is also necessary to discuss the limitations of this study. First, the exploration of the relationship between DAL and hypertension in the present study utilized a cross-sectional design, and the association findings do not represent a causal relationship. Longitudinal studies can be conducted at a later stage to further validate these findings. Second, the calculation of PRAL and NEAP scores relied on mathematical modeling formulas rather than objective measurement assessments, which may not have been integrated for the digestion and absorption of nutrients caused by individual differences [34]. However, it is not easy to measure the acid load of urine in large studies. The PRAL and NEAP scores have been validated and are frequently utilized in epidemiological research, displaying a strong association with acid load as measured in 24 h urine [8,11]. Finally, the CHNS did not collect data reflecting low-grade organic metabolic acidosis, such as serum pH, urine pH, or bicarbonate concentration, but some studies conducted in healthy and CKD populations have shown that DAL correlates well with endogenous acid–base status [14,52,53].
5. Conclusions
To sum up, we discovered a positive correlation between elevated PRAL and hypertension risk in males and participants aged <60 years, and these associations were not statistically significant in females or participants aged ≥60 years. In relation to the connection between NEAP and hypertension, significant associations were found in males, participants aged <60 years, and participants aged ≥60 years. There was a significant nonlinear association in the dose–response relationship between DAL (based on PRAL) and hypertension. Consuming diets that have higher PRAL or NEAP scores could potentially increase the risk of hypertension, while following a low-DAL dietary pattern may prove to be highly beneficial in reducing blood pressure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15214664/s1, Table S1: ORs (and 95% CIs) based on PRAL for hypertension risk; Table S2: ORs (and 95% CIs) based on NEAP for hypertension risk; Table S3: ORs (and 95% CIs) of PRAL, NEAP, and hypertension risk in subjects with physical activity deficit exclusion (adjusted with model 3); Table S4: ORs (and 95% CIs) of PRAL, NEAP, and hypertension risk in nondiabetic subjects (adjusted with model 3); Table S5: ORs (and 95% CIs) of PRAL, NEAP, and hypertension risk in subjects with eGFR ≥ 60 mL/min/1.73 m2 (adjusted with model 3).
Author Contributions
Conceptualization, M.Z., F.L. and Z.Z. (Zongfeng Zhang); methodology, M.Z., F.L. and R.W.; software, M.Z., F.L. and M.S.; validation, M.S. and R.W.; formal analysis, Z.Z. (Zongfeng Zhang); investigation, M.Z. and F.L.; data curation, M.S.; writing—original draft preparation, M.Z. and F.L.; writing—review and editing, M.Z. and F.L.; visualization, Z.Z. (Zongfeng Zhang) and M.S.; supervision, R.W. and Z.Z. (Zongfeng Zhang); funding acquisition, Z.Z. (Zhaofeng Zhang) and Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China, grant number 2022YFF1100104.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety at the Chinese Center for Disease Control and Prevention (2015017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cpc.unc.edu/projects/china/data (accessed on 10 December 2022).
Acknowledgments
This research used data from the China Health and Nutrition Survey (CHNS). We thank the National Institute of Nutrition and Food Safety, the China Center for Disease Control and Prevention, the Carolina Population Center, the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, and R01-HD38700), and the Fogarty International Center, NIH, for the financial support for CHNS data collection and the analysis files from 1989 to 2006, along with both parties plus the China–Japan Friendship Hospital, Ministry of Health, for their support of CHNS 2009 and future surveys.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moick, S.; Sommer, I.; Gartlehner, G. WHO Guideline for the Pharmacological Treatment of Hypertension in Adults. Gesundheitswesen Bundesverb. Arzte Offentlichen Gesundheitsdienstes 2023, 85, 139–142. [Google Scholar] [CrossRef]
- Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [CrossRef]
- Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [CrossRef]
