The Role of Endocrine Disrupting Chemicals in Gestation and Pregnancy Outcomes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
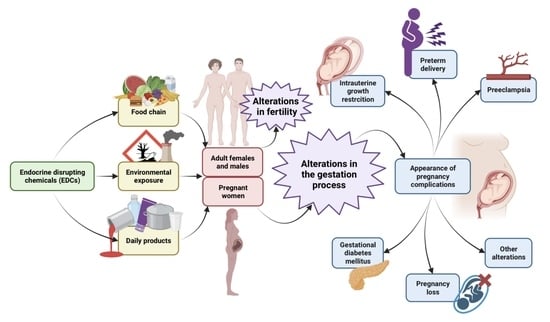
3.1. Endocrine Disruptive Chemicals (EDCs)
3.2. EDCs and Fertility
3.3. EDCs and Pregnancy
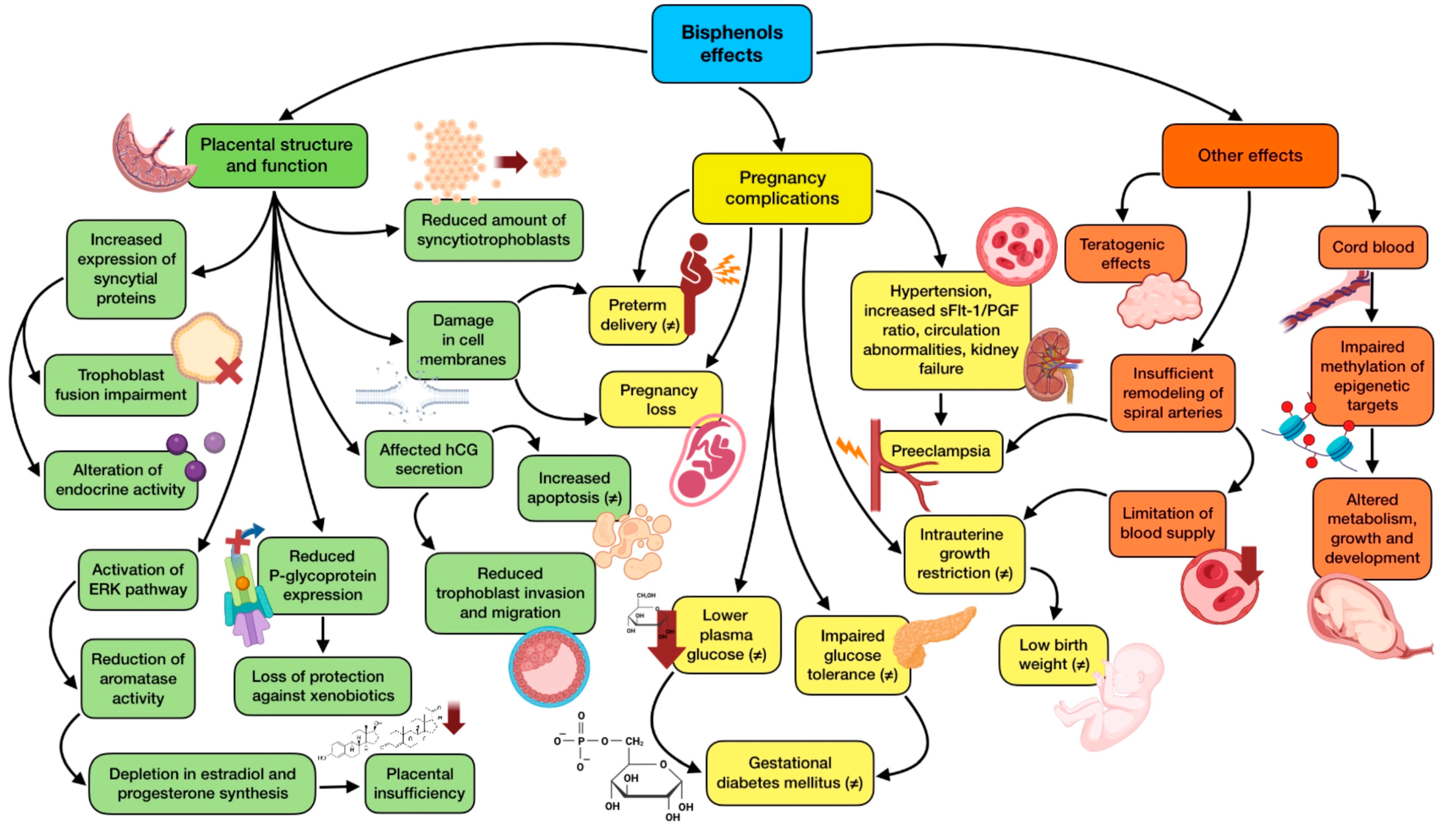
3.3.1. Implications of Bisphenols in Pregnancy
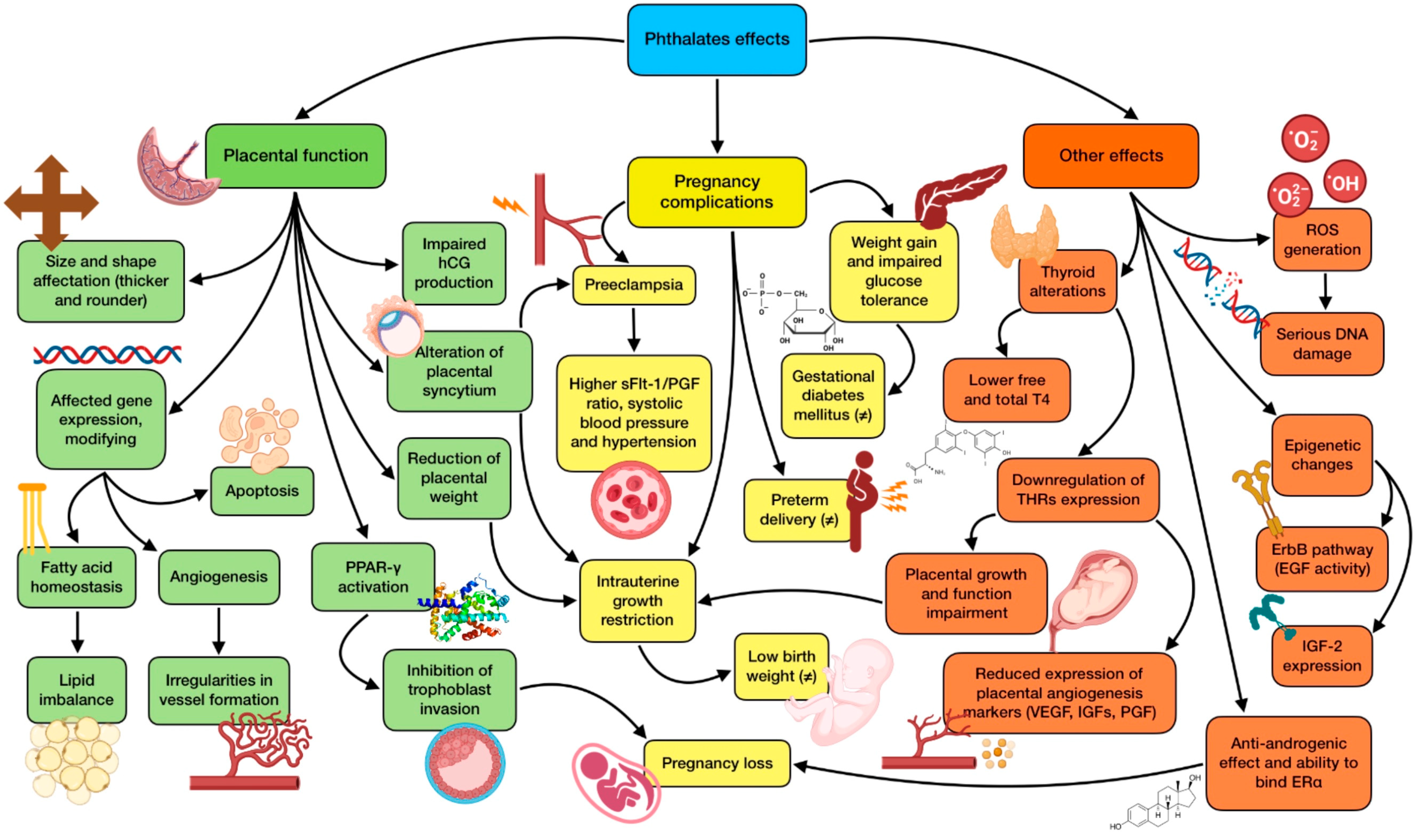
3.3.2. Implication of Phthalates in Pregnancy
3.3.3. Implications of Pesticides in Pregnancy
3.3.4. Implications of Polycyclic Aromatic Hydrocarbons (PAHs) in Pregnancy
3.3.5. Implications of Perfluorinated Compounds (PFCs) in Pregnancy
3.3.6. Implications of Phytoestrogens in Pregnancy
3.3.7. Implications of Parabens in Pregnancy
3.4. EDCs and Advanced Maternal Age
3.5. EDCs Exposure Prevention
4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monneret, C. What is an endocrine disruptor? C. R. Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.R.; Xu, X.L.; Deng, S.L.; Lian, Z.X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, A.; Nuzzo, A.M.; De Amicis, R.; Moretti, L.; Bertoli, S.; Leone, A. Fetal-Maternal Exposure to Endocrine Disruptors: Correlation with Diet Intake and Pregnancy Outcomes. Nutrients 2020, 12, 1744. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Birks, L.; Casas, M.; Garcia, A.M.; Alexander, J.; Barros, H.; Bergström, A.; Bonde, J.P.; Burdorf, A.; Costet, N.; Danileviciute, A.; et al. Occupational Exposure to Endocrine-Disrupting Chemicals and Birth Weight and Length of Gestation: A European Meta-Analysis. Environ. Health Perspect. 2016, 124, 1785–1793. [Google Scholar] [CrossRef]
- Varshavsky, J.; Smith, A.; Wang, A.; Hom, E.; Izano, M.; Huang, H.; Padula, A.; Woodruff, T.J. Heightened susceptibility: A review of how pregnancy and chemical exposures influence maternal health. Reprod. Toxicol. 2020, 92, 14–56. [Google Scholar] [CrossRef]
- Cooke, C.M.; Davidge, S.T. Advanced maternal age and the impact on maternal and offspring cardiovascular health. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H387–H394. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Ghassabian, A.; Trasande, L. Disruption in Thyroid Signaling Pathway: A Mechanism for the Effect of Endocrine-Disrupting Chemicals on Child Neurodevelopment. Front. Endocrinol. 2018, 9, 204. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Piscioli, F.; Pusiol, T. The endocrine disruptors among the environmental risk factors for stillbirth. Sci. Total Environ. 2016, 563–564, 1086–1087. [Google Scholar] [CrossRef] [PubMed]
- Feeley, M.; Brouwer, A. Health risks to infants from exposure to PCBs, PCDDs and PCDFs. Food Addit. Contam. 2000, 17, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Filardi, T.; Panimolle, F.; Lenzi, A.; Morano, S. Bisphenol A and Phthalates in Diet: An Emerging Link with Pregnancy Complications. Nutrients 2020, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Rehman, S.; Usman, Z.; Rehman, S.; AlDraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef]
- James-Todd, T.; Stahlhut, R.; Meeker, J.D.; Powell, S.G.; Hauser, R.; Huang, T.; Rich-Edwards, J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 2012, 120, 1307–1313. [Google Scholar] [CrossRef]
- Ito, R.; Seshimo, F.; Miura, N.; Kawaguchi, M.; Saito, K.; Nakazawa, H. Effect of sterilization process on the formation of mono(2-ethylhexyl)phthalate from di(2-ethylhexyl)phthalate. J. Pharm. Biomed. Anal. 2006, 41, 455–460. [Google Scholar] [CrossRef]
- Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Dec, K.; Styburski, D.; Nowakowska, A.; Gutowska, I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere 2020, 240, 124901. [Google Scholar] [CrossRef]
- Gingrich, J.; Ticiani, E.; Veiga-Lopez, A. Placenta Disrupted: Endocrine Disrupting Chemicals and Pregnancy. Trends Endocrinol. Metab. 2020, 31, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sapbamrer, R.; Prapamontol, T.; Prakobvitayakit, O.; Vaneesorn, Y.; Mangklabruks, A.; Hock, B. Placental transfer of DDT in mother-infant pairs from Northern Thailand. J. Environ. Sci. Health B 2008, 43, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.J.; Wang, Y.; Fu, L.; Shen, R.; Yu, Z.; Wang, H.; Chen, Y.H.; Zhang, C.; Meng, X.H.; et al. Maternal Fenvalerate Exposure Induces Fetal Intrauterine Growth Restriction through Disrupting Placental Thyroid Hormone Receptor Signaling. Toxicol. Sci. 2017, 157, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.H.B.; Polder, A.; Brynildsrud, O.B.; Grønnestad, R.; Karimi, M.; Lie, E.; Manyilizu, W.B.; Mdegela, R.H.; Mokiti, F.; Murtadha, M.; et al. Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood. Environ. Res. 2019, 170, 433–442. [Google Scholar] [CrossRef]
- Siddiqi, M.A.; Laessig, R.H.; Reed, K.D. Polybrominated diphenyl ethers (PBDEs): New pollutants-old diseases. Clin. Med. Res. 2003, 1, 281–290. [Google Scholar] [CrossRef]
- Corsini, E.; Luebke, R.W.; Germolec, D.R.; DeWitt, J.C. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014, 230, 263–270. [Google Scholar] [CrossRef]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef]
- Kolatorova, L.; Vitku, J.; Hampl, R.; Adamcova, K.; Skodova, T.; Simkova, M.; Parizek, A.; Starka, L.; Duskova, M. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 2018, 163, 115–122. [Google Scholar] [CrossRef]
- Pycke, B.F.; Geer, L.A.; Dalloul, M.; Abulafia, O.; Halden, R.U. Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ. Int. 2015, 84, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Tucak, M.; Horvat, D.; Cupic, T.; Krizmanic, G.; Tomas, V.; Ravlic, M.; Popovic, S. Forage Legumes as Sources of Bioactive Phytoestrogens for Use in Pharmaceutics: A Review. Curr. Pharm. Biotechnol. 2018, 19, 537–544. [Google Scholar] [CrossRef] [PubMed]
- López-Rodríguez, D.; Aylwin, C.F.; Delli, V.; Sevrin, E.; Campanile, M.; Martin, M.; Franssen, D.; Gérard, A.; Blacher, S.; Tirelli, E.; et al. Multi- and Transgenerational Outcomes of an Exposure to a Mixture of Endocrine-Disrupting Chemicals (EDCs) on Puberty and Maternal Behavior in the Female Rat. Environ. Health Perspect. 2021, 129, 87003. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Liu, F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021, 262, 127880. [Google Scholar] [CrossRef]
- Mantzouki, C.; Bliatka, D.; Iliadou, P.K.; Margeli, A.; Papassotiriou, I.; Mastorakos, G.; Kousta, E.; Goulis, D.G. Serum Bisphenol A concentrations in men with idiopathic infertility. Food Chem. Toxicol. 2019, 125, 562–565. [Google Scholar] [CrossRef]
- Song, W.; Puttabyatappa, M.; Zeng, L.; Vazquez, D.; Pennathur, S.; Padmanabhan, V. Developmental programming: Prenatal bisphenol A treatment disrupts mediators of placental function in sheep. Chemosphere 2020, 243, 125301. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tang, Y.; Xiong, Y.; Feng, L.; Li, X. Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J. 2019, 33, 2732–2742. [Google Scholar] [CrossRef]
- Kaimal, A.; Al Mansi, M.H.; Dagher, J.B.; Pope, C.; Varghese, M.G.; Rudi, T.B.; Almond, A.E.; Cagle, L.A.; Beyene, H.K.; Bradford, W.T.; et al. Prenatal exposure to bisphenols affects pregnancy outcomes and offspring development in rats. Chemosphere 2021, 276, 130118. [Google Scholar] [CrossRef]
- Ticiani, E.; Gingrich, J.; Pu, Y.; Vettathu, M.; Davis, J.; Martin, D.; Petroff, M.G.; Veiga-Lopez, A. Bisphenol S and Epidermal Growth Factor Receptor Signaling in Human Placental Cytotrophoblasts. Environ. Health Perspect. 2021, 129, 27005. [Google Scholar] [CrossRef]
