Abstract
Maternal weight-status at various time-points may influence child obesity development, however the most critical time-point remains unidentified. We used data from the Healthy Growth Study, a cross-sectional study of 2666 Greek schoolchildren aged 9–13 years, exploring associations between childhood obesity and maternal weight-status at pre-pregnancy, during pregnancy/gestational weight gain, and at the child’s pre-adolescence. Logistic regression analyses examined associations between maternal weight-status being “below” or “above” the recommended cut-off points (WHO BMI thresholds or IOM cut-off points), at the three time-points, individually or combined into weight-status trajectory groups to determine the strongest associations with child obesity in pre-adolescence. Adjusted models found significant associations and the highest odds ratios [95% Confidence Intervals] for mothers affected by obesity before pregnancy (4.16 [2.47, 7.02]), those with excessive gestational weight gain during pregnancy (1.50 [1.08, 2.08]), and those affected by obesity at their child’s pre-adolescence (3.3 [2.29, 4.87]). When combining these weight-status groups, mothers who were above–above–below (3.24 [1.10, 9.55]), and above–above–above (3.07 [1.95, 4.85]) the healthy weight recommendation-based thresholds in each time-point, had a three-fold higher likelihood of child obesity, compared to the below–below–below trajectory group. Maternal obesity across all examined time-points was significantly associated with childhood obesity. Effective childhood obesity preventive initiatives should commence at pre-conception, targeting maternal weight throughout the life-course and childhood developmental stages.
1. Introduction
Obesity is a complicated, prevalent, and multifactorial chronic condition [1]. It has grown to be one of the most critical public health problems worldwide, with 39% of the global adult population being overweight, and 13% obese [2]. In alignment with the global trend, the prevalence of overweight and obesity in Europe is also considerably high, (36% overweight and 17% obese) [3], mainly in the southern European countries [4,5]. In Greece alone, 35% of adults have been reported to be overweight and 27% obese [5,6]. Obesity is also very prevalent in younger ages; according to 2016 data, this clinical condition affects over 124 million children and adolescents (5–19 years of age) globally [2]. In 2019, it was estimated that over 40% of school children in Greece were overweight and obese [4]. Moreover, according to the World Health Organization (WHO) data, in 2016, the rate of obesity alone for children and adolescents in Greece was 13.8% [7].
There are multiple non-modifiable and modifiable risk factors contributing to the development of obesity, with some of these factors falling into both categories. The main non-modifiable risk factors include age, gender, puberty, developmental conditions, heritable factors (genetics and epigenetics), ethnicity, and other factors which may also be considered to be potentially modifiable, i.e., income, education, employment status and neighbourhood composition [1,8,9,10]. Conversely, a wide range of modifiable risk factors such as psychosocial, behavioural, and environmental factors have been associated with obesity, thus contributing to an “obesogenic environment” [11,12]. The latter is considered as a key influence of the modifiable behavioural risk factors that are likely to increase the risk of childhood obesity [1,13]. For children and adolescents, the age of obesity onset is also an important factor influencing long-term health. Obesity at the age of pre-adolescence (9–12 years) increases the risk to develop type 2 diabetes, cardio-metabolic diseases, and mortality [14,15,16].
Studies have also shown that children are at greater risk of overweight or obesity when parents also have an unhealthy BMI [17,18,19] since they are more likely to create and sustain a permanent positive energy balance within the home [1,13]. In this regard, children adopt obesogenic behaviours with early life exposure to sedentary habits, low physical activity levels, increased screen time, and low-quality dietary patterns (i.e., high consumption of energy-dense processed foods and low consumption of vegetables and wholemeal foods) [17,18,19]. Mothers have shown to play a key role of influence, since they are pivotal in setting the food landscape and eating patterns/choices of their children [19,20]. There is also considerable evidence pointing to perinatal factors occurring prior to the child’s conception and risk of obesity [21,22,23], as well as during pregnancy [24,25,26]. Throughout the time-points of before pregnancy and during pregnancy up to the child’s birth, there are several mechanistic pathways occurring early in life that have been proposed to interpret the associations observed between maternal obesity with the occurrence of obesity in offspring. Perinatal factors occurring before pregnancy and in the intrauterine environment have been found to result in physiological and metabolic changes, impacting the long-term health of children [27,28,29].
Recently our research team conducted a systematic literature review [30], which demonstrated that the current evidence does not conclude which maternal exposure (pre-pregnancy or during childhood) has a stronger effect on the development of childhood obesity. Although an extensive volume of literature suggests maternal weight status at individual time-points as a risk factor for childhood obesity or combinations of time-points, for example pre-pregnancy [23,29,31,32,33,34], during pregnancy [35,36], during infancy [37], and during childhood [38,39], only a handful of studies have reported the combined effect of these exposures at different time-points [32,40,41]. There is scarce evidence combining each of these exposure time-points with maternal obesity during the child’s life, i.e., pre-pregnancy, during pregnancy/gestational weight gain, and maternal weight status at their offspring’s pre-adolescence. Furthermore, to our knowledge there have been no studies that have combined pre-pregnancy, gestational weight gain, and maternal weight status at pre-adolescence and examined all possible combinations of these exposures on the likelihood of childhood obesity.
To address the current knowledge gap and to further understand the most appropriate timing of preventative strategies, this study aimed to test associations between childhood obesity and maternal weight status at three different time-points in the life course of mothers, i.e., before pregnancy, during pregnancy (i.e., gestational weight gain), and at their child’s pre-adolescence.
2. Materials and Methods
2.1. Study Design and Population
This secondary analysis used data from the Healthy Growth Study, a nationally representative cross sectional study, with retrospectively collected data on perinatal risk factors, initiated by way of a pilot study in May 2007 and concluded in 2009 [27,42]. The Healthy Growth Study comprised school children attending the 5th and 6th grade (aged 9–13 years, n = 2666; boys n = 1348; girls n = 1318 [with signed consent]). School children were recruited from municipalities within 4 counties across the wider urban area of Athens, Greece; counties included Thessaloniki, Attica, Aitoloakarnania, and Heraklion. Schools were identified and invited to participate in the study (n = 77) as per data obtained from the Greek Ministry of Education. All schools responded positively [27]. A detailed process of sampling for the Healthy Growth Study can be found elsewhere [27,42]. The Healthy Growth Study adhered to the Declaration of Helsinki and the conventions of the Council of Europe on Human Rights and Biomedicine. The study was approved by the Greek Ministry of National Education and the Ethics Committee of Harokopio University of Athens. The present secondary data analysis of the Healthy Growth Study was approved by La Trobe University Human Research Ethics Committee on 29 July 2022 (Ethics Approval Number: HEC22199).
2.2. Family and Sociodemographic Data
Socio-economic and demographic background information of participants and their families was collected during face-to-face interviews at schools with guardians by rigorously trained interviewers with the use of a standardised questionnaire to reduce interviewer bias. Demographic data were collected from parents and guardians, including age of parents, years of education, nationality, and region of residence (i.e., urban vs. rural). Social-related data were also collected, including the primary guardian(s) of the child. Finally, economic characteristics were collected, which included employment status [27].
