Association of Estimated Total Body Iron with All-Cause Mortality in Japanese Hemodialysis Patients: The Miyazaki Dialysis Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Extraction of General Clinical Data and Measurement of Laboratory Indices
2.3. Predictors
2.4. Outcomes
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Baseline Characteristics of Participants
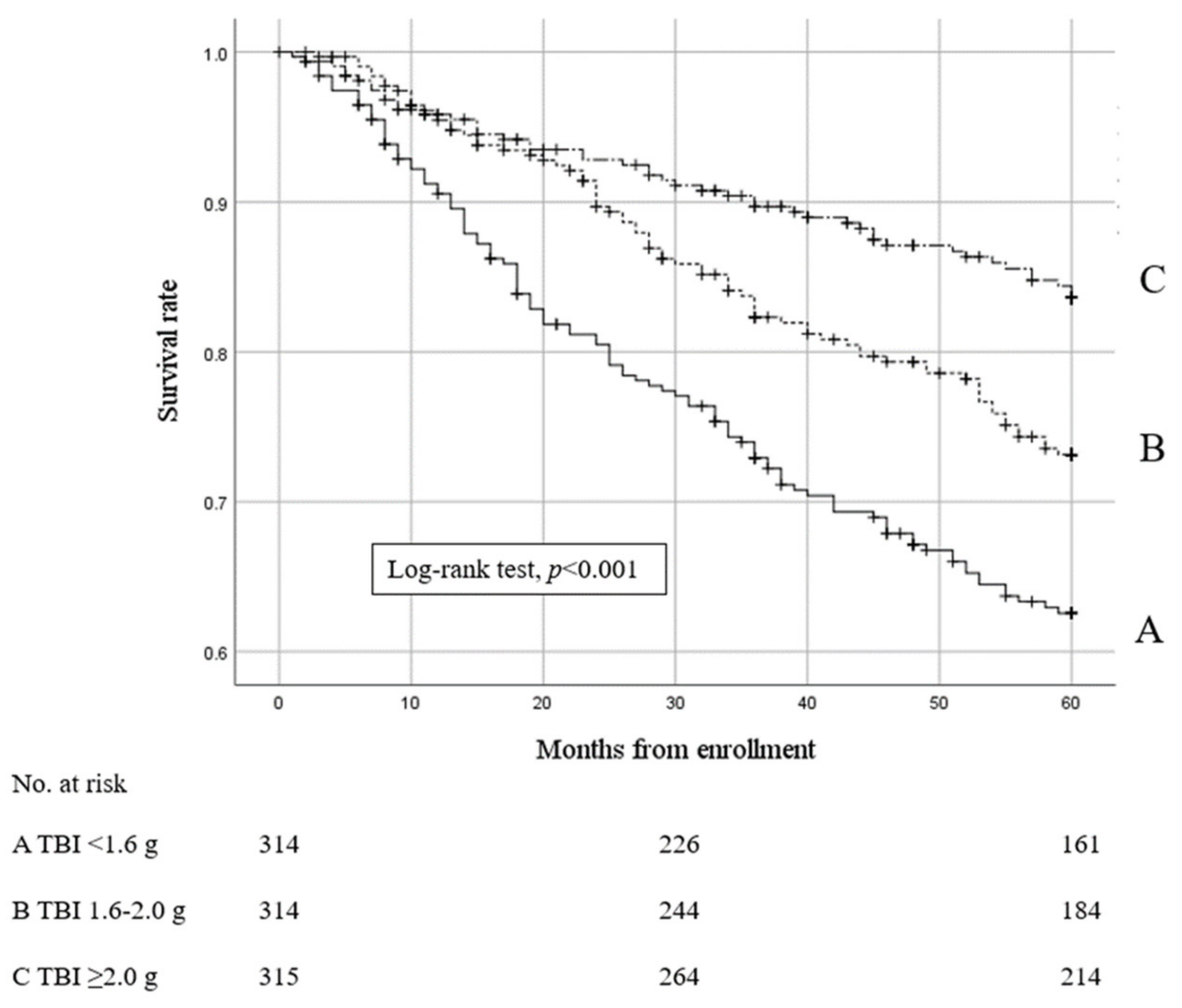
3.2. Effects of TBI on Mortality
3.3. Effects of IS/TBI on Mortality
3.4. Subgroup Analyses
3.5. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daher, R.; Karim, Z. Iron metabolism: State of the art. Transfus. Clin. Biol. 2017, 24, 115–119. [Google Scholar] [CrossRef] [PubMed]
- KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335.