- Harnett, K.G.; Moore, L.G.; Chin, A.; Cohen, I.C.; Lautrup, R.R.; Schuh, S.M. Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development. Curr. Res. Toxicol. 2021, 2, 399–410. [Google Scholar] [CrossRef]
- Narciso, L.; Ietta, F.; Romagnoli, R.; Paulesu, L.; Mantovani, A.; Tait, S. Effects of Bisphenol A on endogenous retroviral envelopes expression and trophoblast fusion in BeWo cells. Reprod. Toxicol. 2019, 89, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Ghasemi, Z.; Khoshhali, M.; Taheri, E.; Dehdashti, B.; Fatehizadeh, A.; Rafiei, N.; Kelishadi, R. Association of maternal exposure to bisphenol A with her β-hCG level and neonatal anthropometric measures. Environ. Sci. Pollut. Res. Int. 2021, 28, 62809–62815. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, J.; Xu, S.; Zhao, H.; Li, Y.; Zhou, Y.; Fang, J.; Liao, J.; Cai, Z.; Xia, W. Bisphenol A and bisphenol S exposures during pregnancy and gestational age—A longitudinal study in China. Chemosphere 2019, 237, 124426. [Google Scholar] [CrossRef] [PubMed]
- Namat, A.; Xia, W.; Xiong, C.; Xu, S.; Wu, C.; Wang, A.; Li, Y.; Wu, Y.; Li, J. Association of BPA exposure during pregnancy with risk of preterm birth and changes in gestational age: A meta-analysis and systematic review. Ecotoxicol. Environ. Saf. 2021, 220, 112400. [Google Scholar] [CrossRef]
- Mustieles, V.; Mínguez-Alarcón, L.; Christou, G.; Ford, J.B.; Dimitriadis, I.; Hauser, R.; Souter, I.; Messerlian, C. Placental weight in relation to maternal and paternal preconception and prenatal urinary phthalate metabolite concentrations among subfertile couples. Environ. Res. 2019, 169, 272–279. [Google Scholar] [CrossRef]
- Kim, S.; Park, E.; Park, E.K.; Lee, S.; Kwon, J.A.; Shin, B.H.; Kang, S.; Park, E.Y.; Kim, B. Urinary Concentrations of Bisphenol Mixtures during Pregnancy and Birth Outcomes: The MAKE Study. Int. J. Environ. Res. Public Health 2021, 18, 10098. [Google Scholar] [CrossRef]
- Philips, E.M.; Santos, S.; Steegers, E.A.P.; Asimakopoulos, A.G.; Kannan, K.; Trasande, L.; Jaddoe, V.W.V. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environ. Int. 2020, 135, 105342. [Google Scholar] [CrossRef]
- Tang, P.; Liang, J.; Liao, Q.; Huang, H.; Guo, X.; Lin, M.; Liu, B.; Wei, B.; Zeng, X.; Liu, S.; et al. Associations of bisphenol exposure with the risk of gestational diabetes mellitus: A nested case-control study in Guangxi, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 25170–25180. [Google Scholar] [CrossRef]
- Derakhshan, A.; Shu, H.; Broeren, M.A.C.; Lindh, C.H.; Peeters, R.P.; Kortenkamp, A.; Demeneix, B.; Bornehag, C.G.; Korevaar, T.I.M. Association of phthalate exposure with thyroid function during pregnancy. Environ. Int. 2021, 157, 106795. [Google Scholar] [CrossRef]
- Philippat, C.; Heude, B.; Botton, J.; Alfaidy, N.; Calafat, A.M.; Slama, R. Prenatal Exposure to Select Phthalates and Phenols and Associations with Fetal and Placental Weight among Male Births in the EDEN Cohort (France). Environ. Health Perspect. 2019, 127, 17002. [Google Scholar] [CrossRef]
- Shoaito, H.; Petit, J.; Chissey, A.; Auzeil, N.; Guibourdenche, J.; Gil, S.; Laprévote, O.; Fournier, T.; Degrelle, S.A. The Role of Peroxisome Proliferator–Activated Receptor Gamma (PPARγ) in Mono(2-ethylhexyl) Phthalate (MEHP)-Mediated Cytotrophoblast Differentiation. Environ. Health Perspect. 2019, 127, 27003. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Chou, W.C.; Waits, A.; Liao, K.W.; Kuo, P.L.; Huang, P.C. Cumulative risk assessment of phthalates exposure for recurrent pregnancy loss in reproductive-aged women population using multiple hazard indices approaches. Environ. Int. 2021, 154, 106657. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sheng, Y.; Liu, B.; Tang, P.; Liu, R.; Wu, L.; Chen, J.; Huang, D.; Liu, S.; Qiu, X. Exposure to phthalates in early pregnancy and the risk of fetal growth restriction: A nested case-control study in a Zhuang Chinese population. Environ. Sci. Pollut. Res. Int. 2022, 29, 57318–57329. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.M.; Keil, A.P.; Buckley, J.P.; Calafat, A.M.; Christenbury, K.E.; Engel, S.M.; O’Brien, K.M.; Rosen, E.M.; James-Todd, T.; Zota, A.R.; et al. Associations Between Prenatal Urinary Biomarkers of Phthalate Exposure and Preterm Birth: A Pooled Study of 16 US Cohorts. JAMA Pediatr. 2022, 176, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mustieles, V.; Yland, J.; Braun, J.M.; Williams, P.L.; Attaman, J.A.; Ford, J.B.; Calafat, A.M.; Hauser, R.; Messerlian, C. Association of Parental Preconception Exposure to Phthalates and Phthalate Substitutes with Preterm Birth. JAMA Netw. Open 2020, 3, e202159. [Google Scholar] [CrossRef] [PubMed]
- Philips, E.M.; Trasande, L.; Kahn, L.G.; Gaillard, R.; Steegers, E.A.P.; Jaddoe, V.W.V. Early pregnancy bisphenol and phthalate metabolite levels, maternal hemodynamics and gestational hypertensive disorders. Hum. Reprod. 2019, 34, 365–373. [Google Scholar] [CrossRef]
- Bedell, S.M.; Lyden, G.R.; Sathyanarayana, S.; Barrett, E.S.; Ferguson, K.K.; Santilli, A.; Bush, N.R.; Swan, S.H.; McElrath, T.F.; Nguyen, R.H.N. First- and Third-Trimester Urinary Phthalate Metabolites in the Development of Hypertensive Diseases of Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 10627. [Google Scholar] [CrossRef]
- Hirke, A.; Varghese, B.; Varade, S.; Adela, R. Exposure to endocrine-disrupting chemicals and risk of gestational hypertension and preeclampsia: A systematic review and meta-analysis. Environ. Pollut. 2023, 317, 120828. [Google Scholar] [CrossRef]
- Shaffer, R.M.; Ferguson, K.K.; Sheppard, L.; James-Todd, T.; Butts, S.; Chandrasekaran, S.; Swan, S.H.; Barrett, E.S.; Nguyen, R.; Bush, N.; et al. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ. Int. 2019, 123, 588–596. [Google Scholar] [CrossRef]
- Yan, D.; Jiao, Y.; Yan, H.; Liu, T.; Yan, H.; Yuan, J. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: A systematic review and meta-analysis. Environ. Health 2022, 21, 53. [Google Scholar] [CrossRef]
- Liang, Q.X.; Lin, Y.; Fang, X.M.; Gao, Y.H.; Li, F. Association Between Phthalate Exposure in Pregnancy and Gestational Diabetes: A Chinese Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Zukin, H.; Eskenazi, B.; Holland, N.; Harley, K.G. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ. Res. 2021, 194, 110712. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Singh, L.; Agarwal, P.; Saroj, R.; Taneja, A. Pesticides exposure through environment and risk of pre-term birth: A study from Agra city. Drug Chem. Toxicol. 2019, 42, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.L.; Neelon, B.; Bloom, M.S.; Buckley, J.P.; Ananth, C.V.; Perera, F.; Vena, J.; Hunt, K. Exploring associations between prenatal exposure to multiple endocrine disruptors and birth weight with exposure continuum mapping. Environ. Res. 2021, 200, 111386. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.F.; Kapidzic, M.; Hamilton, E.G.; Chen, H.; Puckett, K.W.; Zhou, Y.; Ona, K.; Parry, E.; Wang, Y.; Park, J.S.; et al. Genomic Profiling of BDE-47 Effects on Human Placental Cytotrophoblasts. Toxicol. Sci. 2019, 167, 211–226. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Williams, P.L.; Bellavia, A.; Wylie, B.J.; Hacker, M.R.; Kannan, K.; Bloom, M.S.; Hunt, K.J.; Hauser, R.; et al. Polybrominated diphenyl ethers in early pregnancy and preterm birth: Findings from the NICHD Fetal Growth Studies. Int. J. Hyg. Environ. Health 2022, 243, 113978. [Google Scholar] [CrossRef]