2.3. Physical Activity Levels of Children
To calculate the average of total steps per day, participants wore waist-mounted pedometers (vertically aligned with the patella) (Yamax SW-200 Digiwalker, Tokyo, Japan) for 7 days. A standardised interview was used to assess physical activity during leisure time, on two weekdays and one weekend, based on a valid questionnaire [21,43]. Physical activities (both leisure and organised) were grouped into moderate-to-vigorous physical activities (MVPA) (intensity higher than four metabolic equivalents, METs). Organised moderate-to-vigorous physical activities (OMVPA) was a fraction of total MVPA and were defined as activities that took place in an organised manner i.e., under the supervision of a trainer or coach. Screen time was also assessed via reports from children regarding their television/video viewing time and time playing computer/video games and reported by calculating the average screentime over a week. Detailed description of the physical activity data collection methods is reported elsewhere [21,43].
2.4. Perinatal Data
Perinatal data were collected from guardians who recalled and reported the information on their child’s growth during infancy, such as the growth rate during the first 6 months of life. Growth velocity was defined by calculating changes in weight or height of infants over time and was categorised as normal growth, poor growth, and rapid growth as per the International Obesity Task Force (IOTF) cut-offs [44]. Birth weight and gestational age were extracted from each child’s birth certificate and medical record, and were used to calculate birth weight for age z-score, which allowed the classification of children at birth as small for gestational age (SGA, <10th percentile), appropriate for gestational age (AGA, 10 to 89th percentile), and large for gestational age (LGA, ≥90th percentile) [22,27].
Pre-pregnancy data (i.e., parity and history of miscarriages before child’s birth); gestational data (i.e., history of smoking, alcohol use, gestational diabetes mellitus and high blood pressure during pregnancy, type of delivery (vaginal vs. caesarean); and factors occurring at their child’s pre-adolescence, including infant’s feeding practices from birth to 6 months of age, i.e., exclusive breastfeeding vs. use of formula or mixed feeding; history of maternal smoking or alcohol use during breastfeeding; age of the initiation of solids in infants’ diet; and parental smoking at their child’s pre-adolescence were also collected [22,27]. Finally, anthropometric data of parents, which included body weight and height, were also reported by mothers and fathers.
Mothers’ anthropometrics before pregnancy and at their child’s pre-adolescence were used to calculate their body mass index (BMI), which was used to categorise them as ‘underweight’ (<18.5 kg/m2, ‘healthy weight’ (18.50–24.99 kg/m2), ‘overweight’ (25.00–29.99 kg/m2), or ‘obese’ (≥30.00 kg/m2), based on WHO’s weight status classification of adults [45]. Mothers’ gestational weight gain was categorised based on the Institute of Medicine (IOM) classifications of ‘weight gain in mothers with a pre-pregnancy BMI category of underweight (12.5–18 kg), healthy-weight (11.5–16 kg), overweight (7–11.5 kg), and obese (5–9 kg)’ [46].
2.5. Maternal Recommendation-Based Weight Status Trajectory Groups
To create ‘maternal recommendation-based weight status trajectory groups’, we followed the procedure set out by Leonard, Rasmussen, King, and Abrams [41]. According to this procedure, we dichotomised each mother’s self-reported pre-pregnancy BMI (overweight or obese: ≥25 kg/m2 [45]), gestational weight gain from clinical records (excessive based on the IOM guidelines: ≥18 kg if underweight, ≥16 kg if healthy weight, ≥11.5 kg if overweight, or ≥9 kg if obese), and self-reported current maternal BMI (maternal BMI at the child’s current age [pre-adolescence] (overweight or obese: ≥25 kg/m2) [46].
For each timepoint, in the following order, pre-pregnancy, gestational weight gain, and at the child’s pre-adolescence, mothers were then categorised as “above” or “below” the WHO (2000) BMI thresholds or IOM (2009) cut-off points, and these categories were combined into all possible combinations of the eight maternal recommendation-based weight status trajectory groups, assigned as follows: below–below–below (reference group), below–below–above, below–above–below, below–above–above, above–below–below, above–below–above, above–above–below, and above–above–above. For the above category, this has been described in the results as ‘above’ or ‘upper’ threshold. “Above” includes maternal weight status above the WHO classification of adults according to BMI thresholds (overweight/obesity) [45] or IOM recommendations [46]). Below includes maternal weight status as healthy or below the WHO classification of adults [45] according to BMI, or IOM recommendations [46].
2.6. Anthropometric Measurements of Children
For school children with parental signed informed consent, a full medical examination was conducted, which included anthropometric and body composition measurements, blood collection and clinical examination. The physical examination was carried out by two trained members of the research team with identical protocols and equipment used across all schools [27]. Regarding body weight, children were weighed without shoes and in the minimum clothing possible, to the nearest 10 g using a Seca digital scale (Seca Alpha, Model 770, Hamburg, Germany).
Height was measured without shoes, with shoulders relaxed, arms hanging freely, and participants heads aligned in Frankfort plane, to the nearest 0.1 cm using a commercial stadiometer (Leicester Height Measure, Invicta Plastics Ltd., Oadby, UK). The measures of children’s weight and height were converted to BMI using Quetelet’s equation (weight (kg)/height2 (m2)) [47]. Waist circumference (WC) was measured, with the child standing, to the nearest 0.1 cm at the end of a gentle expiration, with the use of a Hoechstmass non-elastic tape. The measuring tape was placed around the waist, half-way between the lower rib margin and the iliac crest. Hip circumference was measured to the nearest 0.1 cm, at the level of greater trochanters and pubic symphysis. Waist-to-Hip ratio (WHR) (waist circumference/hip circumference) was also calculated for each school child [27].
The IOTF BMI-for-age cut-off points were used to categorise school children as “underweight”, “ healthy weight”, “overweight”, or “obese” [44]. Waist circumference measurements were classified based on age and sex-specific percentiles (≥90th percentile) to identify central obesity [48,49,50].
2.7. Statistical Analyses
All analyses were based on the Healthy Growth Study population, which included selected cases, comprising the majority of the total sample, but excluding those with energy intakes of <800 kilocalories (kcal)/day and >3000 kcal/day, as a strategy to exclude under- and over-reporters of dietary intake [51]. Both continuous and categorical variables were used in the current analysis. Logistic regression analysis was used to examine adjusted associations between maternal weight status at each one of the three time-points (i.e., pre-pregnancy, during pregnancy, and at their child’s pre-adolescence) separately or combined into recommendation-based weight status trajectory groups to test all possible combinations of these groups, and the outcome of child obesity. The level of statistical significance was set at p < 0.05 and the reported p-values were two-tailed. All statistical analyses were performed using the IBM SPSS Statistics package, version 28.0, for Windows (IMB, Armonk, NY, USA). Figures were created with the use of Graphpad (Prism), version 9 for Windows (Dotmatics, San Diego, CA, USA).