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I. 2015 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ren. Replace. Ther. 2017, 3, 36. [Google Scholar] [CrossRef]
- Toida, T.; Sato, Y.; Shibata, N.; Kitamura, K.; Fujimoto, S. A randomized control study on the procedure for switching epoetin beta (EPO) to epoetin beta pegol (CERA) in the treatment of renal anemia in maintenance hemodialysis patients. Blood Purif. 2014, 38, 174–179. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kuragano, T.; Nanami, M.; Otaki, Y.; Nonoguchi, H.; Hasuike, Y. Importance of ferritin for optimizing anemia therapy in chronic kidney disease. Am. J. Nephrol. 2010, 32, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Semba, R.D.; Guralnik, J.M.; Ershler, W.B.; Bandinelli, S.; Patel, K.V.; Sun, K.; Woodman, R.C.; Andrews, N.C.; Cotter, R.J.; et al. Proinflammatory state, hepcidin, and anemia in older persons. Blood 2010, 115, 3810–3816. [Google Scholar] [CrossRef]
- Bross, R.; Zitterkoph, J.; Pithia, J.; Benner, D.; Rambod, M.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am. J. Nephrol. 2009, 29, 571–581. [Google Scholar] [CrossRef]
- Kroot, J.J.C.; Hendriks, J.C.M.; Laarakkers, C.M.M.; Klaver, S.M.; Kemna, E.H.J.M.; Tjalsma, H.; Swinkels, D.W. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: Implications for clinical studies. Anal. Biochem. 2009, 389, 124–129. [Google Scholar] [CrossRef]
- Morrison, B.; Shenkin, A.; McLelland, A.; Robertson, D.A.; Barrowman, M.; Graham, S.; Wuga, G.; Cunningham, K.J. Intra-individual variation in commonly analyzed serum constituents. Clin. Chem. 1979, 25, 1799–1805. [Google Scholar] [CrossRef]
- Nicolau, G.Y.; Haus, E.; Lakatua, D.J.; Bogdan, C.; Popescu, M.; Petrescu, E.; Sackett-Lundeen, L.; Swoyer, J.; Adderley, J. Circadian periodicity of the results of frequently used laboratory tests in elderly subjects. Endocrinologie 1983, 21, 3–21. [Google Scholar]
- Wish, J.B. Assessing iron status: Beyond serum ferritin and transferrin saturation. Clin. J. Am. Soc. Nephrol. 2006, 1 (Suppl. S1), S4–S8. [Google Scholar] [CrossRef]
- Sato, M.; Hanafusa, N.; Tsuchiya, K.; Kawaguchi, H.; Nitta, K. Impact of transferrin saturation on all-cause mortality in patients on maintenance hemodialysis. Blood Purif. 2019, 48, 158–166. [Google Scholar] [CrossRef]
- Kuragano, T.; Joki, N.; Hase, H.; Kitamura, K.; Murata, T.; Fujimoto, S.; Fukatsu, A.; Inoue, T.; Itakura, Y.; Nakanishi, T. Low transferrin saturation (TSAT) and high ferritin levels are significant predictors for cerebrovascular and cardiovascular disease and death in maintenance hemodialysis patients. PLoS ONE 2020, 15, e0236277. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Alqahtani, F.; Jaber, B.L. Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: A meta-analysis. Am. J. Nephrol. 2014, 39, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.; Muenz, D.G.; Zee, J.; Bieber, B.; Stengel, B.; Massy, Z.A.; Mansencal, N.; Wong, M.M.Y.; Charytan, D.M.; Reichel, H.; et al. Serum biomarkers of iron stores are associated with increased risk of all-cause mortality and cardiovascular events in nondialysis CKD patients, with or without anemia. J. Am. Soc. Nephrol. 2021, 32, 2020–2030. [Google Scholar] [CrossRef]