- Kelley, A.S.; Banker, M.; Goodrich, J.M.; Dolinoy, D.C.; Burant, C.; Domino, S.E.; Smith, Y.R.; Song, P.X.K.; Padmanabhan, V. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci. Rep. 2019, 9, 5422. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Chen, L.; Li, J.; Wang, Y.; Wang, J.; Meng, G.; Chi, M.; Zhao, Y.; Chen, H.; et al. Structure-based investigation on the association between perfluoroalkyl acids exposure and both gestational diabetes mellitus and glucose homeostasis in pregnant women. Environ. Int. 2019, 127, 85–93. [Google Scholar] [CrossRef]
- Rahman, M.L.; Zhang, C.; Smarr, M.M.; Lee, S.; Honda, M.; Kannan, K.; Tekola-Ayele, F.; Buck Louis, G.M. Persistent organic pollutants and gestational diabetes: A multi-center prospective cohort study of healthy US women. Environ. Int. 2019, 124, 249–258. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Q.; Zhang, L.; Luo, K.; Chen, L.; Zhao, S.; Feng, L.; Zhang, J. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ. Health 2019, 18, 5. [Google Scholar] [CrossRef]
- Awobajo, F.O.; Medobi, E.F.; Abdul, M.W.; Aminu, B.B.; Ojimma, C.T.; Dada, O.G. The effect of genistein on IGF-1, PlGF, sFLT-1 and fetoplacental development. Gen. Comp. Endocrinol. 2022, 329, 114122. [Google Scholar] [CrossRef] [PubMed]
- Uldbjerg, C.S.; Lim, Y.H.; Krause, M.; Frederiksen, H.; Andersson, A.M.; Bräuner, E.V. Sex-specific associations between maternal exposure to parabens, phenols and phthalates during pregnancy and birth size outcomes in offspring. Sci. Total Environ. 2022, 836, 155565. [Google Scholar] [CrossRef] [PubMed]
- Pacyga, D.C.; Talge, N.M.; Gardiner, J.C.; Calafat, A.M.; Schantz, S.L.; Strakovsky, R.S. Maternal diet quality moderates associations between parabens and birth outcomes. Environ. Res. 2022, 214 Pt 3, 114078. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.Z.; Krall, J.R.; Swan, S.H.; Louis, G.M.B. Does Older Age Modify Associations between Endocrine Disrupting Chemicals and Fecundability? Int. J. Environ. Res. Public Health 2022, 19, 8074. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Li, Y.; Zhang, B.; Huo, W.; Zhu, Y.; Wan, Y.; Zheng, T.; Zhou, A.; Chen, Z.; et al. Association between urinary parabens and gestational diabetes mellitus across prepregnancy body mass index categories. Environ. Res. 2019, 170, 151–159. [Google Scholar] [CrossRef]
- Estors Sastre, B.; Campillo Artero, C.; González Ruiz, Y.; Fernández Atuan, R.L.; Bragagnini Rodríguez, P.; Frontera Juan, G.; Gracia Romero, J. Occupational exposure to endocrine-disrupting chemicals and other parental risk factors in hypospadias and cryptorchidism development: A case-control study. J. Pediatr. Urol. 2019, 15, 520.e1–520.e8. [Google Scholar] [CrossRef]
- Patel, S.; Brehm, E.; Gao, L.; Rattan, S.; Ziv-Gal, A.; Flaws, J.A. Bisphenol A Exposure, Ovarian Follicle Numbers, and Female Sex Steroid Hormone Levels: Results From a CLARITY-BPA Study. Endocrinology 2017, 158, 1727–1738. [Google Scholar] [CrossRef]
- Berger, A.; Ziv-Gal, A.; Cudiamat, J.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod. Toxicol. 2016, 60, 39–52. [Google Scholar] [CrossRef]
- Hu, Y.; Yuan, D.Z.; Wu, Y.; Yu, L.L.; Xu, L.Z.; Yue, L.M.; Liu, L.; Xu, W.M.; Qiao, X.Y.; Zeng, R.J.; et al. Bisphenol A Initiates Excessive Premature Activation of Primordial Follicles in Mouse Ovaries via the PTEN Signaling Pathway. Reprod. Sci. 2018, 25, 609–620. [Google Scholar] [CrossRef]
- Mok-Lin, E.; Ehrlich, S.; Williams, P.L.; Petrozza, J.; Wright, D.L.; Calafat, A.M.; Ye, X.; Hauser, R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 2010, 33, 385–393. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, W.; Zhu, W.; Chen, L.; Wang, W.; Tian, Y.; Shen, L.; Zhang, J. Associations of female exposure to bisphenol A with fecundability: Evidence from a preconception cohort study. Environ. Int. 2018, 117, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hannon, P.R.; Peretz, J.; Flaws, J.A. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol. Reprod. 2014, 90, 136. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, T.; Qin, X.S.; Ge, W.; Ma, H.G.; Sun, L.L.; Hou, Z.M.; Chen, H.; Chen, P.; Qin, G.Q.; et al. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol. Biol. Rep. 2014, 41, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Pan, D.; Zheng, X.; Ding, H.; Ma, Z.; Xie, M.; Ge, S. Long-term effects of neonatal exposure to bisphenol A on testes structure and the expression of Boule in testes of male mice. Wei Sheng Yan Jiu 2017, 46, 975–980. [Google Scholar]
- Adoamnei, E.; Mendiola, J.; Vela-Soria, F.; Fernández, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018, 161, 122–128. [Google Scholar] [CrossRef]
- Barakat, R.; Lin, P.P.; Rattan, S.; Brehm, E.; Canisso, I.F.; Abosalum, M.E.; Flaws, J.A.; Hess, R.; Ko, C. Prenatal Exposure to DEHP Induces Premature Reproductive Senescence in Male Mice. Toxicol. Sci. 2017, 156, 96–108. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, W.; Chen, M.; Gu, H.; Tang, Q.; Guo, D.; Chen, T.; Chen, Y.; Lu, C.; Song, L.; et al. From the Cover: Metabolomics Reveals a Role of Betaine in Prenatal DBP Exposure-Induced Epigenetic Transgenerational Failure of Spermatogenesis in Rats. Toxicol. Sci. 2017, 158, 356–366. [Google Scholar] [CrossRef]
- Doyle, T.J.; Bowman, J.L.; Windell, V.L.; McLean, D.J.; Kim, K.H. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol. Reprod. 2013, 88, 112. [Google Scholar] [CrossRef]
- Müller, J.E.; Meyer, N.; Santamaria, C.G.; Schumacher, A.; Luque, E.H.; Zenclussen, M.L.; Rodriguez, H.A.; Zenclussen, A.C. Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci. Rep. 2018, 8, 9196. [Google Scholar] [CrossRef]
- Gingrich, J.; Pu, Y.; Ehrhardt, R.; Karthikraj, R.; Kannan, K.; Veiga-Lopez, A. Toxicokinetics of bisphenol A, bisphenol S, and bisphenol F in a pregnancy sheep model. Chemosphere 2019, 220, 185–194. [Google Scholar] [CrossRef]
- Benachour, N.; Aris, A. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicol. Appl. Pharmacol. 2009, 241, 322–328. [Google Scholar] [CrossRef]
- Mørck, T.J.; Sorda, G.; Bechi, N.; Rasmussen, B.S.; Nielsen, J.B.; Ietta, F.; Rytting, E.; Mathiesen, L.; Paulesu, L.; Knudsen, L.E. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010, 30, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ponniah, M.; Billett, E.E.; De Girolamo, L.A. Bisphenol A increases BeWo trophoblast survival in stress-induced paradigms through regulation of oxidative stress and apoptosis. Chem. Res. Toxicol. 2015, 28, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, A.; Paulesu, L.; Mannelli, C.; Ermini, L.; Romagnoli, R.; Cintorino, M.; Ietta, F. Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell. Endocrinol. 2015, 412, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.W.; Yang, Z.J.; Huang, H.H.; Chang, A.A.; Cheng, Y.C.; Wu, G.J.; Lan, H.C. Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol. Reprod. 2018, 98, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 2015, 36, 699–703. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Kotrogianni, P.; Christopoulos-Timogiannakis, E.; Koutaki, D.; Daskalakis, G.; Papantoniou, N. Bisphenol A and adverse pregnancy outcomes: A systematic review of the literature. J. Matern. Fetal Neonatal Med. 2018, 31, 3320–3327. [Google Scholar] [CrossRef]