2.8. Confounding Variables
According to the available evidence pertaining to factors which might affect the examined associations, specific variables collected for different time-points in the life-course of children and their mothers were included as possible confounders in the regression models [27,29,52]. More specifically, confounding variables related to children’s early life included the three categories of birth weight (i.e., SGA, appropriate for gestational age (AGA) and LGA) and growth velocity from birth to the first 6 months of life (i.e., normal growth, poor growth, and rapid growth); infant feeding history (i.e., exclusive breastfeeding, exclusive formula, mixed feeing); infant’s age of solid foods introduction, in months; and gestational age (in months). Regarding variables that can also serve as confounders and were collected from children in their pre-adolescence, these included their average dietary energy intake (in Kcal/day) and their daily moderate-to-vigorous physical activity (in minutes/day). Regarding confounding variables related to parents, these included mother’s and father’s age (in years), three categories of mother’s and father’s education (i.e., <9 years, 9–12 years, >12 years), mother’s age at birth (in years), and father’s BMI (in kg/m2).
3. Results
3.1. Socio-Demographic and Anthropometric Characteristics
Table 1 presents the socio-demographic characteristics of the study participants in the Healthy Growth Study. Of the 2519 children included, 50.4% were girls. The mean age of children was 11.2 ± 0.67 years.

Table 1.
Socio-demographic descriptive characteristics of study participants in the Healthy Growth Study.
Table 2 presents the anthropometric characteristics of study participants. Girls were taller (p = 0.010) and had a wider hip circumference (p = 0.022) than boys. Boys had higher BMIs and waist-to-hip ratios than girls, i.e., 20.5 ± 3.89 vs. 20.1 ± 3.69 for BMI (p = 0.010), and 87.0 ± 5.71 vs. 85.4 ± 7.28 (p < 0.001) for waist-to-hip ratios, respectively. No other statistically significant gender differences were observed.

Table 2.
Anthropometric characteristics of study participants in the Healthy Growth Study.
3.2. Perinatal Characteristics
Perinatal characteristics of study participants are presented in Table 3. Most children (81.1%) had a gestational age (at birth) of 37 weeks or over, with 80.7% born with an appropriate gestational weight (i.e., AGA), while 7.4% were LGA. With regards to growth velocity, 32.8% of children had a rapid growth and 56% of children had a normal growth. With regards to factors relating to the mother, 15% of mothers had miscarried prior to the child’s pregnancy, and most mothers did not drink alcohol (98.1%), smoke (84.1%), have high blood pressure (94.6%), or have gestational diabetes (95.1%) during pregnancy.

Table 3.
Perinatal descriptive characteristics of study participants in the Healthy Growth Study.
For factors related to children after birth, most children were born by vaginal delivery (71.6%), most children were mixed fed via a combination of breastfeeding and formula (72.7%) and commenced solid foods at 5–6 months of age (66.6%). Regarding mothers, 99.0% reported not drinking alcohol or smoking during breastfeeding, however 39.2% of mothers smoked as the child grew up, and 45.1% of parents smoked at their child’s pre-adolescence. With regards to gender differences, girls had a higher rapid growth velocity than boys 36.3% vs. 29.1%, p = 0.002. Additionally, girls were more likely to have mothers who reported they did not know of their gestational diabetes history during pregnancy, 3.2% vs. 2.8%, p = 0.033.
3.3. Weight Status Prevalence
The prevalence of underweight, healthy weight, overweight, and obesity among participants in the study is presented in Figure 1. The prevalence of overweight and obesity in the total sample of school children was 30.3% and 11.5%, respectively. The prevalence rates of overweight and obesity were higher in boys compared to girls, with 31.0% vs. 29.5% (although not statistically significant) for overweight, and 13.8% vs. 9.3% (p <0.05) for obesity, respectively. The prevalence of underweight, healthy weight, overweight, and obesity among mothers at the various time-points categories are available in Supplementary Figure S1.
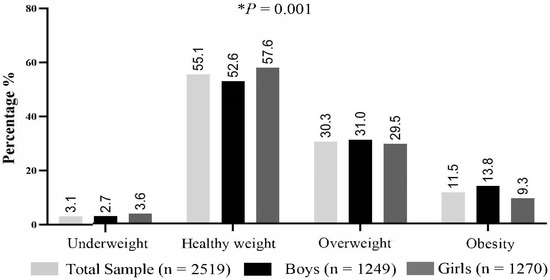
Figure 1.
Child weight status in sample population. Abbreviations: %, percentage; n, sample size. Child weight status as defined by the international body mass index cut-offs for thinness, overweight and obesity (IOTF) [44,53]. The asterisk (*) indicates statistically significant difference between boys and girls.
3.4. Associations between Maternal Weight Status and Child Obesity
The logistic regressions models that assessed the associations between maternal weight status and child obesity status were stratified by gender, while the analyses were also adjusted for a number of potential confounders. The results from the logistic regressions models are presented as odds ratios and 95% confidence intervals in Table 4.

Table 4.
Adjusted a odds ratios for the association of maternal weight status and child obesity status, stratified by sex.
According to the observed associations, maternal pre-pregnancy overweight was associated with an increased likelihood of child obesity, by 1.90 (1.34, 2.70) in the total sample, 1.65 (1.00, 2.72) in boys, and 2.43 (1.45, 4.08) in girls. However significant and even stronger associations were also found with maternal pre-pregnancy obesity, which increased the likelihood of child obesity by 4.16 (2.47, 7.02) in the total sample, 3.55 (1.68, 7.02) in boys, and 5.60 (2.60, 12.08) in girls.
For gestational weight gain, maternal weight gain above the IOM recommendations, was associated with an increased likelihood of childhood obesity by 1.50 (1.08, 2.08) in the total sample and 1.66 (1.07, 3.56) in boys, while no significant associations were observed in girls. Similarly, maternal obesity at their child’s pre-adolescence was associated with an increased likelihood of child obesity by 3.34 (2.29, 4.87) in the total sample, 3.14 (1.89, 5.42) in boys, and 4.18 (2.31, 7.56) in girls.
The highest odds ratios in these associations with child obesity were observed for mothers who were affected by overweight or obesity in each time-point, and more specifically for mothers affected by obesity prior to conception, followed by mothers who were affected by obesity at their child’s pre-adolescence.
3.5. Associations between Recommendation-Based Maternal Weight Trajectory Groups and Child Obesity
The odds ratios and 95% CIs that reflect the associations between recommendation-based maternal weight status trajectories and child obesity status are presented in Figure 2, after adjusting for a number of potential confounders. According to these findings, there were statistically significant positive associations observed for mothers with weight status above the WHO (2000) BMI thresholds or IOM (2009) cut-off points, healthy thresholds in each of the examined time-points, thus showing an increased likelihood for child obesity, i.e., by 3.1 times in the total sample (1.95, 4.85). 2.4 times in boys (1.28, 4.63), and 4.6 times in girls (2.34, 9.12).