- Karaboyas, A.; Morgenstern, H.; Pisoni, R.L.; Zee, J.; Vanholder, R.; Jacobson, S.H.; Inaba, M.; Loram, L.C.; Port, F.K.; Robinson, B.M. Association between serum ferritin and mortality: Findings from the USA, Japan and European dialysis outcomes and practice patterns study. Nephrol. Dial. Transplant. 2018, 33, 2234–2244. [Google Scholar] [CrossRef]
- Shoji, T.; Niihata, K.; Fukuma, S.; Fukuhara, S.; Akizawa, T.; Inaba, M. Both low and high serum ferritin levels predict mortality risk in hemodialysis patients without inflammation. Clin. Exp. Nephrol. 2017, 21, 685–693. [Google Scholar] [CrossRef]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a kidney disease: Improving global outcomes (KDIGO) conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Cable, R.G.; Brambilla, D.; Glynn, S.A.; Kleinman, S.; Mast, A.E.; Spencer, B.R.; Stone, M.; Kiss, J.E.; National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Effect of iron supplementation on iron stores and total body iron after whole blood donation. Transfusion 2016, 56, 2005–2012. [Google Scholar] [CrossRef]
- Bernhart, F.W.; Skeggs, L. The iron content of crystallized human hemoglobin. J. Biol. Chem. 1943, 147, 19–22. [Google Scholar] [CrossRef]
- Kaplan, A.A. Therapeutic plasma exchange: Core curriculum 2008. Am. J. Kidney Dis. 2008, 52, 1180–1196. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, H., Jr.; Mollison, P.L.; Vetter, H. The body/venous hematocrit ratio: Its constancy over a wide hematocrit range. J. Clin. Investig. 1953, 32, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Cao, G.; Rivas, C.; Giani, J.F.; Dominici, F.P. Intravenous iron sucrose reverses anemia-induced cardiac remodeling, prevents myocardial fibrosis, and improves cardiac function by attenuating oxidative/nitrosative stress and inflammation. Int. J. Cardiol. 2016, 212, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Klip, I.T.; Jankowska, E.A.; Enjuanes, C.; Voors, A.A.; Banasiak, W.; Bruguera, J.; Rozentryt, P.; Polonski, L.; van Veldhuisen, D.J.; Ponikowski, P.; et al. The additive burden of iron deficiency in the cardiorenal-anaemia axis: Scope of a problem and its consequences. Eur. J. Heart Fail. 2014, 16, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017, 31, 225–233. [Google Scholar] [CrossRef]
- Awan, A.A.; Walther, C.P.; Richardson, P.A.; Shah, M.; Winkelmayer, W.C.; Navaneethan, S.D. Prevalence, correlates, and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 129–136. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Nolte, I.M.; van der Meer, P.; Bakker, S.J.L.; Gaillard, C.A.J.M. Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol. 2018, 19, 225. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-dependent associations between iron and mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef]
- Bailie, G.R.; Larkina, M.; Goodkin, D.A.; Li, Y.; Pisoni, R.L.; Bieber, B.; Mason, N.; Tong, L.; Locatelli, F.; Marshall, M.R.; et al. Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015, 87, 162–168. [Google Scholar] [CrossRef]