- Chen Zee, E.; Cornet, P.; Lazimi, G.; Rondet, C.; Lochard, M.; Magnier, A.M.; Ibanez, G. Impact of endocrine disrupting chemicals on birth outcomes. Gynecol. Obstet. Fertil. 2013, 41, 601–610. [Google Scholar] [CrossRef]
- Hu, C.Y.; Li, F.L.; Hua, X.G.; Jiang, W.; Mao, C.; Zhang, X.J. The association between prenatal bisphenol A exposure and birth weight: A meta-analysis. Reprod. Toxicol. 2018, 79, 21–31. [Google Scholar] [CrossRef]
- Lee, Y.M.; Hong, Y.C.; Ha, M.; Kim, Y.; Park, H.; Kim, H.S.; Ha, E.H. Prenatal Bisphenol-A exposure affects fetal length growth by maternal glutathione transferase polymorphisms, and neonatal exposure affects child volume growth by sex: From multiregional prospective birth cohort MOCEH study. Sci. Total Environ. 2018, 612, 1433–1441. [Google Scholar] [CrossRef]
- Tang, R.; Chen, M.J.; Ding, G.D.; Chen, X.J.; Han, X.M.; Zhou, K.; Chen, L.M.; Xia, Y.K.; Tian, Y.; Wang, X.R. Associations of prenatal exposure to phenols with birth outcomes. Environ. Pollut. 2013, 178, 115–120. [Google Scholar] [CrossRef]
- Shapiro, G.D.; Dodds, L.; Arbuckle, T.E.; Ashley-Martin, J.; Fraser, W.; Fisher, M.; Taback, S.; Keely, E.; Bouchard, M.F.; Monnier, P.; et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ. Int. 2015, 83, 63–71. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Schantz, S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenet. 2018, 4, dvy022. [Google Scholar] [CrossRef]
- Nahar, M.S.; Liao, C.; Kannan, K.; Harris, C.; Dolinoy, D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 2015, 124, 54–60. [Google Scholar] [CrossRef]
- Montrose, L.; Padmanabhan, V.; Goodrich, J.M.; Domino, S.E.; Treadwell, M.C.; Meeker, J.D.; Watkins, D.J.; Dolinoy, D.C. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics 2018, 13, 301–309. [Google Scholar] [CrossRef]
- Nahar, M.S.; Kim, J.H.; Sartor, M.A.; Dolinoy, D.C. Bisphenol A-associated alterations in the expression and epigenetic regulation of genes encoding xenobiotic metabolizing enzymes in human fetal liver. Environ. Mol. Mutagen. 2014, 55, 184–195. [Google Scholar] [CrossRef]
- Speidel, J.T.; Xu, M.; Abdel-Rahman, S.Z. Bisphenol A (BPA) and bisphenol S (BPS) alter the promoter activity of the ABCB1 gene encoding P-glycoprotein in the human placenta in a haplotype-dependent manner. Toxicol. Appl. Pharmacol. 2018, 359, 47–54. [Google Scholar] [CrossRef]
- Yu, Z.; Han, Y.; Shen, R.; Huang, K.; Xu, Y.Y.; Wang, Q.N.; Zhou, S.S.; Xu, D.X.; Tao, F.B. Gestational di-(2-ethylhexyl) phthalate exposure causes fetal intrauterine growth restriction through disturbing placental thyroid hormone receptor signaling. Toxicol. Lett. 2018, 294, 1–10. [Google Scholar] [CrossRef]
- Petit, J.; Wakx, A.; Gil, S.; Fournier, T.; Auzeil, N.; Rat, P.; Laprévote, O. Lipidome-wide disturbances of human placental JEG-3 cells by the presence of MEHP. Biochimie 2018, 149, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Gao, H.; Huang, K.; Zhang, Y.W.; Cai, X.X.; Yao, H.Y.; Mao, L.J.; Ge, X.; Zhou, S.S.; Xu, Y.Y.; et al. Prenatal phthalate exposure and placental size and shape at birth: A birth cohort study. Environ. Res. 2018, 160, 239–246. [Google Scholar] [CrossRef]
- Wang, X.K.; Agarwal, M.; Parobchak, N.; Rosen, A.; Vetrano, A.M.; Srinivasan, A.; Wang, B.; Rosen, T. Mono-(2-Ethylhexyl) Phthalate Promotes Pro-Labor Gene Expression in the Human Placenta. PLoS ONE 2016, 11, e0147013. [Google Scholar] [CrossRef]
- Gao, F.; Hu, W.; Li, Y.; Shen, H.; Hu, J. Mono-2-ethylhexyl phthalate inhibits human extravillous trophoblast invasion via the PPARγ pathway. Toxicol. Appl. Pharmacol. 2017, 327, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Toft, G.; Jönsson, B.A.; Lindh, C.H.; Jensen, T.K.; Hjollund, N.H.; Vested, A.; Bonde, J.P. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ. Health Perspect. 2012, 120, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Jukic, A.M.; Calafat, A.M.; McConnaughey, D.R.; Longnecker, M.P.; Hoppin, J.A.; Weinberg, C.R.; Wilcox, A.J.; Baird, D.D. Urinary Concentrations of Phthalate Metabolites and Bisphenol A and Associations with Follicular-Phase Length, Luteal-Phase Length, Fecundability, and Early Pregnancy Loss. Environ. Health Perspect. 2016, 124, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Ko, Y.A.; Mukherjee, B.; Meeker, J.D. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ. Int. 2014, 70, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Engel, S.M.; Berkowitz, G.S.; Ye, X.; Silva, M.J.; Zhu, C.; Wetmur, J.; Calafat, A.M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092–1097. [Google Scholar] [CrossRef]
- Suzuki, Y.; Niwa, M.; Yoshinaga, J.; Mizumoto, Y.; Serizawa, S.; Shiraishi, H. Prenatal exposure to phthalate esters and PAHs and birth outcomes. Environ. Int. 2010, 36, 699–704. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Garcia, J.M.; Lin, H.; Wang, Y.; Yan, P.; Wang, L.; Tan, Y.; Luo, J.; Qiu, Z.; et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS ONE 2014, 9, e87430. [Google Scholar] [CrossRef]
- Shoaff, J.R.; Romano, M.E.; Yolton, K.; Lanphear, B.P.; Calafat, A.M.; Braun, J.M. Prenatal phthalate exposure and infant size at birth and gestational duration. Environ. Res. 2016, 150, 52–58. [Google Scholar] [CrossRef]
- Bellavia, A.; Hauser, R.; Seely, E.W.; Meeker, J.D.; Ferguson, K.K.; McElrath, T.F.; James-Todd, T. Urinary phthalate metabolite concentrations and maternal weight during early pregnancy. Int. J. Hyg. Environ. Health 2017, 220, 1347–1355. [Google Scholar] [CrossRef]
- Robledo, C.A.; Peck, J.D.; Stoner, J.; Calafat, A.M.; Carabin, H.; Cowan, L.; Goodman, J.R. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int. J. Hyg. Environ. Health 2015, 218, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Schantz, S.L. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol. Sci. 2018, 166, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.E.; Tolba, M.F.; Rondelli, C.M.; Xu, M.; Abdel-Rahman, S.Z. Oxidative Stress Alters miRNA and Gene Expression Profiles in Villous First Trimester Trophoblasts. BioMed Res. Int. 2015, 2015, 257090. [Google Scholar] [CrossRef] [PubMed]
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Wang, X.; Song, Q.; Xu, H.H.; Zhang, Y.H. Third trimester phthalate exposure is associated with DNA methylation of growth-related genes in human placenta. Sci. Rep. 2016, 6, 33449. [Google Scholar] [CrossRef]
- Tindula, G.; Murphy, S.K.; Grenier, C.; Huang, Z.; Huen, K.; Escudero-Fung, M.; Bradman, A.; Eskenazi, B.; Hoyo, C.; Holland, N. DNA methylation of imprinted genes in Mexican-American newborn children with prenatal phthalate exposure. Epigenomics 2018, 10, 1011–1026. [Google Scholar] [CrossRef]
- Lopez-Espinosa, M.J.; Granada, A.; Carreno, J.; Salvatierra, M.; Olea-Serrano, F.; Olea, N. Organochlorine pesticides in placentas from Southern Spain and some related factors. Placenta 2007, 28, 631–638. [Google Scholar] [CrossRef]
- Docea, A.O.; Vassilopoulou, L.; Fragou, D.; Arsene, A.L.; Fenga, C.; Kovatsi, L.; Petrakis, D.; Rakitskii, V.N.; Nosyrev, A.E.; Izotov, B.N.; et al. CYP polymorphisms and pathological conditions related to chronic exposure to organochlorine pesticides. Toxicol. Rep. 2017, 4, 335–341. [Google Scholar] [CrossRef]