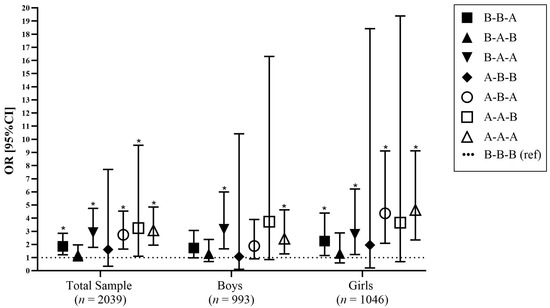
Figure 2.
Adjusted associations of maternal weight status trajectories and childhood obesity. Markers indicate odds ratios and lines indicate 95% CIs, adjusted for confounders. * p < 0.05. Abbreviations: B, below (includes maternal weight status as healthy or below the WHO classification of adults according to BMI [45], or Institute of Medicine (IOM) recommendations [46]); A, above (includes maternal weight status above the WHO classification of adults according to BMI thresholds (overweight/obese) [45] or IOM recommendations [46]). Maternal weight trajectories groups have been combined based on recommended cut-off points for weight status at the following time-points pre-pregnancy and during pregnancy (gestational weight gain [based on the IOM (2009) recommendations [46]]), and, at their child’s pre-adolescence, combined into 8 maternal weight trajectory groups; OR, Odds Ratio; 95%CI, 95% Confidence Interval.
Overall, girls were found to have the highest odds ratio in the above–above–above category (4.62 [2.34, 9.12]). There were specific time-points that, when combined, could also create a higher risk for childhood obesity, compared to children with mothers within the healthy ranges or reference ranges. Specifically, there were higher odds ratios for children born to mothers who were above thresholds for at least one of the maternal time-points investigated. More specifically, for mothers who were above the healthy threshold at their child’s pre-adolescence, the likelihood for childhood obesity increased by 1.9 times in the total sample (1.21, 2.84), 1.7 times in boys (0.98, 3.06), and 2.3 times in girls (1.16, 4.39).
Furthermore, when maternal weight status was above during pregnancy and at their child’s pre-adolescence, the likelihood of childhood obesity increased by 2.9 times in the total sample (1.78, 4.74), 3.2 times in boys (1.67, 5.99), and 2.8 times in girls (1.23, 6.21). For mothers above the healthy threshold before pregnancy and at their child’s pre-adolescence, the likelihood of childhood obesity increased by 2.7 times in the total sample (1.65, 4.54), 1.8 times in boys (0.91, 3.90) and 4.4 times in girls (2.09, 9.12).
4. Discussion
To our knowledge this is the first study to test associations between pre-pregnancy maternal overweight/obesity, excessive gestational weight gain, and maternal overweight/obesity at their child’s pre-adolescence with child obesity. More specifically, the present study reported strong associations and the highest odds ratios for mothers who were above (the WHO (2000) BMI thresholds or IOM (2009) cut-off points) recommendation-based weight status thresholds in each time-point and child obesity. In addition, when these time-points were combined, the highest rates of child obesity were found among women who were above the recommendation-based thresholds in two or all three of the time-points combined, where there was a three-fold increase in the likelihood of child obesity in the total sample. The findings from this study suggest that maternal overweight and obesity across all time-points examined in this study are important risk factors for the development of child and adolescent obesity.
When we compared maternal weight status in association with childhood obesity, we found the highest odds ratios for children, with mothers who were affected by obesity before pregnancy (a four-fold likelihood). This finding is consistent with the results of previous studies, including big data analytics, exploring the association between maternal pre-pregnancy obesity and childhood obesity [29,54,55,56,57]. The study by Leonard, Rasmussen, King, and Abrams [41] examined similar maternal exposures, i.e., pre-pregnancy, gestational weight gain and postpartum weight retention and their associations with child obesity. They found pre-pregnancy overweight/obesity to be associated with more than a two-fold increase in the risk in childhood obesity (aged 6–19 years) [41]. The presence of overweight and obesity prior to pregnancy can be influenced by a number of factors, including nutritional status, increase of fat accumulation, inflammation, and maternal genetic pre-disposition [55,58,59]. Obesity is an inflammatory state associated with insulin resistance and increasing blood glucose levels. After conception, this exposes the growing foetus to an over-nutrition environment in utero [55,58], resulting in a cascade of changes to foetal developmental programming and subsequently increasing the risk of obesity in offspring [29,56].
Excessive gestational weight gain is considered one of the risk factors for child obesity [35,40,60,61,62]. The present study found that children and adolescents were 1.5 times more likely to be affected by obesity if their mothers gained weight above the IOM recommendations during pregnancy. Similar findings were highlighted by Baran et al. [63] who found that mothers’ gestational weight gain was higher in children affected by obesity at ages 7–11, compared to children of healthy weight status. Our findings also support the literature that has previously compared the effect of maternal pre-pregnancy overweight or obesity and excessive gestational weight gain, showing that pre-pregnancy weight status is a stronger predictor for child obesity [29,57]. According to models and hypotheses that attempt to interpret the early origins of obesity and chronic disease, both the presence of maternal pre-pregnancy overweight/obesity and excessive gestational weight gain shape an intrauterine risk environment for the growing foetus, influencing physiological and metabolic adaptions, which can negatively affect the long term health of offspring [64]. These adaptions occur in response to a derivative environment, and their primary aim is to increase the survival of the growing foetus [59]. As postulated by the Thrifty Phenotype hypothesis, these adaptions, or changes to the in-utero programming, increases the foetus capacity to store calories, and therefore increase the likelihood of overweight and obesity in later life and as part of an environment with adequate food availability [59].
The presence of maternal overweight and obesity during childhood has been reported as a risk factor for child obesity [39,65,66,67]. In this context, the present study also showed that maternal overweight/obesity at their child’s pre-adolescence was associated with high odds of child and adolescent obesity, second to those found for the pre-pregnancy. The retrospective cohort study by Vehapoglu, Goknar, Turel, Torun, and Ozgurhan [67] found a 4-fold increase in the likelihood of child obesity when mothers were also affected by obesity. Similarly, a prospective cohort study by Kjaer, Faurholt-Jepsen, Medrano, Elwan, Mehta, Christensen, and Wojcicki [66] reported that obesity in mothers 5 years post-partum was associated with child obesity at 9 years. Mothers are more likely to be the primary caregiver of their child, thus having a key role in influencing the energy-balance-related behaviours of their children [39]; this includes influence over food type and choice, the promotion of physical activity, sleep patterns, screen time, and other lifestyle choices [12,21,27,48]. The factors contributing to the increased weight status of mothers would also have greater influence over the child’s weight status, hence increasing the likelihood of child obesity [37,68]. With mothers affected by overweight and obesity likely creating and sustaining the obesogenic environment, this reinforces the Maternal Resource Hypothesis [69] and further delineates this as a strong risk factor for child and adolescent obesity [70]. This further highlights the importance of maternal weight status on their children’s weight status and the risk of childhood obesity and related comorbidities [71].