- Miskulin, D.C.; Tangri, N.; Bandeen-Roche, K.; Zhou, J.; McDermott, A.; Meyer, K.B.; Ephraim, P.L.; Michels, W.M.; Jaar, B.G.; Crews, D.C.; et al. Intravenous iron exposure and mortality in patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1930–1939. [Google Scholar] [CrossRef]
- Feldman, H.I.; Joffe, M.; Robinson, B.; Knauss, J.; Cizman, B.; Guo, W.; Franklin-Becker, E.; Faich, G. Administration of parenteral iron and mortality among hemodialysis patients. J. Am. Soc. Nephrol. 2004, 15, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Miskulin, D.C.; Zhou, J.; Bandeen-Roche, K.; Michels, W.M.; Ephraim, P.L.; McDermott, A.; Crews, D.C.; Scialla, J.J.; Sozio, S.M.; et al. Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: A comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol. Dial. Transplant. 2015, 30, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Hougen, I.; Collister, D.; Bourrier, M.; Ferguson, T.; Hochheim, L.; Komenda, P.; Rigatto, C.; Tangri, N. Safety of intravenous iron in dialysis: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2018, 13, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; White, C.; Anker, S.D.; Bhandari, S.; Farrington, K.; Kalra, P.A.; McMurray, J.J.V.; Murray, H.; Tomson, C.R.V.; Wheeler, D.C.; et al. Intravenous iron in patients undergoing maintenance hemodialysis. N. Engl. J. Med. 2019, 380, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Canavese, C.; Bergamo, D.; Ciccone, G.; Longo, F.; Fop, F.; Thea, A.; Martina, G.; Piga, A. Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int. 2004, 65, 1091–1098. [Google Scholar] [CrossRef]
- Drüeke, T.; Witko-Sarsat, V.; Massy, Z.; Descamps-Latscha, B.; Guerin, A.P.; Marchais, S.J.; Gausson, V.; London, G.M. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end stage renal disease. Circulation 2002, 106, 2212–2217. [Google Scholar] [CrossRef]
- Kuo, K.L.; Hung, S.C.; Lin, Y.P.; Tang, C.F.; Lee, T.S.; Lin, C.P.; Tarng, D.C. Intravenous ferric chloride hexahydrate supplementation induced endothelial dysfunction and increased cardiovascular risk among hemodialysis patients. PLoS ONE 2012, 7, e50295. [Google Scholar] [CrossRef]
- Kuo, K.L.; Hung, S.C.; Lee, T.S.; Tarng, D.C. Iron sucrose accelerates early atherogenesis by increasing superoxide production and upregulating adhesion molecules in CKD. J. Am. Soc. Nephrol. 2014, 25, 2596–2606. [Google Scholar] [CrossRef]
- Rostoker, G.; Vaziri, N.D.; Fishbane, S. Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs 2016, 76, 741–757. [Google Scholar] [CrossRef]
- Cho, M.E.; Hansen, J.L.; Peters, C.B.; Cheung, A.K.; Greene, T.; Sauer, B.C. An increased mortality risk is associated with abnormal iron status in diabetic and non-diabetic Veterans with predialysis chronic kidney disease. Kidney Int. 2019, 96, 750–760. [Google Scholar] [CrossRef]
- Del Vecchio, L.D.; Ekart, R.; Ferro, C.J.; Malyszko, J.; Mark, P.B.; Ortiz, A.; Sarafidis, P.; Valdivielso, J.M.; Mallamaci, F.; ERA-EDTA European Renal and Cardiovascular Medicine Working (EURECA-m) Group. Intravenous iron therapy and the cardiovascular system: Risks and benefits. Clin. Kidney J. 2021, 14, 1067–1076. [Google Scholar] [CrossRef]