- Mrema, E.J.; Rubino, F.M.; Brambilla, G.; Moretto, A.; Tsatsakis, A.M.; Colosio, C. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 2013, 307, 74–88. [Google Scholar] [CrossRef]
- Pathak, R.; Mustafa, M.; Ahmed, R.S.; Tripathi, A.K.; Guleria, K.; Banerjee, B.D. Association between recurrent miscarriages and organochlorine pesticide levels. Clin. Biochem. 2010, 43, 131–135. [Google Scholar] [CrossRef]
- Dewan, P.; Jain, V.; Gupta, P.; Banerjee, B.D. Organochlorine pesticide residues in maternal blood, cord blood, placenta, and breastmilk and their relation to birth size. Chemosphere 2013, 90, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, Y.; Tang, Q.; Lin, S.; Li, Y.; Hu, X.; Nian, J.; Gu, H.; Lu, Y.; Tang, H.; et al. The association between prenatal exposure to organochlorine pesticides and thyroid hormone levels in newborns in Yancheng, China. Environ. Res. 2014, 129, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Mlynarczuk, J.; Kotwica, J. Changes in the mRNA expression of structural proteins, hormone synthesis and secretion from bovine placentome sections after DDT and DDE treatment. Toxicology 2017, 375, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.K.; Milewicz, T.; Gregoraszczuk, E. DDT and its metabolite DDE alter steroid hormone secretion in human term placental explants by regulation of aromatase activity. Toxicol. Lett. 2007, 173, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, Y.H.; Lee, I.; Kim, W.; Won, S.; Ku, J.L.; Moon, H.B.; Park, J.; Kim, S.; Choi, G.; et al. Prenatal exposure to persistent organic pollutants and methylation of LINE-1 and imprinted genes in placenta: A CHECK cohort study. Environ. Int. 2018, 119, 398–406. [Google Scholar] [CrossRef]
- Murray, J.; Eskenazi, B.; Bornman, R.; Gaspar, F.W.; Crause, M.; Obida, M.; Chevrier, J. Exposure to DDT and hypertensive disorders of pregnancy among South African women from an indoor residual spraying region: The VHEMBE study. Environ. Res. 2018, 162, 49–54. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Al-Doush, I.; Alsabbaheen, A.; Mohamed Gel, D.; Rabbah, A. Levels of DDT and its metabolites in placenta, maternal and cord blood and their potential influence on neonatal anthropometric measures. Sci. Total Environ. 2012, 416, 62–74. [Google Scholar] [CrossRef]
- Kezios, K.L.; Liu, X.; Cirillo, P.M.; Cohn, B.A.; Kalantzi, O.I.; Wang, Y.; Petreas, M.X.; Park, J.S.; Factor-Litvak, P. Dichlorodiphenyltrichloroethane (DDT), DDT metabolites and pregnancy outcomes. Reprod. Toxicol. 2013, 35, 156–164. [Google Scholar] [CrossRef]
- Farhang, L.; Weintraub, J.M.; Petreas, M.; Eskenazi, B.; Bhatia, R. Association of DDT and DDE with birth weight and length of gestation in the Child Health and Development Studies, 1959–1967. Am. J. Epidemiol. 2005, 162, 717–725. [Google Scholar] [CrossRef]
- Ridano, M.E.; Racca, A.C.; Flores-Martín, J.; Camolotto, S.A.; de Potas, G.M.; Genti-Raimondi, S.; Panzetta-Dutari, G.M. Chlorpyrifos modifies the expression of genes involved in human placental function. Reprod. Toxicol. 2012, 33, 331–338. [Google Scholar] [CrossRef]
- Reyna, L.; Flores-Martín, J.; Ridano, M.E.; Panzetta-Dutari, G.M.; Genti-Raimondi, S. Chlorpyrifos induces endoplasmic reticulum stress in JEG-3 cells. Toxicol. In Vitro 2017, 40, 88–93. [Google Scholar] [CrossRef]
- Tang, J.; Zhai, J.X. Distribution of polybrominated diphenyl ethers in breast milk, cord blood and placentas: A systematic review. Environ. Sci. Pollut. Res. Int. 2017, 24, 21548–21573. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, C.; Butt, C.M.; Hoffman, K.; Hammel, S.C.; Miranda, M.L.; Stapleton, H.M. Brominated flame retardants in placental tissues: Associations with infant sex and thyroid hormone endpoints. Environ. Health 2016, 15, 113. [Google Scholar] [CrossRef]
- Zhu, Y.; Tan, Y.Q.; Wang, C.C.; Leung, L.K. The flame retardant 2,2′,4,4′-Tetrabromodiphenyl ether enhances the expression of corticotropin-releasing hormone in the placental cell model JEG-3. Chemosphere 2017, 174, 499–505. [Google Scholar] [CrossRef]
- Serme-Gbedo, Y.K.; Abdelouahab, N.; Pasquier, J.C.; Cohen, A.A.; Takser, L. Maternal levels of endocrine disruptors, polybrominated diphenyl ethers, in early pregnancy are not associated with lower birth weight in the Canadian birth cohort GESTE. Environ. Health 2016, 15, 49. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, S.; Xiang, Y.; Yang, Y.; Li, J.; Shan, Z.; Teng, W. Correlation between Prenatal Exposure to Polybrominated Diphenyl Ethers (PBDEs) and Infant Birth Outcomes: A Meta-Analysis and an Experimental Study. Int. J. Environ. Res. Public Health 2017, 14, 268. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Q.; Cao, Z.; Su, X.; Hua, J.; Zhang, Y.; He, X. Umbilical cord blood PBDEs concentrations in relation to placental size at birth. Chemosphere 2018, 201, 20–24. [Google Scholar] [CrossRef]
- Li, Q.; Kappil, M.A.; Li, A.; Dassanayake, P.S.; Darrah, T.H.; Friedman, A.E.; Friedman, M.; Lambertini, L.; Landrigan, P.; Stodgell, C.J.; et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics 2015, 10, 793–802. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Ge, Q.; Guo, L.; Chen, F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol. Med. Rep. 2015, 12, 527–534. [Google Scholar] [CrossRef]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef]
- Xu, X.; Yekeen, T.A.; Xiao, Q.; Wang, Y.; Lu, F.; Huo, X. Placental IGF-1 and IGFBP-3 expression correlate with umbilical cord blood PAH and PBDE levels from prenatal exposure to electronic waste. Environ. Pollut. 2013, 182, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.G.; El-Gareib, A.W.; Shaker, H.M. Gestational 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) exposure disrupts fetoplacental unit: Fetal thyroid-cytokines dysfunction. Life Sci. 2018, 192, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Small, C.M.; Cheslack-Postava, K.; Terrell, M.; Blanck, H.M.; Tolbert, P.; Rubin, C.; Henderson, A.; Marcus, M. Risk of spontaneous abortion among women exposed to polybrominated biphenyls. Environ. Res. 2007, 105, 247–255. [Google Scholar] [CrossRef][Green Version]
- Tsuji, M.; Aiko, Y.; Kawamoto, T.; Hachisuga, T.; Kooriyama, C.; Myoga, M.; Tomonaga, C.; Matsumura, F.; Anan, A.; Tanaka, M.; et al. Polychlorinated biphenyls (PCBs) decrease the placental syncytiotrophoblast volume and increase Placental Growth Factor (PlGF) in the placenta of normal pregnancy. Placenta 2013, 34, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Kalkunte, S.; Huang, Z.; Lippe, E.; Kumar, S.; Robertson, L.W.; Sharma, S. Polychlorinated biphenyls target Notch/Dll and VEGF R2 in the mouse placenta and human trophoblast cell lines for their anti-angiogenic effects. Sci. Rep. 2017, 7, 39885. [Google Scholar] [CrossRef]
- Wu, K.; Xu, X.; Liu, J.; Guo, Y.; Huo, X. In utero exposure to polychlorinated biphenyls and reduced neonatal physiological development from Guiyu, China. Ecotoxicol. Environ. Saf. 2011, 74, 2141–2147. [Google Scholar] [CrossRef]
- Lee, C.K.; Kang, S.G.; Lee, J.T.; Lee, S.W.; Kim, J.H.; Kim, D.H.; Son, B.C.; Kim, K.H.; Suh, C.H.; Kim, S.Y.; et al. Effects of perfluorooctane sulfuric acid on placental PRL-family hormone production and fetal growth retardation in mice. Mol. Cell. Endocrinol. 2015, 401, 165–172. [Google Scholar] [CrossRef]