- Valenzuela, P.L.; Carrera-Bastos, P.; Gálvez, B.G.; Ruiz-Hurtado, G.; Ordovas, J.M.; Ruilope, L.M.; Lucia, A. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2021, 18, 251–275. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.; Tatakis, F.; Polyzos, D.; Manta, E.; Thomopoulos, C.; Nihoyannopoulos, P.; Tousoulis, D.; Tsioufis, K. Overview of salt restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet for blood pressure reduction. Rev. Cardiovasc. Med. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J. Low-grade metabolic acidosis as a driver of chronic disease: A 21st century public health crisis. Open Heart 2021, 8, e001730. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; Sergi, D.; Colombari, S.; Capatti, E.; Situlin, R.; Biolo, G.; Di Girolamo, F.G.; Lazzer, S.; Šimunič, B.; Pišot, R.; et al. Dietary Acid Load but Not Mediterranean Diet Adherence Score Is Associated with Metabolic and Cardiovascular Health State: A Population Observational Study From Northern Italy. Front. Nutr. 2022, 9, 828587. [Google Scholar] [CrossRef] [PubMed]
- Incollingo Rodriguez, A.C.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef]
- Remer, T.; Dimitriou, T.; Manz, F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am. J. Clin. Nutr. 2003, 77, 1255–1260. [Google Scholar] [CrossRef]
- Osuna-Padilla, I.A.; Leal-Escobar, G.; Garza-García, C.A.; Rodríguez-Castellanos, F.E. Dietary acid load: Mechanisms and evidence of its health repercussions. Nefrologia 2019, 39, 343–354. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Quade, B.N.; Parker, M.D.; Occhipinti, R. The therapeutic importance of acid-base balance. Biochem. Pharmacol. 2021, 183, 114278. [Google Scholar] [CrossRef]
- Krupp, D.; Esche, J.; Mensink, G.B.M.; Klenow, S.; Thamm, M.; Remer, T. Dietary Acid Load and Potassium Intake Associate with Blood Pressure and Hypertension Prevalence in a Representative Sample of the German Adult Population. Nutrients 2018, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Engberink, M.F.; Bakker, S.J.; Brink, E.J.; van Baak, M.A.; van Rooij, F.J.; Hofman, A.; Witteman, J.C.; Geleijnse, J.M. Dietary acid load and risk of hypertension: The Rotterdam Study. Am. J. Clin. Nutr. 2012, 95, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Ji, G.Y.; Jiang, Q.; Wang, P.; Huang, R.; Ma, W.J.; Chen, Z.H.; Peng, J.W. Association between dietary acid load and the risk of hypertension among adults from South China: Result from nutrition and health survey (2015–2017). BMC Public Health 2019, 19, 1599. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Su, C.; Wang, H.; Wang, Z.; Wang, Y.; Zhang, B. Secular Trends in Energy and Macronutrient Intakes and Distribution among Adult Females (1991-2015): Results from the China Health and Nutrition Survey. Nutrients 2018, 10, 115. [Google Scholar] [CrossRef]
- Du, S.; Batis, C.; Wang, H.; Zhang, B.; Zhang, J.; Popkin, B.M. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am. J. Clin. Nutr. 2014, 99, 334–343. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.; Adams, R.; Carnethon, M.; De Simone, G.; Ferguson, T.B.; Flegal, K.; Ford, E.; Furie, K.; Go, A.; Greenlund, K.; et al. Heart disease and stroke statistics—2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009, 119, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Zou, Q.; Wang, H.; Su, C.; Du, W.; Ouyang, Y.; Jia, X.; Wang, Z.; Ding, G.; Zhang, B. Longitudinal association between physical activity and blood pressure, risk of hypertension among Chinese adults: China Health and Nutrition Survey 1991–2015. Eur. J. Clin. Nutr. 2021, 75, 274–282. [Google Scholar] [CrossRef]
- Bardin, T.; Richette, P. Definition of hyperuricemia and gouty conditions. Curr. Opin. Rheumatol. 2014, 26, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Chinese expert consensus on the treatment of hyperuricemia and gout. China Endocrinol. Metab. 2013, 29, 913–920. [CrossRef]
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. S1), S67–S74. [CrossRef] [PubMed]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 63, 713–735. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Okubo, H.; Sasaki, S. Higher dietary acid load is weakly associated with higher adiposity measures and blood pressure in Japanese adults: The National Health and Nutrition Survey. Nutr. Res. 2017, 44, 67–75. [Google Scholar] [CrossRef]
- Chen, S.W.; Chen, Z.H.; Liang, Y.H.; Wang, P.; Peng, J.W. Elevated hypertension risk associated with higher dietary acid load: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2019, 33, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Curhan, G.C.; Forman, J.P. Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension 2009, 54, 751–755. [Google Scholar] [CrossRef]