Most of the current literature has examined the effect of maternal overweight and obesity on child obesity at independent time-points, however there is scarce evidence combinedly examining maternal weight status in different time-points. The present study showed a cumulative positive association when combining maternal overweight and excess weight gain in critical time-points with the likelihood of child obesity, observing the highest odds ratios when there were any two and especially three time-points with maternal weight status above the recommendation-based weight status thresholds combined. The multi-etiological/dual-determination model of child obesity could explain these findings, where genetic and perinatal influences meet the obesogenic environment, all influencing cues of overeating [9,72].
This study further highlights the importance of maternal weight status before, during and after pregnancy on the risk of childhood obesity and places special emphasis on the transitioning of children to adolescence (i.e., on the life stage of pre-adolescence). For children and adolescents, the age of obesity onset is an important factor influencing long-term health [14,15,16]. More specifically, there are important hormonal (i.e., oestradiol and gonadotropin-releasing hormone) [73], and biomarker changes (i.e., serum lipids, glucose, and insulin levels), particularly in girls [74,75,76], occurring in this transitional stage, which may have a stronger influence on metabolism compared to current eating behaviours. With overweight and obesity during childhood, if an individual’s BMI further increases during the pre-adolescence stage, the risk of poor health outcomes is strengthened, and it becomes a further risk if this increase continues into the adolescence [16,77,78]. There are also other environmental changes occurring during this transitional stage, where the sphere of the obesogenic environment expands to social influences [79]. Obesity as well as overweight have long-lasting adverse physical, mental, and emotional effects on children as they move into adolescence and then to adulthood [13]. Previous intervention studies have demonstrated a decrease in the risk of obesity in adolescence and adulthood when there is a reduction in body weight prior to the pre-adolescence stage [16].
In order to curb the prevalence of obesity for both mothers and children, this study provides indications on particular stages throughout the maternal life-course where interventions should take place. For the time-point of pre-conception, it is recommended that future mothers are supported through an integrated approach, including planning and preparation, which should include raising awareness about the importance of a healthy weight status and the impact of weight status above the recommendations on offspring [80]. Primary health, medical practitioners and allied health professionals should aim to support women planning pregnancies to achieve an appropriate body weight prior to conception, on the importance of physical activity, and to provide nutrition education appropriate for both mothers and children [81]. During pregnancy, the immediate environment around the expecting mother grows to a larger network of family and health professionals, who should provide appropriate support to maintain/achieve healthy weight gain throughout pregnancy, i.e., within the IOM recommendations [80,81]. It is equally important to support mothers to achieve a healthy body weight post-partum and manage their weight as their child grows up [82,83]. It is also vitally important to target families as a whole, since a recent umbrella review showed that family interventions that include nutrition and physical activity education are quite successful in improving health outcomes for children [84].
Our findings should be interpreted in light of this study’s strengths and limitations. Regarding strengths, the Healthy Growth Study provides sufficient representativeness, as it was the first large-scale epidemiological study covering the central, northern, southern, and western parts of the Greek territory, and varying social–demographic groups [27]. The sample used pre-adolescent children, which is a pivotal age as it is representative of a transition life stage from childhood to adolescence [79]. To avoid the inaccurate reporting of self-reported data, the Healthy Growth Study used trained researchers and standardised procedures to measure and collect anthropometric data, thus increasing the accuracy of determined weight status among participants. In addition, the current study reported associations that were independent of the confounding effect of third factors. The final strength is the use of longitudinal trajectories for maternal weight status across the life-course which is a recently reported approach in the examination of child obesity [41]; this study is the first to combine not only pre-pregnancy maternal weight status and gestational weight gain, but also maternal weight status at the time-point of their child’s pre-adolescence, providing a comprehensive assessment of the perinatal and obesogenic factors affecting the development of child obesity.
This study also has limitations, with its cross-sectional design representing its main limitation, as it cannot support causality in the observed associations. In addition, and although these findings are also not generalisable to other countries/populations around the world, it is important to note that the prevalence of overweight and obesity in Greece is one of the highest in the world, demonstrating the importance of determining the maternal, perinatal, and environmental factors in this population. Another limitation also lies in the fact that mothers were asked to provide information retrospectively for a period of years prior to the study i.e., pre-pregnancy, which could increase the risk of recall bias and cause a misclassification of weight status and therefore maternal weight status trajectories.
5. Conclusions
The present study provides a clear indication that the presence of maternal obesity in different life stages and specifically before pregnancy, during pregnancy (gestational weight gain), and at the child’s pre-adolescence, as important risk factors for the development of childhood obesity. Future birth cohort studies are needed to thoroughly examine these associations and identify the most critical time-points of maternal weight status and their effect on the development of child obesity. Health practitioners and policy makers should ensure future mothers are targeted early, while continuous messaging should be provided to pregnant women and mothers of children throughout their life-course on the importance of healthy weight maintenance not only for themselves, but for their children too.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15214602/s1, Figure S1: Maternal weight status across the time-points; pre-pregnancy, gestational weight gain, and at their child’s pre-adolescence; Figure S2: Prevalence of childhood obesity, across recommendation-based maternal weight trajectory groups, stratified by sex; Table S1: Behavioural descriptive characteristics of study participants; Table S2: Crude odds ratios for the association of maternal weight status and child obesity status, stratified by sex; Table S3: Crude odds ratios for the association of recommendation-based maternal weight trajectory groups and child obesity status, stratified by sex; Table S4: Adjusted a odds ratios for the association of recommendation-based maternal weight trajectory groups and child obesity status, stratified by sex.