- Li, X.; Ye, L.; Ge, Y.; Yuan, K.; Zhang, Y.; Liang, Y.; Wei, J.; Zhao, C.; Lian, Q.Q.; Zhu, X.; et al. In utero perfluorooctane sulfonate exposure causes low body weights of fetal rats: A mechanism study. Placenta 2016, 39, 125–133. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.S.; Li, W.J.; Liu, C.; Wang, Y.; Sun, K. Reduction of progesterone, estradiol and hCG secretion by perfluorooctane sulfonate via induction of apoptosis in human placental syncytiotrophoblasts. Placenta 2015, 36, 575–580. [Google Scholar] [CrossRef]
- Johnson, P.I.; Sutton, P.; Atchley, D.S.; Koustas, E.; Lam, J.; Sen, S.; Robinson, K.A.; Axelrad, D.A.; Woodruff, T.J. The Navigation Guide—Evidence-based medicine meets environmental health: Systematic review of human evidence for PFOA effects on fetal growth. Environ. Health Perspect. 2014, 122, 1028–1039. [Google Scholar] [CrossRef]
- Negri, E.; Metruccio, F.; Guercio, V.; Tosti, L.; Benfenati, E.; Bonzi, R.; La Vecchia, C.; Moretto, A. Exposure to PFOA and PFOS and fetal growth: A critical merging of toxicological and epidemiological data. Crit. Rev. Toxicol. 2017, 47, 482–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Teng, Y.; Zhang, J.; Yang, L.; Li, J.; Lai, J.; Zhao, Y.; Wu, Y. Association of serum levels of perfluoroalkyl substances with gestational diabetes mellitus and postpartum blood glucose. J. Environ. Sci. 2018, 69, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.C.; Glintborg, D.; Timmermann, C.A.G.; Nielsen, F.; Kyhl, H.B.; Andersen, H.R.; Grandjean, P.; Jensen, T.K.; Andersen, M. Perfluoroalkyl substances and glycemic status in pregnant Danish women: The Odense Child Cohort. Environ. Int. 2018, 116, 101–107. [Google Scholar] [CrossRef]
- Matilla-Santander, N.; Valvi, D.; Lopez-Espinosa, M.J.; Manzano-Salgado, C.B.; Ballester, F.; Ibarluzea, J.; Santa-Marina, L.; Schettgen, T.; Guxens, M.; Sunyer, J.; et al. Exposure to Perfluoroalkyl Substances and Metabolic Outcomes in Pregnant Women: Evidence from the Spanish INMA Birth Cohorts. Environ. Health Perspect. 2017, 125, 117004. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yin, S.; Kelly, B.C.; Liu, W. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Matched Maternal, Cord, and Placenta Samples: A Study of Transplacental Transfer. Environ. Sci. Technol. 2017, 51, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.R.; Zhang, R.; Lian, Z.X.; Deng, S.L.; Yu, K. Estrogen-Receptor Expression and Function in Female Reproductive Disease. Cells 2019, 8, 1123. [Google Scholar] [CrossRef]
- Jeschke, U.; Briese, V.; Richter, D.U.; Bruer, G.; Plessow, D.; Waldschläger, J.; Mylonas, I.; Friese, K. Effects of phytoestrogens genistein and daidzein on production of human chorionic gonadotropin in term trophoblast cells in vitro. Gynecol. Endocrinol. 2005, 21, 180–184. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Butyl paraben promotes apoptosis in human trophoblast cells through increased oxidative stress-induced endoplasmic reticulum stress. Environ. Toxicol. 2018, 33, 436–445. [Google Scholar] [CrossRef]
- Cleary-Goldman, J.; Malone, F.D.; Vidaver, J.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Eddleman, K.A.; Klugman, S.; Dugoff, L.; et al. Impact of maternal age on obstetric outcome. Obstet. Gynecol. 2005, 105, 983–990. [Google Scholar] [CrossRef]
- Khalil, A.; Syngelaki, A.; Maiz, N.; Zinevich, Y.; Nicolaides, K.H. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound Obstet. Gynecol. 2013, 42, 634–643. [Google Scholar] [CrossRef]
- Fuchs, F.; Monet, B.; Ducruet, T.; Chaillet, N.; Audibert, F. Effect of maternal age on the risk of preterm birth: A large cohort study. PLoS ONE 2018, 13, e0191002. [Google Scholar] [CrossRef]
- Wright, D.; Tan, M.Y.; O’Gorman, N.; Poon, L.C.; Syngelaki, A.; Wright, A.; Nicolaides, K.H. Predictive performance of the competing risk model in screening for preeclampsia. Am. J. Obstet. Gynecol. 2019, 220, e191–e199. [Google Scholar] [CrossRef]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef]
- Rydahl, E.; Declercq, E.; Juhl, M.; Maimburg, R.D. Cesarean section on a rise-Does advanced maternal age explain the increase? A population register-based study. PLoS ONE 2019, 14, e0210655. [Google Scholar] [CrossRef]
- Kort, D.H.; Gosselin, J.; Choi, J.M.; Thornton, M.H.; Cleary-Goldman, J.; Sauer, M.V. Pregnancy after age 50: Defining risks for mother and child. Am. J. Perinatol. 2012, 29, 245–250. [Google Scholar] [CrossRef]
- Marozio, L.; Picardo, E.; Filippini, C.; Mainolfi, E.; Berchialla, P.; Cavallo, F.; Tancredi, A.; Benedetto, C. Maternal age over 40 years and pregnancy outcome: A hospital-based survey. J. Matern. Fetal Neonatal Med. 2019, 32, 1602–1608. [Google Scholar] [CrossRef]
- Jolly, M.; Sebire, N.; Harris, J.; Robinson, S.; Regan, L. The risks associated with pregnancy in women aged 35 years or older. Hum. Reprod. 2000, 15, 2433–2437. [Google Scholar] [CrossRef]
- Kahveci, B.; Melekoglu, R.; Evruke, I.C.; Cetin, C. The effect of advanced maternal age on perinatal outcomes in nulliparous singleton pregnancies. BMC Pregnancy Childbirth 2018, 18, 343. [Google Scholar] [CrossRef]
- Vélez, M.P.; Arbuckle, T.E.; Fraser, W.D. Female exposure to phenols and phthalates and time to pregnancy: The Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil. Steril. 2015, 103, 1011–1020.e2. [Google Scholar] [CrossRef]
- Darbre, P.D. Endocrine Disruptors and Obesity. Curr. Obes. Rep. 2017, 6, 18–27. [Google Scholar] [CrossRef]
- Dalfra, M.G.; Burlina, S.; Lapolla, A. Weight gain during pregnancy: A narrative review on the recent evidences. Diabetes Res. Clin. Pract. 2022, 188, 109913. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Romero, E.; Scheringer, M. A review of phthalate pharmacokinetics in human and rat: What factors drive phthalate distribution and partitioning? Drug Metab. Rev. 2019, 51, 314–329. [Google Scholar] [CrossRef]
- Serrano, S.E.; Braun, J.; Trasande, L.; Dills, R.; Sathyanarayana, S. Phthalates and diet: A review of the food monitoring and epidemiology data. Environ. Health 2014, 13, 43. [Google Scholar] [CrossRef]
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R.S. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Betts, K.S. Plastics and food sources: Dietary intervention to reduce BPA and DEHP. Environ. Health Perspect. 2011, 119, A306. [Google Scholar] [CrossRef]
- Rouillon, S.; Deshayes-Morgand, C.; Enjalbert, L.; Rabouan, S.; Hardouin, J.B.; Migeot, V.; Albouy-Llaty, M. Endocrine Disruptors and Pregnancy: Knowledge, Attitudes and Prevention Behaviors of French Women. Int. J. Environ. Res. Public Health 2017, 14, 1021. [Google Scholar] [CrossRef]
- Lane, A.; Goodyer, C.G.; Rab, F.; Ashley, J.M.; Sharma, S.; Hodgson, A.; Nisker, J. Pregnant Women’s perceptions of exposure to brominated flame retardants. Reprod. Health 2016, 13, 142. [Google Scholar] [CrossRef]
- Marie, C.; Lémery, D.; Vendittelli, F.; Sauvant-Rochat, M.P. Perception of Environmental Risks and Health Promotion Attitudes of French Perinatal Health Professionals. Int. J. Environ. Res. Public Health 2016, 13, 1255. [Google Scholar] [CrossRef]

| EDC Group | Molecules | Exposure Sources |
|---|---|---|
| Bisphenols | Bisphenol A (BPA), Bisphenol S (BPS), Bisphenol F (BPF), Bisphenol B (BPB), Bisphenol AF (BPAF), Tetramethyl bisphenol F (TMBPF), Tetrabromo bisphenol A (TBBPA). | Food packaging, bottles, coat metal products, cans, dinnerware, coating powders, medical material, dental sealants, thermal printing papers. |