- Yang, J.; Xing, J.; Zhu, X.; Xie, X.; Wang, L.; Zhang, X. Effects of hypoxia-inducible factor-prolyl hydroxylase inhibitors vs. erythropoiesis-stimulating agents on iron metabolism in non-dialysis-dependent anemic patients with CKD: A network meta-analysis. Front. Endocrinol. 2023, 14, 1131516. [Google Scholar] [CrossRef] [PubMed]
- Rumjon, A.; Sarafidis, P.; Brincat, S.; Musto, R.; Malyszko, J.; Bansal, S.S.; Macdougall, I.C. Serum hemojuvelin and hepcidin levels in chronic kidney disease. Am. J. Nephrol. 2012, 35, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Yokoyama, K.; Yokoo, T.; Shigematsu, T.; Iseki, K.; Tsubakihara, Y. The different association between serum ferritin and mortality in hemodialysis and peritoneal dialysis patients using Japanese nationwide dialysis registry. PLoS ONE 2015, 10, e0143430. [Google Scholar] [CrossRef] [PubMed]
- Besarab, A.; Bolton, W.K.; Browne, J.K.; Egrie, J.C.; Nissenson, A.R.; Okamoto, D.M.; Schwab, S.J.; Goodkin, D.A. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. J. Med. 1998, 339, 584–590. [Google Scholar] [CrossRef]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Johnson, D.W.; Tonelli, M.; Garg, A.X.; Pellegrini, F.; Ravani, P.; Jardine, M.; Perkovic, V.; et al. Meta-analysis: Erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann. Intern. Med. 2010, 153, 23–33. [Google Scholar] [CrossRef]
| All | TBI < 1.6 g | TBI 1.6–2.0 g | TBI ≥ 2.0 g | p-Value * | |
|---|---|---|---|---|---|
| n | 943 | 314 | 314 | 315 | |
| Age (years) | 68 (59–76) | 71 (61–80) | 69 (60–77) | 64 (57–73) | <0.001 |
| Female, n (%) | 399 (42.3) | 227 (72.3) | 112 (35.7) | 60 (19.0) | <0.001 |
| Duration of HD (months) | 83 (38–149) | 97 (43–176) | 84 (41–153) | 62 (34–116) | <0.001 |
| Pre-HD SBP (mmHg) | 155 (142–166) | 154 (141–166) | 155 (144–166) | 155 (141–167) | 0.404 |
| Pre-HD DBP (mmHg) | 77 (71–83) | 76 (70–82) | 77 (71–83) | 79 (72–86) | 0.001 |
| Dry weight (kg) | 53.1 ± 10.7 | 43.3 ± 6.9 | 52.8 ± 6.9 | 62.7 ± 8.5 | <0.001 |
| Inter-dialysis weight gain (%) | 4.5 (3.6–5.7) | 4.7 (3.6–5.9) | 4.6 (3.6–5.6) | 4.3 (3.5–5.4) | 0.009 |
| Vascular access (AVF), n (%) | 860 (91.2) | 280 (89.2) | 284 (90.4) | 296 (94.0) | 0.089 |
| Diabetes mellitus, n (%) | 291 (30.9) | 81 (25.8) | 93 (29.6) | 117 (37.1) | 0.007 |
| History of CVD, n (%) | 227 (24.1) | 80 (25.5) | 70 (22.3) | 77 (24.4) | 0.635 |
| Walk independently, n (%) | 760 (80.6) | 227 (72.3) | 252 (80.3) | 281 (89.2) | <0.001 |
| Smokers, n (%) | 144 (15.3) | 26 (8.3) | 47 (15.0) | 71 (22.5) | <0.001 |
| Medications | |||||
| ESA use, n (%) | 897 (95.1) | 302 (96.2) | 296 (94.3) | 299 (94.9) | 0.528 |
| Iron supplement use, n (%) | 737 (78.2) | 239 (76.1) | 247 (78.7) | 251 (79.7) | 0.537 |
| Antihypertensive drug use, n (%) | 731 (77.5) | 229 (72.9) | 254 (80.9) | 248 (78.7) | 0.047 |
| -ARB use, n (%) | 502 (53.2) | 161 (51.3) | 168 (53.5) | 173 (54.9) | 0.653 |
| -ACEI use, n (%) | 92 (9.8) | 22 (7.0) | 34 (10.8) | 19 (6.0) | 0.128 |
| Laboratory data | |||||
| Hemoglobin (g/dL) | 10.8 (10.0–11.5) | 10.3 (9.6–11.0) | 10.8 (10.2–11.5) | 11.2 (10.6–11.8) | <0.001 |
| TSAT (%) | 23.7 (17.8–31.2) | 22.2 (16.9–29.6) | 25.0 (17.8–32.9) | 24.0 (18.7–32.2) | 0.047 |
| Serum ferritin (ng/dL) | 96.7 (42.5–205.1) | 52.1 (27.4–132.9) | 95.5 (42.0–193.2) | 157 (74.0–273.7) | <0.001 |