- Di Giosia, P.; Giorgini, P.; Stamerra, C.A.; Petrarca, M.; Ferri, C.; Sahebkar, A. Gender Differences in Epidemiology, Pathophysiology, and Treatment of Hypertension. Curr. Atheroscler. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Luis, D.; Huang, X.; Riserus, U.; Sjögren, P.; Lindholm, B.; Arnlöv, J.; Cederholm, T.; Carrero, J.J. Estimated dietary acid load is not associated with blood pressure or hypertension incidence in men who are approximately 70 years old. J. Nutr. 2015, 145, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Anderson, C.A. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv. Chronic Kidney Dis. 2013, 20, 141–149. [Google Scholar] [CrossRef]
- Fernández-Llama, P.; Ayasreh, N.; Calero, F. Hypertension in the elderly: What we need to know. Hipertens. Y Riesgo Vasc. 2021, 38, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Tallheden, T.; Vormann, J. Acid-base conditions regulate calcium and magnesium homeostasis. Magnes. Res. 2009, 22, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Stroup, B.M.; Sawin, E.A.; Murali, S.G.; Binkley, N.; Hansen, K.E.; Ney, D.M. Amino Acid Medical Foods Provide a High Dietary Acid Load and Increase Urinary Excretion of Renal Net Acid, Calcium, and Magnesium Compared with Glycomacropeptide Medical Foods in Phenylketonuria. J. Nutr. Metab. 2017, 2017, 1909101. [Google Scholar] [CrossRef] [PubMed]
- Kesteloot, H.; Tzoulaki, I.; Brown, I.J.; Chan, Q.; Wijeyesekera, A.; Ueshima, H.; Zhao, L.; Dyer, A.R.; Unwin, R.J.; Stamler, J.; et al. Relation of urinary calcium and magnesium excretion to blood pressure: The International Study Of Macro- And Micro-nutrients And Blood Pressure and The International Cooperative Study On Salt, Other Factors, And Blood Pressure. Am. J. Epidemiol. 2011, 174, 44–51. [Google Scholar] [CrossRef]
- Rodrigues Neto Angéloco, L.; Arces de Souza, G.C.; Almeida Romão, E.; Garcia Chiarello, P. Alkaline Diet and Metabolic Acidosis: Practical Approaches to the Nutritional Management of Chronic Kidney Disease. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2018, 28, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Behers, B.J.; Melchor, J.; Behers, B.M.; Meng, Z.; Swanson, P.J.; Paterson, H.I.; Mendez Araque, S.J.; Davis, J.L.; Gerhold, C.J.; Shah, R.S.; et al. Vitamins and Minerals for Blood Pressure Reduction in the General, Normotensive Population: A Systematic Review and Meta-Analysis of Six Supplements. Nutrients 2023, 15, 4223. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Zhou, X.; Cai, J. The Role and Mechanism of Intestinal Flora in Blood Pressure Regulation and Hypertension Development. Antioxid. Redox Signal. 2021, 34, 811–830. [Google Scholar] [CrossRef]
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef]
- McLean, R.M. Measuring population sodium intake: A review of methods. Nutrients 2014, 6, 4651–4662. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.R.C.; He, F.J.; Tan, M.; Cappuccio, F.P.; Neal, B.; Woodward, M.; Cogswell, M.E.; McLean, R.; Arcand, J.; MacGregor, G.; et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J. Clin. Hypertens. 2019, 21, 700–709. [Google Scholar] [CrossRef]
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H.; et al. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Covas, M.I.; Marrugat, J.; Vila, J.; Pena, A.; Alcántara, M.; Masiá, R. Use of a three-day estimated food record, a 72-hour recall and a food-frequency questionnaire for dietary assessment in a Mediterranean Spanish population. Clin. Nutr. 2001, 20, 429–437. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.; Cameron, C.; Butcher, E.; Cook, N.R.; Woodward, M.; Campbell, N.R.C. Comparison of 24-hour urine and 24-hour diet recall for estimating dietary sodium intake in populations: A systematic review and meta-analysis. J. Clin. Hypertens. 2019, 21, 1753–1762. [Google Scholar] [CrossRef]
- Chmielewski, J.; Carmody, J.B. Dietary sodium, dietary potassium, and systolic blood pressure in US adolescents. J. Clin. Hypertens. 2017, 19, 904–909. [Google Scholar] [CrossRef]
- Wang, Y.J.; Yeh, T.L.; Shih, M.C.; Tu, Y.K.; Chien, K.L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Eguchi, M.; Kurotani, K.; Kochi, T.; Pham, N.M.; Ito, R.; Kuwahara, K.; Tsuruoka, H.; Mizoue, T.; Kabe, I.; et al. High dietary acid load is associated with increased prevalence of hypertension: The Furukawa Nutrition and Health Study. Nutrition 2015, 31, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I.; Maher, T.; Hulter, H.N.; Schambelan, M.; Sebastian, A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983, 24, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Angeloco, L.R.N.; Arces de Souza, G.C.; Romão, E.A.; Frassetto, L.; Chiarello, P.G. Association of dietary acid load with serum bicarbonate in chronic kidney disease (CKD) patients. Eur. J. Clin. Nutr. 2020, 74, 69–75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