Author Contributions
Conceptualization, G.M.; methodology, A.M., G.M. and K.S.; validation, G.M.; formal analysis, A.M.; writing—original draft preparation, A.M.; writing—review and editing, A.M., K.S., N.M., E.K., C.A.A., Y.M. and G.M.; visualization, A.M.; supervision, G.M. and K.S.; project administration, A.M. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of La Trobe University Human Research Ethics Committee (HEC22199 and 29 July 2022) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank members of the Healthy Growth Study Group for their valuable contribution to the completion of the study. Healthy Growth Study Group: (i) Harokopio University Research Team/Department of Nutrition and Dietetics: Yannis Manios (Coordinator), George Moschonis (Project Manager), Katerina P. Skenderi, Evangelia Grammatikaki, Odysseas Androutsos, Sofia Tanagra, Alexandra Koumpitski, Paraskevi-Eirini Siatitsa, Anastasia Vandorou, Aikaterini-Efstathia Kyriakou, Vasiliki Dede, Maria Kantilafti, Aliki-Eleni Farmaki, Aikaterini Siopi, Sofia Micheli, Louiza Damianidi, Panagiota Margiola, Despoina Gakni, Vasiliki Iatridi, Christina Mavrogianni, Kelaidi Michailidou, Aggeliki Giannopoulou, Efstathoula Argyri, Konstantina Maragkopoulou, Maria Spyridonos, Eirini Tsikalaki, Panagiotis Kliasios, Anthi Naoumi, Konstantinos Koutsikas, Katerina Kondaki, Epistimi Aggelou, Zoi Krommyda, Charitini Aga, Manolis Birbilis, Ioanna Kosteria, Amalia Zlatintsi, Elpida Voutsadaki, Eleni-Zouboulia Papadopoulou, Zoi Papazi, Maria Papadogiorgakaki, Fanouria Chlouveraki, Maria Lyberi, Nora Karatsikaki-Vlami, Eva Dionysopoulou, Efstratia Daskalou. (ii) Aristotle University of Thessaloniki/Department of Physical Education and Sports Science: Vassilis Mougios, Anatoli Petridou, Konstantinos Papaioannou, Georgios Tsalis, Ananis Karagkiozidis, Konstantinos Bougioukas, Afroditi Sakellaropoulou, Georgia Skouli. (iii) University of Athens/Medical School: George P. Chrousos, Maria Drakopoulou, Evangelia Charmandari, Panagiota Pervanidou.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; ter Horst, K.W.; Serlie, M.J. The pathogenesis of obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 March 2023).
- Eurostat. Body Mass Index (BMI) by Sex, Age and Educational Attainment Level. 2022. Available online: https://ec.europa.eu/eurostat/databrowser/view/HLTH_EHIS_BM1E__custom_1162105/bookmark/table?lang=en&bookmarkId=6f21bd9e-cbe6-4467-9821-2050435af363 (accessed on 18 October 2022).
- Tsolakis, C.; Cherouveim, E.D.; Skouras, A.Z.; Antonakis-Karamintzas, D.; Czvekus, C.; Halvatsiotis, P.; Savvidou, O.; Koulouvaris, P. The Impact of Obesity on the Fitness Performance of School-Aged Children Living in Rural Areas—The West Attica Project. Int. J. Environ. Res. Public Health 2022, 19, 11476. [Google Scholar] [CrossRef]
- World Health Organization. Prevalence of Obesity Among Adults, BMI ≥ 30, Crude; Estimates by Country. 2016. Available online: https://apps.who.int/gho/data/node.main.BMI30C?lang=en (accessed on 18 October 2022).
- World Health Organization. Prevalence of Overweight among Adults, BMI ≥ 25, Age-Standardized; Estimates by Country. 2016. Available online: https://apps.who.int/gho/data/view.main.CTRY2430A?lang=en (accessed on 18 October 2022).
- World Health Organization. Prevalence of Obesity among Children and Adolescents, BMI>+2 Standard Deviation above the Median, Crude; Estimates by Country, among Children Aged 5–19 Years. 2016. Available online: https://apps.who.int/gho/data/view.main.BMIPLUS2C05-19v (accessed on 18 October 2022).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.H. Genetic susceptibility to the “obesogenic” environment: The role of eating behavior in obesity and an appetite for change. Am. J. Clin. Nutr. 2018, 108, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Wahlqvist, M.L.; Krawetz, S.A.; Rizzo, N.S.; Dominguez-Bello, M.G.; Szymanski, L.M.; Barkin, S.; Yatkine, A.; Waterland, R.A.; Mennella, J.A.; Desai, M.; et al. Early-life influences on obesity: From preconception to adolescence. Ann. N. Y. Acad. Sci. 2015, 1347, 1–28. [Google Scholar] [CrossRef]
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary Patterns and Childhood Obesity Risk: A Systematic Review. Obes. Child. 2020, 16, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.; Egger, G.; Raza, F. Dissecting Obesogenic Environments: The Development and Application of a Framework for Identifying and Prioritizing Environmental Interventions for Obesity. Prev. Med. 1999, 29, 563–570. [Google Scholar] [CrossRef]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef]
- Baker, J.L.; Olsen, L.W.; Sørensen, T.I.A. Childhood Body-Mass Index and the Risk of Coronary Heart Disease in Adulthood. N. Engl. J. Med. 2007, 357, 2329–2337. [Google Scholar] [CrossRef]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- Weihrauch-Blüher, S.; Schwarz, P.; Klusmann, J.-H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 2019, 92, 147–152. [Google Scholar] [CrossRef]
- Börnhorst, C.; Russo, P.; Veidebaum, T.; Tornaritis, M.; Molnár, D.; Lissner, L.; Mårild, S.; De Henauw, S.; Moreno, L.A.; Floegel, A.; et al. The role of lifestyle and non-modifiable risk factors in the development of metabolic disturbances from childhood to adolescence. Int. J. Obes. 2020, 44, 2236–2245. [Google Scholar] [CrossRef] [PubMed]
- Güngör, N.K. Overweight and obesity in children and adolescents. J. Clin. Res. Pediatr. Endocrinol. 2014, 6, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Saelens, B.E.P.; Sallis, J.F.P.; Frank, L.D.P.; Couch, S.C.P.R.D.; Zhou, C.P.; Colburn, T.P.; Cain, K.L.M.A.; Chapman, J.M.; Glanz, K.P.M.P.H. Obesogenic Neighborhood Environments, Child and Parent Obesity. Am. J. Prev. Med. 2012, 42, e57–e64. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.O.; Power, T.G.; O’Connor, T.M.; Fisher, J.O.; Micheli, N.E.; Papaioannou, M.A. Maternal feeding style and child weight status among Hispanic families with low-income levels: A longitudinal study of the direction of effects. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 30. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Birbilis, M.; Moschonis, G.; Birbilis, G.; Mougios, V.; Lionis, C.; Chrousos, G.P. Childhood Obesity Risk Evaluation based on perinatal factors and family sociodemographic characteristics: CORE Index. Eur. J. Pediatr. 2013, 172, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Androutsos, O.; Moschonis, G.; Birbilis, M.; Maragkopoulou, K.; Giannopoulou, A.; Argyri, E.; Kourlaba, G. Criterion validity of the Physical Activity Questionnaire for Schoolchildren (PAQ-S) in assessing physical activity levels: The Healthy Growth Study. J. Sports Med. Phys. Fit. 2013, 53, 502–508. [Google Scholar]