| Phthalates (PAEs) | Primary molecules: Diethyl phthalate (DEP), Di(2-ethylhexyl) phthalate (DEHP), Di(2-propylheptyl) phthalate (DPHP), Di-iso-nonyl phthalate (DINP), Benzyl butyl phthalate (BBP), Di-n-butyl phthalate (DBP), Dimethyl phthalate (DMP), Di-n-octyl phthalate (DNOP), Diisononyl cyclohexane dicarboxylate (DINCH). Metabolites: Monoethyl phthalate (MEP), Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), Mono(2-ethylhexyl) phthalate (MEHP), Mono(2-ethyl-5-oxohexyl) phthalate (MEOH), Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), Mono(3-carboxypropyl) phthalate (MCPP), Mono-iso-butyl phthalate (MIBP), Monobutyl phthalate (MBP), Mono-n-butylphthalate (MNBP), Mono-benzyl phthalate (MBZP), Mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), Monocarboxy-isononly phthalate (MCNP), Mono-carboxy-isooctyl phthalate (MCOP). | Food packaging, pharmaceutical coatings, personal care products (perfumes, deodorants, soaps, shampoos, lotions), toys, textiles, building materials, medical equipment. |
| Pesticides | Organochlorine pesticides (OCPs): DDT, hexachlorobenzene, dieldrin, lindane. Organophosphates (OPs): Parathion, Methyl Parathion, Malathion. Pyrethroids: Fenvalerate, Permethrin, Deltamethrin. | Insects, fungus, and weeds control. |
| Polycyclic aromatic hydrocarbons (PAHs) | Polychlorinated biphenyls (PCBs) Polybrominated diphenyl ethers (PBDEs) | Building materials, electrical equipment, paints, textiles, furniture, foams, hydraulic fluids, combustion processes. |
| Perfluorinated alkylated substances (PFASs)/Perfluorinated compounds (PFCs) | Perfluorooctane sulfonate (PFOS), Perfluorooctonoic acid (PFOA), Perfluononanoic acid (PFNA) | Non-stick cookware, firefighting foams, waterproof clothing, personal care products, anti-fouling paints. |
| Parabens | Methyl-paraben, Ethyl-paraben, Propyl-paraben, Butyl-paraben | Personal care products. |
| Phytoestrogens | Isoflavones: Genistein, Daidzein, Glycitein. Coumestans: Coumestrol. Lignan | Natural: soy (and derivate products), beans, other legumes, grains, fruits, vegetables. |
| Reference | EDC | Study Design | Major Findings |
|---|---|---|---|
| Rodríguez-López et al., 2021 [33] | PAEs (DNBP, DEHP), BPA, butylparaben | Animal model | Exposure to an EDC mixture during pregnancy exhibits multi- and transgenerational disruption of sexual maturation (folliculogenesis) and maternal behavior through a hypothalamic epigenetic reprogramming. |
| Liu et al., 2021 [34] | BPA | Animal model | Low BPA doses can reduce mice sperm quality by altering germ cell proliferation, leading to decreased fertility. |
| Mantzouki et al., 2019 [35] | BPA | Observational | High concentrations of BPA could contribute to male infertility (conflicting results). |
| Song et al., 2020 [36] | BPA | Animal model | BPA treatment reduces placental efficiency and fetal weight, increasing inflammatory and oxidative stress biomarkers, through epigenetic changes. |
| Ye et al., 2019 [37] | BPA | Animal model | BPA exposure disrupts trophoblast cell invasion and produces abnormal placental vessel remodeling which leads to preeclampsia-like characteristics. |
| Kaimal et al., 2021 [38] | Bisphenols (BPA, BPS, BPF) | Animal model | Prenatal exposure to BPF affects pregnancy outcomes (increasing spontaneous abortions), BPS alters male anogenital distance, and all three bisphenols alter ovarian function in female offspring. |
| Ticiani et al., 2021 [39] | BPS | In vitro | BPS exposure at environmentally relevant levels might result in placenta dysfunction, affecting fetal development, through a blockage of epidermal growth factor (EGF) binding. |
| Harnett et al., 2021 [40] | Bisphenols (TMBPF, BPA, BPS, BPF) | Animal model | TMBPF is the second-most toxic and teratogenic of the molecules tested (BPAF > TMBPF > BPS > BPA). BPA replacements exert adverse effects on early embryo development, having implications for reproductive health. |
| Narciso et al., 2019 [41] | BPA | In vitro | Placenta is a target organ of BPA, modifying the expression of hormones and proteins related to trophoblast fusion and apoptosis. This could bring possible implications on fetal and pregnancy health. |
| Amin et al., 2021 [42] | BPA | Observational | There is no significant association between BPA levels and anthropometric measurements, nor gestational age. No significant relationship exists between BPA and β-hCG with birth outcomes. This lack of association may be due to the low levels of urinary BPA (adverse effects might be related to higher concentrations) (conflicting results). |
| Huang et al., 2019 [43] | BPA, BPS | Observational | Higher average concentrations of BPA across pregnancy are related to a 1.97-day decrease in the gestation process. BPA levels in three trimesters are also negatively correlated with gestational age and positively associated with preterm birth. |
| Namat et al., 2021 [44] | BPA | Meta-analysis | Higher BPA exposure is related to increased risk of preterm birth and reduced gestational age, suggesting that exposure in the third trimester may be a critical period. |
| Mustieles et al., 2019 [45] | PAEs (DEHP, MEP) | Observational | Certain paternal and maternal urinary phthalates might affect placental weight and birth weight/placental weight ratio. |
| Kim et al., 2021 [46] | Bisphenols (BPA, BPS, BPF) | Observational | There seems to be an inverse association between bisphenol mixtures and birth weight. |
| Philips et al., 2020 [47] | Bisphenols, PAEs | Observational | High maternal urine concentrations of bisphenols in early pregnancy leads to reduced gestational weight in the second half of gestation. No association was found for PAEs. |
| Tang et al., 2023 [48] | Bisphenols (BPA, BPS, BPF, BPB, TBBPA) | Observational | BPS shows a positive effect on the risk of gestational diabetes mellitus, whereas BPA and TBBPA have a negative effect on it. Exposure to the mixture of the five bisphenols was negatively related to the risk of gestational diabetes (conflicting results) |
| Derakhshan et al., 2021 [49] | PAEs (DEHP, DINP, DBP, DINCH) | Observational | Exposure to phthalates may interfere with the thyroid system during gestation, even for those compounds introduced to replace known disruptive phthalates. |
| Philippat et al., 2019 [50] | PAEs (MCNP, MCOP), BPA, parabens | Observational | There is a positive association between the sum parabens and placental weight, also providing preliminary evidence of possible relationship between MCNP, MCOP and both placental weight and placental–to–birth weight ratio. |
| Shoaito et al., 2019 [51] | PAEs (MEHP) | In vitro | MEHP produces significantly lower PPAR-γ activity and less villous cytotrophoblast differentiation, whereas high doses have the opposite effect. MEHP also inhibits hCG production and has significant effects on the mitogen-activated protein kinase (MAPK) pathway. |
| Chang et al., 2021 [52] | PAEs (DEHP) | Observational | Women patients with recurrent pregnancy loss (RPL) have a significantly higher cumulative exposure to phthalates than controls. The risk of RPL is strongly associated with the higher quartiles of DEHP. |