| Heme iron (mg) | 1362 (1151–1586) | 1069 (938–1202) | 1353 (1238–1482) | 1638 (1524–1814) | <0.001 |
| Iron stores (mg) | 490 (328–626) | 323 (216–430) | 492 (363–577) | 682 (552–786) | <0.001 |
| Total body iron (g) | 1.8 (1.5–2.2) | 1.4 (1.3–1.5) | 1.8 (1.7–1.9) | 2.3 (2.2–2.5) | <0.001 |
| ERI | 6.87 (4.62–11.34) | 9.11 (6.47–15.38) | 6.31 (4.32–10.50) | 5.53 (3.55–7.30) | <0.001 |
| Serum BUN (mg/dL) | 66.3 ± 15.6 | 63.8 ± 15.7 | 66.5 ± 15.2 | 68.7 ± 15.5 | <0.001 |
| Serum creatinine (mg/dL) | 10.8 ± 2.8 | 9.4 ± 2.1 | 10.9 ± 2.7 | 12.1 ± 2.9 | <0.001 |
| Serum adjusted Ca (mg/dL) | 9.3 (8.9–9.7) | 9.3 (8.9–9.7) | 9.2 (8.8–9.6) | 9.2 (8.9–9.7) | 0.205 |
| Serum phosphate (mg/dL) | 5.1 (4.4–5.9) | 5.1 (4.3–5.8) | 5.1 (4.4–6.0) | 5.3 (4.5–6.1) | 0.154 |
| Serum iPTH (mg/dL) | 158 (81–277) | 121 (58–214) | 153 (81–280) | 152 (85–250) | 0.036 |
| Serum albumin (g/dL) | 3.8 (3.6–4.1) | 3.7 (3.5–4.0) | 3.8 (3.6–4.1) | 3.9 (3.7–4.1) | <0.001 |
| Serum CRP (mg/dL) | 0.17 (0.06–0.70) | 0.12 (0.05–0.58) | 0.13 (0.05–0.67) | 0.15 (0.08–0.70) | 0.121 |
| Single-pool Kt/V | 1.18 ± 0.21 | 1.29 ± 0.21 | 1.17 ± 0.20 | 1.08 ± 0.16 | <0.001 |
| TBI | Number of Deaths, Per 100 Patient Years | Unadjusted Model | Adjusted Mode l † | Adjusted Model 2 ‡ |
|---|---|---|---|---|
| All-cause | ||||
| T1 | 109, 9.6 | 2.70 (1.91–3.80) | 2.51 (1.72–3.67) | 1.87 (1.13–2.84) |
| T2 | 76, 6.1 | 1.70 (1.18–2.44) | 1.50 (1.04–2.17) | 1.18 (0.76–1.84) |
| T3 | 47, 3.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Cardiovascular | ||||
| T1 | 42, 3.7 | 1.75 (1.09–2.83) | 1.64 (0.96–2.82) | 1.31 (0.63–2.71) |
| T2 | 33, 2.7 | 1.24 (0.75–2.05) | 1.12 (0.67–1.86) | 1.00 (0.58–1.85) |
| T3 | 28, 2.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Infection | ||||
| T1 | 26, 2.3 | 5.09 (2.10–12.37) | 4.22 (1.62–10.97) | 2.05 (0.58–7.30) |
| T2 | 20, 1.6 | 3.51 (1.41–8.73) | 2.94 (1.17–7.36 | 1.53 (0.48–4.85) |
| T3 | 6, 0.5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Other | ||||
| T1 | 41, 3.6 | 3.61 (1.94–6.74) | 3.61 (1.83–7.14) | 2.59 (1.04–6.43) |
| T2 | 23, 1.9 | 1.84 (0.93–3.64) | 1.66 (0.84–3.29) | 1.44 (0.66–3.15) |
| T3 | 13, 1.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| IS/TBI Ratio | Number of Deaths, Per 100 Patient Years | Unadjusted Model | Adjusted Model † | Adjusted Model 2 ‡ |
|---|---|---|---|---|
| All-cause | ||||
| T1 | 48, 4.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| T2 | 73, 6.0 | 1.48 (1.03–2.12) | 1.06 (0.73–1.53) | 1.17 (0.79–1.73) |
| T3 | 111, 8.8 | 2.17 (1.54–3.04) | 1.49 (1.05–2.10) | 1.50 (1.03–2.18) |
| Cardiovascular | ||||
| T1 | 24, 2.0 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| T2 | 32, 2.6 | 1.30 (0.77–2.21) | 1.01 (0.58–1.79) | 1.07 (0.60–1.90) |
| T3 | 47, 3.7 | 1.85 (1.13–3.02) | 1.35 (0.82–2.23) | 1.45 (0.83–2.53) |
| Infection | ||||
| T1 | 8, 0.7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| T2 | 19, 1.6 | 2.31 (1.01–5.29) | 1.47 (0.64–3.39) | 1.43 (0.56–3.63) |
| T3 | 25, 2.0 | 2.95 (1.33–6.54) | 1.76 (0.78–3.95) | 1.57 (0.64–3.86) |
| Other | ||||
| T1 | 16, 1.3 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| T2 | 22, 1.8 | 1.32 (0.69–2.52) | 0.95 (0.50–1.82) | 1.24 (0.62–2.47) |