- Pavlidou, E.; Papandreou, D.; Taha, Z.; Mantzorou, M.; Tyrovolas, S.; Kiortsis, D.N.; Psara, E.; Papadopoulou, S.K.; Yfantis, M.; Spanoudaki, M.; et al. Association of Maternal Pre-Pregnancy Overweight and Obesity with Childhood Anthropometric Factors and Perinatal and Postnatal Outcomes: A Cross-Sectional Study. Nutrients 2023, 15, 3384. [Google Scholar] [CrossRef]
- Ino, T. Maternal smoking during pregnancy and offspring obesity: Meta-analysis. Pediatr. Int. 2010, 52, 94–99. [Google Scholar] [CrossRef]
- Magriplis, E.; Farajian, P.; Panagiotakos, D.B.; Risvas, G.; Zampelas, A. Maternal smoking and risk of obesity in school children: Investigating early life theory from the GRECO study. Prev. Med. Rep. 2017, 8, 177–182. [Google Scholar] [CrossRef]
- Santos da Silva, L.L.; Malta, M.B.; Lourenço, B.H.; Mosquera, P.S.; Araújo Damasceno, A.A.; Neves, P.A.R.; Cardoso, M.A. Maternal pre-pregnancy body mass index, gestational weight gain and child weight during the first 2 years of life in an Amazonian birth cohort. J. Hum. Nutr. Diet. 2023, 36, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Birbilis, M.; Moschonis, G.; Mougios, V.; Manios, Y. Obesity in adolescence is associated with perinatal risk factors, parental BMI and sociodemographic characteristics. Eur. J. Clin. Nutr. 2013, 67, 115–121. [Google Scholar] [CrossRef]
- NCD risk factor collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Patro Golab, B.; Gaillard, R.; Jaddoe, V.W.V.; Charles, M.-A.; Heude, B.; Chatzi, L.; Georgiu, V.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Mannino, A.; Sarapis, K.; Moschonis, G. The Effect of Maternal Overweight and Obesity Pre-Pregnancy and During Childhood in the Development of Obesity in Children and Adolescents: A Systematic Literature Review. Nutrients 2022, 14, 5125. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.d.S.; Matijasevich, A.; Barros, A.J.D.; Menezes, A.M.B.; Schneider, B.C.; Hartwig, F.P.; Barros, F.C.; Wehrmeister, F.C.; Goncalves, H.; Santos, I.S.; et al. Influence of maternal pre-pregnancy nutritional status on offspring anthropometric measurements and body composition in three Brazilian Birth Cohorts. Public Health Nutr. 2021, 24, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.C.; Lanza, H.I.; Anglin, M.D. Trajectory of Adolescent Obesity: Exploring the Impact of Prenatal to Childhood Experiences. J. Child Fam. Stud. 2014, 23, 1090–1101. [Google Scholar] [CrossRef][Green Version]
- Yoshida, S.; Kimura, T.; Noda, M.; Takeuchi, M.; Kawakami, K. Association of maternal prepregnancy weight and early childhood weight with obesity in adolescence: A population-based longitudinal cohort study in Japan. Pediatr. Obes. 2020, 15, e12597. [Google Scholar] [CrossRef]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef]
- Lau, E.Y.; Junxiu, L.; Archer, E.; McDonald, S.M.; Jihong, L. Maternal Weight Gain in Pregnancy and Risk of Obesity among Offspring: A Systematic Review. J. Obes. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Castillo, H.; Santos, I.S.; Matijasevich, A. Relationship between maternal pre-pregnancy body mass index, gestational weight gain and childhood fatness at 6-7 years by air displacement plethysmography. Matern. Child Nutr. 2015, 11, 606–617. [Google Scholar] [CrossRef]
- Téllez-Rojo, M.M.; Trejo-Valdivia, B.; Roberts, E.; Muñoz-Rocha, T.V.; Bautista-Arredondo, L.F.; Peterson, K.E.; Cantoral, A. Influence of post-partum BMI change on childhood obesity and energy intake. PLoS ONE 2019, 14, e0224830. [Google Scholar] [CrossRef]
- Gibson, L.; Allen, K.; Byrne, S.; Clark, K.; Blair, E.; Davis, E.; Zubrick, S. Childhood Overweight and Obesity: Maternal and Family Factors. J. Child Fam. Stud. 2016, 25, 3236–3246. [Google Scholar] [CrossRef]
- Xu, R.Y.; Zhou, Y.Q.; Zhang, X.M.; Wan, Y.P.; Gao, X. A two-year study of parental obesity status and childhood obesity in China. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Kaar, J.L.; Crume, T.; Brinton, J.T.; Bischoff, K.J.; McDuffie, R.; Dabelea, D. Maternal obesity, gestational weight gain, and offspring adiposity: The exploring perinatal outcomes among children study. J. Pediatr. 2014, 165, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.A.; Rasmussen, K.M.; King, J.C.; Abrams, B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am. J. Clin. Nutr. 2017, 106, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Tanagra, S.; Vandorou, A.; Kyriakou, A.E.; Dede, V.; Siatitsa, P.E.; Koumpitski, A.; Androutsos, O.; Grammatikaki, E.; Kantilafti, M.; et al. Social, economic and demographic correlates of overweight and obesity in primary-school children: Preliminary data from the Healthy Growth Study. Public Health Nutr. 2010, 13, 1693–1700. [Google Scholar] [CrossRef]
- Manios, Y.; Kafatos, A.G.; Markakis, G. Physical Activity of 6-Year-Old Children: Validation of Two Proxy Reports. Pediatr. Exerc. Sci. 1998, 10, 176–188. [Google Scholar] [CrossRef]
- Cole, T.J. Establishing A Standard Definition For Child Overweight And Obesity Worldwide: International Survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 894, pp. i–xii, 1–268. [Google Scholar]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines. 2009. Available online: https://nap.nationalacademies.org/resource/12584/Report-Brief---Weight-Gain-During-Pregnancy.pdf (accessed on 14 August 2022).