| Guo et al., 2022 [53] | PAEs (BBP, DEHP, DMP, MBP) | Observational | Exposure to BBP, DEHP, and DMP are significantly positively associated with the risk of fetal growth restriction, while MBP showed a negative relationship, only among girls (conflicting results). |
| Welch et al., 2022 [54] | PAEs | Observational | Increases in urinary levels of phthalate metabolites show an association with higher odds of preterm birth (from 12% to 16%). |
| Zhang et al., 2020 [55] | PAEs (DEHP) | Observational | Maternal higher exposure to DEHP metabolites augment the risk of preterm delivery. |
| Philips et al., 2019 [56] | Bisphenols, PAEs | Observational | Increases in high molecular weight phthalate metabolites are associated with higher early pregnancy sFlt-1/PlGF ratio (related to preeclampsia). BPA is associated with higher intercept and reduced slope of the umbilical and uterine artery PI Z-score. |
| Bedell et al., 2021 [57] | PAEs (MEP, MCPP, MIBP, MBP, DEHP) | Observational | Higher levels of first trimester MEP and MCPP, and third trimester MIBP, are significantly related to diagnosis of pregnancy-induced hypertension. First-trimester MBP and MEP, along with DEHP, are each associated with augmented systolic blood pressure across pregnancy. |
| Hirke et al., 2023 [58] | PAEs (MEP) PFAS | Meta-analysis | Among several PAEs analyzed, MEP is the only one that showed a positive relationship in this regard with gestational hypertension. |
| Shaffer et al., 2019 [59] | PAEs | Observational | There is an association between MEP and gestational diabetes mellitus. Other phthalate metabolites are linked to impaired glucose intolerance, with possible stronger relationships in certain racial/ethnic groups. |
| Yan et al., 2022 [60] | PCBs, BBDEs, PAEs, PFAS | Meta-analysis | Exposure to certain PCBs, PBDEs, PAEs, and PFAS increase the risk of gestational diabetes. |
| Liang et al., 2022 [61] | PAEs (MEHP) | Observational | Patients with gestational diabetes have higher MEHP levels than those in the control group. The diabetes and MEHP dose-response associations are different among pregnant women aged < 35 years and those aged > 35 years. |
| Zukin et al., 2021 [62] | PAEs | Observational | There is no association between prenatal phthalate levels and increased risk of hyperglycemia, impaired glucose tolerance, or gestational diabetes mellitus. However, there is an increased odds of excessive gestational weight gain, a gestational diabetes risk factor (conflicting results). |
| Anand et al., 2019 [63] | OCPs (DDT, lindane) | Observational | Mean levels of pesticides (DDT and lindane) are higher in the placenta of the women with preterm birth. Exposed women are more likely to deliver a preterm baby than not exposed ones. Increasing maternal age reduces the risk of preterm delivery. |
| Pearce et al., 2021 [64] | OCPs, PBDEs, PCBs, PFAS | Observational | EDCs combinations with high PBDEs levels are related to low birth weight, and combinations with high concentrations of PCBs and PFAS are associated with augmented birth weight. |
| Robinson et al., 2019 [65] | PBDEs | In vitro | PBDEs significantly reduce primary villous cytotrophoblasts viability and increased death, also decreasing their ability to migrate and invade, which could adversely impact placental activity. |
| Wang et al., 2022 [66] | PBDEs | Observational | PBDEs are related to shorter gestation and higher risk of certain preterm birth subtypes among no-obese pregnant women. |
| Kelley et al., 2019 [67] | PAEs | Observational | Maternal and cord blood cytokines are differentially associated with individual and mixtures of EDCs, being several of them related to gestational age and birth weight. |
| Liu et al., 2019 [68] | PFAS | Observational | There seems to be a structure-specific association between short-chain PFAS exposure and both gestational diabetes risk and impaired glucose homeostasis in pregnant women. |
| Rahman et al., 2019 [69] | OCPs, PBDEs, PCBs, PFAS | Observational | Environmentally relevant concentrations of PCBs and some PBDEs and PFAS are associated with gestational diabetes. |
| Huang et al., 2019 [70] | PFAS | Observational | Prenatal PFAS exposure seems to be positively associated with the risk of preeclampsia and overall hypertensive disorders of pregnancy. |
| Awobajo et al., 2022 [71] | Genistein | Animal model | Serum IGF-1 and PIGF are increased when genistein is administered at gestational day 12 and 16. However, serum IGF-1 and PIGF levels are reduced in the placenta at gestational day 20. Placental sFLT-1 levels are increased gestational day 12 and 20. The sFL-1/PlGF ratio in exposed placenta samples decreases at gestational day 16 increases at gestational day 20, whereas the opposite is reported in serum (conflicting results) |
| Uldbjerg et al., 2022 [72] | Parabens, PAEs (MEP), BPA | Observational | BPA exposure is associated with lower birth size in male offspring. Similarly, exposure to MEP in male offspring is also related to lower birth weight. No associations are found regarding parabens nor female offspring. |
| Pacyga et al., 2022 [73] | Parabens | Observational | Maternal urinary paraben concentrations are modestly inversely associated with newborn head circumference and gestational length. Methylparaben and propylparaben seem to be inversely related to birth weight, body length, and weight/length ratio just in female offspring. Maternal diet may modify associations between parabens and birth size in a sex-specific way. |
| Pollack et al., 2022 [74] | BPA, PAEs, Benzophenones | Observational | Age does not seem to modify the association between BPA, phthalates, and benzophenone (BP) type UV filters. However, benzophenone 2 (BP-2) and 4-hydroxybenzophenone (4OH-BP) report longer time-to-pregnancy among females ≥ 35 years old, which reflected 39% and 29% decreases in fertility, respectively for each chemical. |
| Li et al., 2019 [75] | Parabens | Observational | Even though no significant association is found in overall population, higher levels of this propyl-paraben are detected among the overweight/obese pregnant women (who are more prone to developing GDM). |
| Estors Sastre et al., 2019 [76] | BPA, PAEs | Observational | Both AMA and parental occupational exposure to EDCs are associated with increased risk of cryptorchidism in the offspring. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puche-Juarez, M.; Toledano, J.M.; Moreno-Fernandez, J.; Gálvez-Ontiveros, Y.; Rivas, A.; Diaz-Castro, J.; Ochoa, J.J. The Role of Endocrine Disrupting Chemicals in Gestation and Pregnancy Outcomes. Nutrients 2023, 15, 4657. https://doi.org/10.3390/nu15214657
Puche-Juarez M, Toledano JM, Moreno-Fernandez J, Gálvez-Ontiveros Y, Rivas A, Diaz-Castro J, Ochoa JJ. The Role of Endocrine Disrupting Chemicals in Gestation and Pregnancy Outcomes. Nutrients. 2023; 15(21):4657. https://doi.org/10.3390/nu15214657
Chicago/Turabian StylePuche-Juarez, Maria, Juan M. Toledano, Jorge Moreno-Fernandez, Yolanda Gálvez-Ontiveros, Ana Rivas, Javier Diaz-Castro, and Julio J. Ochoa. 2023. "The Role of Endocrine Disrupting Chemicals in Gestation and Pregnancy Outcomes" Nutrients 15, no. 21: 4657. https://doi.org/10.3390/nu15214657
APA StylePuche-Juarez, M., Toledano, J. M., Moreno-Fernandez, J., Gálvez-Ontiveros, Y., Rivas, A., Diaz-Castro, J., & Ochoa, J. J. (2023). The Role of Endocrine Disrupting Chemicals in Gestation and Pregnancy Outcomes. Nutrients, 15(21), 4657. https://doi.org/10.3390/nu15214657