| T3 | 39, 3.1 | 2.52 (1.26–4.03) | 1.55 (0.85–2.81) | 1.69 (0.89–3.22) |
| Subgroups | No. of Patients | All-Cause Mortality, aHR (95% CI) | p-Value | p for Interaction * | |
|---|---|---|---|---|---|
| Age, years | <68 | 491 | 1.39 (0.47–4.12) | 0.557 | 0.070 |
| ≥68 | 452 | 1.70 (0.92–3.12) | 0.089 | ||
| Sex | Male | 544 | 1.87 (0.98–3.57) | 0.057 | 0.788 |
| Female | 399 | 1.17 (0.31–2.55) | 0.889 | ||
| Duration of HD, months | <83 | 470 | 1.72 (0.83–3.58) | 0.147 | 0.420 |
| ≥83 | 473 | 2.01 (0.93–4.36) | 0.077 | ||
| Diabetes mellitus | Yes | 291 | 1.26 (0.31–2.74) | 0.499 | 0.192 |
| No | 652 | 3.04 (1.51–6.14) | 0.002 | ||
| Kt/V | <1.17 | 472 | 1.46 (0.74–2.89) | 0.278 | 0.056 |
| ≥1.17 | 471 | 2.25 (0.85–5.94) | 0.101 | ||
| TSAT, % | <23.7 | 472 | 1.30 (0.41–4.09) | 0.652 | 0.091 |
| ≥23.7 | 471 | 2.58 (0.97–6.85) | 0.058 | ||
| Alb, g/dL | <3.8 | 399 | 2.49 (1.28–4.84) | 0.007 | 0.360 |
| >3.8 | 544 | 1.04 (0.52–2.07) | 0.913 | ||
| CRP, mg/dL | <0.17 | 469 | 1.74 (0.72–4.18) | 0.219 | 0.096 |
| ≥0.17 | 474 | 2.22(1.15–4.32) | 0.018 |
| Subgroups | No. of Patients | All-Cause Mortality, aHR (95% CI) | p-Value | p for Interaction * | |
|---|---|---|---|---|---|
| Age, years | <68 | 491 | 2.32 (1.10–4.90) | 0.027 | 0.077 |
| ≥68 | 452 | 1.36 (0.86–2.16) | 0.185 | ||
| Sex | Male | 544 | 1.66 (0.87–3.16) | 0.122 | 0.936 |
| Female | 399 | 1.55 (0.93–2.58) | 0.094 | ||
| Duration of HD, months | <83 | 470 | 1.48 (0.88–2.49) | 0.136 | 0.409 |
| ≥83 | 473 | 1.36 (0.74–2.49) | 0.880 | ||
| Diabetes mellitus | Yes | 291 | 2.14 (1.11–4.13) | 0.023 | 0.711 |
| No | 652 | 1.36 (0.84–2.21) | 0.208 | ||
| Kt/V | <1.17 | 472 | 1.73 (1.01–2.96) | 0.046 | 0.736 |
| ≥1.17 | 471 | 1.60 (0.90–2.83) | 0.109 | ||
| TSAT, % | <23.7 | 472 | 2.41 (1.25–4.63) | 0.009 | 0.887 |
| ≥23.7 | 471 | 1.23 (0.54–2.78) | 0.626 | ||
| Alb, g/dL | <3.8 | 399 | 1.32 (0.79–2.20) | 0.283 | 0.155 |
| >3.8 | 544 | 2.24 (1.23–4.09) | 0.009 | ||
| CRP, mg/dL | <0.17 | 469 | 1.29 (0.71–2.36) | 0.410 | 0.681 |
| ≥0.17 | 474 | 1.36 (0.80–2.31) | 0.255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toida, T.; Sato, Y.; Komatsu, H.; Fujimoto, S. Association of Estimated Total Body Iron with All-Cause Mortality in Japanese Hemodialysis Patients: The Miyazaki Dialysis Cohort Study. Nutrients 2023, 15, 4658. https://doi.org/10.3390/nu15214658
Toida T, Sato Y, Komatsu H, Fujimoto S. Association of Estimated Total Body Iron with All-Cause Mortality in Japanese Hemodialysis Patients: The Miyazaki Dialysis Cohort Study. Nutrients. 2023; 15(21):4658. https://doi.org/10.3390/nu15214658
Chicago/Turabian StyleToida, Tatsunori, Yuji Sato, Hiroyuki Komatsu, and Shouichi Fujimoto. 2023. "Association of Estimated Total Body Iron with All-Cause Mortality in Japanese Hemodialysis Patients: The Miyazaki Dialysis Cohort Study" Nutrients 15, no. 21: 4658. https://doi.org/10.3390/nu15214658
APA StyleToida, T., Sato, Y., Komatsu, H., & Fujimoto, S. (2023). Association of Estimated Total Body Iron with All-Cause Mortality in Japanese Hemodialysis Patients: The Miyazaki Dialysis Cohort Study. Nutrients, 15(21), 4658. https://doi.org/10.3390/nu15214658