- Gadzik, J. “How much should I weigh?”—Quetelet’s equation, upper weight limits, and BMI prime. Conn. Med. 2006, 70, 81–88. [Google Scholar]
- Moschonis, G.; Kalliora, A.C.; Costarelli, V.; Papandreou, C.; Koutoukidis, D.; Lionis, C.; Chrousos, G.P.; Manios, Y. Identification of lifestyle patterns associated with obesity and fat mass in children: The Healthy Growth Study. Public Health Nutr. 2014, 17, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Karatzi, K.; Polychronopoulou, M.C.; Manios, Y. Waist circumference, trunk and visceral fat cutoff values for detecting hyperinsulinemia and insulin resistance in children: The Healthy Growth Study. Eur. J. Nutr. 2016, 55, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Zong, X.n.; Kelishadi, R.; Litwin, M.; Hong, Y.M.; Poh, B.K.; Steffen, L.M.; Galcheva, S.V.; Herter-Aeberli, I.; Nawarycz, T.; et al. International Waist Circumference Percentile Cutoffs for Central Obesity in Children and Adolescents Aged 6 to 18 Years. J. Clin. Endocrinol. Metab. 2020, 105, e1569–e1583. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. The sensitivity and specificity of the Goldberg cut-off for EI:BMR for identifying diet reports of poor validity. Eur. J. Clin. Nutr. 2000, 54, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, N.; Roderick, P.J.; Macklon, N.S.; Alwan, N.A. Predicting childhood overweight and obesity using maternal and early life risk factors: A systematic review. Obes. Rev. 2018, 19, 302–312. [Google Scholar] [CrossRef]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Catalano, P.M.; Farrell, K.; Thomas, A.; Huston-Presley, L.; Mencin, P.; de Mouzon, S.H.; Amini, S.B. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am. J. Clin. Nutr. 2009, 90, 1303–1313. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, J.; Choi, J. Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity. Children 2022, 9, 928. [Google Scholar] [CrossRef]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The association between maternal body mass index and child obesity: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Barker, D.J.P. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Badon, S.E.; Quesenberry, C.P.; Xu, F.; Avalos, L.A.; Hedderson, M.M. Gestational weight gain, birthweight and early-childhood obesity: Between- and within-family comparisons. Int. J. Epidemiol. 2020, 49, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.; Mannan, M.; Doi, S. Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2014, 15, 338–347. [Google Scholar] [CrossRef]
- Sridhar, S.B.; Darbinian, J.; Ehrlich, S.F.; Markman, M.A.; Gunderson, E.P.; Ferrara, A.; Hedderson, M.M. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am. J. Obstet. Gynecol. 2014, 211, e251–e258. [Google Scholar] [CrossRef]
- Baran, J.; Weres, A.; Czenczek-Lewandowska, E.; Leszczak, J.; Kalandyk-Osinko, K.; Łuszczki, E.; Sobek, G.; Mazur, A. Excessive Gestational Weight Gain: Long-Term Consequences for the Child. J. Clin. Med. 2020, 9, 3795. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Kato, R.; Kubota, M.; Yasui, Y.; Hayashi, Y.; Higashiyama, Y.; Nagai, A. Retrospective tracking of young obese children back to birth in Japan: Special attention to the relationship with parental obesity. Asia Pac. J. Clin. Nutr. 2014, 23, 641–650. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Faurholt-Jepsen, D.; Medrano, R.; Elwan, D.; Mehta, K.; Christensen, V.B.; Wojcicki, J.M. Higher Birthweight and Maternal Pre-pregnancy BMI Persist with Obesity Association at Age 9 in High Risk Latino Children. J. Immigr. Minor. Health 2019, 21, 89–97. [Google Scholar] [CrossRef]
- Vehapoglu, A.; Goknar, N.; Turel, O.; Torun, E.; Ozgurhan, G. Risk factors for childhood obesity: Do the birth weight, type of delivery, and mother’s overweight have an implication on current weight status? World J. Pediatr. 2017, 13, 457–464. [Google Scholar] [CrossRef]
- Adane, A.A.; Dobson, A.; Tooth, L.; Mishra, G.D. Maternal preconception weight trajectories are associated with offsprings’ childhood obesity. Int. J. Obes. 2018, 42, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Archer, E. The childhood obesity epidemic as a result of nongenetic evolution: The maternal resources hypothesis. Mayo Clin. Proc. 2015, 90, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Jia, P. Obesogenic environment and childhood obesity. Obes. Rev. 2021, 22, e13158. [Google Scholar] [CrossRef]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Bernasconi, S. A multi-etiological model of childhood obesity: A new biobehavioral perspective for prevention? Ital. J. Pediatr. 2019, 45, 169. [Google Scholar] [CrossRef]
- Zhai, L.; Liu, J.; Zhao, J.; Liu, J.; Bai, Y.; Jia, L.; Yao, X. Association of Obesity with Onset of Puberty and Sex Hormones in Chinese Girls: A 4-Year Longitudinal Study. PLoS ONE 2015, 10, e0134656. [Google Scholar] [CrossRef]
- Altwaijri, Y.A.; Day, R.S.; Harrist, R.B.; Dwyer, J.T.; Ausman, L.M.; Labarthe, D.R. Sexual Maturation Affects Diet–Blood Total Cholesterol Association in Children: Project HeartBeat! Am. J. Prev. Med. 2009, 37, S65–S70. [Google Scholar] [CrossRef]
- Boyne, M.S.; Thame, M.; Osmond, C.; Fraser, R.A.; Gabay, L.; Taylor-Bryan, C.; Forrester, T.E. The effect of earlier puberty on cardiometabolic risk factors in Afro-Caribbean children. J. Pediatr. Endocrinol. Metab. 2014, 27, 453–460. [Google Scholar] [CrossRef]
- Moran, A.; Jacobs, D.R., Jr.; Steinberger, J.; Hong, C.-P.; Prineas, R.; Luepker, R.; Sinaiko, A.R. Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes 1999, 48, 2039–2044. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Srinivasan, S.R.; Xu, J.; Berenson, G.S. Relation of childhood obesity/cardiometabolic phenotypes to adult cardiometabolic profile: The Bogalusa Heart Study. Am. J. Epidemiol. 2012, 176 (Suppl. S7), S142–S149. [Google Scholar] [CrossRef]
- Tirosh, A.; Shai, I.; Afek, A.; Dubnov-Raz, G.; Ayalon, N.; Gordon, B.; Derazne, E.; Tzur, D.; Shamis, A.; Vinker, S.; et al. Adolescent BMI Trajectory and Risk of Diabetes versus Coronary Disease. N. Engl. J. Med. 2011, 364, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Makri, R.; Katsoulis, M.; Fotiou, A.; Kanavou, E.; Stavrou, M.; Richardson, C.; Kanellopoulou, A.; Orfanos, P.; Benetou, V.; Kokkevi, A. Prevalence of Overweight and Obesity and Associated Diet-Related Behaviours and Habits in a Representative Sample of Adolescents in Greece. Children 2022, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.; Barker, M.; Dodd, J.M.; Kumanyika, S.; Norris, S.; Steegers, E.; Stephenson, J.; Thangaratinam, S.; Yang, H. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017, 5, 65–76. [Google Scholar] [CrossRef]
- Shrestha, A.; Prowak, M.; Berlandi-Short, V.M.; Garay, J.; Ramalingam, L. Maternal Obesity: A Focus on Maternal Interventions to Improve Health of Offspring. Front. Cardiovasc. Med. 2021, 8, 696812. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; Flynn, A.C.; Relph, S.A.; O’Keeffe, M.; Poston, L. Lifestyle Interventions in Overweight and Obese Pregnant or Postpartum Women for Postpartum Weight Management: A Systematic Review of the Literature. Nutrients 2018, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; Martyni-Orenowicz, J.; Flynn, A.C.; Poston, L.; O’Keeffe, M. Can antenatal diet and lifestyle interventions influence childhood obesity? A systematic review. Matern Child Nutr. 2018, 14, e12628. [Google Scholar] [CrossRef]
- Chai, L.K.; Collins, C.; May, C.; Brain, K.; Wong See, D.; Burrows, T. Effectiveness of family-based weight management interventions for children with overweight and obesity: An umbrella review. JBI Evid. Synth. 2019, 17, 1341–1427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).